Impact of Harvest Periods on the Physicochemical and Flavour Characteristics of Sichuan Pepper (Zanthoxylum bungeanum Maxim)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Equipment
2.2. Determination of Growth Characteristics
2.2.1. Determination of Moisture Content
2.2.2. Determination of Thousand-Grain Weight
2.2.3. Determination of Fruit Shape Index
2.3. Determination of Colour Difference
2.4. Determination of Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activities
2.4.1. Determination of Total Phenolic Content
2.4.2. Determination of Total Flavonoids
2.4.3. Determination of DPPH Free Radical-Scavenging Activity
2.4.4. Determination of the FRAP Free Radical-Scavenging Activity
2.5. Determination of Amino Acids
2.6. Protein, Lipid, Carbohydrate, and Energy Determination
2.7. E-Tongue Analysis
2.8. HS-GC-MS Analysis
2.8.1. GC Conditions
2.8.2. MS Conditions
2.9. Data Processing
3. Results and Discussion
3.1. Growth Characteristics of Sichuan Peppers at Different Harvesting Periods
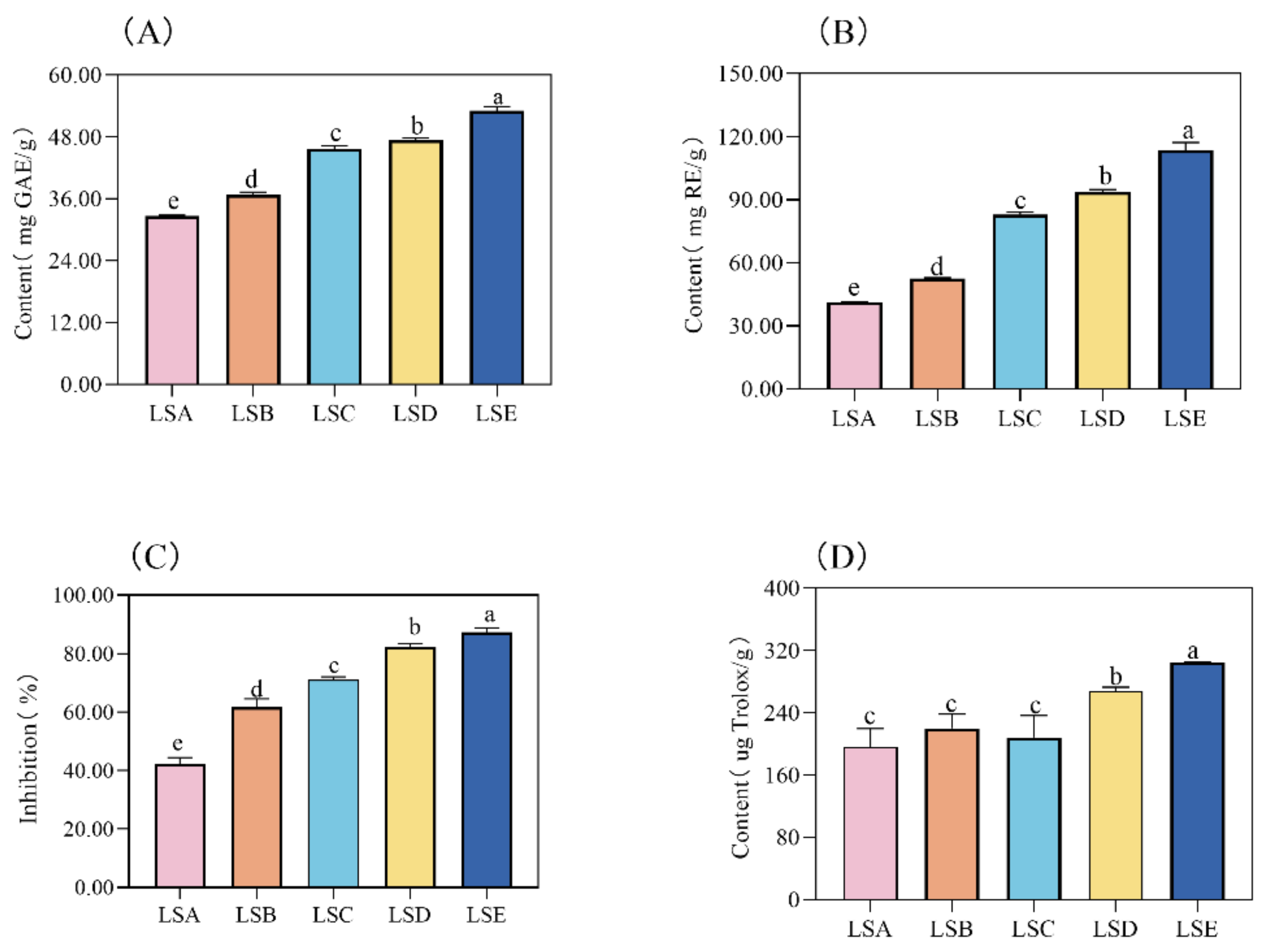
3.2. Analysis of Flavonoids, Total Phenols, and Antioxidant Activity in Sichuan Pepper at Different Harvesting Periods
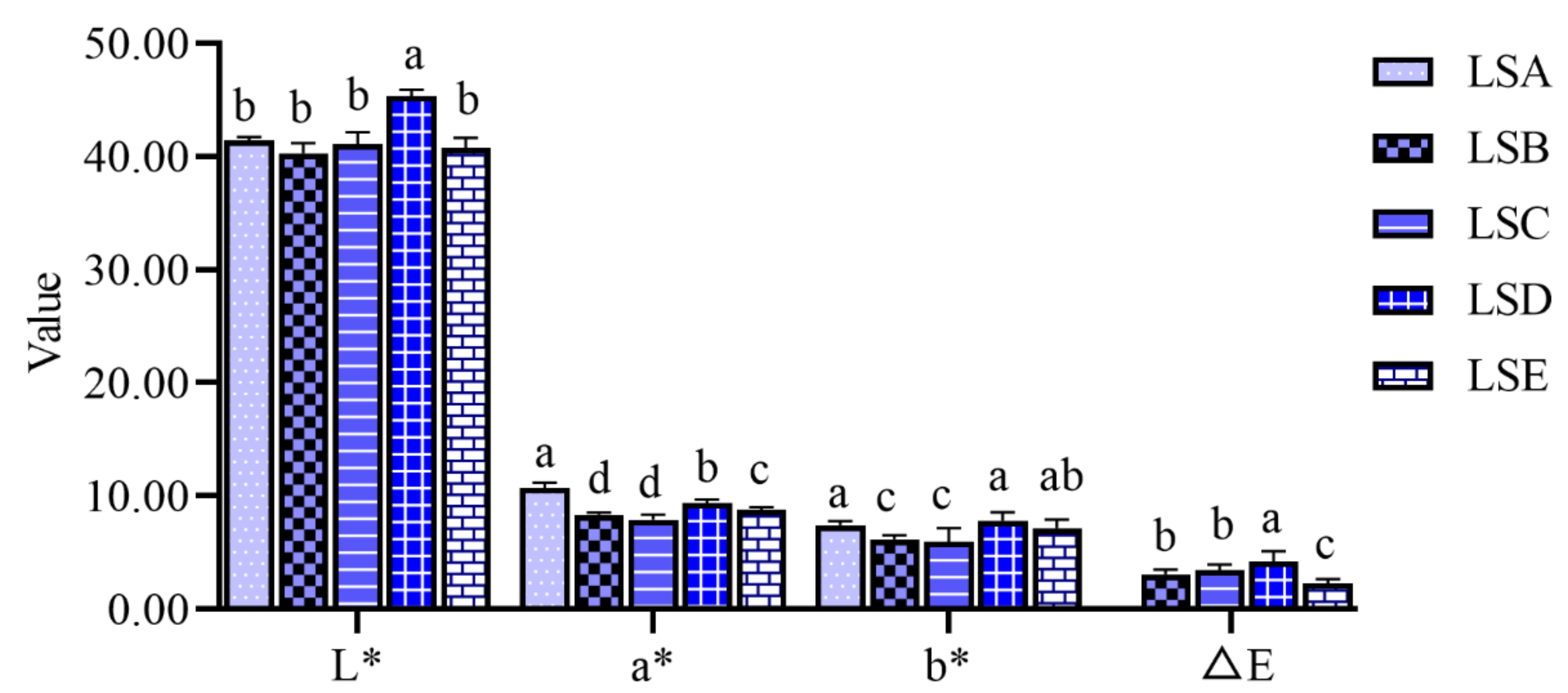
3.3. Analysis of Colour Characteristics of Sichuan Pepper at Different Harvesting Periods
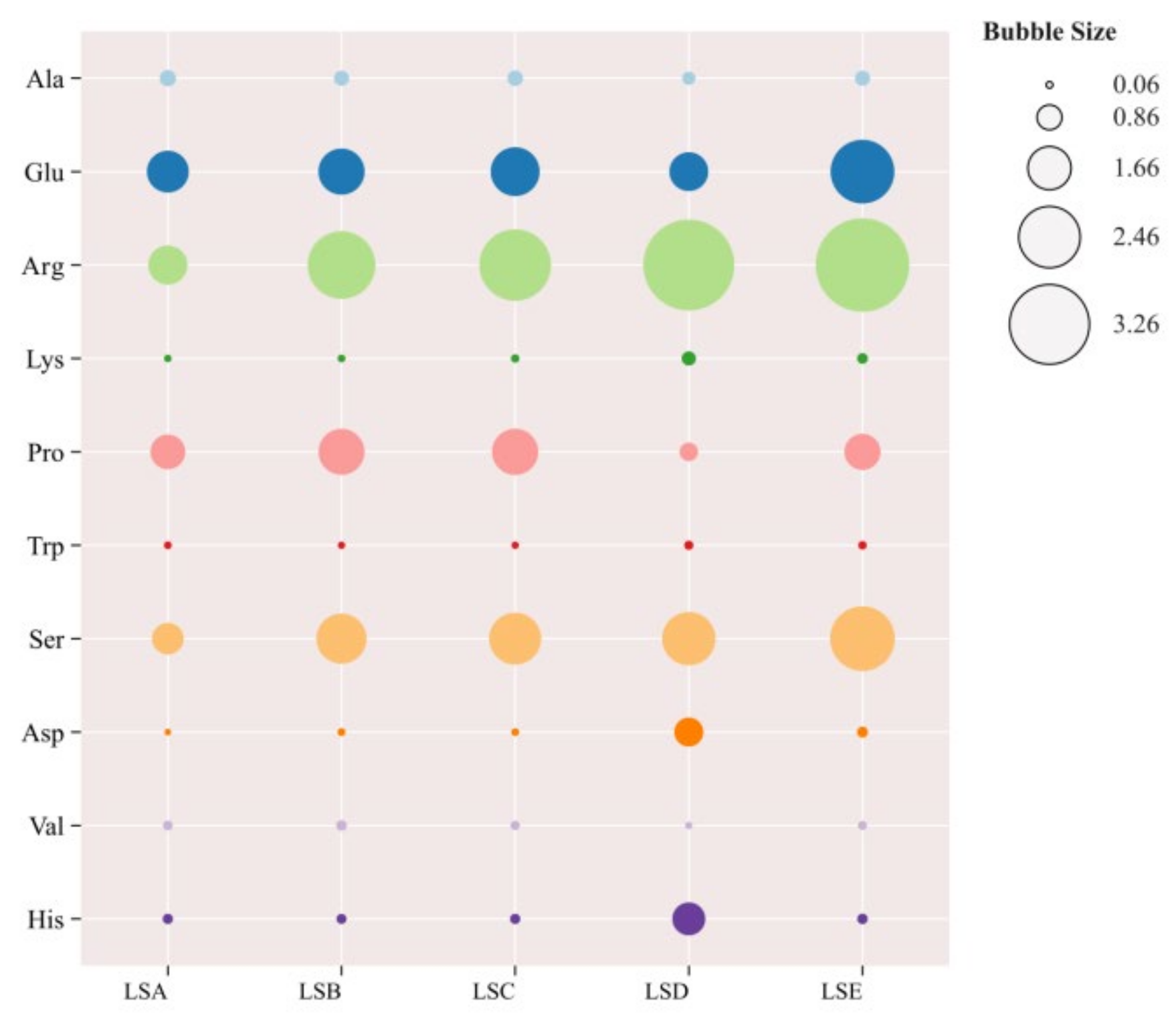
3.4. Analysis of Free Amino Acids in Sichuan Pepper at Different Harvesting Periods
3.5. TAV Analysis of Key Amino Acids in Sichuan Pepper at Different Harvesting Periods
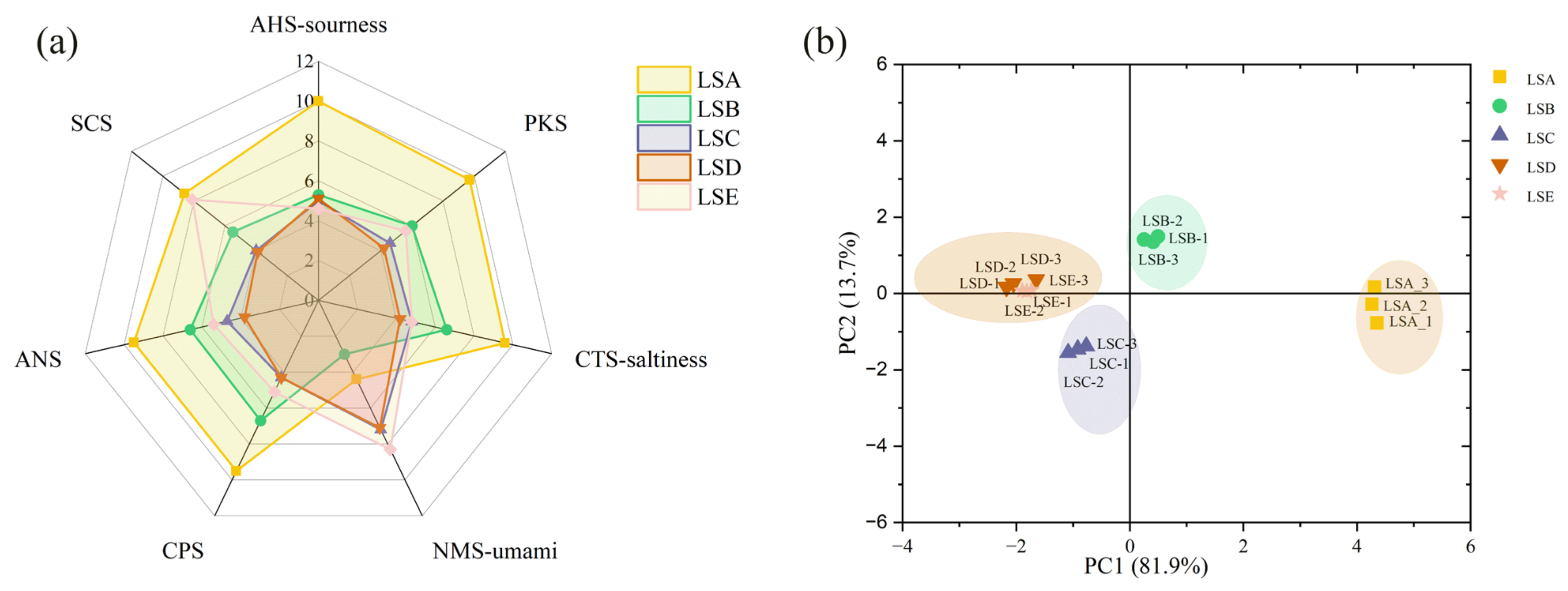
3.6. Analysis of Taste Characteristics of Sichuan Pepper at Different Harvesting Periods Based on E-Tongue Analysis
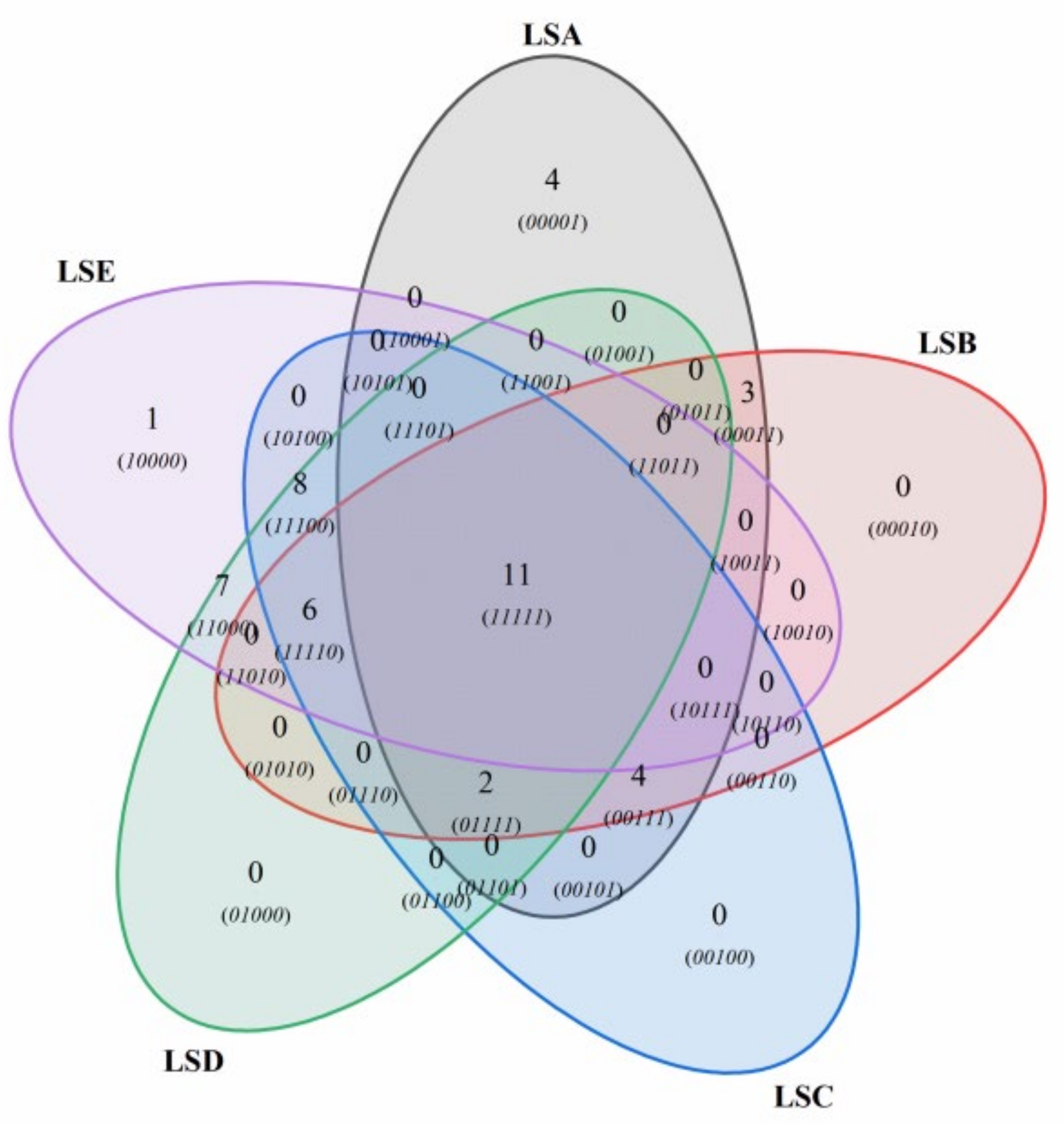
3.7. Analysis of Volatile Compounds in Sichuan Pepper at Different Harvest Periods Through GC-MS
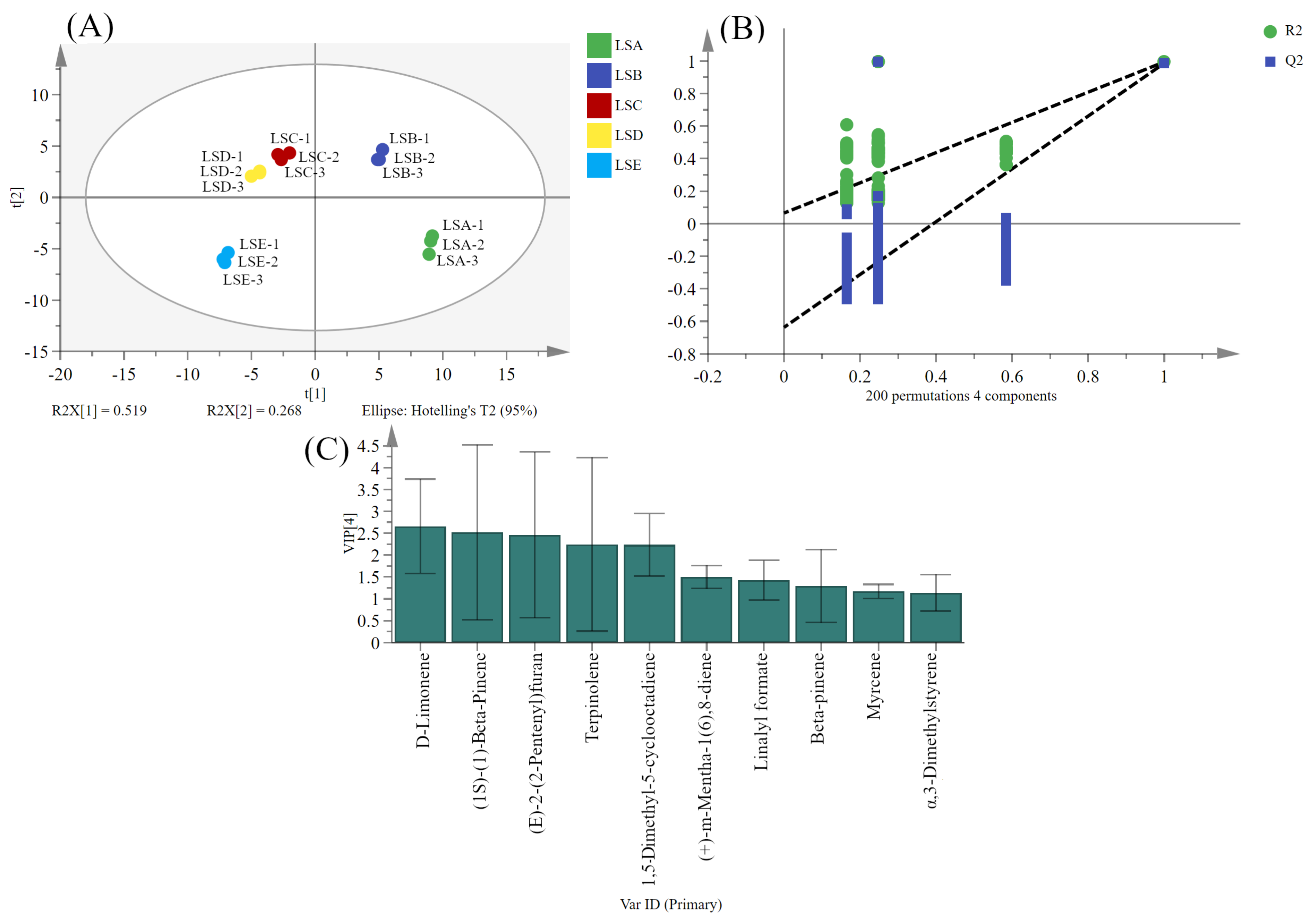
3.8. Multivariate Statistical Analysis to Identify Key Volatile Compounds in Sichuan Pepper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC | gas chromatography |
| MS | mass spectroscopy |
| PLS-DA | partial least squares discriminant analysis |
| PCA | principal component analysis |
| TA | total amino acid |
| TAV | taste activity value |
| VIP | variable importance in projection |
References
- Zhang, D.; Sun, X.; Battino, M.; Wei, X.; Shi, J.; Zhao, L.; Liu, S.; Xiao, J.; Shi, B.; Zou, X. A Comparative Overview on Chili Pepper (Capsicum Genus) and Sichuan Pepper (Zanthoxylum Genus): From Pungent Spices to Pharma-Foods. Trends Food Sci. Technol. 2021, 117, 148–162. [Google Scholar] [CrossRef]
