Peptide-Rich Yeast Fractions from Brewer’s Spent Yeast: A Scalable Fractionation Approach and Their Functional Application in Bakery Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Tomato Pomace By-Product and Wheat Germ
2.3. BSY By-Product
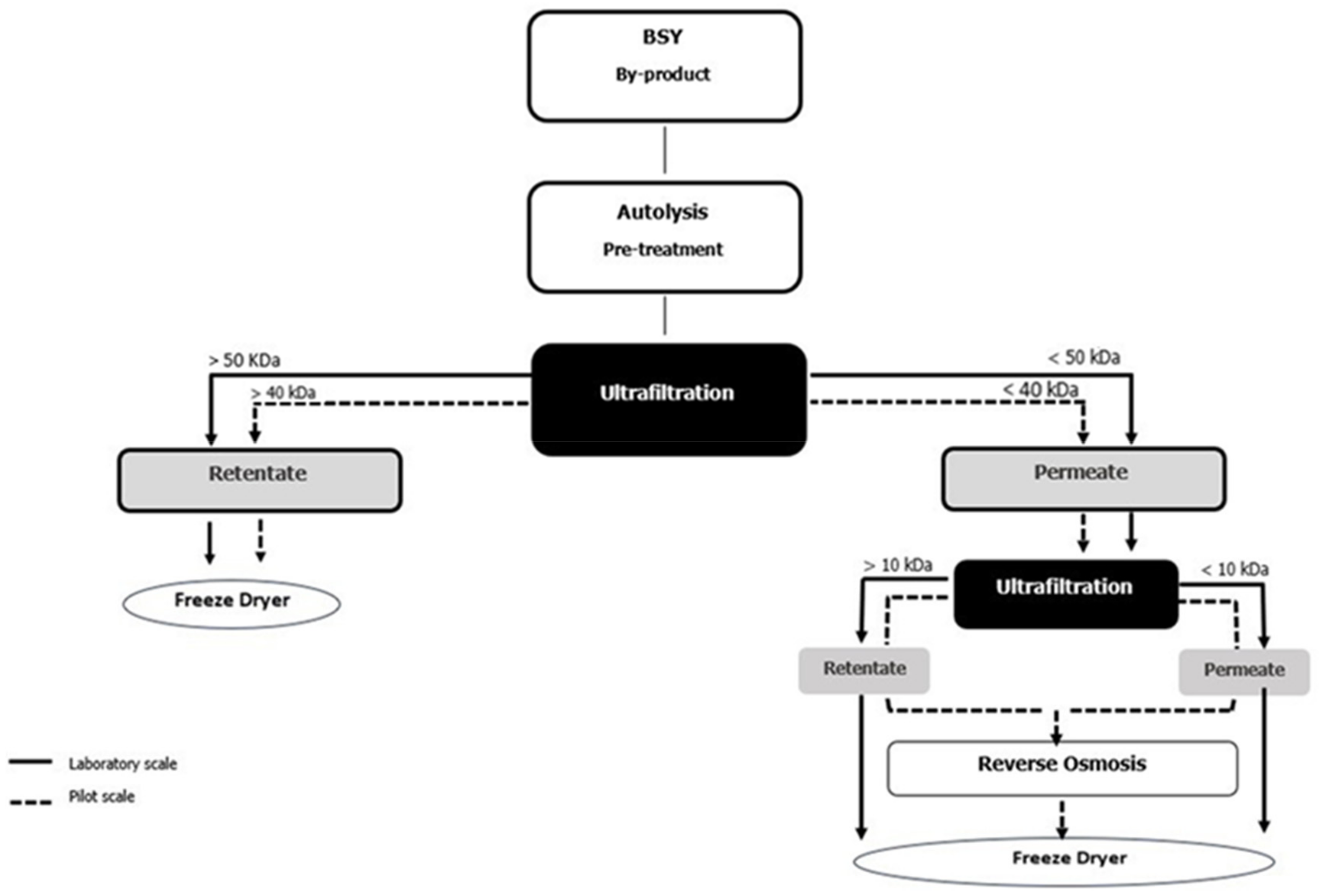
2.4. Optimization and Scale-Up of BSY Fractionation Processes
- Laboratory-Scale Fractions:
- ○
- L/RUF50: retentate from 50 kDa ultrafiltration.
- ○
- L/RUF10: permeate from 50 kDa/retentate from 10 kDa ultrafiltration.
- ○
- L/PUF10: permeate from 10 kDa ultrafiltration.
- Pilot-Scale Fractions:
- ○
- P/RUF40: retentate from 40 kDa ultrafiltration.
- ○
- P/RUF10: permeate from 40 kDa/retentate from 10 kDa ultrafiltration.
- ○
- P/PUF10: permeate from 10 kDa ultrafiltration.
