A Comprehensive Review of the Nutritional and Health-Promoting Properties of Edible Parts of Selected Cucurbitaceae Plants
Abstract
1. Introduction
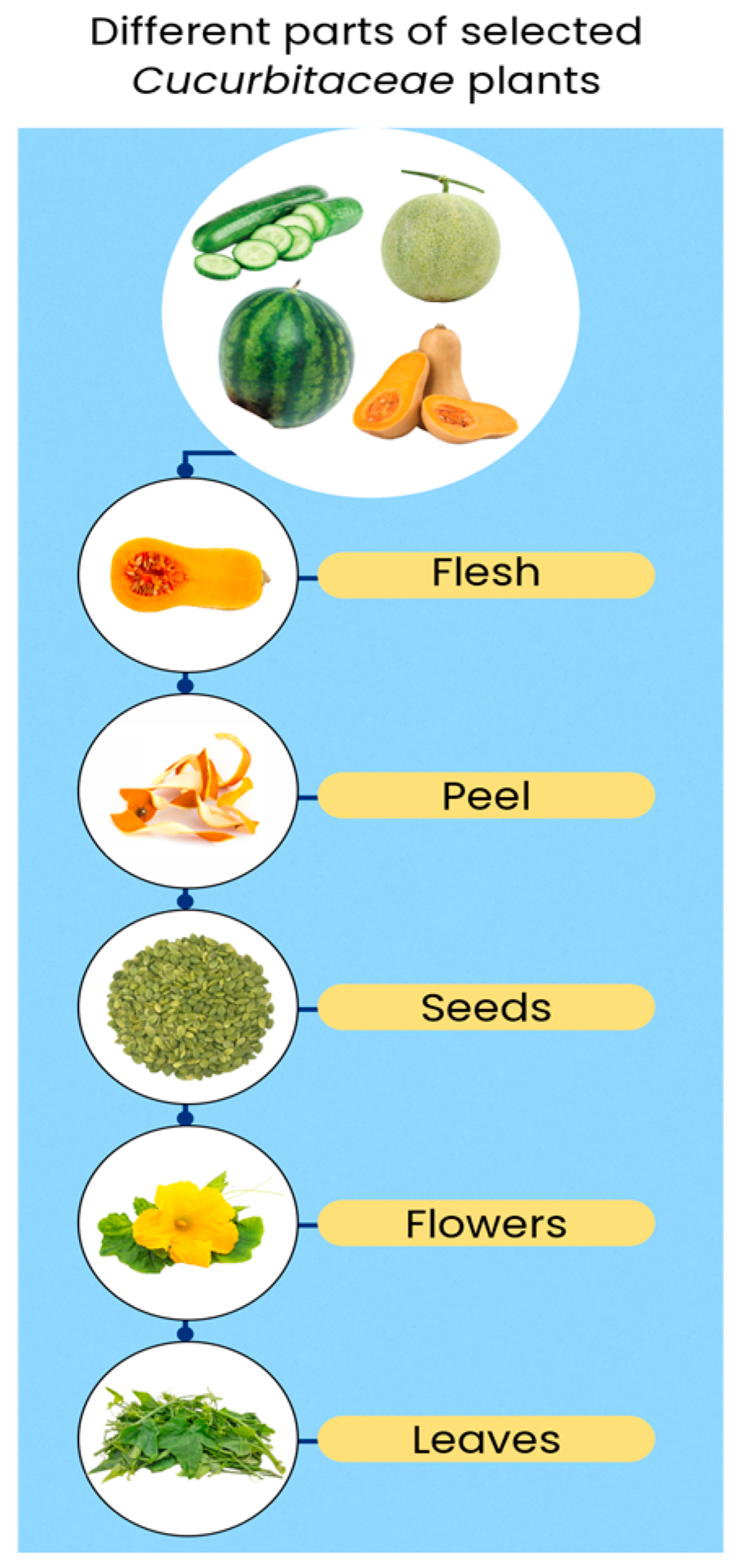
2. General Characteristics of Cucurbitaceae Family Plants
3. Nutritional Value of Cucurbitaceae Family Plants
4. Antinutritional Factors and Toxic Constituents in Cucurbitaceae Family Plants
5. Mineral Composition
6. Phytochemical Composition
| Plant/Part | Bioactive Compounds | References | |
|---|---|---|---|
| Cucumber (C. sativus Linn., C. trigonus Roxb.) | fruits | flavonoids (rutin, quercetin, apigenin, kaempferol) (2.63 mg/kg); phenolics TPC (0.18 mg/kg); carotenoids; alkaloids (0.82 mg/kg); tannins; lignins, saponins; glycosides; terpenoids; cucurbitacins (B, C, E), carbohydrates; steroids; serpentines | [9,35,36,37] |
| peel | flavonoids (14.02 mg QE/g); tannins; saponins; glycosides; anthraquinones; phenolics (23.08 mg GAE/g) | [48,49] | |
| seeds | phenolics (93.5 mg/GAE/g); flavonoids (57.4 mg QE/g); β-carotene (19.46 mg/100 g); tannins; terpenoids; saponins; flavonoids | [16,50,51] | |
| leaves | coumarin A and B; vitexin; isovitexin; orientin; isoorientin; hydroxycinnamic acid | [52] | |
| Melon (C. melo, C. metuliferus) | pulp | saponins; tannins; phenolic compounds (47.2–200 mg GAE/100 g); terpenoids; carotenoids (0.81–1.14 mg β-car/100 g) (lycopene, β-carotene, lutein, phytofluene, phytoene, γ-carotene, ζ-carotene); chlorophylls (4.23–5.06 mg/100 g); flavonoid aglycones (myricetin and quercetin); flavonol glycosides (rutin). | [42,43,44,45] |
| peel | 3-hydroxybenzoic acid (33.5 mg/100 g); apigenin-7-glycoside (29.3 mg/100 g); isovanilic acid (23.7 mg/100 g); m-coumaric acid; oleuropein; luteolin-7-glycoside; flavonoids (95.5 QE/100 g); β-carotene (821.5 μg/100 g); lycopene (64.5 μg/100 g); glycosides (2.19 mg/g); tannins (1.38 mg/g) | [12,41,53,54,55] | |
| seeds | alkaloids (2.54 mg/g); steroids (2.62 mg/g); terpenoids; tanins (2.93 mg/g); glycosides; phenolics TPC (29.39 mg/100 g); flavonoids TFC (20.67 mg/100 g); carotenoids (1.56–130 mg/g); α-tocopherol; γ-tocopherol; sitosterol (3248.48 mg/kg oil); Δ 5-avenasterol (1533.11 mg/kg oil); ferulic acid (134.93 µg/g); vanillic acid (2.31 µg/g oil); caffeic acid (1.98 µg/g oil); oleuropein; pinoresinol | [43,56,57] | |
| leafs | TPC (26.40 mg GAE/g) and TFC (69.7 μg RE/g) | [9,58] | |
| Pumpkin (C. pepo, C. moschata, C. maxima, C. ficifolia) | flesh | carotenoids (171.9–461.9 μg/g) (α- and β-carotene; ζ-carotene; neoxanthin; violaxanthin; lutein; zeaxanthin; taraxanthin; luteoxanthin; auroxanthin; neurosporen; flavoxanthin; 5,6,5′,6′-diepoxy-β-carotene; phytofluene; α-cryptoxanthin; β-cryptoxanthin, tocopherols); phytosterols (β-sitosterol, stigmasterol, campesterol, Δ5-avenasterol); polysaccharides; D-chiro-inositol; myo-inositol; cucurbitacins (C and E) (105 µg/g and 438 µg/g); polyphenolic compounds: p-coumaric acid; p-hydroxybenzoic acid; salicin; stigmast-7,2,2-dien-3-ol; stigmast-7-en-3-ol; carbohydrates: D-chiro-inositol; myoinositol; fagopyritols; sucrose | [4,30,31,36,37,38] |
| peel | flavonoids (0.41 mg CE/100 g) and phenolic compounds (1.83 mg GAE/100 g); carotenoid compounds: β-carotene (39.48–123.19 mg/kg) | [4,30,59] | |
| seeds | phytosterols (265 mg/100 g); squalene; β-sitosterol (0.024–0.038 mg/100 g); stigmasterol (8.40–13.28 mg/100 g); α-tocopherol (0.18–9.66 mg/100 g); β-tocopherol (0.058–1.68 mg/100 g); γ-tocopherol (1.21–62.00 mg/100 g); kaempferol; p-coumaric acid (5.04 mg/kg); ferulic acid; apigenin; quercetin; vanillic acid (9.54 mg/kg); p-hydroxybenzoic acid (27.00 mg/kg); lutein; sinapic acid; tyrosol; vanillin; protocatechuic acid (1.58 mg/kg); fatty acids; primarily oleic; linoleic acids; Δ7-sterols (avenasterol, spinasterol); Δ5-sterol (sitosterol, stigmasterol); campesterol; cempestanol; triterpenoids; sesquiterpenoids; tetraterpenoids (carotenoids); tocopherols | [6,13,60,61,62,63,64,65,66,67] | |
| flowers | carotenoids (29.8 mg/100 g); phenolics (133.26 mg CAE/100 g; 8.09–17.39 µg GAE/mL); flavonoids (0.51–8.23 mg QE/100 g; 2.29–17.134 QE µg/mL) | [16,68,69] | |
| leaves | saponins; tannins; alkaloids; flavonoids; glycosides | [18,63,67] | |
| Watermelon (C. lanatus) | flesh | carotenoids in yellow-fleshed varities: neoxanthin; carotenoids in red-fleshed varities: lycopene, β-carotene, phytofluene, phytoene, γ-carotene, ζ-carotene, and α-carotene (15 mg/kg); lutein; zeaxanthin; phenolics (47.3 TAE/g); non-flavonoid phenolic compounds: 11-hydroxybenzoic acid; 20-hydroxycinnamic acid derivatives; coumarin derivatives: coumarin, aviprin, and obtusoside; L-citrulline (25.5 and 14.2 mg/g); cucurbitacin B, C, D, E, I; cucurbitacin L 2- O–b -glucoside | [39,40,41] |
| peel | polyphenols (63.33 mg TAE/g); alkaloids; phytates; tannins; oxalate; saponins | [8] | |