- Ji, Y.; Li, S.; Ho, C.-T. Chemical Composition, Sensory Properties and Application of Sichuan Pepper (Zanthoxylum Genus). Food Sci. Hum. Wellness 2019, 8, 115–125. [Google Scholar] [CrossRef]
- Ivane, N.M.A.; Haruna, S.A.; Zekrumah, M.; Roméo Elysé, F.K.; Hassan, M.O.; Hashim, S.B.H.; Tahir, H.E.; Zhang, D. Composition, Mechanisms of Tingling Paresthesia, and Health Benefits of Sichuan Pepper: A Review of Recent Progress. Trends Food Sci. Technol. 2022, 126, 1–12. [Google Scholar] [CrossRef]
- Xiong, Q.; Dawen, S.; Yamamoto, H.; Mizuno, M. Alkylamides from Pericarps of Zanthoxylum Bungeanum. Phytochemistry 1997, 46, 1123–1126. [Google Scholar] [CrossRef]
- Yang, L.-C.; Li, R.; Tan, J.; Jiang, Z.-T. Polyphenolics Composition of the Leaves of Zanthoxylum bungeanum Maxim. Grown in Hebei, China, and Their Radical Scavenging Activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, C.; Deng, J.; Dong, P.; Xiong, Y.; Wu, H. Effect of Sichuan Pepper (Zanthoxylum Genus) Addition on Flavor Profile in Fermented Ciba Chili (Capsicum Genus) Using GC-IMS Combined with E-Nose and E-Tongue. Molecules 2023, 28, 5884. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Wang, J.; Zhang, M.; Chen, J.; He, Z.; Qin, F.; Xu, Z.; Cao, D.; Chen, J. Inhibitory Effects of Sichuan Pepper (Zanthoxylum bungeanum) and Sanshoamide Extract on Heterocyclic Amine Formation in Grilled Ground Beef Patties. Food Chem. 2018, 239, 111–118. [Google Scholar] [CrossRef]
- Blanc, N.; Hauchard, D.; Audibert, L.; Gall, E.A. Radical-Scavenging Capacity of Phenol Fractions in the Brown Seaweed Ascophyllum Nodosum: An Electrochemical Approach. Talanta 2011, 84, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Sharma, S.; Kumar, N.; Sharma, U.; Singh, B. Simultaneous Quantification and Identification of Flavonoids, Lignans, Coumarin and Amides in Leaves of Zanthoxylum Armatum Using UPLC-DAD-ESI-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2017, 132, 46–55. [Google Scholar] [CrossRef]
- Li, P.; Zhou, L.; Mou, Y.; Mao, Z. Extraction Optimization of Polysaccharide from Zanthoxylum bungeanum Using RSM and Its Antioxidant Activity. Int. J. Biol. Macromol. 2015, 72, 19–27. [Google Scholar] [CrossRef]
