An Optimized Microwave-Assisted Extraction and Evaluation of Amino Acids Content and Nutritional Value in Chebulae fructus from Different Origins
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.1.1. Instruments
2.1.2. Reagents
2.1.3. Sample Preparation
2.2. Solution Preparation
2.2.1. Standard Solution
2.2.2. Sample Solution
2.3. Chromatographic Condition
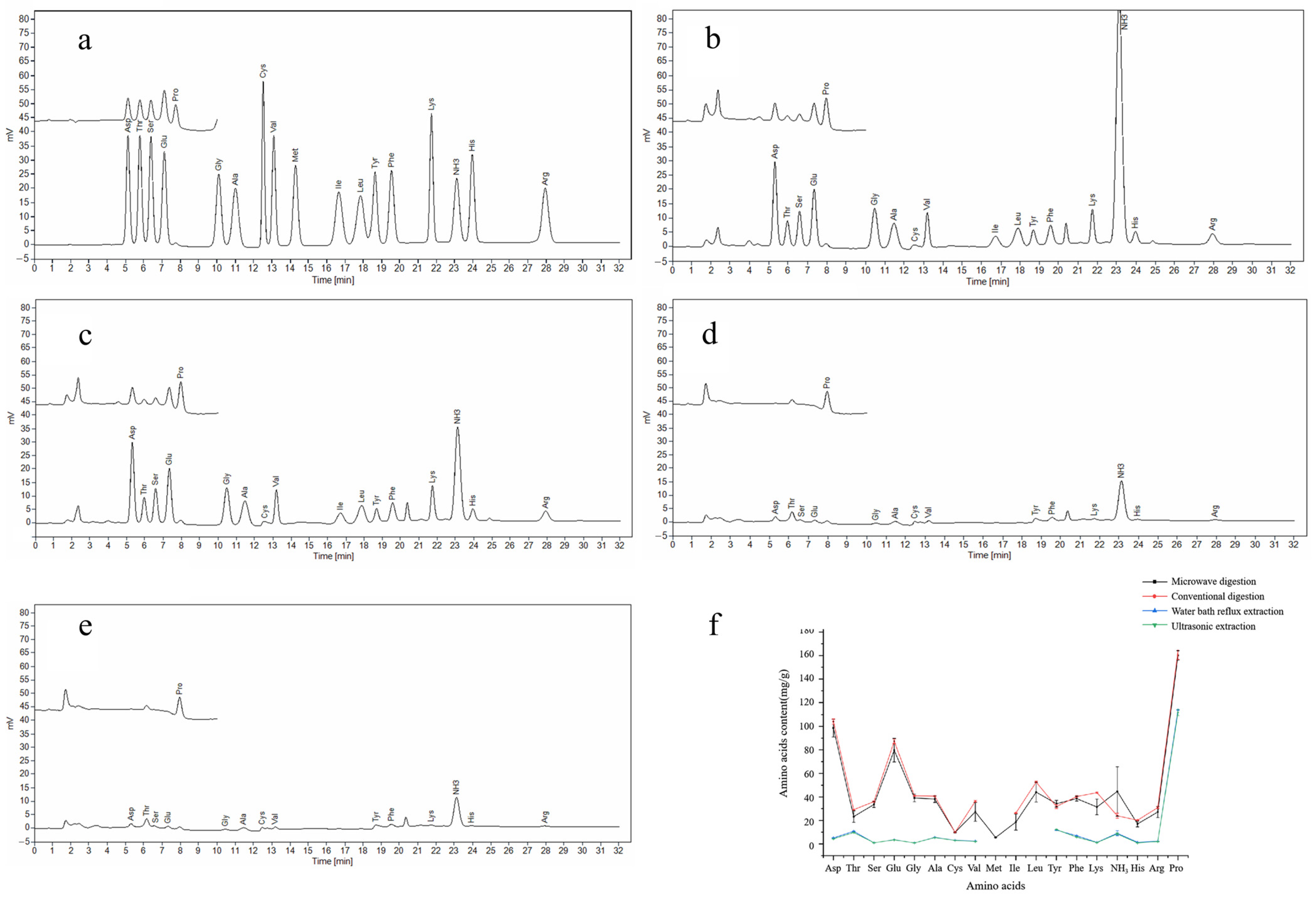
2.4. Comparison of Amino Acid Extraction Methods
2.4.1. Microwave Digestion
2.4.2. Conventional Digestion
2.4.3. Water Bath Reflux Extraction
2.4.4. Ultrasonic Extraction
2.5. Single Factor Test
2.5.1. Digestion Temperature Investigation
2.5.2. Digestion Time Investigation
2.5.3. Liquid–Material Ratio Investigation
2.6. Response Surface Optimization Experiment
2.7. Method Validation
2.7.1. Linearity, LODs, LOQs Experiment
2.7.2. Precision and Repeatability Experiment
2.7.3. Recovery Experiment
2.7.4. Stability Experiment
2.8. Determination and Evaluation of Content of Amino Acids in CF
3. Results and Discussion
3.1. Comparison Results of Amino Acid Extraction Methods
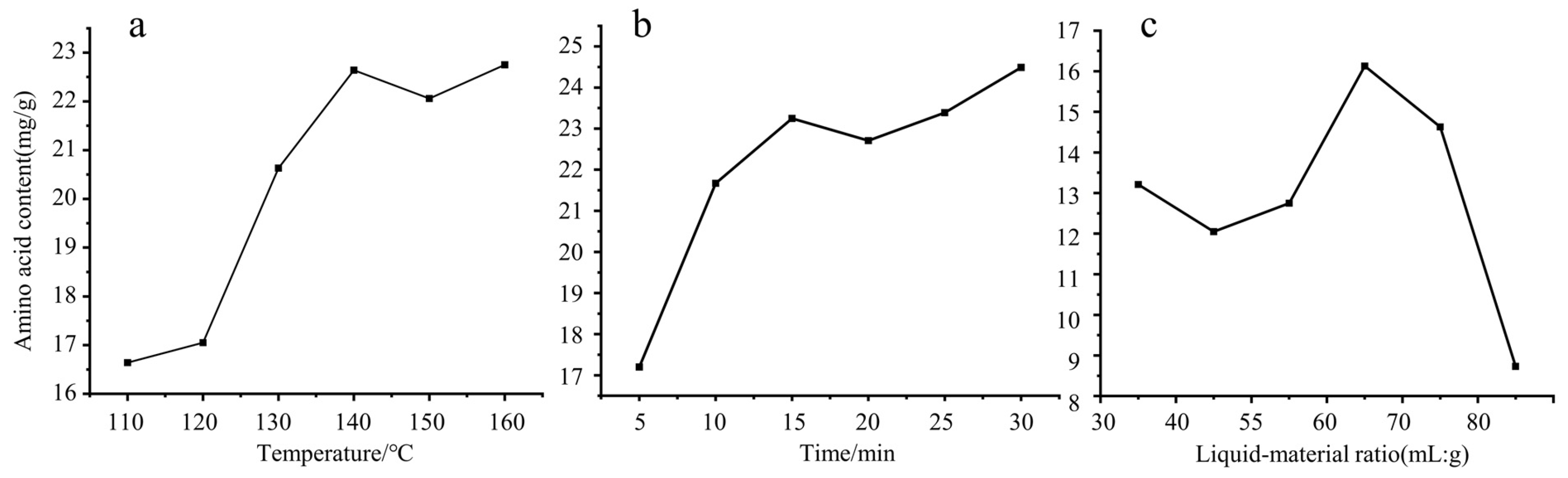
3.2. Results of Single Factor Test
3.2.1. Results of Digestion Temperature
3.2.2. Results of Digestion Time
3.2.3. Results of Liquid-Material Ratio
3.3. Results of RSM Optimization Experiment
3.3.1. Results of Box–Behnken Experimental Design
3.3.2. Results of ANOVA and Significance Test
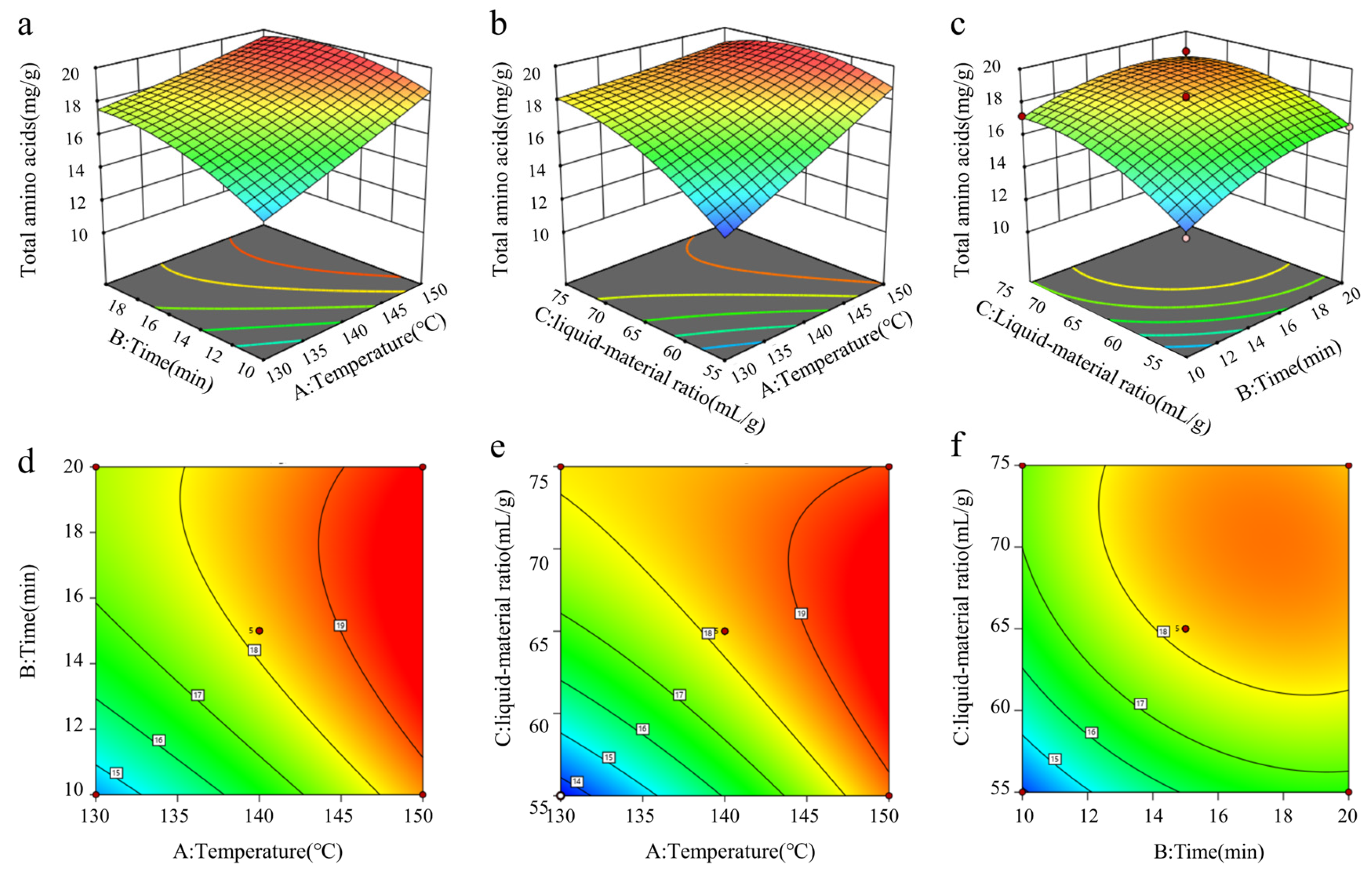
3.3.3. Results of Response Surface Interaction Analysis
3.3.4. Optimization and Verification of Extraction Process
3.4. Method Validation
3.4.1. Results of Linearity, LODs, LOQs
3.4.2. Results of Precision and Repeatability
3.4.3. Results of Recovery
3.4.4. Results of Stability
3.5. Amino Acid Composition Analysis and Nutritional Value Evaluation
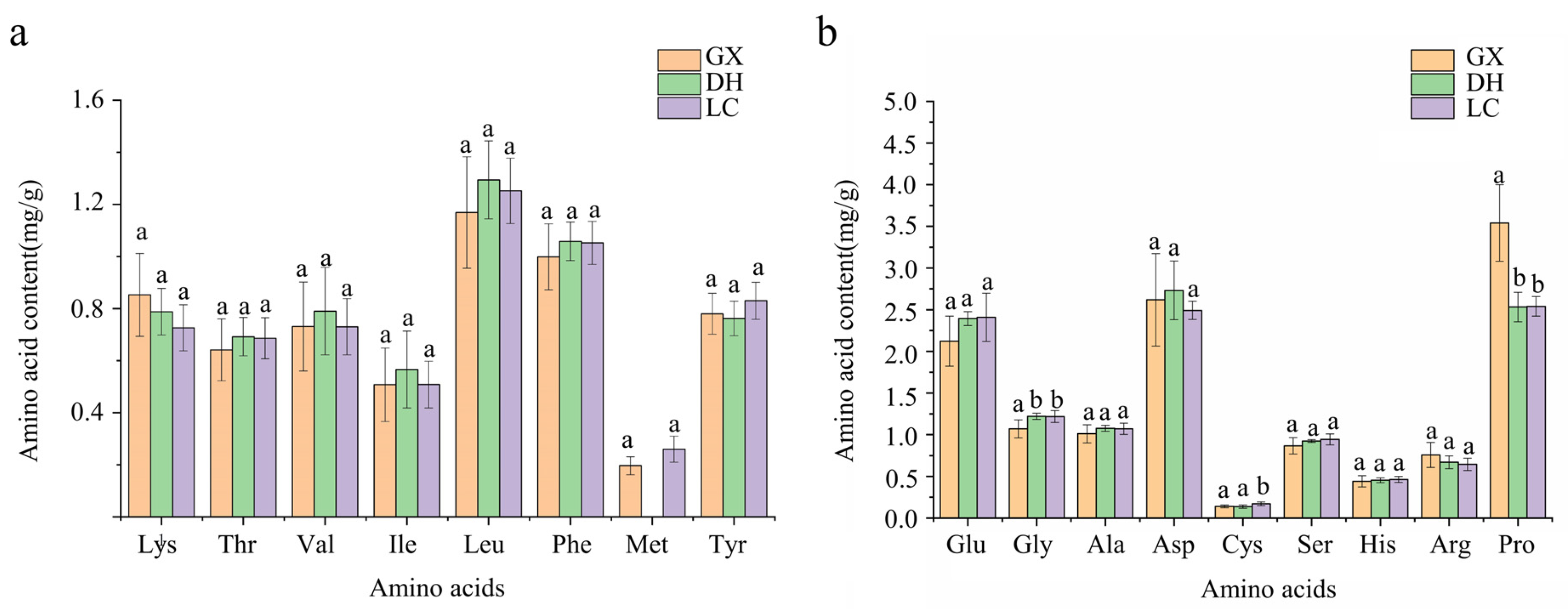
3.5.1. Amino Acid Composition Analysis and Nutritional Value Evaluation of CF from Different Origins
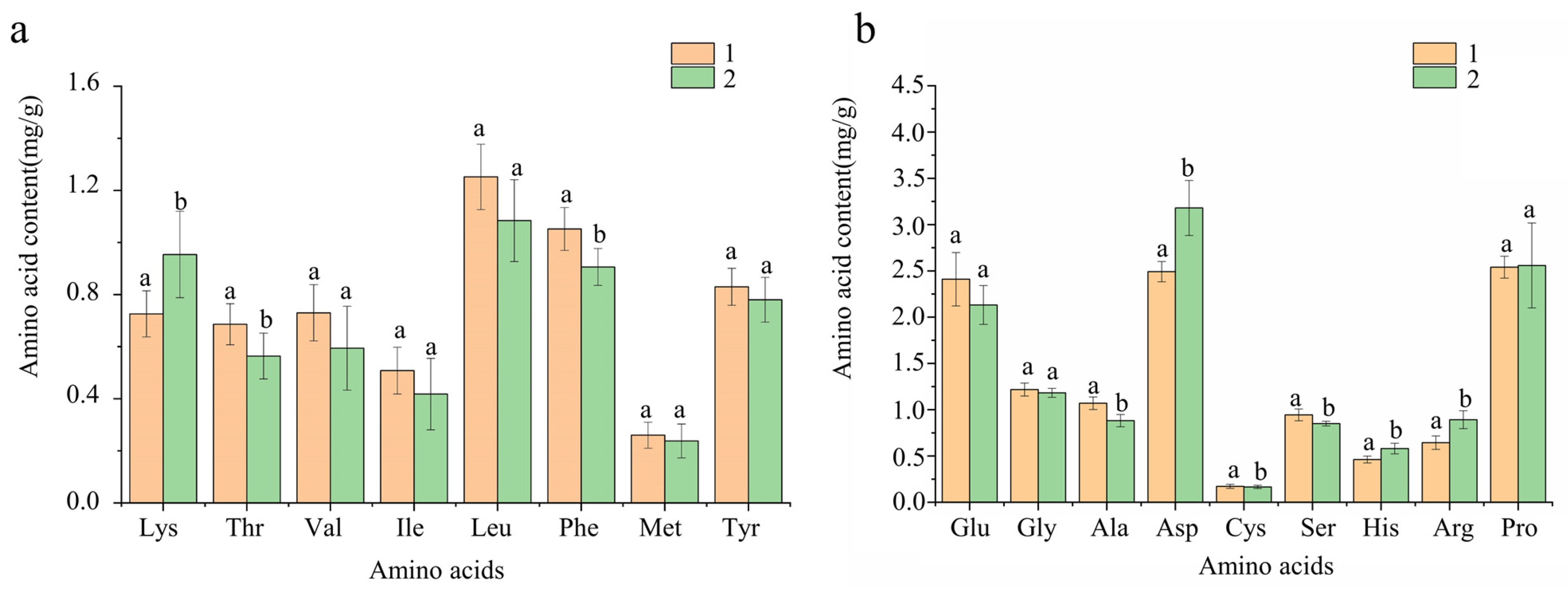
3.5.2. Amino Acids Composition Analysis and Nutritional Value Evaluation of CF in Different Processing States
3.6. Chemometric Analysis
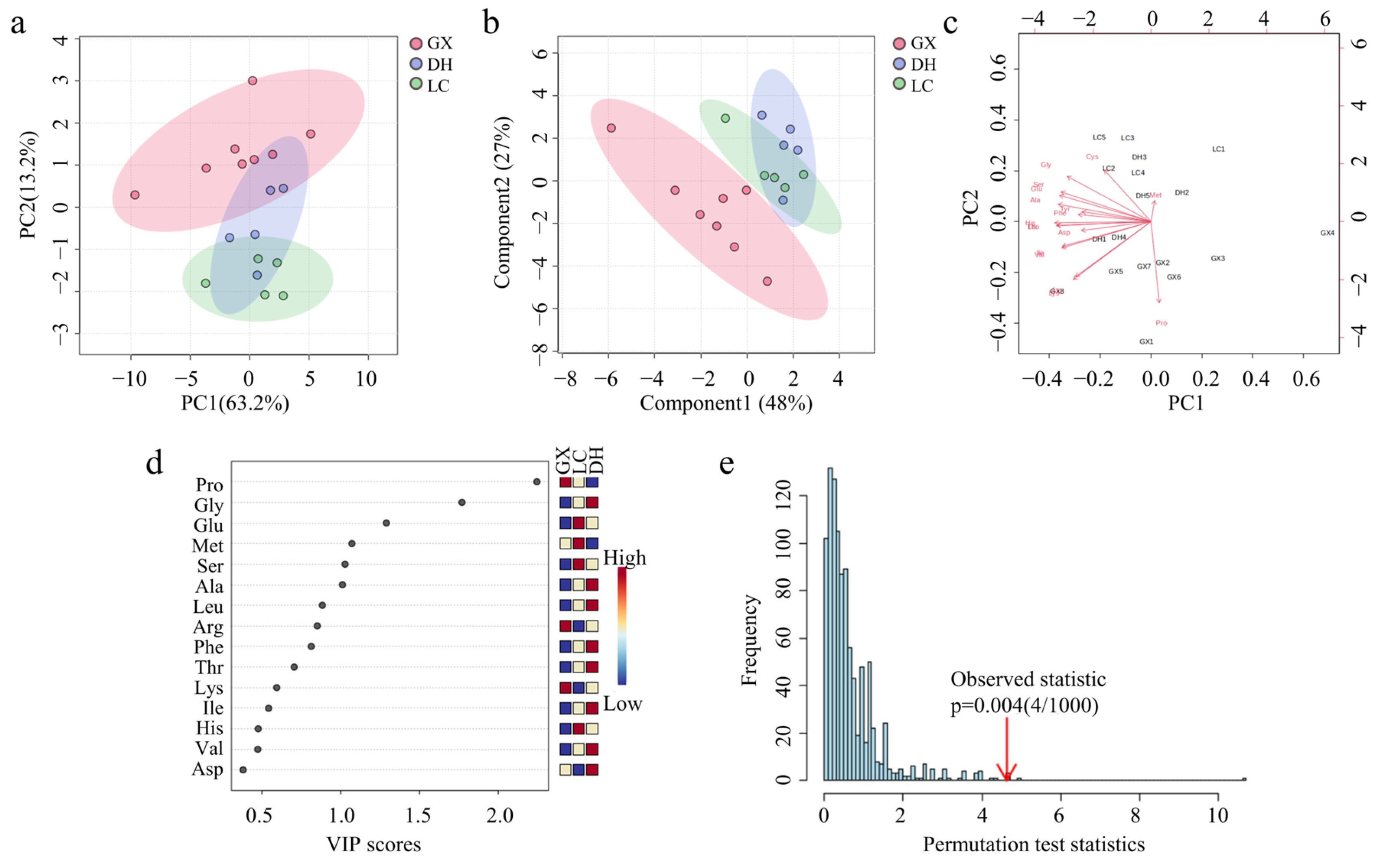
3.6.1. Amino Acid Chemometric Analysis of CF from Different Origins
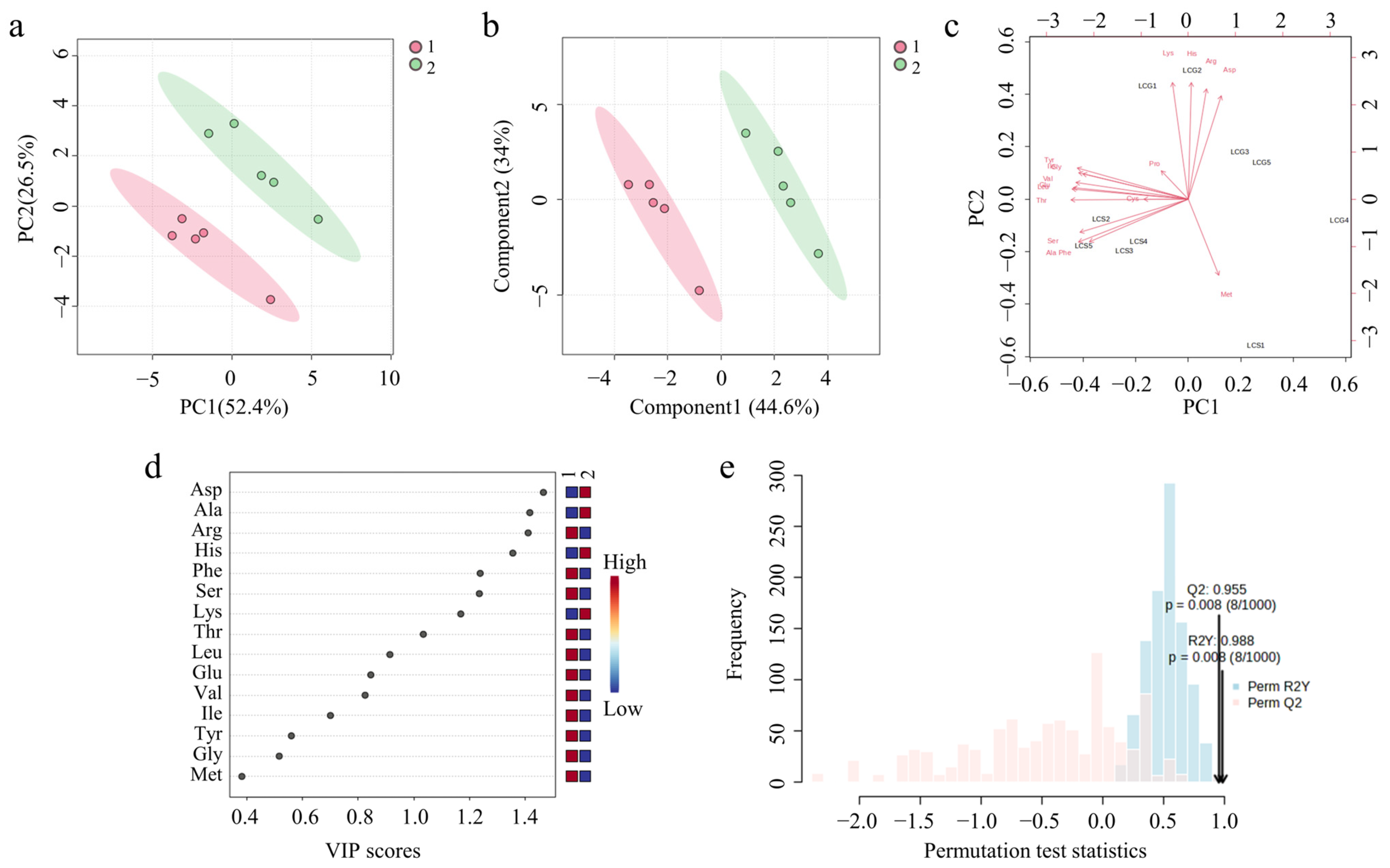
3.6.2. Amino Acid Chemometric Analysis of CF in Different Processing States
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Chen, J.H.; Yao, K.; Yin, Y.L. Research Progress of Terminalia chebula and Its Extract and Their Application Potential in Animal Husbandry. Feed. Ind. 2023, 44, 29–33. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume 1, p. 194. [Google Scholar]
- Yang, Y.H.; Hou, L.Y.; Yang, Y.; Chen, Y.F.; Yu, H. Research progress on components, biological functions and application of Chebulae fructus in aquaculture. Feed. Res. 2022, 45, 144–148. [Google Scholar]