| seeds | phenolics (0.087 mg GAE/g); sinapic acid (152.300 µg/mL); ferulic acid (68.285 µg/mL); 4-hydroxybenzoic acid (59.707 µg/mL); caffeic acid (1.33 μg/g); pinoresinol lignans (and 1.02 μg/g); alkaloids (28.33 mg/g); saponins (16.87 mg/g); flavonoids (0.75 mg/g); alkaloids; tannins; terpenoids; sitosterol (2298.83 mg/kg oil); Δ 5-avenasterol (1319.21 mg/kg seed oil); in seed cakes TFC (721.09 mg); luteolin mg/kg; TPC (1.67 mg GAE/g) | [8,29,48,56,70,71,72] | |
7. Caloric Value, Glycemic Index, and Glycemic Load
8. Health Properties of Cucurbitaceae Family Plants
8.1. Hypoglycemic and Antidiabetic Effect
| Plants | Part of Plant | Biological Activity | References |
|---|---|---|---|
| Pumpkin | Seed oil | anticancer | [7] |
| Seeds | antioxidant | [29,88] | |
| Pumpkin (C. maxima) | Seed (ethanolic extracts) | antidiabetic | [89] |
| Pumpkin (C. moschata) | Flesh + seeds (extract) | anticancer | [90] |
| Pumpkin (C. pepo) | Flesh + wheat flour | hypolipidemic | [91] |
| Seeds (hydroethanolic extract) | anticancer | [3,92] | |
| Leaf (extracts) | antidiabetic | [18] | |
| Seed oil | antidiabetic and hypolipidemic | [18,93] | |
| Peels (ethanolic extracts) | antidiabetic | [84] | |
| [5] | |||
| Seeds (tocopherols extract) | [85] | ||
| Seeds (extract) | hypolipidemic | [94] | |
| Watermelon | Pulp, Seeds, Rind (juice) | antihyperglycemic | [8,95] |
| YellowWatermelon | Flesh | antidiabetic | [96] |
| Watermelon (C. lanatus) | Seeds (ethanolic extract) | cardioprotective | [97] |
| Seeds (peptides) | antioxidant | [39,98,99] | |
| Peel extracts | anticancer and antioxidant | [100,101] | |
| Melon | Seeds (hexane extract) | antidiabetic | [102] |
| Fruits | antioxidant | [103] | |
| Pulp, seeds, peels | [104] | ||
| Seed and leaf (extract) | [58] | ||
| Methanolic extracts | anticancer | [105] | |
| Melon (C. melo L.) | Concentrates | cardioprotective | [106] |
| Horned Melon (Cucumis metuliferus) | fruit | antiulcer, anti-inflammatory | [43] |
| peel | antioxidant and antifungal | [44] | |
| Cucumber (C. sativus) | Fruits (ethanolic extracts) | antihyperglycemic | [107] |
| Seeds (extract) | hypocholesterolemic | [108] | |
| Peel (ethanol extract) | antioxidant | [49] | |
| Pulp, Leaf | [109,110] | ||
| Flowers (acetyl extracts) | anticancer | [9] | |
| Flowers (molecular docking) | antiangiogenic | [111] |
8.2. Hypolipidemic Effect and Cardiovascular Preventive Properties
8.3. Antioxidant Effect
8.4. Anticancer Effect
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Caco-2 | colon adenocarcinoma |
| CAE | chlorogenic acid equivalent |
| CAT | catalase |
| CE | catechin equivalent |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DPP-IV | dipeptidyl peptidase-IV |
| FRAP | ferric-reducing antioxidant power |
| GAE | gallic acid equivalent |
| GI | glycemic index |
| GL | glycemic load |
| GLP-1 | glucose-like peptide-1 |
| GLUT2 | glucose transporter-2 |
| GSH-Px | glutathione peroxidase |
| GST | glutathione S-transferase |
| HDL | high density lipoprotein |
| Hep-2 | human epithelial type-2 cell |
| LDL | low-density lipoprotein |
| NO | nitric oxide |
| QE | quercetin equivalent |
| ROS | reactive oxygen species |
| SGLT1 | sodium glucose cotransporter-1 |
| SOD | superoxide dismutase |
| TAE | tannic acid equivalent |
| TFC | total flavonoids content |
| TG | triglyceride |
| TPC | total phenolic content |
| VCAM | vascular cell adhesion molecule |
References
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef]
- Yiblet, Y. Overview of Cucurbitaceae Families. In Biological and Abiotic Stress in Cucurbitaceae Crops; Haiping, W., Ed.; IntechOpen: London, UK, 2023; pp. 1–112. [Google Scholar]
- Patel, S.; Rauf, A. Edible seeds from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother. 2017, 91, 330–331. [Google Scholar] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; et al. Cucurbita Plants: From Farm to Industry. Appl. Sci. 2019, 9, 3387. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Singha, S.; Kar, A.; Chanda, J.; Banerjee, S.; Dasgupta, B.; Haldar, P.K.; Sharma, N. Therapeutic importance of Cucurbitaceae: A medicinally important family. J. Ethnopharmacol. 2022, 282, 114599. [Google Scholar] [PubMed]
- Singh, A.; Kumar, V. Pumpkin seeds as nutraceutical and functional food ingredient for future: A review. Grain Oil Sci. Technol. 2024, 7, 12–29. [Google Scholar]
- Batool, M.; Ranjha, M.M.A.N.; Roobab, U.; Manzoor, M.F.; Farooq, U.; Nadeem, H.R.; Nadeem, M.; Kanwal, R.; AbdElgawad, H.; Jaouni, S.K.A.; et al. Nutritional Value, Phytochemical Potential, and Therapeutic Benefits of Pumpkin (Cucurbita sp.). Plants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aadil, R.M. An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends Food Sci. Technol. 2021, 114, 275–291. [Google Scholar]
- Tzortzakis, N.; Chrysargyris, A.; Spyridon, A.P. Phytochemicals Content and Health Effects of Cultivated and Underutilized Species of the Cucurbitaceae Family. In Phytochemicals in Vegetables: A Valuable Source of Bioactive Compounds; Petropoulos, S.A., Ferreira, I., Barros, L., Eds.; Bentham Science Publishers: Bussum, The Netherlands, 2018; pp. 99–165. [Google Scholar]
