A Comparative Study on Physicochemical Properties and Biological Activities of Polysaccharides from Coreopsis tinctoria Buds Obtained by Different Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
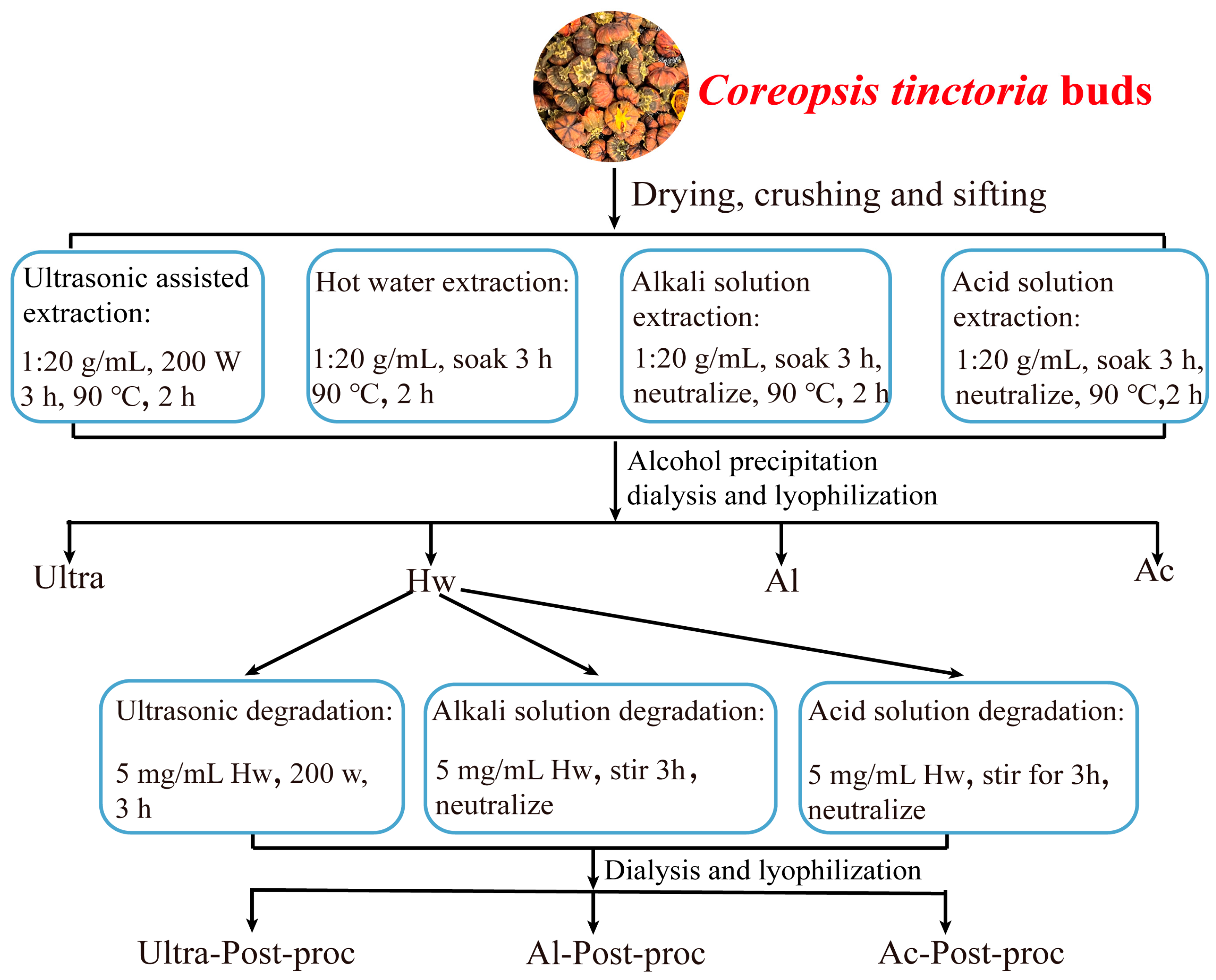
2.2. Preparation of Coreopsis tinctoria Buds Polysaccharides (CTBPs)
2.3. The Composition of CTBPs
2.4. The Molecular Weight (Mw) of CTBPs
2.5. The Particle Size and Zeta Potential of CTBPs
2.6. Spectral Analysis of CTBPs
2.7. Congo Red Experiments
2.8. Water Holding Capacity Analysis
2.9. Oil Holding Capacity Analysis
2.10. Bile Acid Binding Capacity of CTBPs
2.11. Activities Analysis
2.11.1. α-Glucosidase Inhibitory Activities of CTBPs
2.11.2. Antioxidant Capacities of CTBPs
2.11.3. Anti-Glycation Activities of CTBPs
2.12. Statistical Analysis
3. Results and Discussion
3.1. Yields and Composition of CTBPs
3.2. Monosaccharide Composition and Molecular Weight
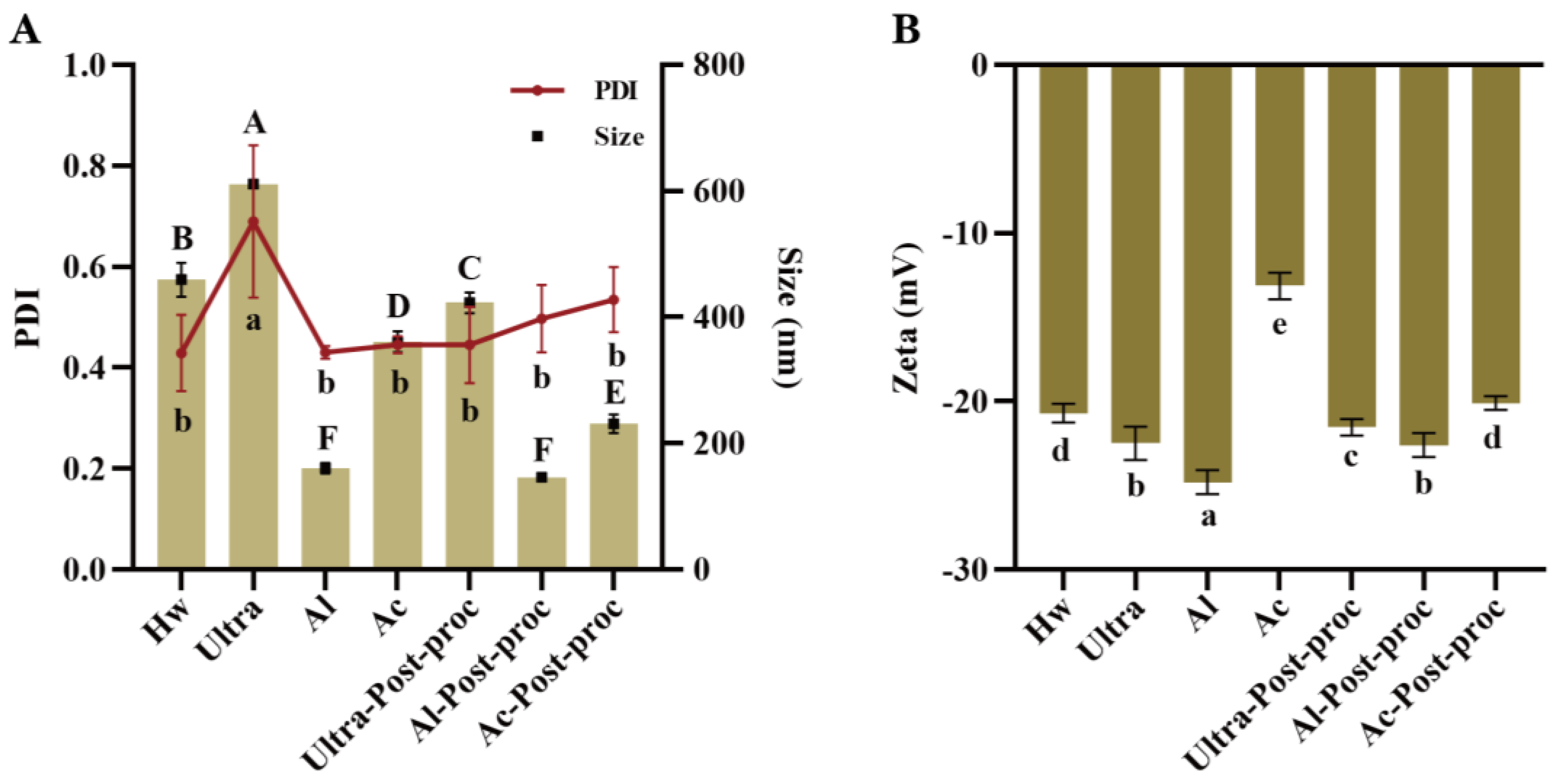
3.3. Particle Size and Zeta Potential Analysis
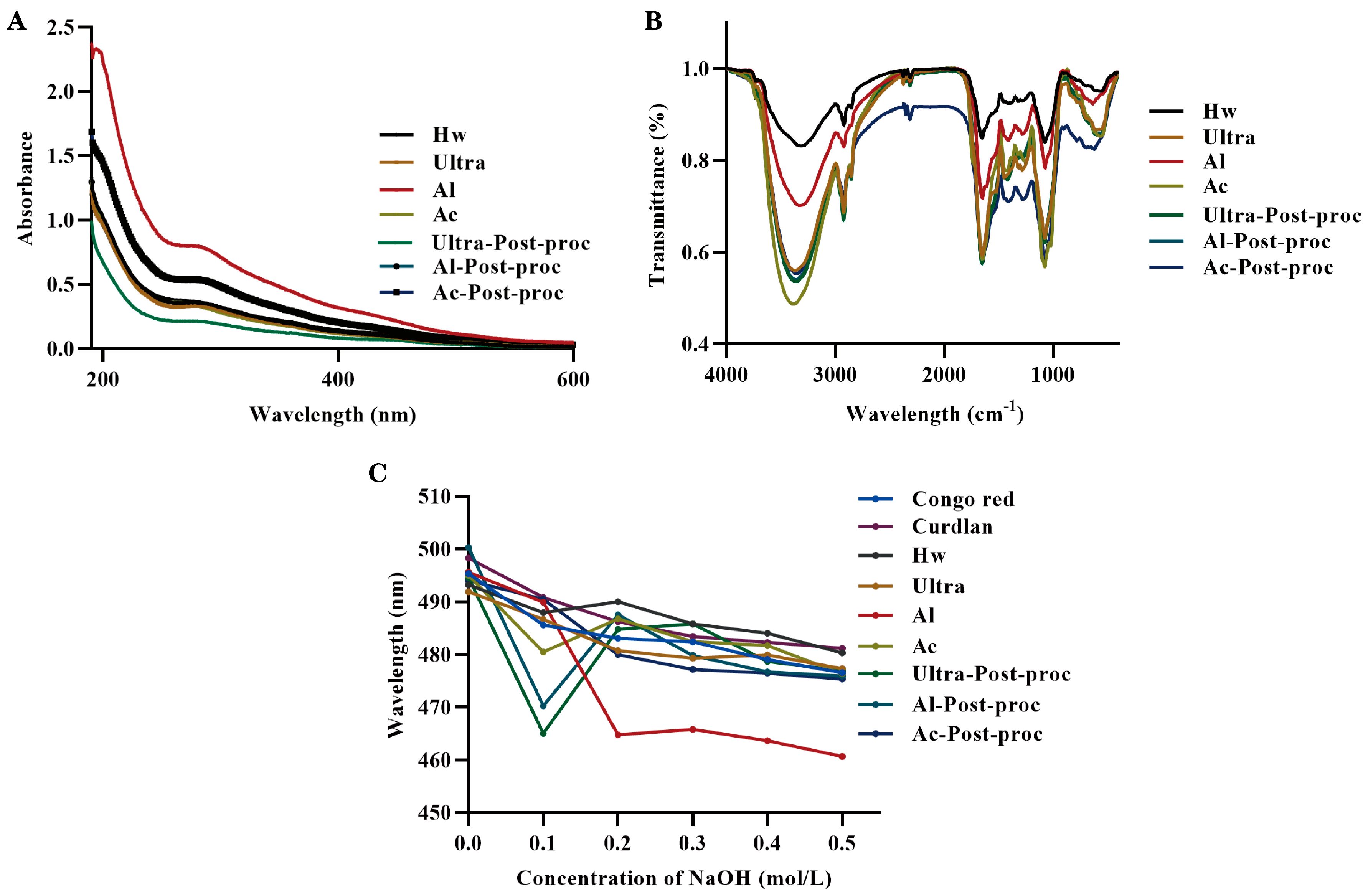
3.4. Spectral Analysis
3.5. Conformational Analysis
3.6. Water and Oil Holding Capacity
3.7. Bile Acid Binding Ability
3.8. Biological Activities
3.8.1. α-Glucosidase Inhibitory Activity
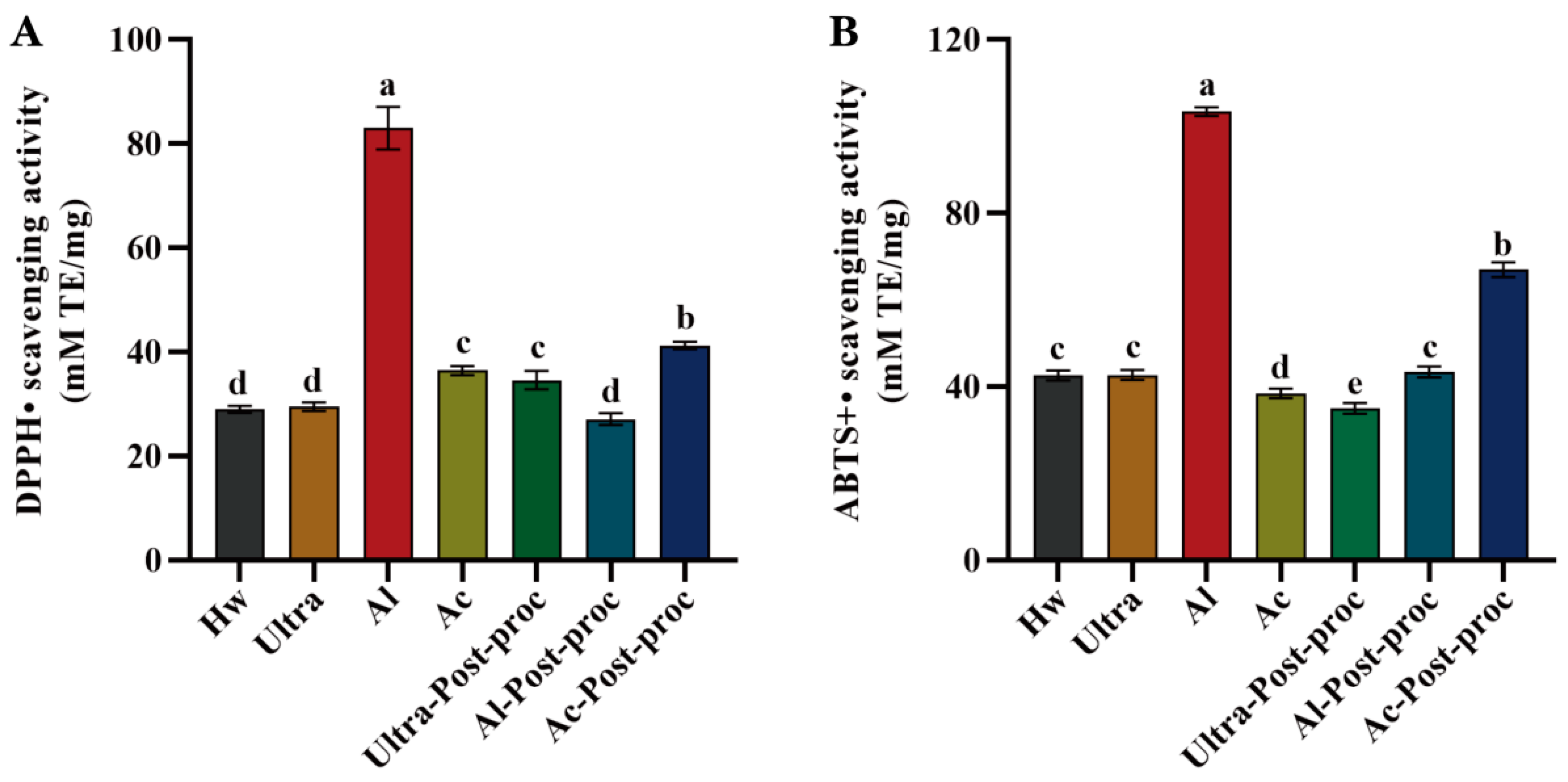
3.8.2. Antioxidant Activities
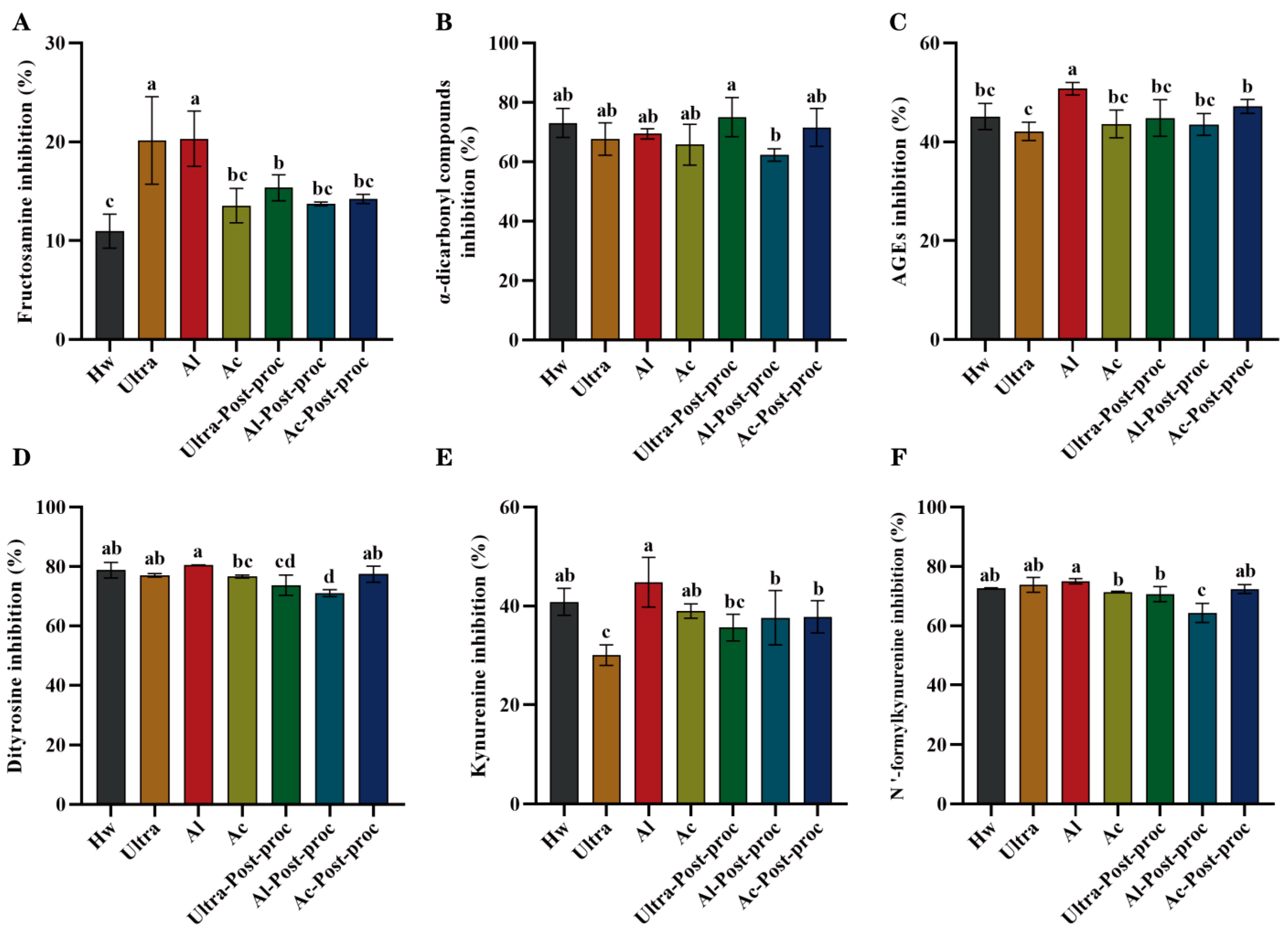
3.8.3. Anti-Glycation Activities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Dharanie, S.; Meghana, C.H.; Lavanya, M.; Baskaran, N.; Elumalai, A.; Vignesh, S. Bioactive food polysaccharides: A review on sources, classification, and its potential health benefits in humans. Food Humanit. 2024, 3, 100451. [Google Scholar] [CrossRef]
- Herburger, K.; Głazowska, S.; Mravec, J. Bricks out of the wall: Polysaccharide extramural functions. Trends Plant Sci. 2022, 27, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, S.; Ding, R.; Li, Y.; Li, C.; Gu, R. Antitumor effects of polysaccharides from medicinal lower plants: A review. Int. J. Biol. Macromol. 2023, 252, 126313. [Google Scholar] [CrossRef]