- Fei, X.; Qi, Y.; Lei, Y.; Hu, H.; Wei, A. Transcriptome and Metabolome Dynamics Explain Aroma Differences between Green and Red Prickly Ash Fruit. Foods 2021, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and Characterization of the Volatile Organic Compounds in Eight Kinds of Huajiao with Geographical Indication of China Using Electronic Nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef] [PubMed]
- Machmudah, S.; Izumi, T.; Sasaki, M.; Goto, M. Extraction of Pungent Components from Japanese Pepper (Xanthoxylum Piperitum DC.) Using Supercritical CO2. Sep. Purif. Technol. 2009, 68, 159–164. [Google Scholar] [CrossRef]
- Luo, J.; Ke, J.; Hou, X.; Li, S.; Luo, Q.; Wu, H.; Shen, G.; Zhang, Z. Composition, Structure and Flavor Mechanism of Numbing Substances in Chinese Prickly Ash in the Genus Zanthoxylum: A Review. Food Chem. 2022, 373, 131454. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Z.; Chen, X.; Guo, Z.; Wen, L.; Kan, J. Evaluation of Bitter Compounds in Zanthoxylum schinifolium Sieb. et Zucc. by Instrumental and Sensory Analyses. Food Chem. 2022, 390, 133180. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Metabolomics Analysis of Camellia Sinensis with Respect to Harvesting Time. Food Res. Int. 2020, 128, 108814. [Google Scholar] [CrossRef]
- Li, W.-T.; Ho, M.-C.; Yang, C. Study on Design Strategy for Sustainable Development of Chinese Solar Term Culture. Sustainability 2018, 10, 4355. [Google Scholar] [CrossRef]
- Tao, S. Cultural Structure of Solar Terms and Traditional Festivals. Natl. Arts 2018, 2018, 37–40. [Google Scholar]
- Han, S.; Zhang, L.; Liu, Y.; Ma, Y.; Yan, J.; Li, L. A Data Sample Division Method for Wind Power Prediction Based on China’s 24 Solar Terms. Int. Trans. Electr. Energy Syst. 2020, 30, e12342. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Wu, F.; Xie, G.; Shan, Z.; Liu, X. Flavor Profile Variations of Huangjiu Brewed in Different Traditional Chinese Solar Terms. Food Qual. Saf. 2022, 6, fyac037. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Hou, X.; Wu, H.; Li, Q.; Li, S.; Luo, Q.; Li, M.; Liu, X.; Shen, G.; et al. Physicochemical, antibacterial, and biodegradability properties of green Sichuan pepper (Zanthoxylum armatum DC.) essential oil incorporated starch films. LWT 2022, 161, 113392. [Google Scholar]
- Liu, J.; Wan, J.; Zhang, Y.; Hou, X.; Shen, G.; Li, S.; Luo, Q.; Li, Q.; Zhou, M.; Liu, X.; et al. The establishment of comprehensive quality evaluation model for flavor characteristics of green Sichuan pepper (Zanthoxylum armatum DC.) in Southwest China. Food Chem. X 2023, 18, 100721. [Google Scholar] [PubMed]
