Increased Temperature Effects During Fruit Growth and Maturation on the Fruit Quality, Sensory and Antioxidant Properties of Raspberry (Rubus idaeus L.) cv. Heritage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Treatments
2.3. Harvest
2.4. Fruit Quality Assessments
2.5. Analyses of Antioxidant Capacity Related to Polyphenolic Compounds
2.5.1. Extraction of Polyphenolic Compounds
2.5.2. Antioxidant Capacity (ORAC)
2.5.3. Total Polyphenol Content (TPC)
2.5.4. Total Flavonoid Content (TFC)
2.5.5. Total Anthocyanin Content (AC)
2.6. Vitamin C Analysis
2.7. Sensory Analyses
2.8. Statistical Analysis
3. Results
3.1. Weather Conditions and Temperature Regimes
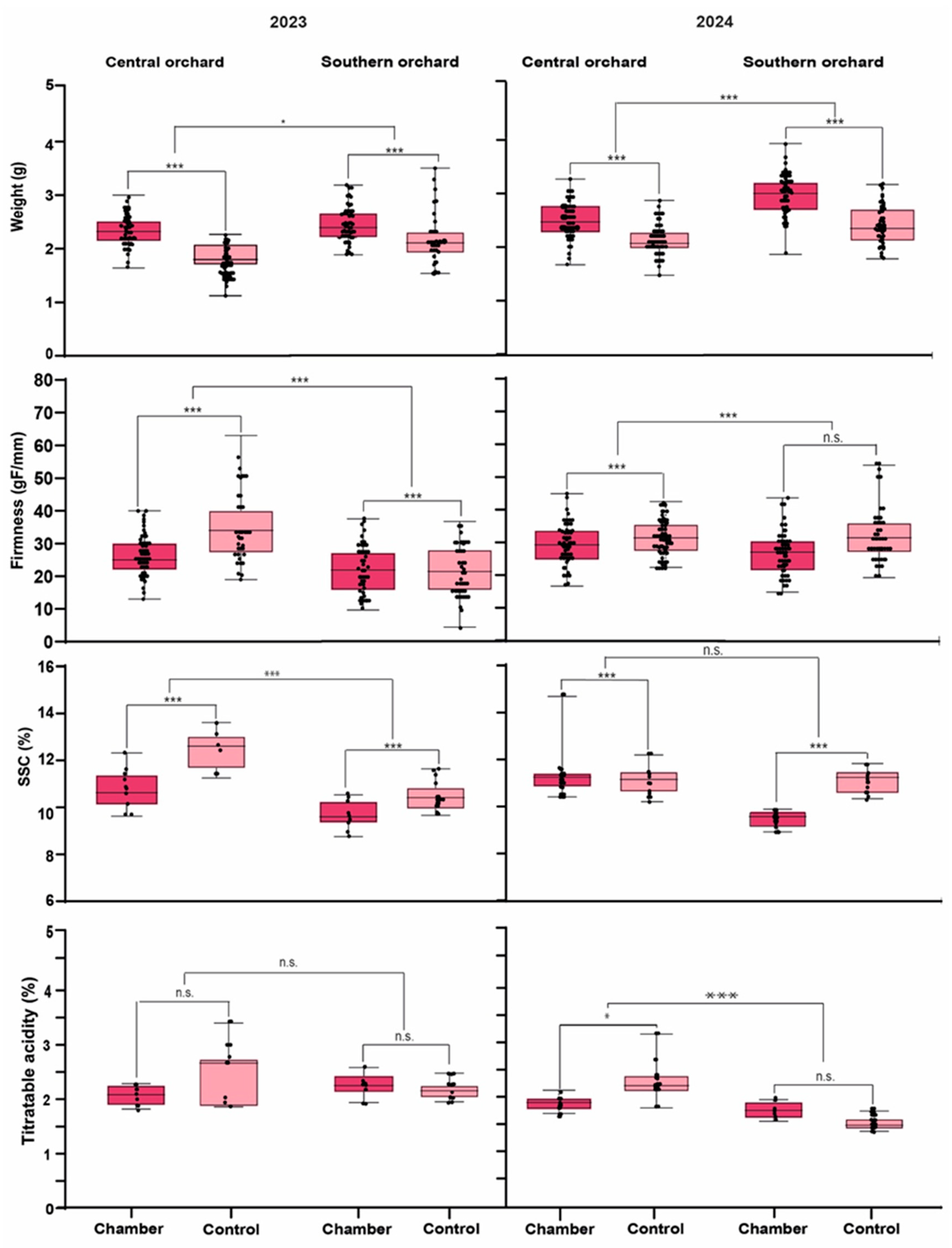
3.2. Effect of Higher Temperature on Fruit Quality Traits
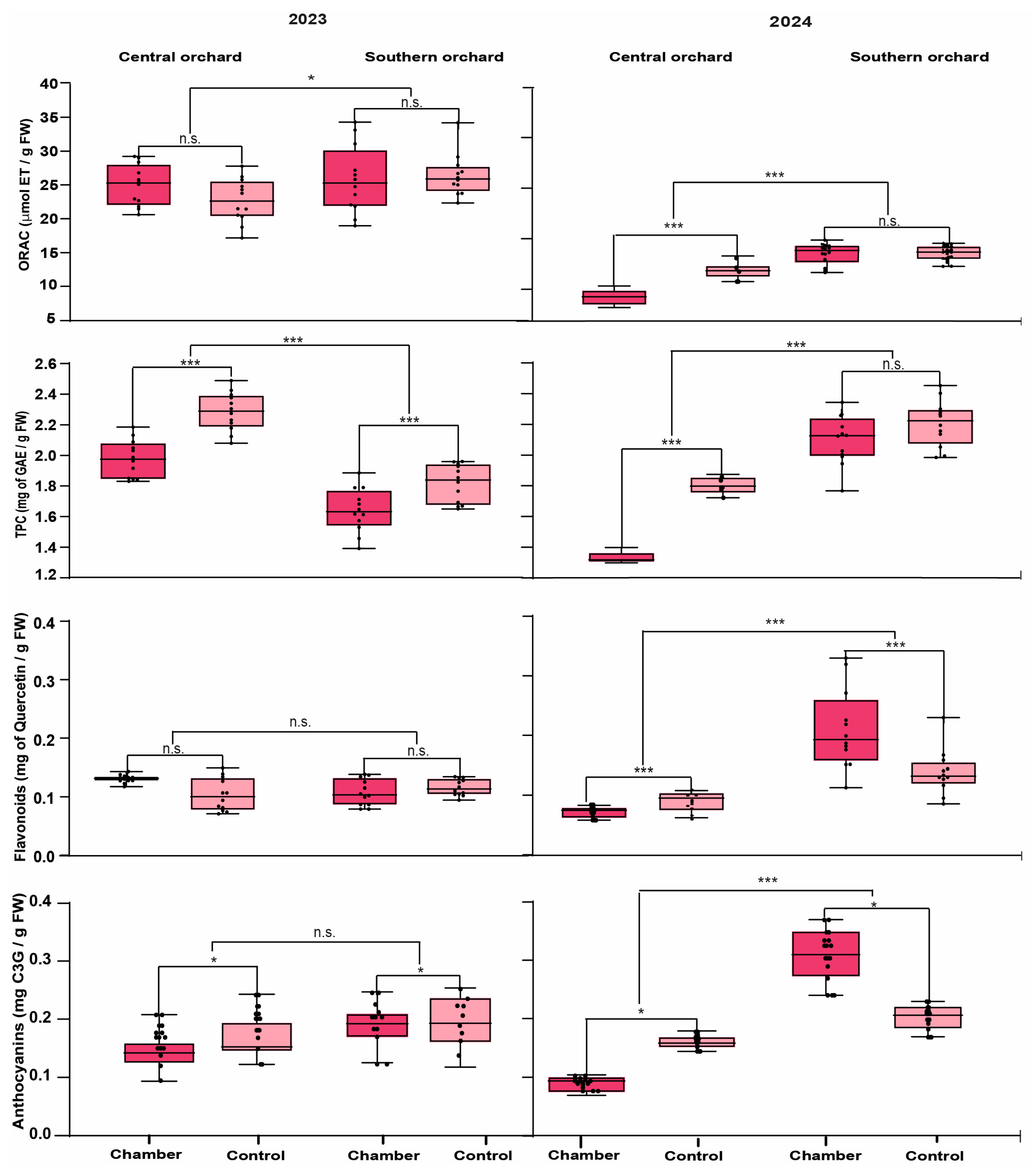
3.3. Effect of Higher Temperature on Antioxidant Properties
3.4. Vitamin C
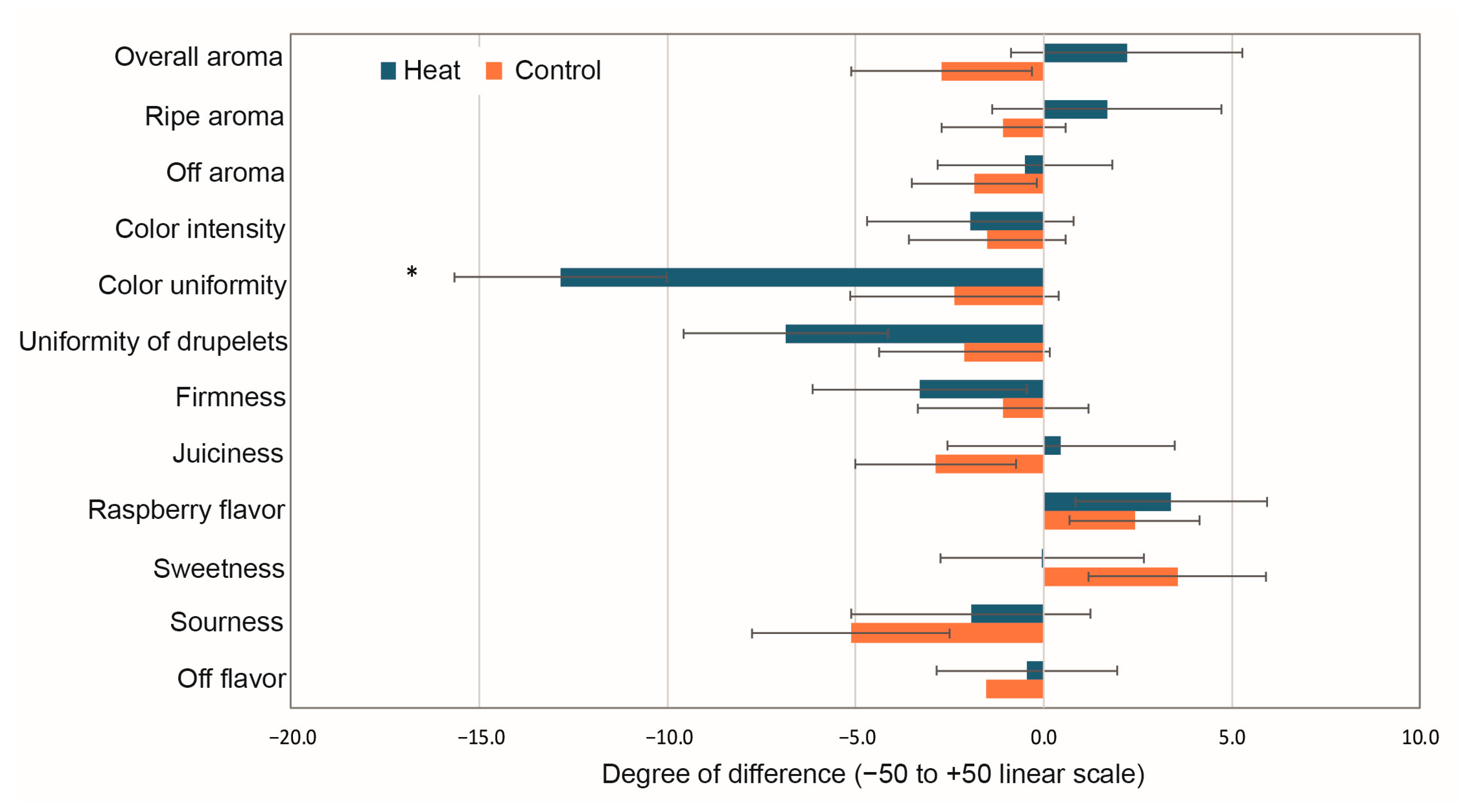
3.5. Sensory Analyses
4. Discussion
4.1. Fruit Quality
4.2. Antioxidant Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baselice, A.; Colantuoni, F.; Lass, D.; Nardone, G.; Stasi, A. Trends in EU consumers’ attitude towards fresh-cut fruit and vegetables. Food Qual. Prefer. 2017, 59, 87–96. [Google Scholar] [CrossRef]
- Shah, H.; Singh, Z.; Kaur, J.; Hasan, M.; Woodward, A.; Afrifa-Yamoah, E. Trends in maintaining postharvest freshness and quality of rubus berries. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4600–4643. [Google Scholar] [CrossRef] [PubMed]
- Chilealimentos. Available online: https://chilealimentos.com/wp-content/uploads/2023/09/AntonioDominguez_compressed.pdf (accessed on 8 March 2024).
- International Raspberry Organization. Available online: https://www.internationalraspberry.net (accessed on 15 January 2025).