2.5. Nutritional Composition
2.6. Water- and Oil-Holding Capacity (WHC and OHC)
2.7. Color Measurement
2.8. Water Activity (aw) Measurement
2.9. Peptide Profile
Molecular Weight Distribution
2.10. Bioactivity Characterization
2.10.1. Extraction of Phenolic Compounds
2.10.2. Total Phenolic Content
Folin–Ciocalteu Assay
2.10.3. Antioxidant Activity
ABTS Assay
ORAC Assay
2.11. Crackers Preparation
2.12. Chemical and Bioactive Properties of Crackers
2.13. Physical and Technological Properties of Crackers
2.13.1. Water Activity and Color Measurement
2.13.2. Density
2.13.3. Texture
2.13.4. Scanning Electron Microscopy
2.14. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the BSY By-Products
3.2. Evaluation of BSY Process Scale-Up Efficiency
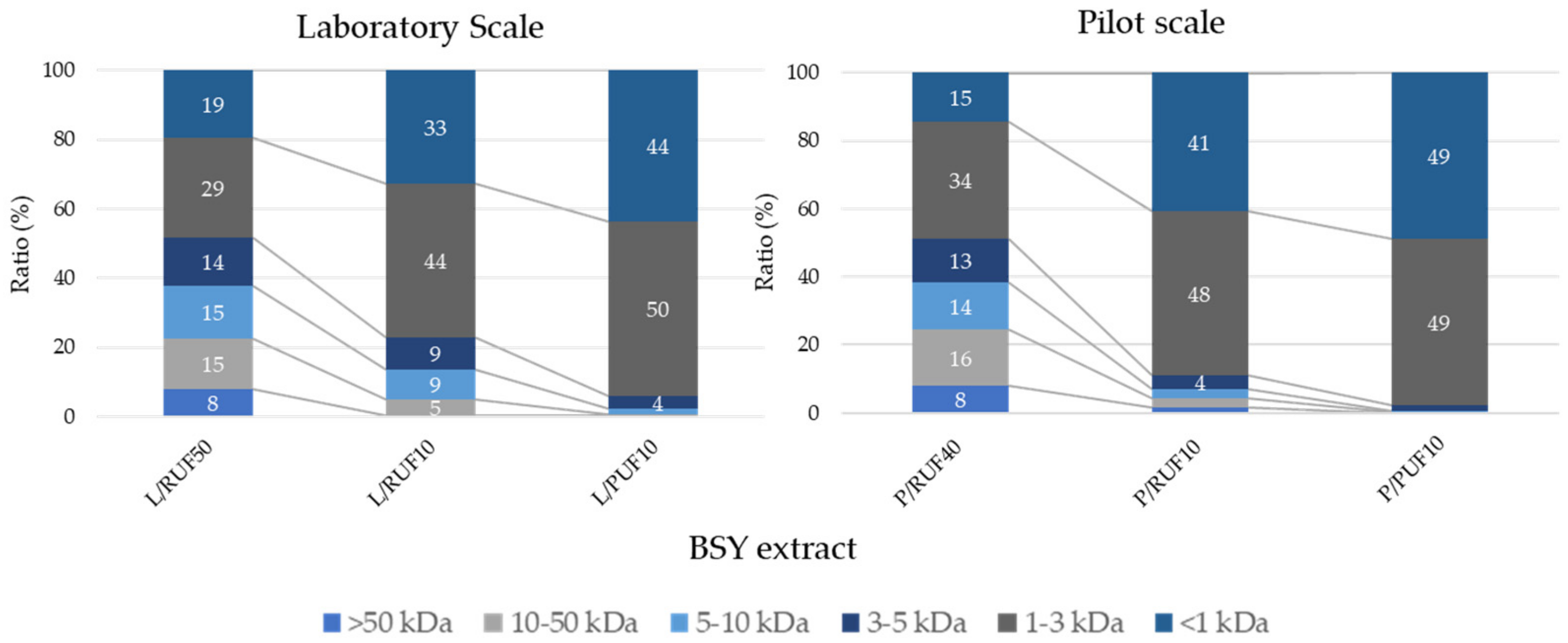
3.2.1. BSY Peptide Profile
3.2.2. Yield, Protein Content, Color Measurement, and Technological Properties
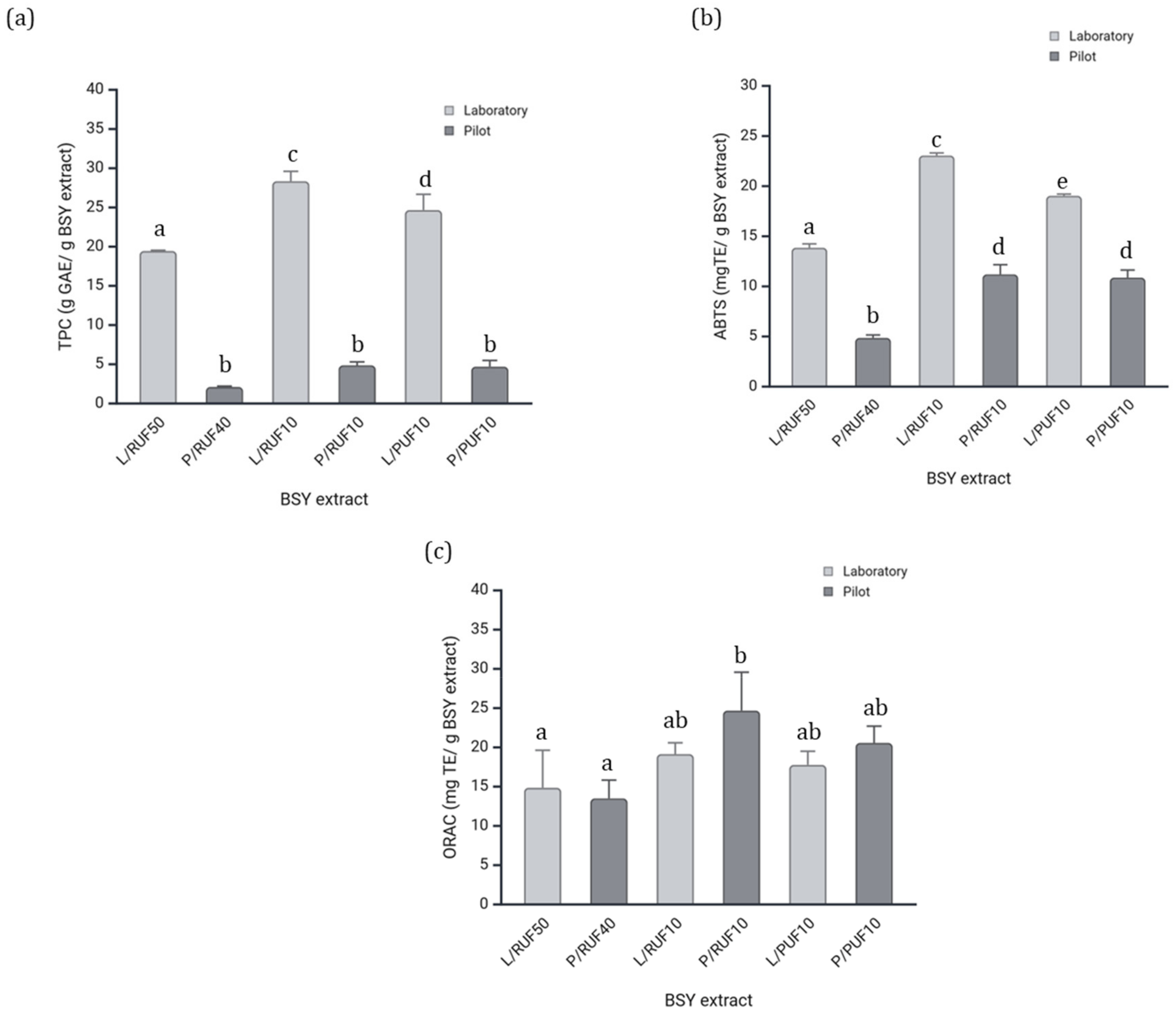
3.2.3. Total Phenolic Content and Antioxidant Capacity