- Zhuang, C.; Wang, Y.; Zhang, Y.; Xu, N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int. J. Biol. Macromol. 2018, 115, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Sun, H.; Chen, Y.; Guo, H.; Liu, Y.; Li, Y.; Lu, C.; Wang, X. Antioxidant and hypoglycemic activity of tea polysaccharides with different degrees of fermentation. Int. J. Biol. Macromol. 2023, 228, 224–233. [Google Scholar] [CrossRef]
- Yue, B.; Zong, G.; Tao, R.; Wei, Z.; Lu, Y. Crosstalk between traditional Chinese medicine-derived polysaccharides and the gut microbiota: A new perspective to understand traditional Chinese medicine. Phytother. Res. 2022, 36, 4125–4138. [Google Scholar] [CrossRef]
- Wei, H.; Shi, Y.; Yuan, Z.; Huang, Z.; Cai, F.; Zhu, J.; Zhang, W.; Li, J.; Xiong, Q.; Wang, Y.; et al. Isolation, Identification, and Anti-Inflammatory Activity of Polysaccharides of Typha angustifolia. Biomacromolecules 2021, 22, 2451–2459. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, L.; Wang, D.; Wang, D.; Wen, C.; Han, B.; Ouyang, Z. Characterization and anti-tumor activity of a polysaccharide isolated from Dendrobium officinale grown in the Huoshan County. Chin. Med. 2018, 13, 47. [Google Scholar] [CrossRef]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.-Q.; Cao, J.-J.; Liu, R.; Chen, H.-Q. Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef]
- Gong, P.; Pei, S.; Long, H.; Yang, W.; Yao, W.; Li, N.; Wang, J.; Zhao, Y.; Chen, F.; Xie, J.; et al. Potential inhibitory effect of Auricularia auricula polysaccharide on advanced glycation end-products (AGEs). Int. J. Biol. Macromol. 2024, 262, 129856. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Cao, Y.; Huang, J.; Lin, H.; Zhao, T.; Liu, L.; Shen, P.; Julian McClements, D.; Chen, J.; et al. Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: Comparison of hot water and ultrasound-assisted extraction methods. Food Chem. 2023, 401, 134156. [Google Scholar] [CrossRef] [PubMed]