- Barthakur, N.N.; Arnold, N.P. Nutritive value of the chebulic myrobalan (Terminalia chebula Retz.) and its potential as a food source. Food Chem. 1991, 40, 213–219. [Google Scholar]
- Song, Y.; Gong, H. Untargeted Metabolomic Profiling of Fructus Chebulae and Fructus Terminaliae Billericae. Appl. Sci. 2024, 14, 3123. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Olaniyan, O.T.; Emeruwa, C.N.; Saha, S.; Saso, L.; Tucci, P. An insight into role of amino acids as antioxidants via NRF2 activation. Amino Acids 2024, 56, 23. [Google Scholar]
- Dyachenko, E.I.; Bel’skaya, L.V. The Role of Amino Acids in Non-Enzymatic Antioxidant Mechanisms in Cancer: A Review. Metabolites 2023, 14, 28. [Google Scholar] [CrossRef]
- Galezowska, G.; Ratajczyk, J.; Wolska, L. Determination of amino acids in human biological fluids by high-performance liquid chromatography: Critical review. Amino Acids 2021, 53, 993–1009. [Google Scholar]
- Xu, W.H.; Zhong, C.C.; Zou, C.P.; Wang, B.; Zhang, N. Analytical methods for amino acid determination in organisms. Amino Acids 2020, 52, 1071–1088. [Google Scholar]
- Han, J.; Sun, R.; Huang, C.; Xie, H.; Gao, X.; Yao, Q.; Yang, P.; Li, J.; Gong, Z. Effects of Different Carbon and Nitrogen Ratios on Yield, Nutritional Value, and Amino Acid Contents of Flammulina velutipes. Life 2024, 14, 598. [Google Scholar] [CrossRef]
- Sharma, S.; Zhang, X.M.; Azhar, G.; Patyal, P.; Verma, A.; Grishma, K.C.; Wei, J.Y. Valine improves mitochondrial function and protects against oxidative stress. Biosci. Biotechnol., Biochem. 2024, 88, 168–176. [Google Scholar]
- Chiang, C.J.; Huang, Z.C.; Ta, T.; Chao, Y.P. Deciphering glutamate and aspartate metabolism to improve production of succinate in Escherichia coli. J. Taiwan Inst. Chem. Eng. 2022, 136, 104417. [Google Scholar]
- Zhang, B.B.; Lv, Z.P.; Li, Z.; Wang, W.W.; Li, G.; Guo, Y.M. Dietary L-arginine Supplementation Alleviates the Intestinal Injury and Modulates the Gut Microbiota in Broiler Chickens Challenged by Clostridium perfringens. Front. Microbiol. 2018, 9, 1716. [Google Scholar]
- Yin, Y.J.; Gong, S.M.; Han, M.M.; Wang, J.Z.; Shi, H.J.; Jiang, X.J.; Guo, L.; Duan, Y.H.; Guo, Q.P.; Chen, Q.H.; et al. Leucine regulates lipid metabolism in adipose tissue through adipokine-mTOR-SIRT1 signaling pathway and bile acid-microbe axis in a finishing pig model. Anim. Nutr. 2024, 16, 158–173. [Google Scholar]
- Hoare, M.; Tan, R.; Welle, K.A.; Swovick, K.; Hryhorenko, J.R.; Ghaemmaghami, S. Methionine Alkylation as an Approach to Quantify Methionine Oxidation Using Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2024, 35, 433–440. [Google Scholar]
- Chandran, S.; Binninger, D.; Napolitano, A. Role of Oxidative Stress, Methionine Oxidation and Methionine Sulfoxide Reductases (MSR) in Alzheimer’s Disease. Antioxidants 2023, 13, 21. [Google Scholar] [CrossRef]
