Evaluating Ice-Temperature Storage Efficacy on Volatile Compounds in Blue Honeysuckle (Lonicera caerulea L.) by Combining GC-IMS and GC-MS
Abstract
1. Introduction
2. Materials and Methods
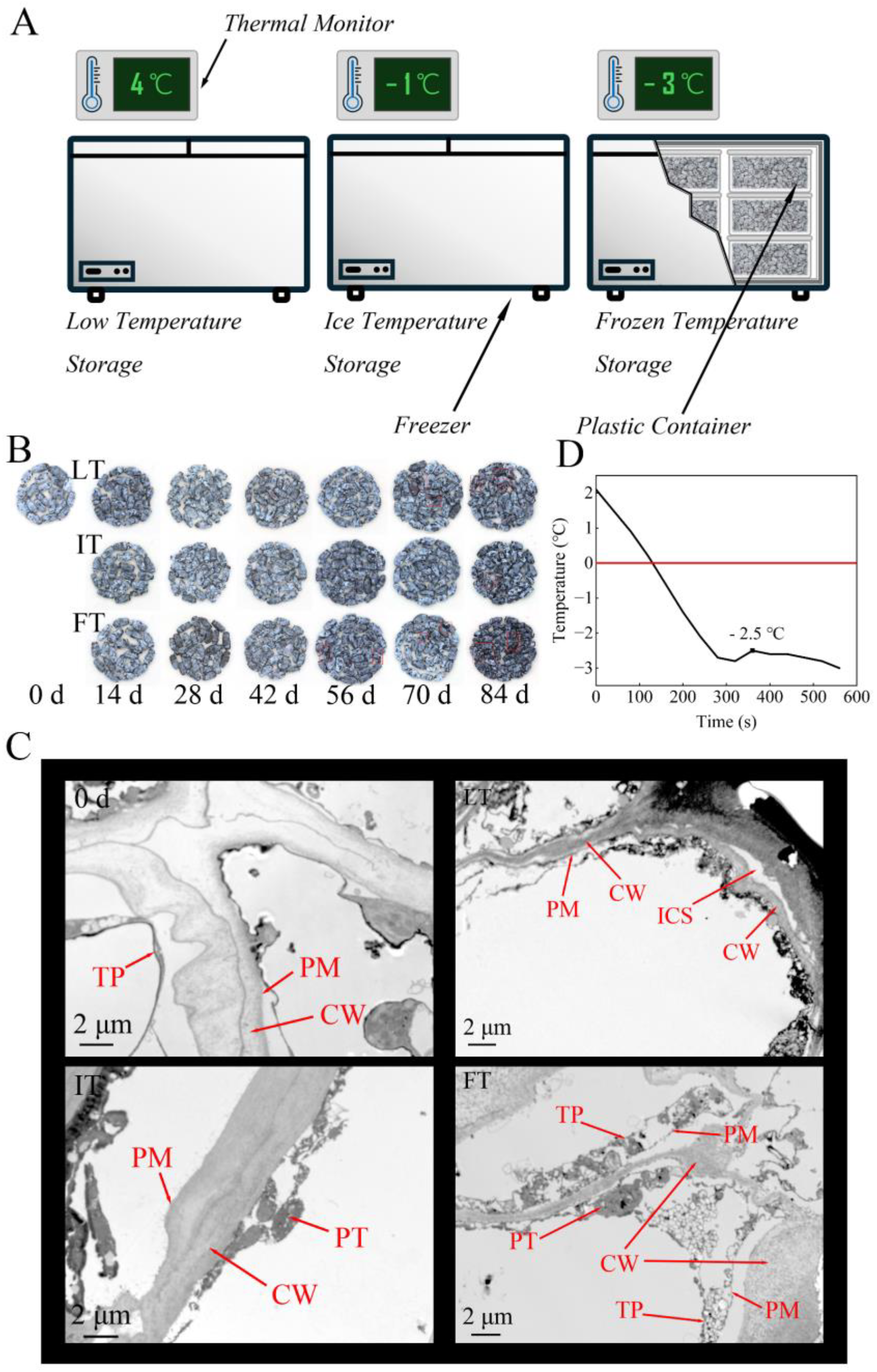
2.1. Plant Materials and Storage Conditions
2.2. Determination of Freezing Point
2.3. Determination of VOCs by GC-IMS
2.4. Determination of VOCs by GC-MS
2.5. Determination of Quality Attributes
2.6. TEM Analysis
2.7. Statistical Analysis
3. Results
3.1. Ultrastructural Analysis by TEM
3.2. Freezing Point of Blue Honeysuckle
3.3. Analysis of Quality Attributes
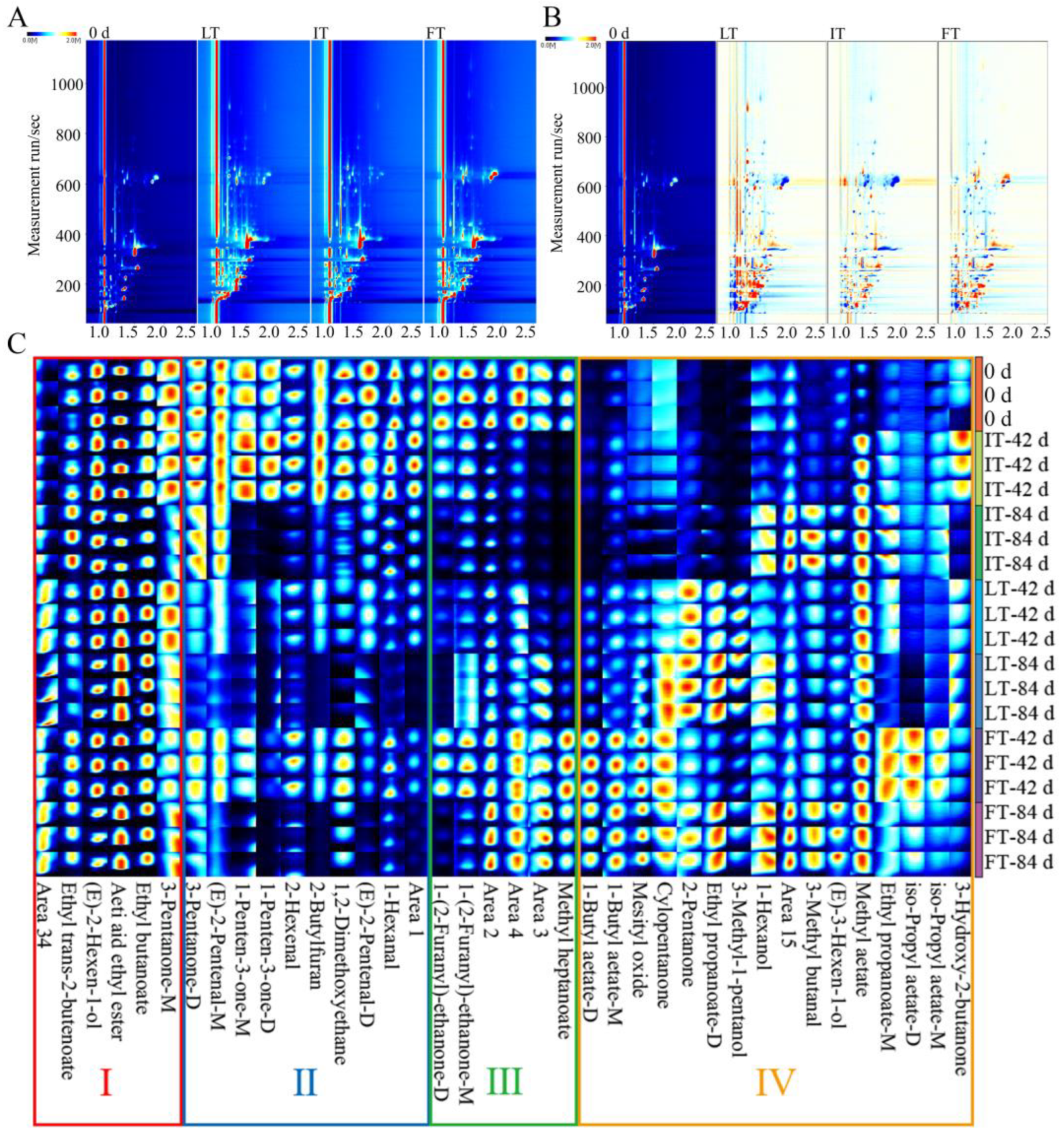
3.4. Analysis of the VOCs Under Different Storage Temperatures by GC-IMS
3.4.1. Analysis of Topographic Plots
3.4.2. Analysis of Fingerprint Spectrum
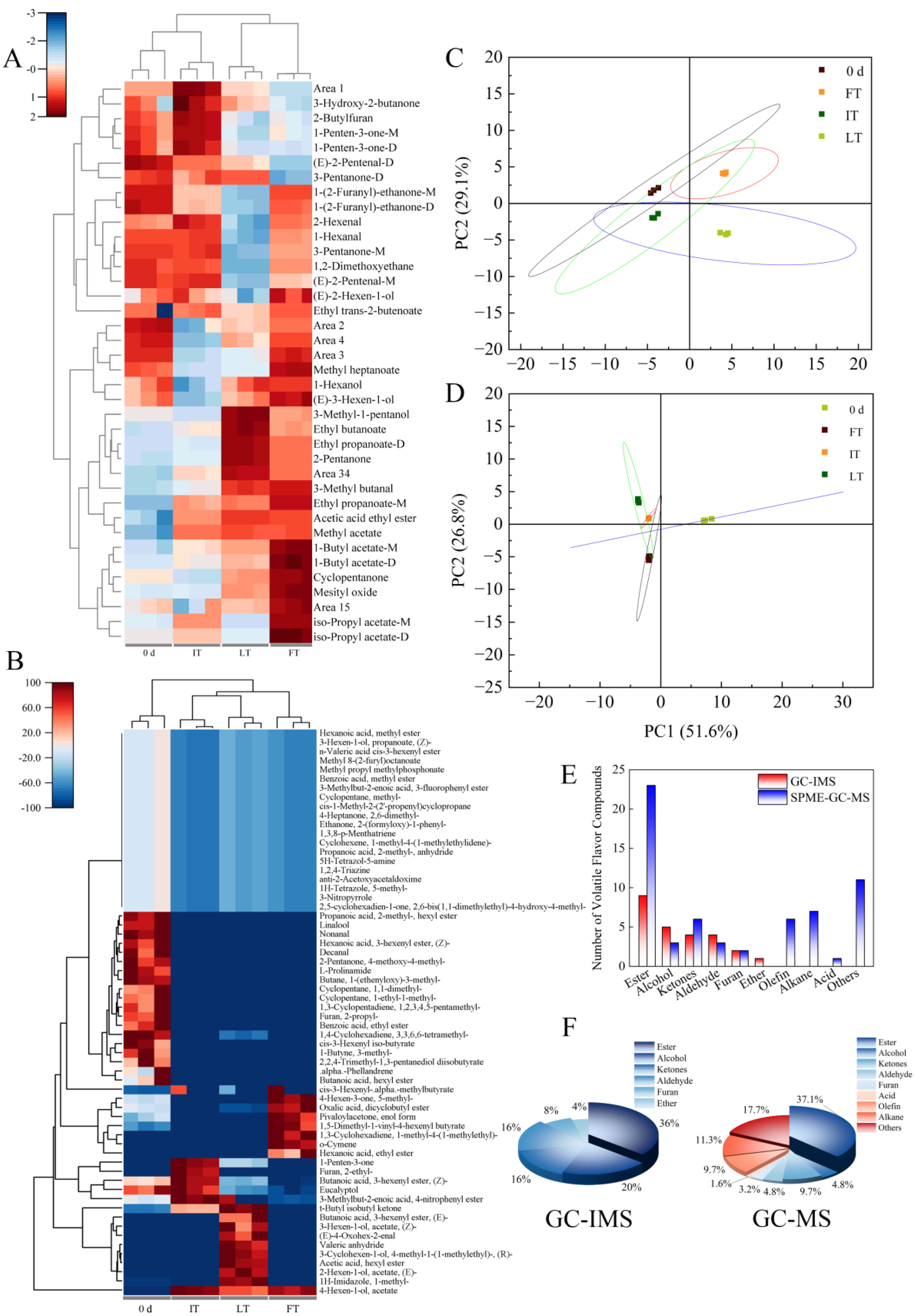
3.4.3. Principal Component Analysis
3.5. Analysis of the VOCs Under Different Storage Temperatures by GC-MS
3.6. Comparative Analysis of GC-IMS and GC-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, R.; Szakiel, A. Phytochemical characteristics and potential therapeutic properties of blue honeysuckle Lonicera caerulea L. (Caprifoliaceae). J. Herb. Med. 2019, 16, 100237. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Haskap Berries (Lonicera caerulea L.)—A Critical Review of Antioxidant Capacity and Health-Related Studies for Potential Value-Added Products. Food Bioprocess Technol. 2014, 7, 1541–1554. [Google Scholar]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S. Effect of dried powder preparation process on polyphenolic content and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L. var. kamtschatica). LWF-Food Sci. Technol. 2016, 67, 214–222. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.-J. Lonicera caerulea: An updated account of its phytoconstituents and health-promoting activities. Trends Food Sci. Technol. 2021, 107, 130–149. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.C.; Kim, J.S. In vitro Screening for Antioxidant, Antimicrobial, and Antidiabetic Properties of Some Korean Native Plants on Mt. Halla, Jeju Island. Indian J Pharm Sci 2015, 77, 668–674. [Google Scholar]
- Jin, X.-H.; Ohgami, K.; Shiratori, K.; Suzuki, Y.; Koyama, Y.; Yoshida, K.; Ilieva, I.; Tanaka, T.; Onoe, K.; Ohno, S. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2006, 82, 860–867. [Google Scholar] [CrossRef]
- Park, M.; Lee, C.; Lee, H.-J. Effects of Lonicera caerulea extract on adipocyte differentiation and adipogenesis in 3T3-L1 cells and mouse adipose-derived stem cells (MADSCs). J. Nutr. Health 2019, 52, 17–25. [Google Scholar] [CrossRef]
- Wu, S.; He, X.; Wu, X.; Qin, S.; He, J.; Zhang, S.; Hou, D.-X. Inhibitory effects of blue honeysuckle (Lonicera caerulea L) on adjuvant-induced arthritis in rats: Crosstalk of anti-inflammatory and antioxidant effects. J. Funct. Foods 2015, 17, 514–523. [Google Scholar] [CrossRef]