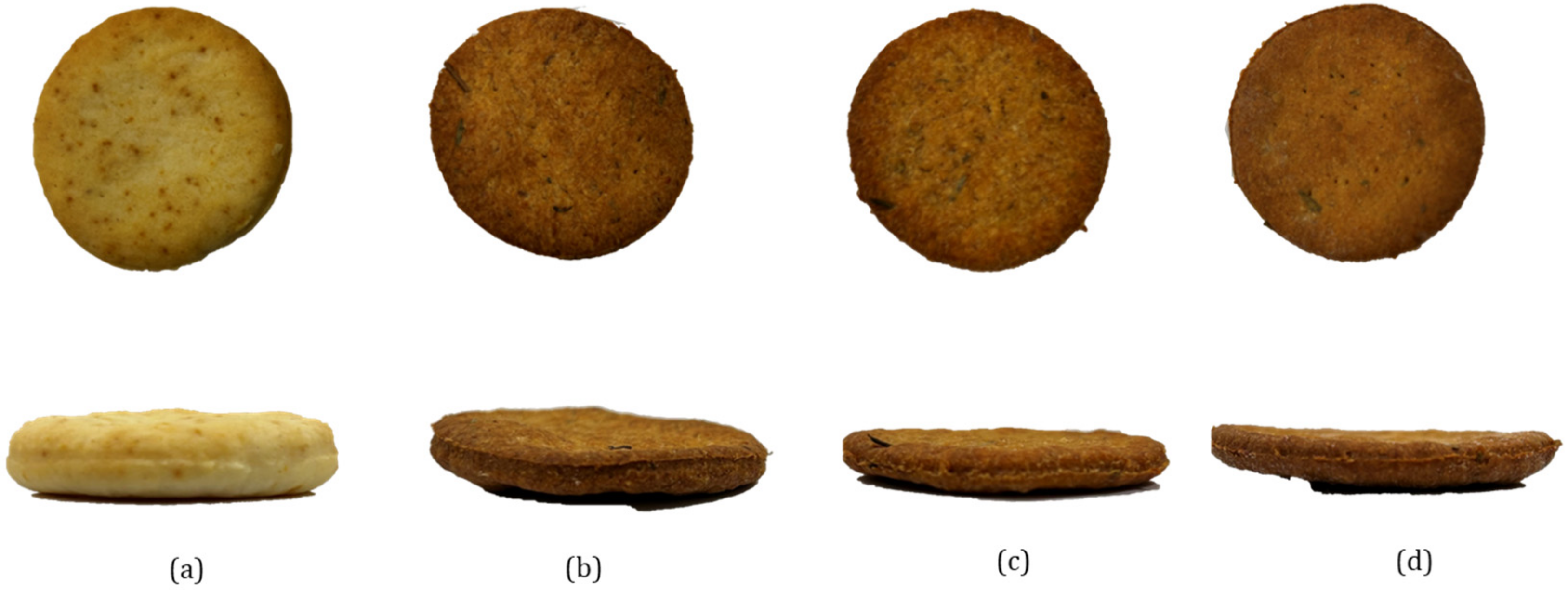
3.3. Properties of Crackers with BSY Fraction
3.3.1. Chemical Analysis of Crackers
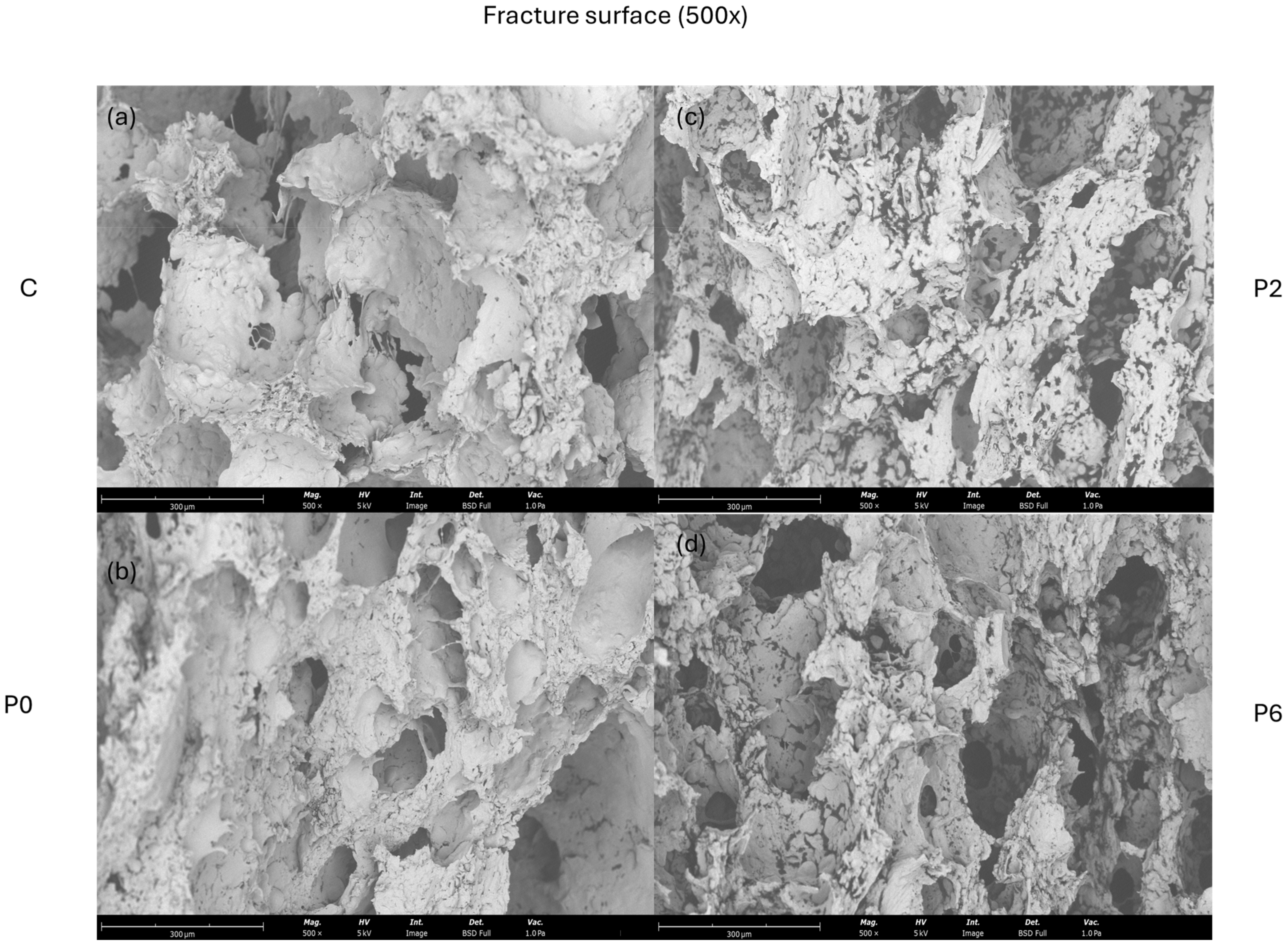
3.3.2. Physical Characteristics of Crackers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Antioxidant activity |
| AAPH | 2,20-azobis (2-methylpropionamidine) dihydrochloride |
| ABTS | Diammonium salt (2, 2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) |
| AOXs | Antioxidant assays |
| BSY | Brewer’s spent yeast |
| DW | Dry weight |
| GAEs | Gallic acid equivalents |
| HPSEC | High-Performance Size Exclusion Chromatography |
| KDa | Kilodaltons |
| L/PUF10 | Permeate from 10 kDa ultrafiltration at laboratory scale |
| L/RUF10 | Permeate from 50 kDa/retentate from 10 kDa ultrafiltration at laboratory scale |
| L/RUF50 | Retentate from 50 kDa ultrafiltration at laboratory scale |
| MWCO | Molecular weight cut-off |
| OHC | Oil-holding capacity |
| ORAC | Oxygen radical absorbance capacity |
| P/PUF10 | Permeate from 10 kDa ultrafiltration at pilot scale |
| P/RUF10 | Permeate from 40 kDa/retentate from 10 kDa ultrafiltration at pilot scale |
| P/RUF40 | Retentate from 40 kDa ultrafiltration at pilot scale |
| PB | Phosphate buffer |
| PTFE | Polytetrafluoroethylene |
| TDF | Total dietary fiber |
| TEs | Trolox equivalents |
| TPC | Total phenolic content |
| UF | Ultrafiltration |
| WHC | Water-holding capacity |
References
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of protein-rich extracts from spent brewer’s yeast (Saccharomyces cerevisiae): An overview. Biomass Convers. Biorefin. 2022, 15, 1771–1793. [Google Scholar]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of brewery wastes in food industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef]
- 360iResearch, E.R. by Brewer’s Spent Yeast Market Worth $2.17 Billion by 2030, Growing at a CAGR of 5.83%. 2024. Available online: https://www.openpr.com/news/3394525/brewer-s-spent-yeast-market-worth-2-17-billion-by-2030-growing (accessed on 15 July 2024).