- Wang, P.Y.; Shuang, F.F.; Yang, J.X.; Jv, Y.X.; Hu, R.Z.; Chen, T.; Yao, X.H.; Zhao, W.G.; Liu, L.; Zhang, D.Y. A rapid and efficient method of microwave-assisted extraction and hydrolysis and automatic amino acid analyzer determination of 17 amino acids from mulberry leaves. Ind. Crops Prod. 2022, 186, 115271. [Google Scholar]
- Mao, Y.J.; Robinson, J.P.; Binner, E.R. Current status of microwave-assisted extraction of pectin. Chem. Eng. J. 2023, 473, 145261. [Google Scholar]
- Li, Q.Q.; Li, H.Y.; Lu, G.F.; Zhou, H.L.; Xu, Z.B. Improvement of National Standard Method for Determination of 16Kinds of Amino Acids in Food. Phys. Test. Chem. Anal. Part B (Chem. Anal.) 2022, 58, 77–83. [Google Scholar]
- Maghfiroh, A.D.; Yanti, R.; Hidayat, C. Microwave-assisted unfermented cocoa bean: Improving flavor precursor after acetic acid submersion. J. Food Sci. Tech. 2024, 61, 279–289. [Google Scholar]
- Temtrirath, K. A UHPLC-PDA method for the quantitative analysis of total amino acids in infant formula with microwave-assisted acid hydrolysis. Chem. Pap. 2022, 76, 3065–3076. [Google Scholar]
- Barut, Y.T.; Koç, G.C.; Ergün, A.R.; Bozkir, H.; Pandiselvam, R. Effect of different roasting methods on the proximate by composition, flow properties, amino acid compositions, colour, texture, and sensory profile of the chickpeas. Int. J. Food Sci. Technol. 2023, 58, 482–492. [Google Scholar]
- Lin, H.B.; Yu, X.Y.; Fang, J.X.; Lu, Y.H.; Liu, P.; Xing, Y.G.; Wang, Q.; Che, Z.M.; He, Q. Flavor Compounds in Pixian Broad-Bean Paste: Non-Volatile Organic Acids and Amino Acids. Molecules 2018, 23, 1299. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, B.T.; Cai, Z.X.; Yan, J.; Ma, R.J.; Yu, M.L. Amino Acid Profiles in Peach (Prunus persica L.) Fruit. Foods 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Du, R.R.; Yang, D.T.; Jiang, G.J.; Song, Y.R.; Yin, X.Q. An Approach for In Situ Rapid Detection of Deep-Sea Aromatic Amino Acids Using Laser-Induced Fluorescence. Sensors 2020, 20, 1330. [Google Scholar] [CrossRef]
- Awchi, M.; Sinues, P.; Datta, A.N.; García-Gómez, D.; Singh, K.D. UHPLC-MS/MS-Based Identity Confirmation of Amino Acids Involved in Response to and Side Effects from Antiseizure Medications. J. Promot. Res. 2023, 22, 990–995. [Google Scholar]
- Aredes, R.S.; Peixoto, F.C.; Sphaier, L.A.; Marques, F.F.D. Evaluation of craft beers through the direct determination of amino acids by capillary electrophoresis and principal component analysis. Food Chem. 2021, 344, 128572. [Google Scholar]
- Yang, F.; Li, X. Uncertainty evaluation for determination of free amino acids in soy sauce by automatic amino acid analyzer. China Brew. 2022, 41, 234–238. [Google Scholar]