- Nuerxiati, R.; Wei, L.; Mutailifu, P.; Abuduwaili, A.; Paierhati, P.; Lei, C.; Zhiyan, Y.; Yufan, W.; Yili, A. The structural characteristic of acidic-degraded polysaccharides from seeds of Plantago ovata Forssk and its biological activity. Int. J. Biol. Macromol. 2024, 262, 129494. [Google Scholar] [CrossRef]
- Zhou, C.; He, S.; Gao, S.; Huang, Z.; Wang, W.; Hong, P.; Jia, R.-B. Effects of Ultrasound-Assisted Treatment on Physicochemical Properties and Biological Activities of Polysaccharides from Sargassum. Foods 2024, 13, 3941. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Li, Z.-R.; Liao, B.; Zhao, M.; Zhou, C.; Luo, D.; Jia, R.-B. Gradient ethanol extracts of Coreopsis tinctoria buds: Chemical and in vitro fermentation characteristics. Food Chem. 2025, 464, 141894. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, J.; Zhang, Q.; Qing, D.; Yan, C. Structural elucidation and bioactivities of a novel arabinogalactan from Coreopsis tinctoria. Carbohydr. Polym. 2019, 219, 219–228. [Google Scholar] [CrossRef]
- Yuan, Q.; Fu, Y.; Xiang, P.-Y.; Zhao, L.; Wang, S.-P.; Zhang, Q.; Liu, Y.-T.; Qin, W.; Li, D.-Q.; Wu, D.-T. Structural characterization, antioxidant activity, and antiglycation activity of polysaccharides from different chrysanthemum teas. RSC Adv. 2019, 9, 35443–35451. [Google Scholar]
- Hu, L.; Liu, R.; Wu, T.; Sui, W.; Zhang, M. Structural Properties of Homogeneous Polysaccharide Fraction Released from Wheat Germ by Hydrothermal Treatment. Carbohydr. Polym. 2020, 240, 116238. [Google Scholar] [CrossRef]
- Yuan, Q.; He, Y.; Xiang, P.-Y.; Wang, S.-P.; Cao, Z.-W.; Gou, T.; Shen, M.-M.; Zhao, L.; Qin, W.; Gan, R.-Y.; et al. Effects of simulated saliva-gastrointestinal digestion on the physicochemical properties and bioactivities of okra polysaccharides. Carbohydr. Polym. 2020, 238, 116183. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, Y.; Zhang, R.; Bai, Y.; Dong, L.; Liu, L.; Jia, X.; Wang, G.; Zhang, M. Structural characterization and in vitro gastrointestinal digestion and fermentation of litchi polysaccharide. Int. J. Biol. Macromol. 2019, 140, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Nagar, S. Studies on Tinospora cordifolia monosugars and correlation analysis of uronic acids by spectrophotometric methods and GLC. Carbohydr. Polym. 2014, 99, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zheng, Y.; Jia, R.-B.; Luo, D.; Chen, C.; Zhao, M. Fucus vesiculosus polysaccharide alleviates type 2 diabetes in rats via remodeling gut microbiota and regulating glycolipid metabolism-related gene expression. Int. J. Biol. Macromol. 2023, 248, 126504. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.; Wang, W.; Li, X.; Ding, X. Ultrasound assisted complexation of soybean peptide aggregates and soluble soybean polysaccharide: pH optimization, structure characterization, and emulsifying behavior. Food Res. Int. 2025, 201, 115546. [Google Scholar] [CrossRef]
- Geng, X.; Guo, D.; Wu, B.; Wang, W.; Zhang, D.; Hou, S.; Bau, T.; Lei, J.; Xu, L.; Cheng, Y.; et al. Effects of different extraction methods on the physico-chemical characteristics and biological activities of polysaccharides from Clitocybe squamulosa. Int. J. Biol. Macromol. 2024, 259, 129234. [Google Scholar] [CrossRef]
- Wang, X.; Su, Y.; Su, J.; Xue, J.; Zhang, R.; Li, X.; Li, Y.; Ding, Y.; Chu, X. Optimization of Enzyme−Assisted Aqueous Extraction of Polysaccharide from Acanthopanax senticosus and Comparison of Physicochemical Properties and Bioactivities of Polysaccharides with Different Molecular Weights. Molecules 2023, 28, 6585. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, Q.; Lin, S.; Liu, W.; Du, G.; Zhao, L.; Zhang, Q.; Lin, D.-R.; Liu, Y.-T.; Qin, W.; et al. Physicochemical characteristics and biological activities of polysaccharides from the leaves of different loquat (Eriobotrya japonica) cultivars. Int. J. Biol. Macromol. 