- Forney, C.F.; Jordan, M.A.; Pennell, K.M.; Fillmore, S. Controlled atmosphere storage impacts fruit quality and flavor chemistry of five cultivars of highbush blueberry (Vaccinium corymbosum). Postharvest Biol. Technol. 2022, 194, 112073. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Bian, S.; Hong, S.-B.; Xu, K.; Zang, Y.; Zheng, W. Melatonin improves the storage quality of rabbiteye blueberry (Vaccinium ashei) by affecting cuticular wax profile. Food Chem. X 2023, 21, 101106. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Z.; Wang, J.; Zhu, S.; Huang, D. Nitric oxide modulates sugar metabolism and maintains the quality of red raspberry during storage. Sci. Hortic. 2019, 256, 108611. [Google Scholar] [CrossRef]
- Ye, S.; Chen, J.; Cao, S.; Luo, D.; Ba, L. Thymol application delays the decline of fruit quality in blueberries via regulation of cell wall, energy and membrane lipid metabolism. Food Chem. 2024, 458, 140193. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Su, S.; Guo, K.; Wang, L.; Tang, Z.; Huo, J.; Song, H. Characterization of key aroma-active compounds in blue honeysuckle (Lonicera caerulea L.) berries by sensory-directed analysis. Food Chem. 2023, 429, 136821. [Google Scholar] [CrossRef] [PubMed]
- Kupska, M.; Chmiel, T.; Jędrkiewicz, R.; Wardencki, W.; Namieśnik, J. Comprehensive two-dimensional gas chromatography for determination of the terpenes profile of blue honeysuckle berries. Food Chem. 2014, 152, 88–93. [Google Scholar] [CrossRef]
- Liu, D.-K.; Xu, C.-C.; Guo, C.-X.; Zhang, X.-X. Sub-zero temperature preservation of fruits and vegetables: A review. J. Food Eng. 2020, 275, 109881. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; Yang, J.; Sun, Q.; Li, K.; Prakash, S.; Dong, X. Preservation strategies for processed grass carp products: Analyzing quality and microbial dynamics during chilled and ice temperature storage. Food Chem. X 2024, 23, 101428. [Google Scholar] [CrossRef]
- Zhao, H.; Shu, C.; Fan, X.; Cao, J.; Jiang, W. Near-freezing temperature storage prolongs storage period and improves quality and antioxidant capacity of nectarines. Sci. Hortic. 2018, 228, 196–203. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, W.; Wang, B.; Shen, J.; Zhao, H.; Jiang, W. Near freezing point storage compared with conventional low temperature storage on apricot fruit flavor quality (volatile, sugar, organic acid) promotion during storage and related shelf life. Sci. Hortic. 2019, 249, 100–109. [Google Scholar] [CrossRef]