- Gao, L.; Xu, P.; Ren, J. A sensitive and economical method for simultaneous determination of D/L- amino acids profile in foods by HPLC-UV: Application in fermented and unfermented foods discrimination. Food Chem. 2023, 410, 135382. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, Z.C.; Zhang, D.S. Effects of Amino Acid Neurotransmitters on Nervous System and Research of Their Content Determination. Acta Neuropharmacol. 2021, 11, 16–20. [Google Scholar]
- Zhang, H.; Song, Q.; Lin, J.; Fei, Z.X. Determination of 16 amino acids in fresh Morchella esculenta of Yunnan province by amino acid automatic analyzer. J. Food Saf. Qual. 2019, 10, 7564–7569. [Google Scholar]
- Wen, X.P.; Yu, H.M.; Cai, D.L.; Fan, S.M.; Liu, W.W.; Yang, C.Z.; Lin, Y. Detection of total amino acids of Pholidota cantonensis Rolfe. from Fujian province by ninhydrin colorimetric method. J. Bengbu Med. Coll. 2024, 49, 238–243. [Google Scholar]
- Cen, Z.Y.; Su, J.; Gao, L.X.; Lü, L.E.; Huang, X.M. Optimization of the extraction process of free amino acids from Moringa oleifera leaves by response surface methodology. Feed. Res. 2021, 44, 85–89. [Google Scholar]
- Ren, X.Y.; Pei, X.J.; Zhang, S.Y.; Xie, L.H.; Du, W.B.; Suo, R. Determination of Amino Acids in Feed by High Performance Liquid Chromatography with Microwave Hydrolysis Derivatization. J. Instrum. Anal. 2017, 36, 1255–1259. [Google Scholar]
- Ren, L.Y.; Liu, B.; Bao, S.X.; Ding, W.; Zhang, Y.M.; Hou, X.C.; Lin, C.; Chen, B. Recovery of Li, Ni, Co and Mn from spent lithium-ion batteries assisted by organic acids: Process optimization and leaching mechanism. Int. J. Miner. Metall. Mater. 2024, 31, 518–530. [Google Scholar]
- Sakthieswaran, N.; Moorthy, N.; Renisha, M.; Chinnadurai, M. Optimization of Strength Properties of Reactive Powder Concrete by Response Surface Methodology. J. Shanghai Jiaotong Univ. Sci. 2023, 31, 518–530. [Google Scholar]
- Gündogdu, T.K.; Deniz, I.; Çaliskan, G.; Sahin, E.S.; Azbar, N. Experimental design methods for bioengineering applications. Crit. Rev. Biotechnol. 2016, 36, 368–388. [Google Scholar]
- Veljkovic, V.B.; Velickovic, A.V.; Avramovic, J.M.; Stamenkovic, O.S. Modeling of biodiesel production: Performance comparison of Box-Behnken, face central composite and full factorial design. Chin. J. Chem. Eng. 2019, 27, 1690–1698. [Google Scholar] [CrossRef]
- Weber, P. Determination of amino acids in food and feed by microwave hydrolysis and UHPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2022, 1209, 123429. [Google Scholar]
- Li, Z.Q.; Hong, T.N.; Shen, G.H.; Gu, Y.T.; Guo, Y.Z.; Han, J. Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet-Waxy Corn from Three Different Regions of China. Nutrients 2022, 14, 3887. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.Q.; Fan, X.L.; Jin, P.F. Optimization of extraction process of 15 bioactive compounds from Qing Fei Yi Huo tablets using response surface methodology. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2023, 1223, 123720. [Google Scholar]