2019, 135, 274–281. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, Y.; Zhang, F.; Zheng, S.; Wang, L.; Bai, J.; Yang, Y. Preparation of Ribes nigrum L. polysaccharides-stabilized selenium nanoparticles for enhancement of the anti-glycation and alpha-glucosidase inhibitory activities. Int. J. Biol. Macromol. Struct. Funct. Interact. 2023, 253, 127122. [Google Scholar]
- Baker, J.R.; Zyzak, D.V.; Thorpe, S.R.; Baynes, J.W. Mechanism of fructosamine assay: Evidence against role of superoxide as intermediate in nitroblue tetrazolium reduction. Clin. Chem. 1994, 39, 2460–2465. [Google Scholar] [CrossRef]
- Mitchel, R.E.J.; Birnboim, H.C. The use of Girard-T reagent in a rapid and sensitive method for measuring glyoxal and certain other α-dicarbonyl compounds. Anal. Biochem. 1977, 81, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-amylase, α-glucosidase and pancreatic lipase by phenolic compounds of Rumex maderensis (Madeira sorrel). Influence of simulated gastrointestinal digestion on hyperglycaemia-related damage linked with aldose reductase activity and protein glycation. LWT 2020, 118, 108727. [Google Scholar] [CrossRef]
- Ding, H.; Ni, M.; Zhang, G.; Liao, Y.; Hu, X.; Zhang, Y.; Gong, D. The inhibition of oleanolic acid on protein non-enzymatic glycation. LWT 2020, 125, 109253. [Google Scholar] [CrossRef]
- Dou, Z.-M.; Chen, C.; Huang, Q.; Fu, X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. Int. J. Biol. Macromol. 2021, 183, 1548–1559. [Google Scholar] [CrossRef]
- Lu, X. Changes in the structure of polysaccharides under different extraction methods. eFood 2023, 4, e82. [Google Scholar] [CrossRef]