- Muthoni, J.; Hussein, S. Minor cucurbits from Africa: Horned melon (Cucumis metuliferus E. Mey. Ex Naudin). Aust. J. Crop Sci. 2024, 18, 723–730. [Google Scholar] [CrossRef]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean diet: Citrus, cucumber and grape. J. Prev. Med. Hyg. NLM (Medlin.) 2022, 63, E21–E27. [Google Scholar]
- Silva, M.A.; Albuquerque, T.G.; Alves, R.C.; Oliveira, M.B.P.P.; Costa, H.S. Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods? Trends Food Sci. Technol. 2020, 98, 181–189. [Google Scholar]
- Peričin, D.; Krimer, V.; Trivić, S.; Radulović, L. The distribution of phenolic acids in pumpkin’s hull-less seed, skin, oil cake meal, dehulled kernel and hull. Food Chem. 2009, 113, 450–456. [Google Scholar] [CrossRef]
- Sinkovič, L.; Kolmanič, A. Elemental composition and nutritional characteristics of cucurbita pepo subsp. Pepo seeds, oil cake and pumpkin oil*. J. Elem. 2021, 26, 97–107. [Google Scholar]
- Kizildogan, Z.; Zengin, E.; Musa Özcan, M. Nutritional Evaluation, Physico-Chemical Characteristics, Fatty Acid Composition and Mineral Contents of Some Vegetable Seed and Oils. Anal. Chem. Lett. 2011, 1, 318–324. [Google Scholar]
- Bieżanowska-Kopeć, R.; Ambroszczyk, A.M.; Piatkowska, E.; Leszczyńska, T. Nutritional Value and Antioxidant Activity of Fresh Pumpkin Flowers (Cucurbita sp.) Grown in Poland. Appl. Sci. 2022, 12, 6673. [Google Scholar] [CrossRef]
- Mohd, A.M.; Mohammed, A.M.; Balarabe Idris, M.; Abdulrasheed, A. The Mineral Composition and Proximate Analysis of T. occidentalis (Fluted pumpkin) Leaves Consumed in Kano Metropolis, Northern Nigeria. Am. Chem. Sci. J. 2016, 1, 1–4. [Google Scholar]
- Isara, R.D.S.; Gunathilaka, M.D.T.L. Pumpkin seeds and leaves. Univeristy Colombo Rev. 2023, 4, 31–47. [Google Scholar]
- Mallek-Ayadi, S.; Bahloul, N.; Baklouti, S.; Kechaou, N. Bioactive compounds from Cucumis melo L. fruits as potential nutraceutical food ingredients and juice processing using membrane technology. Food Sci. Nutr. 2022, 10, 2922. [Google Scholar]
- Niyi, O.H.; Jonathan, A.A.; Ibukun, A.O. Comparative Assessment of the Proximate, Mineral Composition and Mineral Safety Index of Peel, Pulp and Seeds of Cucumber (Cucumis sativus). Open J. Appl. Sci. 2019, 09, 691–701. [Google Scholar]
- Arsov, A.; Tsigoriyna, L.; Batovska, D.; Armenova, N.; Mu, W.; Zhang, W.; Petrov, K.; Petrov, P. Bacterial Degradation of Antinutrients in Foods: The Genomic Insight. Foods 2024, 13, 2408. [Google Scholar] [CrossRef]
- Zieniuk, B.; Pawełkowicz, M. Recent advances in the application of cucurbitacins as anticancer agents. Metabolites 2023, 13, 1081. [Google Scholar] [CrossRef]
- Varela, C.; Melim, C.; Naves, B.G.; Sharifi- Rad, J.; Calina, D.; Mamurova, A.; Cebral, C. Cucurbitacins as potential anticancer agents: New insights on molecular mechanisms. J. Transl. Med. 2022, 20, 630. [Google Scholar]
- Momanyi, V.N. The Toxic Effects of Cucurbitacin in Paddy Melon (Cucumis myriocarpus) on Rats. Int. J. Res. Rev. 2016, 3, 1–5. [Google Scholar]
- Abiola, C.; Ekunrin, M.F. Effect of Fermentation on the Microbial, Nutrient and Anti-nutrient Contents of Melon (Cucumis melo L.) Husk. Microbiol. J. 2015, 6, 9–14. [Google Scholar]
- Bamidele, T.O.; Sunday, H.G.; Mathew, A.; Ombugadu, J.; Maryam, A. Evaluation of the Phytochemicals, Nutritional and Anti-nutritional Compositions of Fresh, Sprouted and Toasted Citrullus lanatus (Watermelon) Seed Extracts. Asian J. Biochem. Genet. Mol. Biol. 2021, 7, 11–19. [Google Scholar]
- Burris, K.P. Melons and cucumbers. The Produce Contamination Problem: Causes and Solutions, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 97–142. [Google Scholar]
- Nkoana, D.K.; Mashilo, J.; Shimelis, H.; Ngwepe, R.M. Nutritional, phytochemical compositions and natural therapeutic values of citron watermelon (Citrullus lanatus var. citroides): A Review. S. Afr. J. Bot. 2022, 145, 65–77. [Google Scholar]
- Jaroszewska, A.; Jedrejek, D.; Sobolewska, M.; Kowalska, I.; Dzięcioł, M. Mineral, Nutritional, and Phytochemical Composition and Baking Properties of Teff and Watermelon Seed Flours. Molecules 2023, 28, 3255. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Rimac Brnčić, S.; Badanjak Sabolović, M.; Šic Žlabur, J.; Marović, R.; Brnčić, M. Carotenoid Content and Profiles of Pumpkin Products and By-Products. Molecules 2023, 28, 858. [Google Scholar] [CrossRef]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Celina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef]
- Jaswir, I.; Shahidan, N.; Othman, R.; Hasim, Y.Z.H.Y.; Octavianti, F.; Salleh, M.N. Effects of season and storage period on accumulation of individual carotenoids in pumpkin flesh (Cucurbita moschata). J. Oleo Sci. 2014, 63, 761–767. [Google Scholar]
- Kulczyński, B.; Gramza-Michałowska, A. The profile of carotenoids and other bioactive molecules in various pumpkin fruits (Cucurbita maxima Duchesne) cultivars. Molecules 2019, 24, 3212. [Google Scholar] [CrossRef]