- Wu, Y.R.; Chen, G.R.; Lei, J.D.; Wang, L.Y. Optimization of Ultrasonic Extraction Combined with Ultrafiltration for Purification of Mulberry Leaf Protein and Its Nutritional Evaluation. Sci. Technol. Food Ind. 2023, 44, 236–245. [Google Scholar]
- Zhan, Q.; Zhang, W.W.; Yang, X.H.; Feng, Y.N. Identification of regenerated protein fibers with an amino acid analysis. China Dye. Finish. 2014, 40, 42–44. [Google Scholar]
- Wang, Y.J.; Wang, Y.F.; Luan, D.L. Thermal degradation characteristics of amino acids in rainbow trout fillets during traditional high temperature short time processing and microwave processing. J. Food Meas. Charact. 2023, 17, 1940–1952. [Google Scholar]
- Mu, H.; Luo, R.M.; Li, Y.L. Effects of Heat Treatment on Flavor and Volatile Components of Maillard Reaction Products from Bovine Bone Hydrolysate. Zhongguo Shipin Xuebao 2023, 23, 181–191. [Google Scholar]
- Paw, J.K.S.; Kiong, T.S.; Kamarulzaman, M.K.; Adam, A.; Hisham, S.; Kadirgama, K.; Ramasamy, D.; Yaw, C.T.; Yusop, A.F.; Yusaf, T.; et al. Advancing renewable fuel integration: A comprehensive response surface methodology approach for internal combustion engine performance and emissions optimization. Heliyon 2023, 9, e22238. [Google Scholar]
- Xia, H.M.; Zhang, H.W.; Bai, Y.X.; Liu, H.; Zhang, L.L.; Wang, H.Y. Evaluation of the Quality of Different Batches of Terminalia chebula Retz. Based on HPLC Fingerprint Technology and Grey Relational Analysis. Chin. J. Mod. Appl. Pharm. 2020, 37, 1448–1453. [Google Scholar]
- Li, G.W.; Suo, C.X.; Wu, W.P.; Pan, L.Y.; Hu, Q.P.; He, J.Y.; Sun, D.M. Multi-indicative component ananlysis of different species of Terminalia L. based on multivariate statistical analysis. Nat. Prod. Res. Dev. 2021, 33, 23–33. [Google Scholar]
- Zhao, X.; Zhang, B.B.; Luo, Z.; Yuan, Y.; Zhao, Z.H.; Liu, M.J. Composition Analysis and Nutritional Value Evaluation of Amino Acids in the Fruit of 161 Jujube Cultivars. Plants 2023, 12, 1744. [Google Scholar] [CrossRef]
- Zhao, S.Q.; He, M.; Liang, X.X.; Li, X.; Liao, L. Effects of the different processed products from Terminalia chebula Retz on the morphology and distribution of argentaffin cells in the digestive tract of mice. Chin. Vet. Sci. 2018, 46, 1048–1056. [Google Scholar]
- Hao, J.; Ju, C.G.; Zhang, Q.; An, Y.Y.; Wang, W. Study on processing technology and extraction technology of ellagic acid from Terminalia chebula by sand-scorch. J. Pharm. Res. 2023, 42, 247–250. [Google Scholar]
- Mao, J.Z.; Wang, Z.S. Basic Tibetan Medicine Processing Science; China Press of Traditional Chinese Medicine: Beijing, China, 2011. [Google Scholar]
- Wu, L.G.; Wang, A.N.; Shen, R.L.; Qu, L.B. Effect of processing on the contents of amino acids and fatty acids, and glucose release from the starch of quinoa. Food Sci. Nutr. 2020, 8, 4877–4887. [Google Scholar] [PubMed]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; Tar’an, B.; House, J.D. Thermal processing methods differentially affect the protein quality of Chickpea (Cicer arietinum). Food Sci. Nutr. 2020, 8, 2950–2958. [Google Scholar] [PubMed]