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Habschied, K.; Kulisic, B.; Barkow, I.; Tišma, M. Valorization of Spent Brewer’s Yeast for the Production of High-Value Products, Materials, and Biofuels and Environmental Application. Fermentation 2023, 9, 208. [Google Scholar] [CrossRef]
- Horn, P.A.; Zeni, A.L.B.; Cavichioli, N.; Winter, E.; Batista, K.Z.S.; Vitali, L.; de Almeida, E.A. Chemical profile of craft brewer’s spent yeast and its antioxidant and antiproliferative activities. Eur. Food Res. Technol. 2023, 249, 2001–2015. [Google Scholar] [CrossRef]
- da Silva Araújo, V.B.; de Melo, A.N.F.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; de Souza, E.L.; Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar]
- Zhu, L.; Wang, J.; Feng, Y.; Yin, H.; Lai, H.; Xiao, R.; He, S.; Yang, Z.; He, Y. Process Optimization, Amino Acid Composition, and Antioxidant Activities of Protein and Polypeptide Extracted from Waste Beer Yeast. Molecules 2022, 27, 6825. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Odila Pereira, J.; Ferreira, C.; Faustino, M.; Durão, J.; Pereira, A.M.; Oliveira, C.M.; Pintado, M.E.; Carvalho, A.P. Spent Yeast Valorization for Food Applications: Effect of Different Extraction Methodologies. Foods 2022, 11, 4002. [Google Scholar] [CrossRef]
- Amorim, M.; Marques, C.; Pereira, J.O.; Guardão, L.; Martins, M.J.; Osório, H.; Moura, D.; Calhau, C.; Pinheiro, H.; Pintado, M. Antihypertensive effect of spent brewer yeast peptide. Process Biochem. 2019, 76, 213–218. [Google Scholar] [CrossRef]
- Amorim, M.M.; Pereira, J.O.; Monteiro, K.M.; Ruiz, A.L.; Carvalho, J.E.; Pinheiro, H.; Pintado, M. Antiulcer and antiproliferative properties of spent brewer’s yeast peptide extracts for incorporation into foods. Food Funct. 2016, 7, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Gautério, G.V.; Silvério, S.I.D.C.; Egea, M.B.; Lemes, A.C. Β-Glucan From Brewer’S Spent Yeast As a Techno-Functional Food Ingredient. Front. Food Sci. Technol. 2022, 2, 1074505. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent Brewer’s yeast as a source of insoluble β-glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Oliveira, R.; Martins, Z.E.; Sousa, J.B.; Ferreira, I.M.P.L.V.O.; Diniz, C. The health-promoting potential of peptides from brewing by-products: An up-to-date review. Trends Food Sci. Technol. 2021, 118, 143–153. [Google Scholar] [CrossRef]
- Djouadi, A.; Sales, J.R.; Carvalho, M.O.; Raymundo, A. Development of healthy protein-rich crackers using Tenebrio molitor flour. Foods 2022, 11, 702. [Google Scholar] [CrossRef]
- Gangola, M.P.; Ramadoss, B.R.; Jaiswal, S.; Fabek, H.; Tulbek, M.; Anderson, G.H.; Chibbar, R.N. Nutritional composition and in vitro starch digestibility of crackers supplemented with faba bean whole flour, starch concentrate, protein concentrate and protein isolate. Foods 2022, 11, 645. [Google Scholar] [CrossRef]
- Amorim, M.; Pereira, J.O.; Gomes, D.; Dias, C. Nutritional ingredients from spent brewer ’ s yeast obtained by hydrolysis and selective membrane fi ltration integrated in a pilot process. J. Food Eng. 2016, 185, 42–47. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, C.; Coscueta, E.R.; Fernandes, J.; Pereira, R.N.; Teixeira, J.A.; Rodrigues, A.S.; Pintado, M.E. Bioactivity and Bioaccessibility of Bioactive Compounds in Gastrointestinal Digestion of Tomato Bagasse Extracts. Foods 2022, 11, 1064. [Google Scholar] [CrossRef]
- Coelho, M.C.; Ghalamara, S.; Campos, D.; Ribeiro, T.B.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M. Tomato Processing By-Products Valorisation through Ohmic Heating Approach. Foods 2023, 12, 818. [Google Scholar] [CrossRef]
- De Vasconcelos, M.C.B.M.; Bennett, R.; Castro, C.; Cardoso, P.; Saavedra, M.J.; Rosa, E.A. Study of composition, stabilization and processing of wheat germ and maize industrial by-products. Ind. Crops Prod. 2013, 42, 292–298. [Google Scholar] [CrossRef]