- Liu, F.; Chen, H.; Qin, L.; Al-Haimi, A.A.N.M.; Xu, J.; Zhou, W.; Zhu, S.; Wang, Z. Effect and characterization of polysaccharides extracted from Chlorella sp. by hot-water and alkali extraction methods. Algal Res. 2023, 70, 102970. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Cui, F.-J.; Sun, L.; Zan, X.-Y.; Sun, W.-J. Recent advances in Ganoderma lucidum polysaccharides: Structures/bioactivities, biosynthesis and regulation. Food Biosci. 2023, 56, 103281. [Google Scholar] [CrossRef]
- Tang, Y.; Wei, Z.; He, X.; Ling, D.; Qin, M.; Yi, P.; Liu, G.; Li, L.; Li, C.; Sun, J. A comparison study on polysaccharides extracted from banana flower using different methods: Physicochemical characterization, and antioxidant and antihyperglycemic activities. Int. J. Biol. Macromol. 2024, 264, 130459. [Google Scholar] [CrossRef]
- Li, Y.; Kong, B.; Liu, Q.; Xia, X.; Chen, H. Improvement of the emulsifying and oxidative stability of myofibrillar protein prepared oil-in-water emulsions by addition of zein hydrolysates. Process Biochem. 2017, 53, 116–124. [Google Scholar] [CrossRef]
- Yang, X.; Chen, F.; Huang, G. Extraction and analysis of polysaccharide from Momordica charantia. Ind. Crops Prod. 2020, 153, 112588. [Google Scholar] [CrossRef]
- Hamed, M.; Coelho, E.; Bastos, R.; Evtuguin, D.V.; Ferreira, S.S.; Lima, T.; Vilanova, M.; Sila, A.; Coimbra, M.A.; Bougatef, A. Isolation and identification of an arabinogalactan extracted from pistachio external hull: Assessment of immunostimulatory activity. Food Chem. 2022, 373, 131416. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ma, M.; Yang, Y.; Liu, Z.; Liu, S.; Hong, T.; Ni, H.; Jiang, Z. Structural characterization and antioxidant activity of polysaccharides extracted from Porphyra haitanensis by different methods. Int. J. Biol. Macromol. 2023, 242, 125003. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Q.; Duan, X.; Tang, T.; Ke, Y.; Zhang, L.; Li, C.; Liu, A.; Su, Z.; Hu, B. Antioxidant and anticoagulant activities of mycelia polysaccharides from Catathelasma ventricosum after sulfated modification. Ind. Crops Prod. 2018, 112, 53–60. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Yan, X.; Yang, Y.; Li, Z.; Jiang, Z.; Ni, H. Hypoglycaemic effect of all-trans astaxanthin through inhibiting α-glucosidase. J. Funct. Foods 2020, 74, 104168. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [PubMed]