- Zhong, L.Y.; Cui, M.N.; Yang, M.; Gong, Q.F. Modern researches on effect of processing of Chinese herb medicine on Chinese medical properties. Zhongguo Zhong Yao Za Zhi 2019, 44, 5109–5113. [Google Scholar]
- Zhang, C. Effects of Different Processing Methods on Content of Gllic Acid in Chebulae fructus. Shandong J. Tradit. Chin. Med. 2014, 33, 668–670. [Google Scholar]
- An, Y.Y.; Ju, C.G.; Zhang, Q.; Hao, J.; Chen, J.M.; Wang, W. Content changes of chemical constituents and comparison of the anti-ulcerative colitis effect of processed products of Terminalia chebula at different temperatures. China Pharm. 2022, 33, 332–337. [Google Scholar]
- Zhou, Y.Y.; Chen, H.J.; Xue, J.; Wu, N.; Yuan, J.H.; Liu, X.H.; Zou, L.S.; Chen, C.H.; Cai, Z.C.; Yang, W.; et al. Quality evaluation of Lysimachiae Herba from different habitats based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Zhongguo Zhong Yao Za Zhi 2023, 48, 4663–4674. [Google Scholar]
| NO. | Sample | Collection Region | Processed or Not |
|---|---|---|---|
| 1 | GX-1 | Guangxi Province (GX) | unprocessed |
| 2 | GX-2 | Guangxi Province (GX) | unprocessed |
| 3 | GX-3 | Guangxi Province (GX) | unprocessed |
| 4 | GX-4 | Guangxi Province (GX) | unprocessed |
| 5 | GX-5 | Guangxi Province (GX) | unprocessed |
| 6 | GX-6 | Guangxi Province (GX) | unprocessed |
| 7 | GX-7 | Guangxi Province (GX) | unprocessed |
| 8 | GX-8 | Guangxi Province (GX) | unprocessed |
| 9 | DH-1 | Dehong prefecture, Yunnan province (DH) | unprocessed |
| 10 | DH-2 | Dehong prefecture, Yunnan province (DH) | unprocessed |
| 11 | DH-3 | Dehong prefecture, Yunnan province (DH) | unprocessed |
| 12 | DH-4 | Dehong prefecture, Yunnan province (DH) | unprocessed |
| 13 | DH-5 | Dehong prefecture, Yunnan province (DH) | unprocessed |
| 14 | LC-1 | Lincang City, Yunnan province (LC) | unprocessed |
| 15 | LC-2 | Lincang City, Yunnan province (LC) | unprocessed |
| 16 | LC-3 | Lincang City, Yunnan province (LC) | unprocessed |
| 17 | LC-4 | Lincang City, Yunnan province (LC) | unprocessed |
| 18 | LC-5 | Lincang City, Yunnan province (LC) | unprocessed |
| 19 | LC-6 | Lincang City, Yunnan province (LC) | processed |
| 20 | LC-7 | Lincang City, Yunnan province (LC) | processed |
| 21 | LC-8 | Lincang City, Yunnan province (LC) | processed |
| 22 | LC-9 | Lincang City, Yunnan province (LC) | processed |
| 23 | LC-10 | Lincang City, Yunnan province (LC) | processed |
| Step | Temperature/°C | Time/min | Pressure/atm |
|---|---|---|---|
| 1 | 80 | 3 | 10 |
| 2 | 120 | 3 | 20 |
| 3 | 150 | 18 | 30 |
| Time (min) | %B1 | %B2 | %B3 | %B4 | %B5 | %B6 | %R1 | %R2 | %R3 | Pump 2 Flow Rate (mL/min) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 100 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | 0 | 0.35 |