- Gallegos, J.; Arce, C.; Jordano, R.; Arce, L.; Medina, L.M. Target identification of volatile metabolites to allow the differentiation of lactic acid bacteria by gas chromatography-ion mobility spectrometry. Food Chem. 2017, 220, 362–370. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Dong, W.; Tang, Y.; Hu, R.; Yu, X.; Chen, X. Characterization of the volatile flavour compounds in Yunnan Arabica coffee prepared by different primary processing methods using HS-SPME/GC-MS and HS-GC-IMS. LWF-Food Sci. Technol. 2024, 192, 115717. [Google Scholar] [CrossRef]
- Fan, C.; Shi, X.; Pan, C.; Zhang, F.; Zhou, Y.; Hou, X.; Hui, M. GC-IMS and GC/Q-TOFMS analysis of Maotai-flavor baijiu at different aging times. LWF-Food Sci. Technol. 2024, 192, 115744. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Detection and analysis of volatile flavor compounds in different varieties and origins of goji berries using HS-GC-IMS. LWF-Food Sci. Technol. 2023, 187, 115322. [Google Scholar] [CrossRef]
- Fan, X.; Xi, Y.; Zhao, H.; Liu, B.; Cao, J.; Jiang, W. Improving fresh apricot (Prunus armeniaca L.) quality and antioxidant capacity by storage at near freezing temperature. Sci. Hortic. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Qin, G.; Meng, X. Chitosan disrupts Penicillium expansum and controls postharvest blue mold of jujube fruit. Food Control 2014, 41, 56–62. [Google Scholar] [CrossRef]
- Zhou, D.; Li, R.; Zhang, H.; Chen, S.; Tu, K. Hot air and UV-C treatments promote anthocyanin accumulation in peach fruit through their regulations of sugars and organic acids. Food Chem. 2020, 309, 125726. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Sang, Y.; Ma, Y.; Guo, M.; Bai, G.; Cheng, S.; Chen, G. Influences of ice-temperature storage on cell wall metabolism and reactive oxygen metabolism in Xinjiang (Diaogan) apricot. Postharvest Biol. Technol. 2021, 180, 111614. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Zhao, L.; Shen, T.; Sun, J.; Chen, H.; Kong, Q.; Nawaz, M.A.; Bie, Z. Melon fruit sugar and amino acid contents are affected by fruit setting method under protected cultivation. Sci. Hortic. 2017, 214, 288–294. [Google Scholar] [CrossRef]
- Guo, L.; Qiao, J.; Gong, C.; Wei, J.; Li, J.; Zhang, L.; Qin, D.; Huo, J. C3G quantified method verification and quantified in blue honeysuckle (Lonicera caerulea L.) using HPLC–DAD. Heliyon 2023, 9, e14685. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, T.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Known and potential health benefits and mechanisms of blueberry anthocyanins: A review. Food Biosci. 2023, 55, 103050. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2021, 88, 104864. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Sardar, H.; Saddiq, B. Tragacanth gum coating modulates oxidative stress and maintains quality of harvested apricot fruits. Int. J. Biol. Macromol. 2020, 163, 2439–2447. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Gul, K.; Wani, M.H.; Langowski, H. Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packag. Shelf Life 2014, 1, 86–99. [Google Scholar] [CrossRef]