- Fu, M.; Wang, Y.; Li, M.; Lai, Y.; Hu, Q.; Wang, Z.; Liu, W.; Wang, H.; Zhang, D.; Fu, M.; et al. Improving the fruit quality of Sichuan-pepper (Zanthoxylum bungeanum Maxim.) ‘Dahongpao’ by optimized GA 3 treatments. Chil. J. Agric. Res. 2023, 83, 602–615. [Google Scholar]
- Zhou, Y.; Zhang, Z.; He, Y.; Gao, P.; Zhang, H.; Ma, X. Integration of Electronic Nose, Electronic Tongue, and Colorimeter in Combination with Chemometrics for Monitoring the Fermentation Process of Tremella Fuciformis. Talanta 2024, 274, 126006. [Google Scholar] [CrossRef] [PubMed]
- Han, C.E.; Chan, S.W.; Yap, C.Y.; Tan, P.L.; Lin, D.; He, L.; Xu, C. Valorization of Jackfruit (Artocarpus heterophllus) Rags As a Functional Ingredient in Sandwich Cookies: Sensory, Physicochemical, and Antioxidant Properties. J. Culin. Sci. Technol. 2024, 1–15. [Google Scholar] [CrossRef]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Total Phenolic, Flavonoid Contents, and Antioxidant Activities of Fruit, Seed, and Bark Extracts of Zanthoxylum armatum DC. Sci. World J. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Saikaew, K.; Lertrat, K.; Meenune, M.; Tangwongchai, R. Effect of High-Pressure Processing on Colour, Phytochemical Contents and Antioxidant Activities of Purple Waxy Corn (Zea mays L. Var. Ceratina) Kernels. Food Chem. 2018, 243, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, X.; Guo, H.; Bai, J.; Xiao, Y.; Fu, Y.; Wu, Y.; Wan, H.; Huang, Y.; Gao, H. Metabolomics and Metatranscriptomics Reveal the Influence Mechanism of Endogenous Microbe (Staphylococcus succinus) Inoculation on the Flavor of Fermented Chili Pepper. Int. J. Food Microbiol. 2023, 406, 110371. [Google Scholar] [CrossRef]
- Ping, C.; Deng, X.; Guo, Z.; Luo, W.; Li, X.; Xin, S. Characterizing the Flavor Profiles of Linjiangsi Broad Bean (Vicia faba L.) Paste Using Bionic Sensory and Multivariate Statistics Analyses Based on Ripening Time and Fermentation Environment. Food Chem. X 2024, 23, 101677. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Laghi, L.; Deng, J.; Dao, X.; Tang, J.; Ji, L.; Zhu, C.; Picone, G. Characterization of Flavor Profile of “Nanx Wudl” Sour Meat Fermented from Goose and Pork Using Gas Chromatography-Ion Mobility Spectrometry (GC-IMS) Combined with Electronic Nose and Tongue. Foods 2023, 12, 2194. [Google Scholar] [CrossRef]