| 2.5 | 100 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | 0 | |
| 2.6 | 0 | 100 | 0 | 0 | 0 | 0 | 50 | 50 | 0 | |
| 4.5 | 0 | 100 | 0 | 0 | 0 | 0 | 50 | 50 | 0 | |
| 4.6 | 0 | 0 | 100 | 0 | 0 | 0 | 50 | 50 | 0 | |
| 12.8 | 0 | 0 | 100 | 0 | 0 | 0 | 50 | 50 | 0 | |
| 12.9 | 0 | 0 | 0 | 100 | 0 | 0 | 50 | 50 | 0 | |
| 27.0 | 0 | 0 | 0 | 100 | 0 | 0 | 50 | 50 | 0 | |
| 27.1 | 0 | 0 | 0 | 0 | 0 | 100 | 50 | 50 | 0 | |
| 32.0 | 0 | 0 | 0 | 0 | 0 | 100 | 50 | 50 | 0 | |
| 32.1 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | |
| 33.0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | |
| 33.1 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
| 34.0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
| 34.1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
| 37.0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
| 37.1 | 100 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | 0 | |
| 53.0 | 100 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | 0 |
| Level | Factors | ||
|---|---|---|---|
| A: Temperature/℃ | B: Time/min | C: Liquid–Material Ratio (mL:g) | |
| −1 | 130 | 10 | 55:1 |
| 0 | 140 | 15 | 65:1 |
| +1 | 150 | 20 | 75:1 |
| NO. | Factors | Amino Acid Content mg/g | ||
|---|---|---|---|---|
| A/°C | B/min | C/mL:g | ||
| 1 | 130 | 10 | 65:1 | 14.56 |
| 2 | 150 | 10 | 65:1 | 18.73 |
| 3 | 130 | 20 | 65:1 | 17.36 |
| 4 | 150 | 20 | 65:1 | 19.46 |
| 5 | 130 | 15 | 55:1 | 13.80 |
| 6 | 150 | 15 | 55:1 | 18.92 |
| 7 | 130 | 15 | 65:1 | 17.90 |
| 8 | 150 | 15 | 75:1 | 18.82 |
| 9 | 140 | 10 | 55:1 | 13.55 |
| 10 | 140 | 20 | 55:1 | 16.57 |
| 11 | 140 | 10 | 75:1 | 17.20 |
| 12 | 140 | 20 | 75:1 | 18.69 |
| 13 | 140 | 15 | 65:1 | 18.37 |
| 14 | 140 | 15 | 65:1 | 18.31 |
| 15 | 140 | 15 | 65:1 | 17.66 |
| 16 | 140 | 15 | 65:1 | 18.36 |
| 17 | 140 | 15 | 65:1 | 18.38 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 51.57 | 9 | 5.73 | 44.31 | <0.0001 | ** |
| A-Temperature | 18.92 | 1 | 18.92 | 146.31 | <0.0001 | ** |
| B-Time | 8.08 | 1 | 8.08 | 62.47 | <0.0001 | ** |
| C-liquid–material ratio | 11.93 | 1 | 11.93 | 92.21 | <0.0001 | ** |
| AB | 1.07 | 1 | 1.07 | 8.26 | 0.0239 | * |
| AC | 4.4 | 1 | 4.4 | 34.02 | 0.0006 | ** |
| BC | 0.5803 | 1 | 0.5803 | 4.49 | 0.0719 | - |
| A2 | 0.0313 | 1 | 0.0313 | 0.2417 | 0.638 | - |
| B2 | 2.51 | 1 | 2.51 | 19.44 | 0.0031 | ** |
| C2 | 3.74 | 1 | 3.74 | 28.93 | 0.001 | ** |
| Residual | 0.9053 | 7 | 0.1293 | |||
| Lack of Fit | 0.5148 | 3 | 0.1716 | 1.76 | 0.2938 | - |
| Pure Error | 0.3905 | 4 | 0.0976 | |||
| Cor Total | 52.48 | 16 | ||||
| R2 | 0.9827 | Radj2 | 0.9606 |
| NO. | Amino Acid | Linear Equation | R2 | LODs ng/mL | LOQs ng/mL | Precision RSD (%) |
|---|---|---|---|---|---|---|
| 1 | Asp | y = 1343.7x − 0.2637 | 0.9999 | 86.94 | 26.08 | 0.47 |
| 2 | Thr | y = 1200.9x − 0.0466 | 0.9999 | 80.00 | 24.00 | 0.34 |