- Vicent, V.; Ndoye, F.-T.; Verboven, P.; Nicolaï, B.; Alvarez, G. Modeling ice recrystallization in frozen carrot tissue during storage under dynamic temperature conditions. J. Food Eng. 2020, 278, 109911. [Google Scholar] [CrossRef]
- Meethaworn, K.; Luckanatinwong, V.; Zhang, B.; Chen, K.; Siriphanich, J. Off-flavor caused by cold storage is related to induced activity of LOX and HPL in young coconut fruit. LWF-Food Sci. Technol. 2019, 114, 108329. [Google Scholar] [CrossRef]
- Zhang, C.; Hua, Y.; Li, X.; Kong, X.; Chen, Y. Key volatile off-flavor compounds in peas (Pisum sativum L.) and their relations with the endogenous precursors and enzymes using soybean (Glycine max) as a reference. Food Chem. 2020, 333, 127469. [Google Scholar] [CrossRef]
- Chen, J.L.; Wu, J.H.; Wang, Q.; Deng, H.; Hu, X.S. Changes in the Volatile Compounds and Chemical and Physical Properties of Kuerle Fragrant Pear (Pyrus serotina Reld) during Storage. J. Agric. Food Chem. 2006, 54, 8842–8847. [Google Scholar] [CrossRef]
- Lara, I.; Miró, R.; Fuentes, T.; Sayez, G.; Graell, J.; López, M. Biosynthesis of volatile aroma compounds in pear fruit stored under long-term controlled-atmosphere conditions. Postharvest Biol. Technol. 2003, 29, 29–39. [Google Scholar] [CrossRef]
- Mortzfeld, F.B.; Hashem, C.; Vranková, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and Industrial Application of these Valuable Flavor and Fragrance Compounds. Biotechnol. J. 2020, 15, 2000064. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2018, 27, 30–36. [Google Scholar] [CrossRef]
- Sebzalli, Y.; Wang, X. Knowledge discovery from process operational data using PCA and fuzzy clustering. Eng. Appl. Artif. Intell. 2002, 14, 607–616. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWF-Food Sci. Technol. 2021, 137, 110478. [Google Scholar] [CrossRef]
- Shvartsburg, A.A. Ion Mobility Spectrometry (IMS) and Mass Spectrometry; Lindon, J.C., Traner, G., Koppenaal, D., Eds.; Academic Press: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Arce, L.; Menéndez, M.; Garrido-Delgado, R.; Valcárcel, M. Sample-introduction systems coupled to ion-mobility spectrometry equipment for determining compounds present in gaseous, liquid and solid samples. TrAC Trends Anal. Chem. 2008, 27, 139–150. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Liu, W.; Li, B.; Chen, S.; Wang, H.; Li, Y.; Li, J. Characterization and exploration of dynamic variation of volatile compounds in vine tea during processing by GC-IMS and HS-SPME/GC–MS combined with machine learning algorithm. Food Chem. 2024, 460, 140580. [Google Scholar] [PubMed]