- Cardoso, R.V.C.; Fernandes, Â.; Pinela, J.; Dias, M.I.; Pereira, C.; Pires, T.C.S.P.; Carocho, M.; Vasallo, E.F.; Ferreira, I.C.F.R.; Barros, L. Valorization of cereal by-products from the milling industry as a source of nutrients and bioactive compounds to boost resource-use efficiency. Agronomy 2021, 11, 972. [Google Scholar] [CrossRef]
- Liadakis, G.; Katsouli, M.; Chanioti, S.; Giannou, V.; Tzia, C. Identification, quantification, and characterization of tomato processing by-products. In Tomato Processing by-Products; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–32. [Google Scholar]
- Coelho, M.C.; Costa, C.; Roupar, D.; Silva, S.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Modulation of the Gut Microbiota by Tomato Flours Obtained after Conventional and Ohmic Heating Extraction and Its Prebiotic Properties. Foods 2023, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Ribeiro, T.B.; Oliveira, C.; Batista, P.; Castro, P.; Monforte, A.R.; Rodrigues, A.S.; Teixeira, J.; Pintado, M. In vitro gastrointestinal digestion impact on the bioaccessibility and antioxidant capacity of bioactive compounds from tomato flours obtained after conventional and ohmic heating extraction. Foods 2021, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources-A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Estivi, L.; Bertuglia, K.; Ivanova, N.; Jukić, M.; Komlenić, D.K.; Lukinac, J.; Hidalgo, A. Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers. Antioxidants 2022, 11, 2087. [Google Scholar] [CrossRef]
- Gün, D.; Çelekli, A.; Bozkurt, H. Nutritional and Sensory Optimization of Functional Crackers with the Incorporation of Arthrospira platensis. Preprints 2024, 2024071555. [Google Scholar]
- Jiang, C.; Wang, R.; Liu, X.; Wang, J.; Zheng, X.; Zuo, F. Effect of Particle Size on Physicochemical Properties and in vitro Hypoglycemic Ability of Insoluble Dietary Fiber From Corn Bran. Front. Nutr. 2022, 9, 951821. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis; AOAC: San Diego, CA, USA, 1990. [Google Scholar]
- ISO International Organization for Standardization. 2009. Available online: https://www.iso.org/ (accessed on 1 June 2024).
- ISO International Organization for Standardization. 2013. Available online: https://www.iso.org/ (accessed on 1 June 2024).
- Norma NP 518; Norma Portuguesa: Lisbon, Portugal, 1986.
- Amorim, M.; Pinheiro, H.; Pintado, M. Valorizaion of spent brewer’s yeast: Optimization of hydrolysis process towards the generation of stable ACE-inhibitory peptides. LWT 2019, 111, 77–84. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.; Pintado, M. Are olive pomace powders a safe source of bioactives and nutrients ? J. Sci. Food Agric. 2020, 101, 1963–1978. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Martins Vilas-Boas, A.; Brandão, T.R.S.; Silva, C.L.M.; Azevedo, M.; Pintado, M. Impact of Roasting Temperature and Seed Presence on Carob Flour (Ceratonia siliqua L.): Physical, Chemical, and Functional Properties. ACS Food Sci. Technol. 2023, 3, 1890–1902. [Google Scholar]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Pectin extracted from thermally treated olive oil by-products: Characterization, physico-chemical properties, invitro bile acid andglucose binding. Food Hydrocoll. 2015, 43, 311–321. [Google Scholar] [CrossRef]
- Mala, T.; Piayura, S.; Itthivadhanapong, P. Characterization of dried pineapple (Ananas comosus L.) peel powder and its application as a novel functional food ingredient in cracker product. Futur. Foods 2024, 9, 100322. [Google Scholar] [CrossRef]
- Cunha, M.F.; Coscueta, E.R.; Marques, R.; Neto, J.; Almada, F.; Gonçalves, D.; Pintado, M. Exploring Bioactivities and Peptide Content of Body Mucus from the Lusitanian Toadfish Halobatrachus didactylus. Molecules 2023, 28, 6458. [Google Scholar] [CrossRef] [PubMed]