| 3 | Ser | y = 1075.8x − 0.9075 | 0.9999 | 70.61 | 21.18 | 0.32 |
| 4 | Glu | y = 1476.7x + 0.3726 | 0.9999 | 112.8 | 33.83 | 0.34 |
| 5 | Gly | y = 751.04x + 0.0118 | 0.9999 | 79.48 | 23.84 | 0.78 |
| 6 | Ala | y = 868.63x + 1.5939 | 0.9999 | 122.6 | 36.77 | 1.07 |
| 7 | Cys | y = 2483.2x − 2.673 | 0.9995 | 119.2 | 35.77 | 0.70 |
| 8 | Val | y = 1179.8x − 0.4082 | 0.9999 | 84.10 | 25.23 | 0.73 |
| 9 | Met | y = 1518.4x − 0.3548 | 0.9999 | 152.5 | 45.75 | 0.68 |
| 10 | Ile | y = 1332.3x − 0.9097 | 0.9999 | 187.9 | 56.37 | 0.56 |
| 11 | Leu | y = 1245.7x + 4.1616 | 0.9997 | 219.7 | 65.92 | 1.91 |
| 12 | Tyr | y = 1880.2x − 0.8519 | 0.9998 | 213.6 | 64.08 | 1.70 |
| 13 | Phe | y = 1720x − 4.2533 | 0.9999 | 171.5 | 51.44 | 0.58 |
| 14 | Lys | y = 1516.2x − 2.3157 | 0.9998 | 84.75 | 25.42 | 0.45 |
| 15 | His | y = 1585.9x − 1.2187 | 0.9999 | 140.1 | 42.03 | 1.44 |
| 16 | Arg | y = 1779.4x − 2.8503 | 0.9999 | 251.8 | 75.53 | 0.54 |
| 17 | Pro | y = 1188.4x − 2.3913 | 0.9999 | 887.0 | 266.10 | 0.40 |
| NO. | Amino Acids | Amount of the Standards/mg | Recovery Rate/% | RSD/% |
|---|---|---|---|---|
| 1 | Asp | 0.28 | 114.1 | 4.53 |
| 2 | Thr | 0.30 | 96.26 | 2.86 |
| 3 | Ser | 0.26 | 100.3 | 2.79 |
| 4 | Glu | 0.37 | 100.3 | 4.17 |
| 5 | Gly | 0.19 | 96.60 | 3.33 |
| 6 | Ala | 0.22 | 97.40 | 2.78 |
| 7 | Cys | 0.60 | 99.90 | 0.47 |
| 8 | Val | 0.29 | 94.64 | 3.39 |
| 9 | Met | 0.37 | 93.94 | 0.70 |
| 10 | Ile | 0.33 | 95.00 | 2.48 |
| 11 | Leu | 0.33 | 95.64 | 4.04 |
| 12 | Tyr | 0.45 | 96.58 | 1.25 |
| 13 | Phe | 0.41 | 95.80 | 1.21 |
| 14 | Lys | 0.37 | 94.52 | 2.88 |
| 15 | His | 0.39 | 95.27 | 1.05 |
| 16 | Arg | 0.44 | 95.27 | 2.01 |
| 17 | Pro | 0.29 | 103.4 | 5.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Li, Y.; Shen, Y.; Wang, Y.; Yu, J.; Xiang, X.; Yang, L.; He, D. An Optimized Microwave-Assisted Extraction and Evaluation of Amino Acids Content and Nutritional Value in Chebulae fructus from Different Origins. Foods 2025, 14, 1166. https://doi.org/10.3390/foods14071166
Xu Z, Li Y, Shen Y, Wang Y, Yu J, Xiang X, Yang L, He D. An Optimized Microwave-Assisted Extraction and Evaluation of Amino Acids Content and Nutritional Value in Chebulae fructus from Different Origins. Foods. 2025; 14(7):1166. https://doi.org/10.3390/foods14071166
Chicago/Turabian StyleXu, Zhiqi, Yan Li, Yuan Shen, Yiwu Wang, Jialing Yu, Xinxin Xiang, Lin Yang, and Dan He. 2025. "An Optimized Microwave-Assisted Extraction and Evaluation of Amino Acids Content and Nutritional Value in Chebulae fructus from Different Origins" Foods 14, no. 7: 1166. https://doi.org/10.3390/foods14071166
APA StyleXu, Z., Li, Y., Shen, Y., Wang, Y., Yu, J., Xiang, X., Yang, L., & He, D. (2025). An Optimized Microwave-Assisted Extraction and Evaluation of Amino Acids Content and Nutritional Value in Chebulae fructus from Different Origins. Foods, 14(7), 1166. https://doi.org/10.3390/foods14071166