- Feng, T.; Sun, J.; Song, S.; Wang, H.; Yao, L.; Sun, M.; Wang, K.; Chen, D. Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chem. X 2022, 15, 100423. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, J.; Wang, J.; Wang, X.; Du, D. Recent development of HS-GC-IMS technology in rapid and non-destructive detection of quality and contamination in agri-food products. TrAC Trends Anal. Chem. 2021, 144, 116435. [Google Scholar] [CrossRef]
- Qiao, Y.; Bi, J.; Chen, Q.; Wu, X.; Jin, X.; Gou, M.; Yang, X.; Purcaro, G. Rapid and sensitive quantitation of DDMP (2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one) in baked red jujubes by HS-SPME-GC-MS/MS. Food Control 2022, 135, 108820. [Google Scholar] [CrossRef]

| Category | No. | Compound | CAS# | MW | RI | RT [sec] | Dt [a.u.] | Aroma Characteristics |
|---|---|---|---|---|---|---|---|---|
| Esters (9) | 1 | Acetic acid ethyl ester | 141-78-6 | 88.1 | 614.8 | 148.578 | 1.33298 | Fruity |
| 2 | Methyl acetate | 79-20-9 | 74.1 | 540.2 | 120.785 | 1.18873 | Special aroma | |
| 3 | Ethyl butanoate | 105-54-4 | 116.2 | 790.6 | 268.747 | 1.55056 | Fresh aroma, Fruity | |
| 4 | Methyl heptanoate | 106-73-0 | 144.2 | 1020 | 615.058 | 1.80562 | Berry, Iris, Fruity | |
| 5 | Mesityl oxide | 141-79-7 | 98.1 | 784.7 | 263.245 | 1.44037 | Fruity | |
| 6 | 1-Butyl acetate-M | 123-86-4 | 116.2 | 805.8 | 283.67 | 1.2346 | Fruity | |
| 1-Butyl acetate-D | 123-86-4 | 116.2 | 805.8 | 283.67 | 1.2346 | Fruity | ||
| 7 | Ethyl propanoate-M | 105-37-3 | 102.1 | 708.7 | 196.933 | 1.15528 | Pineapple | |
| Ethyl propanoate-D | 105-37-3 | 102.1 | 708.7 | 196.933 | 1.15528 | Pineapple | ||
| 8 | iso-Propyl acetate-M | 108-21-4 | 102.1 | 655.9 | 166.56 | 1.15934 | Special fruity aroma | |
| iso-Propyl acetate-D | 108-21-4 | 102.1 | 655.9 | 166.56 | 1.15934 | Special fruity aroma | ||
| 9 | Ethyl trans-2-butenoate | 623-70-1 | 114.1 | 838.9 | 318.841 | 1.1776 | Spicy, Rum, Jackfruit | |
| Alcohols (5) | 10 | 1-Hexanol | 111-27-3 | 102.2 | 873.5 | 360.257 | 1.63716 | Fruity |
| 11 | 3-Hydroxy-2-butanone | 513-86-0 | 88.1 | 737.7 | 220.007 | 1.35493 | Milk | |
| 12 | 3-Methyl-1-pentanol | 589-35-5 | 102.2 | 841.6 | 321.92 | 1.59607 | Spicy, Cocoa, Wine | |
| 13 | (E)-3-Hexen-1-ol | 928-97-2 | 100.2 | 859.9 | 343.432 | 1.24638 | Grass | |
| 14 | (E)-2-Hexen-1-ol | 928-95-0 | 100.2 | 853.9 | 336.164 | 1.51193 | Pleasant odor | |
| Ketones (4) | 15 | Cyclopentanone | 120-92-3 | 84.1 | 783 | 261.57 | 1.32827 | Mint |
| 16 | 2-Pentanone | 107-87-9 | 86.1 | 700.5 | 190.91 | 1.39642 | Spicy, Fruity | |
| 17 | 3-Pentanone-M | 96-22-0 | 86.1 | 695.7 | 187.418 | 1.11127 | - | |
| 3-Pentanone-D | 96-22-0 | 86.1 | 695.7 | 187.418 | 1.11127 | - | ||
| 18 | 1-Penten-3-one-D | 1629-58-9 | 84.1 | 682.8 | 179.511 | 1.30614 | Tangerine, Onion | |
| 1-Penten-3-one-M | 1629-58-9 | 84.1 | 682.8 | 179.511 | 1.30614 | Tangerine, Onion | ||