- Zhang, Z.; Chen, Y.; Deng, P.; He, Z.; Qin, F.; Chen, Q.; Wang, Z.; Pan, H.; Chen, J.; Zeng, M. Research progress on generation, detection and inhibition of multiple hazards—Acrylamide, 5-hydroxymethylfurfural, advanced glycation end products, methylimidazole—In baked goods. Food Chem. 2024, 431, 137152. [Google Scholar] [CrossRef]
- Sun, L.; Su, X.; Zhuang, Y. Preparation, characterization and antiglycation activities of the novel polysaccharides from Boletus snicus. Int. J. Biol. Macromol. 2016, 92, 607–614. [Google Scholar] [CrossRef]
- Anis, M.A.; Sreerama, Y.N. Inhibition of protein glycoxidation and advanced glycation end-product formation by barnyard millet (Echinochloa frumentacea) phenolics. Food Chem. 2020, 315, 126265. [Google Scholar] [CrossRef]



| Sample | Hw | Ultra | Al | Ac | Ultra-Post-Proc | Al-Post-Proc | Ac-Post-Proc |
|---|---|---|---|---|---|---|---|
| Yield (%) | 5.51 ± 0.16 c | 6.42 ± 0.10 b | 7.72 ± 0.09 a | 4.26 ± 0.16 d | - | - | - |
| Carbohydrate (%) | 64.96 ± 0.65 e | 67.47 ± 0.57 d | 78.79 ± 0.62 a | 76.37 ± 0.68 b | 53.86 ± 0.52 f | 64.74 ± 0.65 e | 71.87 ± 0.39 c |
| Total phenol (mg GAE/g) | 75.74 ± 0.14 d | 76.74 ± 0.16 c | 81.69 ± 0.70 a | 70.60 ± 1.31 e | 76.98 ± 0.21 c | 78.33 ± 0.43 b | 78.85 ± 0.40 b |
| Protein (%) | 4.20 ± 0.06 d | 4.40 ± 0.08 c | 4.82 ± 0.10 a | 4.72 ± 0.02 b | 3.94 ± 0.02 e | 3.88 ± 0.02 ef | 3.83 ± 0.05 f |
| Uronic acid | 7.27 ± 0.04 f | 8.26 ± 0.02 d | 10.41 ± 0.07 b | 13.07 ± 0.13 a | 6.99 ± 0.02 g | 7.79 ± 0.09 e | 8.84 ± 0.23 c |
| Monosaccharide composition (%) | |||||||
| Fucose | 0.71 | 0.59 | 0.64 | 0.58 | 0.68 | 0.82 | 0.67 |
| Rhamnose | 2.77 | 2.02 | 2.42 | 3.04 | 2.23 | 2.47 | 2.71 |
| Arabinose | 26.81 | 28.10 | 26.69 | 28.18 | 28.76 | 27.53 | 26.87 |