- Graham, J.; Simpson, C. Developmental Transitions to Fruiting in Red Raspberry. In The Genomes of Rosaceous Berries and Their Wild Relatives; Hytönen, T., Graham, J., Harrison, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 3, pp. 199–212. [Google Scholar] [CrossRef]
- Popović, T.; Šarić, B.; Martačić, J.D.; Arsić, A.; Jovanov, P.; Stokić, E.; Mišan, A.; Mandić, A. Potential health benefits of blueberry and raspberry pomace as functional food ingredients: Dietetic intervention study on healthy women volunteers. Front. Nutr. 2022, 9, 969996. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Karlidag, H.; Şengül, M. Characterization of Red Raspberry (Rubus idaeus L.) Genotypes for Their Physicochemical Properties. J. Food Sci. 2009, 74, 575–579. [Google Scholar] [CrossRef]
- Kassim, A.; Poette, J.; Paterson, A.; Zait, D.; McCallum, S.; Woodhead, M.; Smith, K.; Hackett, C.; Graham, J. Environmental and seasonal influences on red raspberry anthocyanin antioxidant contents and identification of quantitative traitsloci (QTL). Mol. Nutr. Food Res. 2009, 53, 625–634. [Google Scholar] [CrossRef]
- Akhatou, I.; Domínguez, R.G.; Recamales, A.F. Investigation of the effect of genotype and agronomic conditions on metabolomic profiles of selected strawberry cultivars with different sensitivity to environmental stress. Plant Physiol. Biochem. 2016, 101, 14–22. [Google Scholar] [CrossRef]
- Marchi, P.; Carvalho, I.; Pereira, I.; Rosa, T.; Hohn, D.; Szareski, V.; Reisser, C.; Junior, A. Yield and quality of primocane-fruiting raspberry grown under plastic cover in southern Brazil. Sci. Agric. 2019, 76, 481–486. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Nešović, M.; Ćirić, I.; Tešić, Ž.; Pezo, L.; Tosti, T.; Gašić, U.; Dojčinović, B.; Lončar, B.; Meland, M. Chemical fruit profiles of different raspberry cultivars grown in specific Norwegian agroclimatic conditions. Horticulturae 2022, 8, 765. [Google Scholar] [CrossRef]
- Kotuła, M.; Kapusta-Duch, J.; Smoleń, S.; Doskočil, I. Phytochemical composition of the fruits and leaves of raspberries (Rubus idaeus L.)—Conventional vs. organic and those wild grown. Appl. Sci. 2022, 12, 11783. [Google Scholar] [CrossRef]
- Kishor, P.; Guddimalli, R.; Kulkarni, J.; Singam, P.; Somanaboina, A.; Nandimandalam, T.; Patil, S.; Polavarapu, R.; Suravajhala, P.; Sreenivasulu, N.; et al. Impact of Climate Change on Altered Fruit Quality with Organoleptic, Health Benefit, and Nutritional Attributes. J. Agric. Food Chem. 2023, 71, 17510–17527. [Google Scholar] [CrossRef]
- Abbas, S.; Mayo, Z. Impact of temperature and rainfall on rice production in Punjab, Pakistan. Environ. Dev. Sustain. 2021, 23, 1706–1728. [Google Scholar] [CrossRef]
- Asfew, M.; Mitiku, F.; Gemechu, A.; Bekele, Y.; Lemma, T. Do climate change and political instability affect crop production in sub-Saharan Africa countries? J. Agric. Food Res. 2023, 12, 100576. [Google Scholar] [CrossRef]
- Chandio, A.; Ozturk, I.; Akram, W.; Ahmad, F.; Mirani, A. Empirical analysis of climate change factors affecting cereal yield: Evidence from Turkey. Environ. Sci. Pollut. Res. 2020, 27, 11944–11957. [Google Scholar] [CrossRef] [PubMed]
- Pechan, P.; Bohle, H.; Obster, F. Reducing Vulnerability of Fruit Orchards to Climate Change. Agric. Syst. 2023, 210, 103713. [Google Scholar] [CrossRef]
- Swanson, J.; Carlson, J.; Fernández-Fernández, F.; Finn, C.; Graham, J.; Weber, C.; Sargent, D. Blackberries and Raspberries. In Genetics, Genomics and Breeding of Berries, 1st ed.; Folta, M., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 64–105. [Google Scholar] [CrossRef]