- Zakaria Eleiwa, D.; Mohamed, S.A.; Eleiwa, N.Z.; Omar Bakr, R. Phytochemical and Pharmacological Screening of Seeds and Fruits Pulp of Cucurbita moschata Duchesne Cultivated in Egypt. Int. J. Pharmacogn. Phytochem. 2014, 29, 2051–7858. [Google Scholar]
- Yang, R.Y.; Lin Bsc, S.; Kuo, G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008, 17, 275–279. [Google Scholar]
- Jessica, G.G.; Mario, G.L.; Alejandro, Z.; Cesar, A.P.J.; Ivan, J.V.E.; Ruben, R.R.; Javier, A.A.F. Chemical characterization of a hypoglycemic extract from Cucurbita ficifolia bouche that induces liver glycogen accumulation in diabetic mice. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 218–230. [Google Scholar] [PubMed]
- Tripathi, J.; Variyar, A.P.; Mishra, P.K.; Variyar, P.S. Impact of radiation processing on the stability of cucurbitacin glycosides in ready-to-cook (RTC) pumpkin during storage. LWT—Food Sci. Technol. 2016, 73, 239–242. [Google Scholar]
- Xia, T.; Wang, Q. D-chiro-Inositol found in Cucurbita ficifolia (Cucurbitaceae) fruit extracts plays the hypoglycaemic role in streptozocin-diabetic rats. J. Pharm. Pharmacol. 2006, 58, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Zamuz, S.; Munekata, P.E.S.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Grassi, S.; Piro, G.; Lee, J.M.; Zheng, Y.; Fei, Z.; Dalessandro, G.; Giovanni, J.J.; Lenucci, M. Comparative genomics reveals candidate carotenoid pathway regulators of ripening watermelon fruit. BMC Genom. 2013, 14, 1–20. [Google Scholar]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and beyond color: Comparative overview of functional quality of tomato and watermelon fruits. Front Plant Sci. 2019, 10, 455060. [Google Scholar]
- Das, K.; Gajanan Basole, S.; Shivakumar, P.; Kumar, S.; Saradar, B. Phytochemical screening of Cucumis melo L.; fruits (Cucurbitaceae) Licensed under a Creative Commons Attribution 4.0 International License. Plants Second. Metab. 2024, 3, 57–65. [Google Scholar]
- Šeregelj, V.; Šovljanski, O.; Šaponjac, V.T.; Vulić, J.; Ćetković, G.; Markov, S.; Čanadanović-Brunet, J. Horned Melon (Cucumis metuliferus E. Meyer Ex. Naudin)—Current Knowledge on Its Phytochemicals, Biological Benefits, and Potential Applications. Processes 2022, 10, 94. [Google Scholar] [CrossRef]
- Šovljanski, O.; Šeregelj, V.; Pezo, L.; Šaponjac, V.T.; Vulić, J.; Cvanić, T.; Čanadanović-Brunet, J. Horned Melon Pulp, Peel, and Seed: New Insight into Phytochemical and Biological Properties. Antioxidants 2022, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L. A fruit to discover: Cucumis metuliferus E.Mey Ex Naudin (Kiwano). Clin. Nutr. Metab. 2018, 1, 1–2. [Google Scholar]
- Gajc-Wolska, J.; Kowalczyk, K.; Bujalski, D. The effect of cultivation term, substrate and cultivar on Chemical composition of cucumber fruit (Cucumis sativus L.) in greenhouse production. Acta Hortic. 2010, 877, 239–244. [Google Scholar]
- Gopalakrishnan, S.B.; Kalaiarasi, T.; Subramanian, R. Comparative DFT Study of Phytochemical Constituents of the Fruits of Cucumis trigonus Roxb. and Cucumis sativus Linn. J. Comput. Methods Phys. 2014, 2014, 623235. [Google Scholar]
- Adamu, A.U.; Abdulmumin, Y.; Mustapha, R.K. Green Synthesis, Characterization and Phytochemicals Analysis of Silver Nano-Particles Using Aqueous Peel Extract of Cucumis sativus. J. Mater. Environ. Sci. 2021, 2021, 1627–1636. [Google Scholar]
- Yunusa, A.K.; Dandago, A.; Sa’adatu Mukhtar, I.; Abdullahi, N.; Rilwan, A.; Barde, A. Total phenolic content and antioxidant capacity of different parts of cucumber (Cucumis sativus L.). Acta Univ. Cibiniensis Ser. E Food Technol. 2018, 22, 13–20. [Google Scholar]
- Ifeoma, P.O.; Azubike, J.O.; Ifeanyi, V.E.; Precious, O.O.; Osuagwu, C.C.; Collins, C.; Okwudili, O.; Austin, U.A.; Okechukwu, J. Phytochemical and Proximate Composition of Cucumber (Cucumis sativus) Seed Oil. Int. J. Res. Sci. Innov. 2021, 08, 244–250. [Google Scholar]
- Achikanu, C.E.; Ani, O.N.; Akpata, E.I. Proximate, vitamin and phytochemical composition of cucumis metuliferus seed. Int. J. Food Sci. Nutr. 2020, 5, 20–24. [Google Scholar]
- McNally, D.J.; Wurms, K.V.; Labbé, C.; Bélanger, R.R. Synthesis of C-glycosyl flavonoid phytoalexins as a site-specific response to fungal penetration in cucumber. Physiol. Mol. Plant Pathol. 2003, 63, 293–303. [Google Scholar]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Characterization, phenolic compounds and functional properties of Cucumis melo L. peels. Food Chem. 2017, 221, 1691–1697. [Google Scholar]
- Sabino, L.B.S.; Gonzaga, M.L.C.; Soares, D.J.; Lima, A.C.S.; Lima, J.S.S.; Almeida, M.M.B.; De Sousa, P.H.M.; De Figueiredo, R.W. Bioactive compounds, antioxidant activity, and minerals in flours prepared with tropical fruit peels. Acta Aliment. 2015, 44, 520–526. [Google Scholar] [CrossRef]
- Ezekaibeya, A.C.; Nnenna, A.O.; Kenechukwu, O.C. Proximate, Phytochemical and Vitamin Compositions of Cucumis metuliferus (Horned melon) Rind. J. Complement. Altern. Med. Res. 2020, 9, 40–50. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Meddeb, W.; Msaada, K.; Hamdi, S. Chemical composition and bioactive compounds of Cucurbitaceae seeds: Potential sources for new trends of plant oils. Process Saf. Environ. Prot. 2019, 127, 73–81. [Google Scholar] [CrossRef]