| Aldehydes (4) | 19 | 1-Hexanal | 66-25-1 | 100.2 | 798.2 | 276.055 | 1.28327 | Fruity, Vegetable aroma |
| 20 | 2-Hexenal | 505-57-7 | 98.1 | 858.9 | 342.224 | 1.17677 | Leaf aroma | |
| 21 | 3-Methyl butanal | 590-86-3 | 86.1 | 654.6 | 165.965 | 1.40331 | Chocolate, Cocoa, Fruity (diluted) | |
| 22 | (E)-2-Pentenal-M | 1576-87-0 | 84.1 | 749.3 | 230.009 | 1.10366 | - | |
| (E)-2-Pentenal-D | 1576-87-0 | 84.1 | 749.3 | 230.009 | 1.10366 | - | ||
| Furans (2) | 23 | 2-Butylfuran | 4466-24-4 | 124.2 | 893.4 | 386.729 | 1.17853 | - |
| 24 | 1-(2-Furanyl)-ethanone-M | 1192-62-7 | 110.1 | 912.1 | 415.535 | 1.12334 | Fruity, Sweet | |
| 1-(2-Furanyl)-ethanone-D | 1192-62-7 | 110.1 | 912.1 | 415.535 | 1.12334 | Fruity, Sweet | ||
| Ether (1) | 25 | 1,2-Dimethoxyethane | 110-71-4 | 90.1 | 635.4 | 157.328 | 1.09696 | Pungent odor, Sweet |
| Category | NO. | Compound | CAS | Concentration (ng/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 d | 42 d | 84 d | ||||||||
| CK | FT | IT | LT | FT | IT | LT | ||||
| Esters (23) | 1 | Hexanoic acid, methyl ester | 106-70-7 | - | - | - | - | - | 0.64±0.05 | - |
| 2 | Hexanoic acid, ethyl ester | 123-66-0 | 2.02 ± 0.06 | - | 7.73 ± 0.13 | 12.42 ± 0.38 | 54.98 ± 1.89 | 1.14 ± 0.31 | 47.78 ± 4.47 | |
| 3 | Acetic acid, hexyl ester | 142-92-7 | 28.11 ± 0.81 | 286.26 ± 0.00 | 219.94 ± 0.00 | 79.92 ± 0.00 | 815.90 ± 21.07 | 26.11 ± 4.85 | 91.57 ± 7.18 | |
| 4 | Propanoic acid, 2-methyl-, hexyl ester | 2349-07-7 | 2.19 ± 0.16 | - | - | - | - | 0.83 ± 0.11 | - | |
| 5 | 2-Hexen-1-ol, acetate, (E)- | 2497-18-9 | 23.64 ± 0.80 | 185.20 ± 7.58 | 195.76 ± 4.78 | 157.42 ± 2.69 | 367.94 ± 17.46 | 6.07 ± 0.88 | 27.28 ± 3.26 | |
| 6 | Butanoic acid, hexyl ester | 2639-63-6 | 1.95 ± 0.08 | 4.56 ± 0.20 | - | - | - | - | - | |
| 7 | 3-Hexen-1-ol, propanoate, (Z)- | 33467-74-2 | - | - | - | - | 7.53 ± 1.19 | - | - | |
| 8 | n-Valeric acid cis-3-hexenyl ester | 35852-46-1 | - | - | - | - | - | 1.20 ± 0.44 | - | |
| 9 | 3-Hexen-1-ol, acetate, (Z)- | 3681-71-8 | 70.33 ± 2.51 | - | - | - | - | - | - | |
| 10 | Methyl 8-(2-furyl)-octanoate | 38199-50-7 | - | - | - | - | - | 4.17 ± 0.93 | - | |
| 11 | cis-3-Hexenyl iso-butyrate | 41519-23-7 | 6.31 ± 0.83 | - | - | - | - | - | - | |
| 12 | Butanoic acid, 3-hexenyl ester, (E)- | 53398-84-8 | 17.30 ± 1.16 | 37.22 ± 2.60 | - | 2.76 ± 0.45 | 78.20 ± 3.18 | 2.93 ± 0.61 | - | |
| 13 | cis-3-Hexenyl-alpha-methylbutyrate | 53398-85-9 | 1.07 ± 0.20 | 5.08 ± 0.73 | - | - | 24.95 ± 1.87 | - | - | |
| 14 | Methyl propyl methylphosphonate | 683-25-0 | - | - | - | - | - | - | 564.37 ± 14.33 | |
| 15 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 6846-50-0 | 3.04 ± 0.25 | - | - | - | 9.24 ± 0.75 | 0.87 ± 0.10 | - | |
| 16 | 4-Hexen-1-ol, acetate | 72237-36-6 | - | - | 415.24 ± 17.20 | 154.24 ± 5.87 | - | 21.35 ± 2.16 | 83.12 ± 3.19 | |