- Osatuke, A.; Pritts, M. Strawberry flavor is influenced by the air temperature differential during fruit development but not management practices. Agronomy 2021, 11, 606. [Google Scholar] [CrossRef]
- Bradish, C.; Perkins-Veazie, P.; Fernandez, G.; Xie, G.; Jia, W. Comparison of Flavonoid Composition of Red Raspberries (Rubus idaeus L.) Grown in the Southern United States. J. Agric. Food Chem. 2012, 60, 5779–5786. [Google Scholar]
- Ozgen, M.; Wyzgoski, F.; Tulio, A.; Gazula, A.; Miller, A.R.; Scheerens, J.; Reese, R.; Wright, S. Antioxidant capacity and phenolic antioxidants of midwestern black raspberries grown for direct markets are influenced by production site. HortScience 2008, 43, 2039–2047. [Google Scholar] [CrossRef]
- Fuentealba, C.; Álvarez, F.; Veas, S.; Salazar, M.; Romero, D.; Ayala-Raso, A.; Alvaro, J.; Valdenegro, M.; Figueroa, C.; Fuentes, L. Differences in primary metabolism related to quality of raspberry (Rubus idaeus L.) fruit under open field and protected soilless culture growing conditions. Front. Plant Sci. 2024, 14, 1324066. [Google Scholar] [CrossRef]
- Myers, S.; Wessells, K.; Kloog, I.; Zanobetti, A.; Schwartz, J. Effect of increased concentrations of atmospheric carbon dioxide on the global threat of zinc deficiency: A modelling study. Lancet Glob. Health 2015, 3, 639–645. [Google Scholar] [CrossRef]
- Smith, M.; Golden, C.; Myers, S. Potential rise in iron deficiency due to future anthropogenic carbon dioxide emissions. GeoHealth 2017, 1, 248–257. [Google Scholar] [CrossRef]
- Ziska, L.; Namuco, O.; Moya, T.; Quilang, J. Growth and yield response of field-grown tropical rice to increasing carbon dioxide and air temperature. Agron. J. 1997, 89, 45–53. [Google Scholar] [CrossRef]
- Martre, P.; Dueri, S.; Guarin, J.R.; Ewert, F.; Webber, H.; Calderini, D.; Molero, G.; Reynolds, M.; Miralles, D.; Garcia, G.; et al. Global needs for nitrogen fertilizer to improve wheat yield under climate change. Nat. Plants 2024, 10, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, 1012. [Google Scholar] [CrossRef]
- Alaba, O.A.; Bechami, S.; Chen, Y.-Y.; Gara, T.W.; Perkins, B.; Zhang, Y. Will global warming reduce the nutritional quality of wild blueberries? Clim. Change Ecol. 2024, 8, 100088. [Google Scholar]
- Lizana, X.; Avila, A.; Tolaba, A.; Martinez, J. Field Responses of Potato to Increased Temperature during Tuber Bulking: Projection for Climate Change Scenarios, at High-Yield Environments of Southern Chile. Agric. Meteorol. 2017, 239, 192–201. [Google Scholar] [CrossRef]
- Álvarez, F.; Moya, M.; Rivera-Mora, C.; Zúñiga, P.; Jara-Cornejo, K.; Muñoz, P.; Ayala-Raso, A.; Munné-Bosch, S.; Figueroa, C.; Figueroa, N.; et al. Abscisic Acid Synthesis and Signaling during the Ripening of Raspberry (Rubus idaeus ‘Heritage’) Fruit. Plants 2023, 12, 1882. [Google Scholar] [CrossRef]
- García, J.; González, G.; Ciordia, M. El Cultivo del Frambueso, 1st ed.; SERIDA: Asturias, Spain, 2014; pp. 1–77. [Google Scholar]
- Zuñiga, P.; Castañeda, Y.; Arrey-Salas, O.; Fuentes, L.; Aburto, F.; Figueroa, C. Methyl jasmonate applications from flowering to ripe fruit stages of strawberry (Fragaria × ananassa ‘Camarosa’) reinforce the fruit antioxidant response at post-harvest. Front. Plant Sci. 2020, 11, 538. [Google Scholar] [CrossRef]
- Valdenegro, M.; Bernales, M.; Knox, M.; Vinet, R.; Caballero, E.; Ayala-Raso, A.; Kučerová, D.; Kumar, R.; Viktorová, J.; Ruml, T.; et al. Characterization of Fruit Development, Antioxidant Capacity, and Potential Vasoprotective Action of Peumo (Cryptocarya alba), a Native Fruit of Chile. Antioxidants 2021, 10, 1997. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.; Wrolstad, R.E.; Eisele, T.; Giusti, M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.; Kupina, S.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Sion, M. Genetic diversity, antioxidant activities, and anthocyanin contents in lingonberry. Int. J. Fruit Sci. 2009, 9, 185–199. [Google Scholar] [CrossRef]