- Olubunmi, I.P.; Olajumoke, A.A.; Bamidele, J.A.; Omlara, O.F. Phytochemical Composition and in vitro Antioxidant Activity of Golden Melon (Cucumis melo L.) Seeds for Functional Food Application. Int. J. Biochem. Res. Rev. 2019, 25, 1–13. [Google Scholar] [CrossRef]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Asif, M.; Naqvi, S.A.R.; Sherazi, T.A.; Ahmad, M.; Zahoor, A.F.; Shahzad, S.A.; Hussain, Z.; Mahmood, H.; Mahmood, N. Antioxidant, antibacterial & antiproliferative activities of pumpkin (cucurbit) peel & puree extracts -An in vitro study. Pak. J. Pharm. Sci. 2017, 30, 1327–1334. [Google Scholar] [PubMed]
- Srbinoska, M.; Hrabovski, N.; Rafajlovska, V.; Sinadinović-Fišer, S. Characterization of the seed and seed extracts of the pumpkins Cucurbita maxima D. and Cucurbita pepo l. from Macedonia. Maced. J. Chem. Chem. Eng. 2012, 31, 65–78. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef]
- Phillips, K.M.; Ruggio, D.M.; Ashraf-Khorassani, M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J. Agric. Food Chem. 2005, 53, 9436–9445. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-ŁȨtowska, A.; Kucharska, A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overview on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Van Camp, J.; Trawka, A.; Verhé, R. Phenolic compounds and some quality parameters of pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2010, 112, 208–217. [Google Scholar] [CrossRef]
- Rabrenović, B.B.; Dimić, E.B.; Novaković, M.M.; Tešević, V.V.; Basić, Z.N. The most important bioactive components of cold pressed oil from different pumpkin (Cucurbita pepo L.) seeds. LWT—Food Sci. Technol. 2014, 55, 521–527. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [PubMed]
- Zhou, C.L.; Mi, L.; Hu, X.Y.; Zhu, B.H. Evaluation of three pumpkin species: Correlation with physicochemical, antioxidant properties and classification using SPME-GC–MS and E-nose methods. J. Food Sci. Technol. 2017, 54, 3118–3131. [Google Scholar] [PubMed]
- Ghosh, P.; Rana, S.S. Physicochemical, nutritional, bioactive compounds and fatty acid profiling of Pumpkin flower (Cucurbita maxima), as a potential functional food. SN Appl. Sci. 2021, 3, 1–14. [Google Scholar]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar]
- Irabor, G.E.; Ebhoaye, J.E.; Odia, A. Qualitative and quantitative screening of some phytochemical compounds in watermelon (Citrullus lanatus) seeds cultivated in esan west local government area of edo state. Int. J. Eng. Sci. Technol. 2020, 5, 268–273. [Google Scholar] [CrossRef]
- Rekha, G.; Rose, A.L.; Rose, D.A.L. Phytochemical, Minerals and Physicochemical Properties of Watermelon Seed Oil. Int. J. Innov. Sci. Res. Technol. 2018, 3, 966–972. [Google Scholar]
- Oboh, G.; Ademiluyi, A.O.; Ogunsuyi, O.B.; Oyeleye, S.I.; Dada, A.F.; Boligon, A.A. Cabbage and cucumber extracts exhibited anticholinesterase, antimonoamine oxidase and antioxidant properties. J. Food Biochem. 2017, 41, 12358. [Google Scholar]
- Badr, S.E.A.; Shaaban, M.; Elkholy, Y.M.; Helal, M.H.; Hamza, A.S.; Masoud, M.S.; Safty, M.M.E. Chemical composition and biological activity of ripe pumpkin fruits (Cucurbita pepo L.) cultivated in Egyptian habitats. Nat. Prod. Res. 2011, 25, 1524–1539. [Google Scholar] [PubMed]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Quddood, M.Y.; et al. A Comprehensive review of functional ingredients, especially bioactive compounds present in pumpkin peel, flesh and seeds, and their health benefits. Food Chem. Adv. 2022, 1, 100067. [Google Scholar]
- Altaf, A.; Sharma, H.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Manzoor, M. Phytochemical Potential and Biological Activity of Pumpkin: A Review. Food Saf. Health 2025, 1–14. [Google Scholar]
- Fatima, H.; Hussain, A.; Ambreen; Kabir, K.; Arshad, F.; Aysha, A.; Bibi, B.; Ahned, A.; Najam, A.; Firdous, N.; et al. Pumpkin seeds; an alternate and sustainable source of bioactive compounds and nutritional food formulations. J. Food Compos. Anal. 2025, 137, 106954. [Google Scholar]
- Zhang, G.; Guo, J.; Guo, J. A sustainable approach in pumpkin seed oil processing line: Recent advances in pumpkin seed oil and o il processing by-products. Food Chem. 2025, 26, 102259. [Google Scholar]
- Muhammed, H.M.; Ojukwu, P.; Hamza, U.I.; Yahaya, I.; Ndayako, H.H.; Maali, A.M. Phytochemical screening and antimicrobial activities of vernonia amygdalina (Bitter leaf), telfairia occidentalis (Pumpkin leaf) and ocimum gratissimum (Scent leaf). J. Plant Dev. 2020, 27, 55–61. [Google Scholar]
- Krauze-Baranowska, M.; Cisowski, W. Flavonoids from some species of the genus Cucumis. Biochem. Syst. Ecol. 2001, 29, 321–324. [Google Scholar]
- Glycemic Index. n.d. Home. Available online: https://glycemic-index.net (accessed on 12 February 2025).