| 17 | 1,5-Dimethyl-1-vinyl-4-hexenyl butyrate | 78-36-4 | - | - | - | 4.84 ± 0.98 | - | - | - | |
| 18 | Benzoic acid, methyl ester | 93-58-3 | - | - | - | - | - | 1.44 ± 0.22 | - | |
| 19 | Benzoic acid, ethyl ester | 93-89-0 | - | - | - | 2.85 ± 0.26 | - | - | - | |
| 20 | 3-Methylbut-2-enoic acid, 4-nitrophenyl ester | 1000307-59-8 | - | - | - | 28.38 ± 1.89 | 90.81 ± 7.19 | - | - | |
| 21 | Oxalic acid, dicyclobutyl ester | 1000309-69-5 | - | 4.60 ± 1.30 | - | - | - | - | - | |
| 22 | 3-Methylbut-2-enoic acid, 3-fluorophenyl ester | 1000331-15-0 | - | - | - | - | - | 5.68 ± 0.39 | - | |
| 23 | Hexanoic acid, 3-hexenyl ester, (Z)- | 31501-11-8 | 1.00 ± 0.23 | - | - | - | - | - | - | |
| Alkanes (7) | 24 | Cyclopentane, methyl- | 96-37-7 | - | - | - | - | 78.50 ± 4.51 | - | - |
| 25 | 2-Pentanone, 4-methoxy-4-methyl- | 107-70-0 | 0.87 ± 0.21 | - | - | - | - | - | - | |
| 26 | Cyclopentane, 1,1-dimethyl- | 1638-26-2 | 1.14 ± 0.18 | - | - | - | - | - | - | |
| 27 | Butanoic acid, 3-hexenyl ester, (Z)- | 16491-36-4 | - | - | 20.36 ± 1.34 | - | - | - | - | |
| 28 | Cyclopentane, 1-ethyl-1-methyl- | 16747-50-5 | 1.23 ± 0.61 | - | - | - | - | - | - | |
| 29 | Butane, 1-(ethenyloxy)-3-methyl- | 39782-38-2 | 0.85 ± 0.10 | - | - | - | - | - | - | |
| 30 | cis-1-Methyl-2-(2′-propenyl)-cyclopropane | 76588-97-1 | - | - | - | - | 491.30 ± 26.82 | - | - | |
| Ketones (6) | 31 | 4-Heptanone, 2,6-dimethyl- | 108-83-8 | - | - | - | - | - | 9.86 ± 1.37 | - |
| 32 | 4-Hexen-3-one, 5-methyl- | 13905-10-7 | 1.34 ± 0.29 | 3.43 ± 0.41 | - | - | - | - | - | |
| 33 | t-Butyl isobutyl ketone | 14705-50-1 | - | - | - | 20.06 ± 1.35 | - | - | - | |
| 34 | 1-Penten-3-one | 1629-58-9 | 17.83 ± 2.30 | - | - | - | - | - | - | |
| 35 | Ethanone, 2-(formyloxy)-1-phenyl- | 55153-12-3 | - | - | - | - | - | 4.23 ± 0.32 | - | |
| 36 | Pivaloylacetone, enol form | 1000202-24-3 | - | 6.44 ± 0.57 | - | - | - | - | - | |
| Olefins (6) | 37 | 1,3,8-p-Menthatriene | 18368-95-1 | - | - | - | - | - | - | 155.85 ± 12.49 |
| 38 | 1,4-Cyclohexadiene, 3,3,6,6-tetramethyl- | 2223-54-3 | - | - | - | 1.44 ± 0.28 | - | - | - | |
| 39 | 1,3-Cyclopentadiene, 1,2,3,4,5-pentamethyl- | 4045-44-7 | 1.59 ± 0.34 | - | - | - | - | - | - | |
| 40 | Cyclohexene, 1-methyl-4-(1-methylethylidene)- | 586-62-9 | - | - | - | - | - | - | 27.94 ± 2.98 | |
| 41 | Alpha-Phellandrene | 99-83-2 | 3.32 ± 0.66 | - | - | - | - | - | - | |
| 42 | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- | 99-86-5 | - | - | - | 8.76 ± 1.21 | - | - | 86.24 ± 5.25 | |
| Aldehydes (3) | 43 | Decanal | 112-31-2 | 0.91 ± 0.19 | - | - | - | - | - | - |
| 44 | Nonanal | 124-19-6 | 1.84 ± 0.51 | - | - | - | - | - | - | |
| 45 | (E)-4-Oxohex-2-enal | 1000374-04-2 | 62.05 ± 3.31 | 10.34 ± 1.08 | 99.42 ± 4.67 | 37.52 ± 2.16 | 21.04 ± 1.59 | 26.78 ± 1.47 | - | |