- Siegelman, H.W.; Hendrick, J.P. The molar extinction coefficient of cyanidin-3-glucoside. Plant Physiol. 1958, 33, 135–139. [Google Scholar] [CrossRef]
- Van De Velde, F.; Pirovani, M.; Cámara, M.; Güemes, D.R.; Bernardi, C. Optimization and validation of a UV-HPLC method for vitamin C determination in strawberries (Fragaria ananassa Duch.), using experimental designs. Food Anal. Methods 2012, 5, 1097–1104. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- AOAC. Vitamins. In Official Methods of Analysis, 16th ed.; Deutsch, M., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995; pp. 16–18. [Google Scholar]
- Meilgaard, M.; Civille, G.; Carr, B. Sensory Evaluation Techniquqes, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Plotto, A.; Chandler, C.; Whitaker, V.; Jouquand, C.; Bai, J.; Narciso, J.; Baldwin, E.; Blanco-Díaz, M.; Ritenour, M.; Li, J. Sensory testing tips and procedures to evaluate quality of stored and minimally processed fruits and vegetables. Acta Hortic. 2016, 1141, 47–56. [Google Scholar] [CrossRef]
- Lippi, N.; Senger, E.; Karhu, S.; Mezzetti, B.; Cianciabella, M.; Denoyes, B.; Soenmez, D.; Fidelis, M.; Gatti, E.; Hoefer, M.; et al. Development and Validation of a Multilingual Lexicon as a Key Tool for the Sensory Analyses and Consumer Tests of Blueberry and Raspberry Fruit. Agriculture 2023, 13, 314. [Google Scholar] [CrossRef]
- Bazgaou, A.; Fatnassi, H.; Bouharroud, R.; Ezzaeri, K.; Gourdo, L.; Wifaya, A.; Demrati, H.; Elame, F.; Carreno-Ortega, A.; Bekkaoui, A.; et al. Effect of active solar heating system on microclimate, development, yield and fruit quality in greenhouse tomato production. Renew. Energy 2021, 165, 237–250. [Google Scholar] [CrossRef]
- Fraga, K.; Rangel, J.; Campostrini, E.; Salinas, I.; Hueso, J.; Cuevas, J. Leaf age does not justify its early removal in Carica papaya L. Ann. Appl. Biol. 2019, 176, 26–35. [Google Scholar] [CrossRef]
- Funt, R.; Hall, H. Raspberries, 3rd ed.; CABI: Boston, MA, USA, 2013; pp. 133–155. [Google Scholar]
- Gouot, J.; Smith, J.P.; Holzapfel, B.; Walker, A.; Barril, C. Impact of short temperature exposure of Vitis vinifera L. cv. Shiraz grapevine bunches on berry development, primary metabolism and tannin accumulation. J. Exp. Bot. 2019, 168, 103866. [Google Scholar] [CrossRef]
- Giongo, L.; Ajelli, M.; Poncetta, P.; Ramos-García, M.; Sambo, P.; Farneti, B. Raspberry texture mechanical profiling during fruit ripening and storage. Postharvest Biol. Technol. 2019, 149, 177–186. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.; Su, G.; Zhang, M.; Grierson, D.; Chen, K. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.; Cervilla, L.; Rios, J.; Blasco, B.; Sanchez-Rodriguez, E.; Romero, L.; Ruiz, J. Environmental conditions affect pectin solubilization in cherry tomato fruits grown in two experimental Mediterranean greenhouses. Environ. Exp. Bot. 2009, 67, 320–327. [Google Scholar] [CrossRef]
- Biasi, R.; Brunori, E.; Ferrara, C.; Salvati, L. Assessing impacts of climate change on phenology and quality traits of Vitis vinifera L.: The contribution of local knowledge. Plants 2019, 8, 121. [Google Scholar] [CrossRef]