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of the Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Hashem Dabaghian, F.; Kamalinejad, M.; Shojaei, A.; Fard, M.A. Presenting anti-diabetic plants in Iranian traditional medicine. J. Diabetes Endocrinol. 2012, 3, 70–76. [Google Scholar]
- Bharti, S.K.; Kumar, A.; Sharma, N.K.; Prakash, O.; Jaiswal, S.K.; Krishnan, S.; Gupta, A.K.; Kumar, A. Tocopherol from seeds of Cucurbita pepo against diabetes: Validation by in vivo experiments supported by computational docking. J. Formos. Med. Assoc. 2013, 112, 676–690. [Google Scholar] [CrossRef]
- Marbun, N.; Sitorus, P.; Sinaga, S.M. Antidiabetic effects of pumpkin (Cucurbita moschata durch) flesh and seeds extracts in streptozotocin induced mice. Asian J. Pharm. Clin. Res. 2018, 11, 91–93. [Google Scholar]
- Laulloo, S.B.J.; Bhowon, M.G.; Jalloo, Y. Literature review: Anti-diabetic potential of some selected edible vegetables in tropical region. Longhua Chin. Med. 2021, 4, 33. [Google Scholar]
- Chen, L.; Long, R.; Huang, G.; Huang, H. Extraction and antioxidant activities in vivo of pumpkin polysaccharide. Ind. Crops Prod. 2020, 146, 112199. [Google Scholar]
- Jane Monica, S.; John, S.; Madhanagopal, R.; Sivaraj, C.; Khusro, A.; Arumugam, P.; Gajdács, M.; Lydia, D.E.; Sahibzada, M.U.K.; Alghamdi, S.; et al. Chemical composition of pumpkin (Cucurbita maxima) seeds and its supplemental effect on Indian women with metabolic syndrome. Arab. J. Chem. 2022, 15, 103985. [Google Scholar]
- Moccia, S.; Russo, M.; Durante, M.; Lenucci, M.S.; Mita, G.; Russo, G.L. A carotenoid-enriched extract from pumpkin delays cell proliferation in a human chronic lymphocytic leukemia cell line through the modulation of autophagic flux. Curr. Res. Biotechnol. 2020, 2, 74–82. [Google Scholar]
- Sh Ali, W. Nutrition with Pumpkin (Cucrbita pepo) Cake as Lowering Cholesterol in Rats. Middle East J. Appl. Sci. 2015, 5, 10–18. [Google Scholar]
- Medjakovic, S.; Hobiger, S.; Ardjomand-Woelkart, K.; Bucar, F.; Jungbauer, A. Pumpkin seed extract: Cell growth inhibition of hyperplastic and cancer cells, independent of steroid hormone receptors. Fitoterapia 2016, 110, 150–156. [Google Scholar]
- Gossell-Williams, M.; Hyde, C.; Hunter, T.; Simms-Stewart, D.; Fletcher, H.; McGrowder, D.; Walters, C.A. Improvement in HDL cholesterol in postmenopausal women supplemented with pumpkin seed oil: Pilot study. Climacteric 2011, 14, 558–564. [Google Scholar]
- Proboningsih, J.; Wirjatmadi, B.; Kuntoro, K.; Adriani, M. Expression of VCAM in Male Wistar Rats (Rattus norvegicus) with Hypercholesterolemia Supplemented with Pumpkin Seeds (Cucurbita moschata Duch) Extract. Health Notions 2018, 2, 648–654. [Google Scholar]
- Sorour, H.A.; Selim, M.M.; EL-Sayed EL-Moselhy, L.; Ahmed, S.G. Ameliorative Effect of Watermelon rind ingestion on the Pancreas of Diabetic Female Albino Rat (Histological, immunohistochemical and morphometric study). Egypt. J. Histol. 2019, 42, 10–22. [Google Scholar]
- Mustapha Jibril, M.; Mohd Ghazali, H.; Sabri Pak Dek, M.; Shazini Ramli, N.; Abdul-Hamid, A.; Haniff Jaafar, A.; Karrupan, J.; Sabo, M. Antidiabetic Antioxidant and Phytochemical Profile of Yellow-Fleshed Seeded Watermelon (Citrullus lantus) Extracts. J. Food Nutr. Res. 2019, 7, 82–95. [Google Scholar]
- Karikpo, C.O.L.; Bartimaeus, E.S.; Holy, B. Evaluation of the Cardioprotective Effect of Citrullus lanatus (Watermelon) Seeds in Streptozotocin Induced Diabetic Albino Rats. Asian J. Biochem. Genet. Mol. Biol. 2019, 1, 1–6. [Google Scholar]
- Hong, M.Y.; Hartig, N.; Kaufman, K.; Hooshmand, S.; Figueroa, A.; Kern, M. Watermelon consumption improves inflammation and antioxidant capacity in rats fed an atherogenic diet. Nutr. Res. 2015, 35, 251–258. [Google Scholar] [PubMed]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar]
- Feizy, J.; Jahani, M.; Ahmadi, S. Antioxidant activity and mineral content of watermelon peel. J. Food Bioprocess Eng. 2020, 3, 35–40. [Google Scholar]
- Dammak, M.I.; Salem, Y.B.; Belaid, A.; Mansour, H.B.; Hammami, S.; Le Cerf, D.; Majdoub, H. Partial characterization and antitumor activity of a polysaccharide isolated from watermelon rinds. Int. J. Biol. Macromol. 2019, 136, 632–641. [Google Scholar]