- León-González, M.E.; Gómez-Mejía, E.; Rosales-Conrado, N.; Madrid-Albarrán, Y. Residual brewing yeast as a source of polyphenols: Extraction, identification and quantification by chromatographic and chemometric tools. Food Chem. 2018, 267, 246–254. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Campos, D.A.; Nunes, C.; Ribeiro, S.; Nunes, J.; Oliveira, A.; Pintado, M. Polyphenol extraction by different techniques for valorisation of non-compliant portuguese sweet cherries towards a novel antioxidant extract. Sustainability 2020, 12, 5556. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content, and in vitro antioxidant activity of red wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as functional ingredients in savory food products: Application to wheat crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef]
- Almeida, D.; Machado, D.; Sousa, S.; Seabra, C.L.; Barbosa, J.C.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Effect of emulsification/internal gelation-based microencapsulation on the viability of Akkermansia muciniphila upon prolonged storage and simulated gastrointestinal passage. Food Hydrocoll. Health 2022, 2, 100084. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Abbasifard, M.; Kazerooni, K.; Taghipour Khaje Sharifi, G.; Bagheri-Hosseinabadi, Z.; Hajizadeh, M.R. Influence of blood trace elements on immune responses and adverse symptoms subsequent to Sinopharm COVID-19 vaccination. Sci. Rep. 2024, 14, 29481. [Google Scholar] [CrossRef] [PubMed]
- Wołonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postepy Hig. Med. Dosw. (Online) 2016, 70, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar]
- Ferreira, I.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends food Sci. Technol. 2010, 21, 77–84. [Google Scholar]
- Yadav, M.; Ahmadi, Y.; Chiu, F.-C. Food and bioprocessing industry. In Handbook of Polymer Nanocomposites for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 295–324. [Google Scholar]
- Wijethunga, A.M.; Emenike, C. Separation methods for food protein purification and analysis. Explor. Foods Foodomics 2024, 2, 391–407. [Google Scholar]
- Abejón, R.; Belleville, M.P.; Sanchez-Marcano, J.; Garea, A.; Irabien, A. Optimal design of industrial scale continuous process for fractionation by membrane technologies of protein hydrolysate derived from fish wastes. Sep. Purif. Technol. 2018, 197, 137–146. [Google Scholar]
- Zhang, B.; Kotsalis, G.; Khan, J.; Xiong, Z.; Igou, T.; Lan, G.; Chen, Y. Backwash sequence optimization of a pilot-scale ultrafiltration membrane system using data-driven modeling for parameter forecasting. J. Memb. Sci. 2020, 612, 118464. [Google Scholar] [CrossRef]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey protein hydrolysates as a source of bioactive peptides for functional foods–Biotechnological facilitation of industrial scale-up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Vollet, G.; Pereira, V.; Teixeira, M.; Di, M.; Martínez, J.; Belleville, M.; Dupas, M. Ultrafiltration performance of spent brewer’s yeast protein hydrolysate: Impact of pH and membrane material on fouling. J. Food Eng. 2021, 302, 110569. [Google Scholar] [CrossRef]
- Martínez, J.; Silva, L.P.S. Scale-up of extraction processes. In Natural Product Extraction: Principles and Applications; Royal Society of Chemistry: London, UK, 2022. [Google Scholar]
- Fawzya, Y.N.; Nursatya, S.M.; Susilowati, R.; Chasanah, E.; Soedirman, U.J. Characteristics of Fish Protein Hydrolysate from Yellowstripe Scad (Selaroides leptolepis) Produced by a Local Microbial Protease. E3S Web Conf. 2020, 147, 03017. [Google Scholar]
- Guan, X.; Yao, H.; Chen, Z.; Shan, L.; Zhang, M. Some functional properties of oat bran protein concentrate modified by trypsin. Food Chem. 2007, 101, 163–170. [Google Scholar]
- Hamzah, N.; Leo, C.P. State-of-the-art Membrane Processing of Solution Rich in Phenolic Compounds. J. Appl. Membr. Sci. Technol. 2023, 27, 1–10. [Google Scholar]
- Conidi, C.; Egea-Corbacho, A.; Cassano, A. A combination of aqueous extraction and polymeric membranes as a sustainable process for the recovery of polyphenols from olive mill solid wastes. Polymers 2019, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) study. Front. Nutr. 2022, 9, 882458. [Google Scholar]