- Xyrafis, E.; Deloire, A.; Petoumenou, D.; Paraskevopoulos, I.; Biniari, K. The unique and extreme vineyard of Santorini Island (Cyclades). IVES Tech. Rev. 2021. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Moukarzel, R.; Parker, A.; Schelezki, O.; Gregan, S.; Jordan, B. Bunch microclimate influence amino acids and phenolic profiles of Pinot Noir grape berries. Front. Plant Sci. 2023, 14, 1162062. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Castellarin, S. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario–A Review. Front. Plant Sci. 2021, 12, 262. [Google Scholar] [CrossRef]
- Dominguez, D.; Cirrincione, M.; Deis, L.; Martínez, L. Impacts of Climate Change-Induced Temperature Rise on Phenology, Physiology, and Yield in Three Red Grape Cultivars: Malbec, Bonarda, and Syrah. Plants 2024, 13, 3219. [Google Scholar] [CrossRef]
- Leolini, L.; Moriondo, M.; Romboli, Y.; Gardiman, M.; Costafreda-Aumedes, S.; Costafreda-Aumedes, S.; Bindi, M.; Granchi, L.; Brilli, L. Modelling Sugar and Acid Content in Sangiovese Grapes under Future Climates: An Italian Case Study. Clim. Res. 2019, 78, 211–224. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. 2018, 38, 66. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Sandhu, A.; Edirisinghe, I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef]
- Rao, A.; Snyder, D. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef]
- Schulz, M.; Chim, J. Nutritional and Bioactive Value of Rubus Berries. Food. Biosci. 2019, 31, 100438. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; Dos Santos, A.; Chalfun-Júnior, A.; Peres, L.; Zhao, J.; Benedito, V. Understanding the genetic regulation of anthocyanin biosynthesis in plants–tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef]
- Liang, Z.; Sang, M.; Fan, P.; Wu, B.; Wang, L.; Duan, W.; Li, S. Changes of polyphenols, sugars, and organic acid in 5 Vitis genotypes during berry ripening. J. Food Sci. 2011, 76, C1231–C1238. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Q.; Yan, T.; Yu, B.; Wang, Q.; Wang, D. Sugar and anthocyanins: A scientific exploration of sweet signals and natural pigments. Plant Sci. 2025, 353, 112409. [Google Scholar] [CrossRef]
- Freeman, B.; Stocks, J.; Eggett, D.; Parker, T. Antioxidant and phenolic changes across one harvest season and two storage conditions in primocane raspberries (Rubus idaeus L.) grown in a hot, dry climate. HortScience 2011, 46, 236–239. [Google Scholar] [CrossRef]
- Balasooriya, B.; Dassanayake, K.; Ajlouni, S. High temperature effects on strawberry fruit quality and antioxidant contents. Acta Hortic. 2020, 1278, 225–234. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Pieri, P.; Charon, J.; Pillet, J.; Hilbert, G.; Renaud, C.; Gomès, E.; Delrot, S.; Lecourieux, D. Dissecting the biochemical and transcriptomic effects of a locally applied heat treatment on developing cabernet sauvignon grape berries. Front. Plant. Sci. 2017, 8, 53. [Google Scholar] [CrossRef]
- Arrizabalaga, M.; Morales, F.; Oyarzun, M.; Delrot, S.; Gomès, E.; Irigoyen, J.; Hilbert, G.; Pascual, I. Tempranillo Clones Differ in the Response of Berry Sugar and Anthocyanin Accumulation to Elevated Temperature. Plant Sci. 2018, 267, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Grau, O.; Chen, H.; Janssens, I.; Ciais, P.; Piao, S.; Penuelas, J. Changes in nutrient concentrations of leaves and roots in response to global change factors. Glob. Change Biol. 2017, 23, 3849–3856. [Google Scholar] [CrossRef]
- Gallie, D.R. L-ascorbic acid: A multifunctional molecule supporting plant growth and development. Scientifica 2013, 1, 795964. [Google Scholar] [CrossRef]

| Year | 2023 | 2024 | ||||||
|---|---|---|---|---|---|---|---|---|
| Orchard | Central | Southern | Central | Southern | ||||