- Chen, L.; Kang, Y.H. In vitro inhibitory effect of oriental melon (Cucumis melo L. var. makuwa Makino) seed on key enzyme linked to type 2 diabetes: Assessment of anti-diabetic potential of functional food. J. Funct. Foods 2013, 5, 981–986. [Google Scholar]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorization of melon fruit (Cucumis melo L.) by-products: Phytochemical and Biofunctional properties with Emphasis on Recent Trends and Advances. Trends Food Sci. Technol. 2020, 99, 507–519. [Google Scholar]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC–ESI(–)–MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar]
- Ibrahim, S.; Al Haidari, R.; Mohamed, G.; Elkhayat, E.; Moustafa, M. Cucumol A: A cytotoxic triterpenoid from Cucumis melo seeds. Rev. Bras. De Farmacogn. 2016, 26, 701–704. [Google Scholar]
- Carillon, J.; Jover, B.; Cristol, J.P.; Rouanet, J.M.; Richard, S.; Virsolvy, A. Dietary supplementation with a specific melon concentrate reverses vascular dysfunction induced by cafeteria diet. Food Nutr. Res. 2016, 60, 1–9. [Google Scholar]
- Sharmin, R.; Khan, M.R.I.; Akhtar Most, A.; Alim, A.; Islam, M.A.; Anisuzzaman, A.S.M.; Ahmed, M. Hypoglycemic and Hypolipidemic Effects of Cucumber, White Pumpkin and Ridge Gourd in Alloxan Induced Diabetic Rats. J. Sci. Res. 2012, 5, 161–170. [Google Scholar]
- Soltani, R.; Hashemi, M.; Farazmand, A.; Asghari, G.; Heshmat-Ghahdarijani, K.; Kharazmkia, A.; Ghanadian, S.M. Evaluation of the Effects of Cucumis sativus Seed Extract on Serum Lipids in Adult Hyperlipidemic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Food Sci. 2017, 82, 214–218. [Google Scholar]
- Insanu, M.; Zahra, A.A.; Sabila, N.; Silviani, V.; Haniffadli, A.; Rizaldy, D.; Fidrianny, I. Phytochemical and Antioxidant Profile: Cucumber Pulp and Leaves Extracts. Open Access Maced. J. Med. Sci. 2022, 10, 616–622. [Google Scholar]
- Nasrin, F.; Aktar, F.; Rashid, M.A. Anti-inflammatory and Antioxidant Activities of Cucumis sativus Leaves. Bangladesh Pharm. J. 2015, 18, 169–173. [Google Scholar]
- Sharma, N.; Akhtar, S.; Jamal, Q.; Kamal, M.; Khan, M.; Siddiqui, M.; Sayeed, U. Elucidation of Antiangiogenic Potential of Vitexin Obtained from Cucumis sativus Targeting Hsp90 Protein: A Novel Multipathway Targeted Approach to Restrain Angiogenic Phenomena. Med. Chem. (Shariqah (United Arab Emir.)) 2017, 13, 282–291. [Google Scholar]
- Pereira, C.; Lourenço, V.; Menezes, R.; Brites, C. Rice compounds with impact on diabetes control. Foods 2021, 10, 1992. [Google Scholar]
- Busuioc, A.C.; Costea, G.; Botezatu, A.V.D.; Furdui, B.; Dinica, R.M. Cucumis metuliferus L. Fruits Extract with Antioxidant, Anti-Inflammatory, and Antidiabetic Properties as Source of Ursolic Acid. Separations 2023, 10, 274. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Y.H.; Suh, J.K. Roasting processed oriental melon (Cucumis melo L. var. makuwa Makino) seed influenced the triglyceride profile and the inhibitory potential against key enzymes relevant for hyperglycemia. Food Res. Int. 2014, 56, 236–242. [Google Scholar]
- Poduri, A.; Rateri, D.L.; Saha, S.K.; Saha, S.; Daugherty, A. Citrullus lanatus ‘sentinel’ (watermelon) extract reduces atherosclerosis in LDL receptor-deficient mice. J. Nutr. Biochem. 2013, 24, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hiamed, H. Hypocholesterolemic effects of watermelon fruit rind on rats. Nutr. Food Sci. 2018, 48, 836–845. [Google Scholar] [CrossRef]
- Bölek, S. Determination of in Vitro Antioxidant Activity and Bioactive Compounds of Kiwano Seeds. In Proceedings of the International Conference on Research in Health Sciences, Kuala Lumpur, Malaysia, 15–20 May 2020. [Google Scholar]
- Men, X.; Choi, S.I.; Han, X.; Kwon, H.Y.; Jang, G.W.; Choi, Y.E.; Park, S.-M.; Lee, O.-K. Physicochemical, nutritional and functional properties of Cucurbita moschata. Food Sci. Biotechnol. 2021, 30, 171. [Google Scholar] [CrossRef]
- Devidas, C.; Karad, V.; Deshmukh, C.D.; Jain, A.; Tambe, M.S. Phytochemical and Pharmacological profile of Citrullus lanatus (THUNB). Biolife—Int. Q. J. Biol. Sci. 2015, 3, 483–488. [Google Scholar]
- Chan, K.T.; Li, K.; Liu, S.L.; Chu, K.H.; Toh, M.; Xie, W.D. Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett. 2010, 289, 46–52. [Google Scholar] [CrossRef]
- Sadou, H.; Sabo, H.; Alma, M.M.M.; Mahamane, S.; Claude-louis, L. Chemical content of the seeds and physico-chemical characteristic of the seed oils from citrullus colocynthis, coccinia grandis, cucumis metuliferus and cucumis prophetarum of niger. Bull. Chem. Soc. Ethiop. 2007, 21, 323–330. [Google Scholar] [CrossRef]