- He, L.; Yi, Y.-W.; Xu, C.-J.; Deng, J.; Wu, H.-C.; Qiao, M.-F.; Chan, S.W.; Hu, J.-X. Impacts of Thermal Sterilization on Volatile Substances in Xiongzhang Tofu Seasoning Packets According to Electronic Nose and Gas Chromatography-Mass Spectrometry. LWT 2024, 207, 116631. [Google Scholar] [CrossRef]
- Shi, J.; Fei, X.; Hu, Y.; Liu, Y.; Wei, A. Identification of Key Genes in the Synthesis Pathway of Volatile Terpenoids in Fruit of Zanthoxylum bungeanum Maxim. Forests 2019, 10, 328. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Karmakar, I.; Haldar, S.; Chakraborty, M.; Chaudhury, K.; Dewanjee, S.; Haldar, P.K. Regulation of Apoptosis through Bcl-2/Bax Proteins Expression and DNA Damage by Zanthoxylum alatum. Pharm. Biol. 2016, 54, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, B.; An, Y.; Yang, G.; Liao, S.; Kan, J. Comprehensive Study on Nutritional, Flavour and Metabolite Characteristics of Green Prickly Ash (Zanthoxylum schinifoliums) at Different Picking Stages. Int. J. Food Sci. Technol. 2022, 57, 7961–7973. [Google Scholar] [CrossRef]
- Pagliarini, E.; Proserpio, C.; Spinelli, S.; Lavelli, V.; Laureati, M.; Arena, E.; Di Monaco, R.; Menghi, L.; Gallina Toschi, T.; Braghieri, A.; et al. The Role of Sour and Bitter Perception in Liking, Familiarity and Choice for Phenol-Rich Plant-Based Foods. Food Qual. Prefer. 2021, 93, 104250. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Wang, C.; Han, F.; Chen, X.; Zhao, A.; Wang, D. Time-Series Based Metabolomics Reveals the Characteristics of the Color-Related Metabolites during the Different Coloration Stages of Zanthoxylum bungeanum Peel. Food Res. Int. 2022, 155, 111077. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Simal-Gandara, J.; Mei, J.; Ma, W.; Peng, Q.; Shi, Y.; Xu, Q.; Lin, Z.; Lv, H. Insight into the Pigmented Anthocyanins and the Major Potential Co-Pigmented Flavonoids in Purple-Coloured Leaf Teas. Food Chem. 2021, 363, 130278. [Google Scholar] [CrossRef]
- Cai, W.; Wang, Y.; Hou, Q.; Zhang, Z.; Tang, F.; Shan, C.; Yang, X.; Guo, Z. Rice Varieties Affect Bacterial Diversity, Flavor, and Metabolites of Zha-Chili. Food Res. Int. 2021, 147, 110556. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Ding, S.; Ye, M.; Jiang, L.; Wang, R. Changes in Free Amino Acids of Fermented Minced Peppers during Natural and Inoculated Fermentation Process Based on HPLC-MS/MS. J. Food Sci. 2020, 85, 2803–2811. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E. Storage Proteins of Vegetative Plant Tissues. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 303–322. [Google Scholar] [CrossRef]