| Treatment | Control | Chamber | Control | Chamber | Control | Chamber | Control | Chamber |
| Average T° (°C) | 18.91 | 20.45 | 13.16 | 17.55 | 18.97 | 22.63 | 12.30 | 16.25 |
| Max T° (°C) | 35.59 | 38.55 | 28.73 | 37.03 | 33.5 | 46.59 | 28.82 | 36.01 |
| Min T° (°C) | 4.96 | 7.47 | 1.96 | 6.63 | 5.42 | 5.72 | −2.31 | 1.40 |
| Relative Humidity (%) | 66.41 | 66.07 | 83.07 | 84.53 | 74.19 | 70.97 | 86.65 | 82.92 |
| p—Value 1 | |||||
|---|---|---|---|---|---|
| Quality Parameters | |||||
| Year | Effect | Weight (g) | Firmness (gF/mm) | SSC (%) | TA (%) |
| 2023 | Orchard | 0.0109 * | 1.55 × 10−5 *** | 9.92 × 10−10 *** | n.s. |
| Heat Treatment | 1.01 × 10−11 *** | 1.51 × 10−11 *** | 2.30 × 10−7 *** | n.s. | |
| Orchard × Heat Treatment | 0.0158 * | n.s. | 0.0206 * | 0.00139 ** | |
| 2024 | Orchard | 1.48 × 10−8 *** | 0.000386 *** | n.s. | 4.64 × 10−8 *** |
| Heat Treatment | <2 × 10−16 *** | 4.62 × 10−6 *** | 4.61 × 10−10 *** | 0.0375 * | |
| Orchard × Heat Treatment | 0.0329 * | n.s. | 0.0128 * | 2.36 × 10−6 *** | |
| p—Value 1 | |||||
|---|---|---|---|---|---|
| Antioxidant Parameters | |||||
| Year | Effect | ORAC (μmol ET/g FW) | TPC (mg GAE/g FW) | Flavonoids (mg de Quercetin/g FW) | Anthocyanins (mg C3G/g FW) |
| 2023 | Orchard | 0.0393 * | 6.88 × 10−14 *** | n.s. | n.s. |
| Heat Treatment | n.s. | 5.42 × 10−8 *** | n.s. | 0.0367 * | |
| Orchard × Heat Treatment | n.s. | n.s. | 0.00156 ** | n.s. | |
| 2024 | Orchard | <2 × 10−16 *** | <2 × 10−16 *** | 1.74 × 10−14 *** | <2 × 10−16 *** |
| Heat Treatment | 3.61 × 10−11 *** | 3.10 × 10−14 *** | n.s | 0.0164 * | |
| Orchard × Heat Treatment | 2.24 × 10−10 *** | 7.68 × 10−10 *** | 9.15 × 10−7 *** | <2 × 10−16 *** | |
| Vitamin C (mg/100 g FW) | ||||
|---|---|---|---|---|
| Year | Orchard | Temperature | ||
| 2023 | Central | 24.04 a* | Heat | 16.77 a |
| Southern | 16.81 a | Control | 24.08 a | |
| 2024 | Central | 24.73 a | Heat | 17.26 b |
| Southern | 17.99 b | Control | 25.47 a | |
| p—Value 1 | ||
|---|---|---|
| Vitamin C (mg/100 g FW) | ||
| Year | Effect | Vitamin C (mg/100 g FW) |
| 2023 | Orchard | n.s. |
| Heat Treatment | n.s. | |
| Orchard × Heat Treatment | n.s. | |
| 2024 | Orchard | 0.0354 * |
| Heat Treatment | 0.0151 * | |
| Orchard × Heat Treatment | n.s. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, F.; Salazar, M.; Fuentes, L.; Calderini, D.; Jerez, A.; Contreras, C. Increased Temperature Effects During Fruit Growth and Maturation on the Fruit Quality, Sensory and Antioxidant Properties of Raspberry (Rubus idaeus L.) cv. Heritage. Foods 2025, 14, 1201. https://doi.org/10.3390/foods14071201
Aguilar F, Salazar M, Fuentes L, Calderini D, Jerez A, Contreras C. Increased Temperature Effects During Fruit Growth and Maturation on the Fruit Quality, Sensory and Antioxidant Properties of Raspberry (Rubus idaeus L.) cv. Heritage. Foods. 2025; 14(7):1201. https://doi.org/10.3390/foods14071201
Chicago/Turabian StyleAguilar, Francisca, Martina Salazar, Lida Fuentes, Daniel Calderini, Alejandro Jerez, and Carolina Contreras. 2025. "Increased Temperature Effects During Fruit Growth and Maturation on the Fruit Quality, Sensory and Antioxidant Properties of Raspberry (Rubus idaeus L.) cv. Heritage" Foods 14, no. 7: 1201. https://doi.org/10.3390/foods14071201
APA StyleAguilar, F., Salazar, M., Fuentes, L., Calderini, D., Jerez, A., & Contreras, C. (2025). Increased Temperature Effects During Fruit Growth and Maturation on the Fruit Quality, Sensory and Antioxidant Properties of Raspberry (Rubus idaeus L.) cv. Heritage. Foods, 14(7), 1201. https://doi.org/10.3390/foods14071201