| Components (%) | Pumpkin (C. moschata, C. pepo, C. maxima) | Watermelon (C. lantus, C. vulgaris) | Melon (C. melo) | Horned Melon (C. metuliferus) | Cucumber (C. sativus) | References | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flesh | Peel | Seeds | Flowers (Various Species) | Leaves (T. occidentalis) | Flesh | Peel | Seeds | Flesh | Peel | Seeds | Flesh | Peel | Seeds | Flesh | Peel | Seeds | ||
| Carbohydrate | 2.62–48.40 | 4.37–20.68 | 6.37–37.9 | 3.02–3.90 | 26.82 | 3.10–8.00 | 42–65 | 6.06–21.20 | 0.50–1.31 | 3.05–57.90 | 8.00–39.00 | 7.56 | 54.80 | 50.2 | 52.70 | 33.70 | 50.10 | [4,8,9,12,15,16,17,19,20] |
| Protein | 0.20–15.50 | 0.92–23.95 | 14.31–38.0 | 1.14–1.50 | 56.00 | 0.12–0.60 | 6.77 | 34.00–35.00 | 3.24 | 0.80–3.05 | 14.91–29.90 | 1.78–1.80 | 2.95 | 2.63 | 15.90 | 26.50 | 38.88 | |
| Lipid | 0.04–0.42 | 0.31–6.57 | 21.9–54.9 | 0.21–0.33 | 2.00 | 0.05–0.10 | 0.92 | 26.83–46.78 | 1.10 | 1.58–2.12 | 35.60–47.00 | 0.03–1.26 | 8.89 | 15.4 | 0.13 | 1.44 | 95.79 | |
| Fiber | 0.37–11.25 | 0.13–33.92 | 1.00–16.15 | - | - | 0.20–0.60 | 24 | 2.00–3.00 | 8.83 | 41.69 | 5.51–24.75 | 4–4.20 | 11.30 | 19.2 | 6.77 | 8.86 | 3.00 | |
| Ash | 0.34–6.64 | 0.63–10.65 | 3.0–5.50 | 0.85–1.92 | 7.73 | 0.28–1.13 | 3.07–13.20 | 3.00 | 2.40 | 3.67 | 1.50–4.83 | - | - | - | 11.60 | 7.85 | 9.34 | |
| Moisture | 18.03–96.77 | 9.76–93.59 | 1.80–7.40 | - | 7.45 | 90.82–95.00 | 95.63 | 8.00–9.00 | 83.05 | 16.95 | 4.27–7.78 | 89–96.0 | 18.4 | 7.31 | 12.90 | 21.70 | 12.30 | |
| Mineral Composition | Pumpkin (C. moschata, C. pepo, C. maxima) (mg/100 g) | Watermelon (C. lantus, C. vulgaris) | Melon (C. melo) (mg/100 g) | Horned Melon (C. metuliferus) (mg/100 g) | Cucumber (C. sativus) (mg/100 g) | References | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flesh | Peel | Seeds | Flowers (Various Species and Varieties) | Leaves (T. occidentalis) | Flesh (mg/g) | Peel (mg/g) | Seeds (mg/100 g) | Flesh | Peel | Seeds | Flesh | Peel | Seeds | Flesh | Peel | Seeds | ||
| Calcium | 21 | 1.360 | 8.44–141.00 | 13.78–37.77 | 75.0 | 1.87 | 3.10 | 0.16 | 855.25 | 14.69–4201.4 | 8.34–806.4 | 13–17 | - | 247 | 139 | 168 | 177 | [6,7,12,16,17,19,20,28] |
| Iron | 0.8 | 4.004 | 3.75–1676 | 1.26–5.29 | 3.7 | 0.34 | 0.22 | 3.71 | 1.82 | 0.4–3.4 | 2.69–81.17 | 0.50–1.13 | - | 10.90 | 7.80 | 7.39 | 9.08 | |
| Magnesium | 12 | 3.353 | 67.41–2385.00 | 17.72–35.83 | 33.0 | 1.85 | 2.72 | 0.15 | 328.75 | 13.27–389.65 | 101.71–3299.27 | 16.2–40 | - | 289 | 70.8 | 64.1 | 64.4 | |
| Phosphorous | 44 | 1.419 | 47.68–1471.24 | - | - | 4.64 | 7.81 | 0.17 | - | - | - | 37–50 | - | 44.70 | - | - | - | |
| Potassium | 340 | 687.47 | 103.12–4300.00 | 199.73–349.66 | 352.0 | 18.53 | 88.67 | 3.57 | 2113.75 | 110.39–1791.9 | 509.8–9548.33 | 123–302 | - | 1174 | 437 | 454 | 541 | |
| Sodium | 1.0 | 9,652 | 0.69–189.81 | - | 89.7 | - | - | - | 137.58 | 8.54–277.9 | 41.22–386.13 | 2–5.60 | - | 247 | 151 | 113 | 156 | |
| Zinc | 0.32 | 0.150 | 1.09–14.14 | 0.48–0.78 | 19.3 | 0.038 | 0.045 | 3.71 | 0.7 | 0.23–2.3 | 2.34–44.03 | 0.20–0.48 | - | 1.70 | 5.27 | 2.66 | 5.46 | |
| Copper | 0.127 | 0.025 | 0.30–89.84 | - | 36.4 | 0.028 | 0.005 | 0.38 | 0.2 | 0.07–8.9 | 0.53–15.9 | 0.10 | - | 5.40 | 2.49 | 1.69 | 2.21 | |
| Manganese | 0.125 | 0.360 | 0.06–8.90 | 0.18–0.38 | - | 0.057 | 0.095 | 0.02 | 0.48 | 0.41–4.1 | 1.25–15.20 | 0.039 | - | - | 0.79 | 0.4 | 0.56 | |
| Selenium | 0.3 µg | - | 0.13–1.91 | - | - | - | - | - | - | - | - | 13–17 | - | 247 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borecka, M.; Karaś, M. A Comprehensive Review of the Nutritional and Health-Promoting Properties of Edible Parts of Selected Cucurbitaceae Plants. Foods 2025, 14, 1200. https://doi.org/10.3390/foods14071200
Borecka M, Karaś M. A Comprehensive Review of the Nutritional and Health-Promoting Properties of Edible Parts of Selected Cucurbitaceae Plants. Foods. 2025; 14(7):1200. https://doi.org/10.3390/foods14071200
Chicago/Turabian StyleBorecka, Magdalena, and Monika Karaś. 2025. "A Comprehensive Review of the Nutritional and Health-Promoting Properties of Edible Parts of Selected Cucurbitaceae Plants" Foods 14, no. 7: 1200. https://doi.org/10.3390/foods14071200
APA StyleBorecka, M., & Karaś, M. (2025). A Comprehensive Review of the Nutritional and Health-Promoting Properties of Edible Parts of Selected Cucurbitaceae Plants. Foods, 14(7), 1200. https://doi.org/10.3390/foods14071200