| Galactose | 22.07 | 26.15 | 25.05 | 23.69 | 19.58 | 32.78 | 29.07 |
| Glucose | 35.07 | 31.44 | 32.86 | 20.47 | 36.00 | 20.58 | 28.11 |
| Mannose | 2.79 | 3.15 | 3.80 | 3.20 | 3.18 | 4.28 | 3.96 |
| Xylose | 1.48 | 4.00 | 4.26 | 2.40 | 4.01 | 5.34 | 4.49 |
| Galacturonic acid | 7.13 | 3.29 | 3.14 | 17.34 | 4.54 | 4.89 | 2.68 |
| Glucuronic acid | 1.15 | 1.26 | 1.14 | 1.10 | 1.01 | 1.31 | 1.44 |
| Molecular weight (kDa) | |||||||
| Mw | 6.62 × 105 | 1.77 × 105 | 4.01 × 105 | 1.95 × 105 | 1.45 × 105 | 1.69 × 105 | 2.26 × 105 |
| Mn | 6.01 × 105 | 6.98 × 104 | 1.30 × 105 | 7.48 × 104 | 5.39 × 104 | 6.74 × 104 | 8.01 × 104 |
| Mw/Mn | 1.10 | 2.54 | 3.11 | 2.61 | 2.68 | 2.51 | 2.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Li, W.; Yin, M.; Jia, R.-B.; Zhou, C.; Pan, X.; Liao, B. A Comparative Study on Physicochemical Properties and Biological Activities of Polysaccharides from Coreopsis tinctoria Buds Obtained by Different Methods. Foods 2025, 14, 1168. https://doi.org/10.3390/foods14071168
Gao S, Li W, Yin M, Jia R-B, Zhou C, Pan X, Liao B. A Comparative Study on Physicochemical Properties and Biological Activities of Polysaccharides from Coreopsis tinctoria Buds Obtained by Different Methods. Foods. 2025; 14(7):1168. https://doi.org/10.3390/foods14071168
Chicago/Turabian StyleGao, Shang, Weipei Li, Moupan Yin, Rui-Bo Jia, Chunxia Zhou, Xinhui Pan, and Bingwu Liao. 2025. "A Comparative Study on Physicochemical Properties and Biological Activities of Polysaccharides from Coreopsis tinctoria Buds Obtained by Different Methods" Foods 14, no. 7: 1168. https://doi.org/10.3390/foods14071168
APA StyleGao, S., Li, W., Yin, M., Jia, R.-B., Zhou, C., Pan, X., & Liao, B. (2025). A Comparative Study on Physicochemical Properties and Biological Activities of Polysaccharides from Coreopsis tinctoria Buds Obtained by Different Methods. Foods, 14(7), 1168. https://doi.org/10.3390/foods14071168