- Fei, X.; Hu, H.; Luo, Y.; Shi, Q.; Wei, A. Widely Targeted Metabolomic Profiling Combined with Transcriptome Analysis Provides New Insights into Amino Acid Biosynthesis in Green and Red Pepper Fruits. Food Res. Int. 2022, 160, 111718. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Flavor Compounds of Traditional Fermented Bean Condiments: Classes, Synthesis, and Factors Involved in Flavor Formation. Trends Food Sci. Technol. 2023, 133, 160–175. [Google Scholar] [CrossRef]
- Yang, Q.; Mei, X.; Wang, Z.; Chen, X.; Zhang, R.; Chen, Q.; Kan, J. Comprehensive Identification of Non-Volatile Bitter-Tasting Compounds in Zanthoxylum bungeanum Maxim. by Untargeted Metabolomics Combined with Sensory-Guided Fractionation Technique. Food Chem. 2021, 347, 129085. [Google Scholar] [CrossRef] [PubMed]
- Greger, H. Alkamides: A Critical Reconsideration of a Multifunctional Class of Unsaturated Fatty Acid Amides. Phytochem. Rev. 2016, 15, 729–770. [Google Scholar] [CrossRef]
- Salman, S.; Yılmaz, C.; Gökmen, V.; Özdemir, F. Effects of Fermentation Time and Shooting Period on Amino Acid Derivatives and Free Amino Acid Profiles of Tea. LWT 2021, 137, 110481. [Google Scholar] [CrossRef]
- Gunlu, A.; Gunlu, N. Taste Activity Value, Free Amino Acid Content and Proximate Composition of Mountain Trout (Salmo Trutta Macrostigma Dumeril, 1858) Muscles. Iran. J. Fish. Sci. 2014, 13, 58–72. [Google Scholar]
- Zhu, C.; Yang, Z.; Lu, X.; Yi, Y.; Tian, Q.; Deng, J.; Jiang, D.; Tang, J.; Laghi, L. Effects of Saccharomyces Cerevisiae Strains on the Metabolomic Profiles of Guangan Honey Pear Cider. LWT 2023, 182, 114816. [Google Scholar] [CrossRef]
- Yin, X.; Xu, X.; Zhang, Q.; Xu, J. Rapid Determination of the Geographical Origin of Chinese Red Peppers (Zanthoxylum bungeanum Maxim.) Based on Sensory Characteristics and Chemometric Techniques. Molecules 2018, 23, 1001. [Google Scholar] [CrossRef]
| Sample ID | Harvesting Time | Solar Term * | Average Highest Temperature (°C) | Average Lowest Temperature (°C) | Average Humidity (%) | Average Rainfall (mm/h) | Average Ground Wind Speed (m/s) |
|---|---|---|---|---|---|---|---|
| LSA | 24 July 2022 | Greater Heat | 29 | 19 | 69.5 | 0.32 | 1.44 |
| LSB | 7 August 2022 | Beginning of Autumn | 26 | 18 | 81.0 | 0.53 | 0.81 |
| LSC | 22 August 2022 | End of Heat | 28 | 18 | 74.0 | 0.31 | 0.91 |
| LSD | 6 September 2022 | White Dew | 26 | 17 | 77.8 | 0.37 | 1.23 |