| Alcohols (3) | 46 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)- | 20126-76-5 | 3.35 ± 0.11 | - | - | 147.08 ± 5.06 | - | - | - |
| 47 | Eucalyptol | 470-82-6 | 13.63 ± 1.49 | 4.42 ± 0.38 | 14.62 ± 1.30 | 6.71 ± 0.46 | 9.20 ± 0.89 | 27.53 ± 3.09 | - | |
| 48 | Linalool | 78-70-6 | 2.35 ± 0.14 | - | - | - | 26.74 ± 1.15 | 2.09 ± 0.33 | - | |
| Furan (2) | 49 | Furan, 2-ethyl- | 3208-16-0 | 16.67 ± 1.03 | - | - | 2.33 ± 0.35 | - | 3.50 ± 0.69 | - |
| 50 | Furan, 2-propyl- | 4229-91-8 | 1.48 ± 0.60 | - | - | - | - | - | - | |
| Acid (1) | 51 | Propanoic acid, 2-methyl-, anhydride | 97-72-3 | - | - | - | - | - | 24.51 ± 0.95 | - |
| Others (11) | 52 | 5H-Tetrazol-5-amine | 1000273-02-0 | - | - | - | - | - | - | 38.84 ± 1.15 |
| 53 | Valeric anhydride | 2082-59-9 | 27.30 ± 1.82 | 4.40 ± 0.50 | 57.17 ± 1.25 | 53.14 ± 0.00 | 45.03 ± 2.57 | 0.87 ± 0.32 | - | |
| 54 | 1,2,4-Triazine | 290-38-0 | - | - | - | - | 24.38 ± 2.13 | - | - | |
| 55 | anti-2-Acetoxyacetaldoxime | 37858-07-4 | - | - | - | - | 17.94 ± 3.48 | - | - | |
| 56 | 1H-Tetrazole, 5-methyl- | 4076-36-2 | - | - | - | - | 85.14 ± 0.52 | - | - | |
| 57 | 3-Nitropyrrole | 5930-94-9 | - | - | - | - | 26.43 ± 0.78 | - | - | |
| 58 | 1-Butyne, 3-methyl- | 598-23-2 | 5.18 ± 0.83 | - | - | - | - | - | - | |
| 59 | 1H-Imidazole, 1-methyl- | 616-47-7 | - | 281.83 ± 16.97 | - | - | - | - | - | |
| 60 | L-Prolinamide | 7531-52-4 | 0.83 ± 0.13 | - | - | - | - | - | - | |
| 61 | 2,5-cyclohexadien-1-one, 2,6-bis(1,1-dimethylethyl)-4-hydroxy-4-methyl- | 1000401-12-0 | - | - | - | - | 16.74 ± 1.04 | - | - | |
| 62 | o-Cymene | 527-84-4 | - | - | - | 8.69 ± 0.46 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Jia, X.; Li, J.; Zhang, P.; Qin, D.; Wu, D.; Chen, T.; Huo, J. Evaluating Ice-Temperature Storage Efficacy on Volatile Compounds in Blue Honeysuckle (Lonicera caerulea L.) by Combining GC-IMS and GC-MS. Foods 2025, 14, 1205. https://doi.org/10.3390/foods14071205
Li T, Jia X, Li J, Zhang P, Qin D, Wu D, Chen T, Huo J. Evaluating Ice-Temperature Storage Efficacy on Volatile Compounds in Blue Honeysuckle (Lonicera caerulea L.) by Combining GC-IMS and GC-MS. Foods. 2025; 14(7):1205. https://doi.org/10.3390/foods14071205
Chicago/Turabian StyleLi, Tianbo, Xiaoyu Jia, Jiangkuo Li, Peng Zhang, Dong Qin, Di Wu, Tong Chen, and Junwei Huo. 2025. "Evaluating Ice-Temperature Storage Efficacy on Volatile Compounds in Blue Honeysuckle (Lonicera caerulea L.) by Combining GC-IMS and GC-MS" Foods 14, no. 7: 1205. https://doi.org/10.3390/foods14071205
APA StyleLi, T., Jia, X., Li, J., Zhang, P., Qin, D., Wu, D., Chen, T., & Huo, J. (2025). Evaluating Ice-Temperature Storage Efficacy on Volatile Compounds in Blue Honeysuckle (Lonicera caerulea L.) by Combining GC-IMS and GC-MS. Foods, 14(7), 1205. https://doi.org/10.3390/foods14071205