| LSE | 21 September 2022 | Autumn Equinox | 24 | 17 | 81.2 | 0.41 | 0.82 |
| Harvest Periods | Moisture Content (%) | Thousand- Grain Weight (g) | Transverse Diameter (mm) | Longitudinal Diameter (mm) | Fruit Shape Index | Energy (kcal) | Protein (g/100 g) | Fat (g/100 g) | Carbohydrates (100/g) |
|---|---|---|---|---|---|---|---|---|---|
| LSA | 9.23 ± 0.11 c | 1.42 ± 0.02 d | 3.75 ± 0.46 a | 4.29 ± 0.30 c | 0.94 ± 0.12 b | 337.33 ± 2.05 c | 9.50 ± 0.22 a | 5.30 ± 0.14 d | 64.33 ± 1.07 b |
| LSB | 10.68 ± 0.17 b | 1.47 ± 0.05 cd | 4.33 ± 0.17 ab | 4.56 ± 0.32 bc | 1.07 ± 0.10 ab | 352.00 ± 2.16 b | 7.03 ± 0.48 b | 6.70 ± 0.08 c | 66.17 ± 0.25 a |
| LSC | 10.70 ± 0.06 b | 1.58 ± 0.12 c | 4.44 ± 0.19 c | 4.20 ± 0.32 c | 1.12 ± 0.12 a | 326.67 ± 2.36 d | 7.63 ± 0.05 b | 5.57 ± 0.25 d | 60.23 ± 0.83 c |
| LSD | 10.57 ± 0.14 b | 1.93 ± 0.06 b | 4.58 ± 0.29 bc | 4.73 ± 0.58 b | 1.16 ± 0.04 a | 358.67 ± 2.05 a | 9.97 ± 0.24 a | 9.47 ± 0.09 a | 57.90 ± 0.80 d |
| LSE | 15.89 ± 0.03 a | 2.57 ± 0.04 a | 4.08 ± 0.57 ab | 5.28 ± 0.34 a | 1.19 ± 0.09 a | 334.00 ± 4.90 c | 6.00 ± 0.45 c | 7.20 ± 0.33 b | 60.73 ± 0.90 c |
| FAA | Taste Characteristics | Content (mg/100 g) | ||||
|---|---|---|---|---|---|---|
| LSA | LSB | LSC | LSD | LSE | ||
| Cysteine | Aromatic | 4.39 ± 0.28 b | 4.32 ± 0.15 b | 4.67 ± 0.37 b | 9.29 ± 0.44 a | 3.89 ± 0.46 b |
| Phenylalanine | 2.48 ± 0.33 ab | 2.83 ± 0.45 a | 1.91 ± 0.18 b | 3.52 ± 0.42 a | 2.59 ± 0.34 ab | |
| Tyrosine | 3.77 ± 0.39 b | 3.09 ± 0.17 bc | 2.32 ± 0.52 c | 6.58 ± 0.42 a | 2.62 ± 0.04 c | |
| Arginine | Bitter | 73.81 ± 0.35 c | 136.42 ± 1.84 b | 145.04 ± 5.01 b | 187.38 ± 6.99 a | 193.17 ± 1.16 a |
| Lysine | 4.84 ± 0.16 e | 5.57 ± 0.15 d | 6.79 ± 0.12 c | 19.52 ± 0.17 a | 12.09 ± 0.32 b | |
| Leucine | 2.40 ± 0.14 ab | 2.45 ± 0.55ab | 1.79 ± 0.28 b | 2.62 ± 0.28 a | 2.14 ± 0.29 ab | |
| Tryptophan | 9.49 ± 0.06 c | 8.52 ± 0.22 d | 8.43 ± 0.38 d | 15.74 ± 0.27 a | 13.00 ± 0.25 b | |
| Valine | 7.51 ± 0.61 b | 9.33 ± 0.29 a | 6.72 ± 0.24 bc | 3.60 ± 0.30 d | 6.24 ± 0.24 c | |
| Isoleucine | 2.76 ± 0.14 a | 3.09 ± 0.21 a | 2.23 ± 0.26 a | 3.21 ± 0.85 a | 2.57 ± 0.36 a | |
| Proline | Sweet | 385.68 ± 4.24 b | 533.45 ± 0.41 a | 535.59 ± 3.04 a | 174.42 ± 5.62 c | 406.23 ± 16.92 b |
| Serine | 172.39 ± 1.30 d | 293.51 ± 1.61 c | 306.11 ± 2.14 bc | 314.88 ± 5.60 b | 389.30 ± 13.98 a | |
| Threonine | 7.16 ± 0.42 bc | 11.28 ± 0.87 a | 11.15 ± 0.88 a | 6.44 ± 0.46 c | 8.41 ± 0.89 b | |
| Histidine | 4.41 ± 0.21 b | 4.24 ± 0.46 b | 4.36 ± 0.21 b | 24.12 ± 1.01 a | 4.58 ± 0.64 b | |
| Alanine | 28.80 ± 0.97 a | 25.97 ± 1.65 a | 26.99 ± 0.99 a | 20.37 ± 0.34 b | 25.73 ± 3.76 a | |
| Glycine | 1.71 ± 0.13 c | 2.28 ± 0.25 abc | 1.98 ± 0.17 bc | 3.27 ± 0.58 a | 2.68 ± 0.36 ab | |
| Glutamic Acid | Umami | 48.03 ± 0.07 c | 53.72 ± 0.33 b | 57.35 ± 2.76 b | 43.99 ± 1.74 d | 76.86 ± 0.14 a |
| Aspartic Acid | 6.10 ± 0.08 d | 11.73 ± 0.32 c | 10.86 ± 0.98 c | 103.84 ± 1.45 a | 25.35 ± 4.11 b | |
| Asparagine | 13.83 ± 4.93 bc | 24.50 ± 0.82 a | 21.12 ± 1.37 ab | 11.62 ± 1.57 c | 21.77 ± 1.86 ab | |
| Sum Content (Aromatic) | - | 10.64 ± 0.41 b | 10.25 ± 0.64 b | 8.89 ± 0.92 b | 19.38 ± 0.93 a | 9.10 ± 0.54 b |
| Sum Content (Bitter) | - | 100.81 ± 0.80 c | 165.38 ± 2.07 b | 170.99 ± 5.63 b | 232.07 ± 5.85 a | 229.21 ± 1.55 a |
| Sum Content (Sweet) | - | 600.15 ± 3.84 c | 870.74 ± 3.73 a | 886.18 ± 5.26 a | 543.49 ± 1.46 d | 836.93 ± 24.60 b |
| Sum Content (Umami) | - | 67.95 ± 4.80 d | 89.95 ± 0.83 c | 89.33 ± 3.40 c | 159.44 ± 2.76 a | 123.98 ± 5.58 b |
| TA | - | 779.55 ± 2.10 d | 1136.31 ± 2.92 b | 1155.39 ± 10.22 b | 954.39 ± 7.67 c | 1199.22 ± 21.94 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Yi, Y.; Jia, H.; Xu, C.; Qiao, M.; Cai, X.; Leong, S.Y.; Singaram, N.; Chan, S.W.; Peng, H. Impact of Harvest Periods on the Physicochemical and Flavour Characteristics of Sichuan Pepper (Zanthoxylum bungeanum Maxim). Foods 2025, 14, 1155. https://doi.org/10.3390/foods14071155
He L, Yi Y, Jia H, Xu C, Qiao M, Cai X, Leong SY, Singaram N, Chan SW, Peng H. Impact of Harvest Periods on the Physicochemical and Flavour Characteristics of Sichuan Pepper (Zanthoxylum bungeanum Maxim). Foods. 2025; 14(7):1155. https://doi.org/10.3390/foods14071155
Chicago/Turabian StyleHe, Lian, Yuwen Yi, Hongfeng Jia, Chengjian Xu, Mingfeng Qiao, Xuemei Cai, Sze Ying Leong, Nallammai Singaram, Sook Wah Chan, and Hua Peng. 2025. "Impact of Harvest Periods on the Physicochemical and Flavour Characteristics of Sichuan Pepper (Zanthoxylum bungeanum Maxim)" Foods 14, no. 7: 1155. https://doi.org/10.3390/foods14071155
APA StyleHe, L., Yi, Y., Jia, H., Xu, C., Qiao, M., Cai, X., Leong, S. Y., Singaram, N., Chan, S. W., & Peng, H. (2025). Impact of Harvest Periods on the Physicochemical and Flavour Characteristics of Sichuan Pepper (Zanthoxylum bungeanum Maxim). Foods, 14(7), 1155. https://doi.org/10.3390/foods14071155