- Yoo-Min, C.; So-Heon, J.; HER, M.; Soon-Ho, Y.I.M. Correlation of antioxidant and radical scavenging activity in Hydrangea macrophylla L. extract from various cultivars. Food Sci. Technol. 2023, 43, e00019. [Google Scholar]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar]
- Bacha, A.A.; Suhail, M.; Awwad, F.A.; Ismail, E.A.A.; Ahmad, H. Role of dietary fiber and lifestyle modification in gut health and sleep quality. Front. Nutr. 2024, 11, 1324793. [Google Scholar]
- Serna, J.; Bergwitz, C. Importance of dietary phosphorus for bone metabolism and healthy aging. Nutrients 2020, 12, 3001. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, J.; Kumar, S.; Singh, P. Metabolism of Macro-elements (Calcium, Magnesium, Sodium, Potassium, Chloride and Phosphorus) and Associated Disorders. In Clinical Applications of Biomolecules in Disease Diagnosis: A Comprehensive Guide to Biochemistry and Metabolism; Springer: Berlin/Heidelberg, Germany, 2024; pp. 177–203. [Google Scholar]
- O’Donnell, M.; Mente, A.; Yusuf, S. Sodium intake and cardiovascular health. Circ. Res. 2015, 116, 1046–1057. [Google Scholar]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Impact of new ingredients obtained from brewer’s spent yeast on bread characteristics. J. Food Sci. Technol. 2018, 55, 1966–1971. [Google Scholar] [CrossRef]
- Silva, F.D.; dos Santos Garcia, V.A.; Vanin, F.M.; Yoshida, C.M.P.; de Carvalho, R.A. Application of Tomato Byproduct in Food Products—A Review. Food Sci. Eng. 2023, 4, 103–115. [Google Scholar]
- Dachana, K.B.; Rajiv, J.; Indrani, D.; Prakash, J. Effect of dried moringa (Moringa oleifera lam) leaves on rheological, microstructural, nutritional, textural and organoleptic characteristics of cookies. J. Food Qual. 2010, 33, 660–677. [Google Scholar]
| Ingredients | C (%) | P0 (%) | P2 (%) | P6 (%) |
|---|---|---|---|---|
| Wheat Flour | 60.5 | 24.0 | 22.0 | 18.0 |
| Baking Powder | 1.5 | 1.5 | 1.5 | 1.5 |
| Salt | 1.0 | 1.0 | 1.0 | 1.0 |
| Sugar | 1.0 | 1.0 | 1.0 | 1.0 |
| Vegetable Oil (sunflower oil) | 7.5 | 7.5 | 7.5 | 7.5 |
| Water | 28.5 | 22.0 | 22.0 | 22.0 |
| Tomate Pomace Flour (<250 µm) | 4.0 | 4.0 | 4.0 | |
| Thyme | 0.1 | 0.1 | 0.1 | |
| P/RUF 10 | 2.0 | 6.0 | ||
| Wheat Germ | 38.9 | 38.9 | 38.9 |
| Chemical Composition (g/100 g DW) | BSY | |
|---|---|---|
| Proximate composition | Moisture | 84.1 ± 0.05 |
| Ash | 5.52 ± 0.02 | |
| Proteins | 43.6 ± 0.45 | |
| Fat | 2.51 ± 0.01 | |
| Carbohydrates | 48.4 ± 0.48 | |
| Energy a | 390.59 | |
| Macrominerals (mg/100 g DW) | Ca | 43.9 ± 6.48 |
| K | 220 ± 2.70 | |
| Na | 2.92 ± 0.06 | |
| Mg | 27.3 ± 1.69 | |
| P | 167 ± 5.71 | |
| Trace minerals (mg/100 g DW) | Al | 0.02 ± 0.01 |
| Cu | 0.21 ± 0.00 | |
| Fe | 1.18 ± 0.09 | |
| Mn | 0.06 ± 0.01 | |
| Zn | 0.53 ± 0.04 |
| Parameters | L/RUF50 | L/RUF10 | L/PUF10 | P/RUF40 | P/RUF10 | P/PUF10 |
|---|---|---|---|---|---|---|
| Yield (% DW) | 23.76 a | 6.25 b | 7.54 c | 17.77 d | 0.70 e | 0.08 f |
| Protein (% DW) | 27.06 ± 0.92 a | 31.12 ± 0.90 b | 28.5 ± 0.39 ab | 22.55 ± 2.85 c | 16.27 ± 0.51 d | 18.01 ± 1.18 d |
| L* | 24.29 ± 0.63 bc | 37.88 ± 2.54 a | 33.75 ± 2.14 a | 24.16 ± 0.99 bc | 20.99 ± 0.48 c | 25.87 ± 2.33 b |
| a* | 4.14 ± 0.20 bc | 4.92 ± 0.33 b | 5.78 ± 0.14 a | 3.93 ± 0.13 c | 2.53 ± 0.04 d | 3.60 ± 0.61 c |
| b* | 7.16 ± 0.31 c | 10.79 ± 0.66 b | 12.69 ± 0.54 a | 6.90 ± 0.21 c | 0.94 ± 0.16 e | 2.56 ± 0.44 d |
| WHC (g water/g) | ND a | ND a | ND a | 1.12 ± 0.11 b | ND a | ND a |
| OHC (g oil/g) | 4.08 ± 0.23 a | 3.36 ± 0.62 ab | 3.15 ± 0.75 b | 1.35 ± 0.02 c | 0.45 ± 0.14 c | 1.02 ± 0.14 c |
| Parameters | C (%) | P0 (%) | P2 (%) | P6 (%) |
|---|---|---|---|---|
| Moisture (%) | 15.77 ± 6.95 a | 13.49 ± 1.97 a | 18.07 ± 0.95 a | 19.79 ± 6.97 a |
| Ash (%) | 1.94 ± 0.01 c | 3.30 ± 0.04 b | 3.41 ± 0.06 a | 3.05 ± 0.10 b |
| Protein (%) | 10.39 ± 0.61 b | 23.61 ± 1.05 a | 24.88 ± 1.31 a | 26.52 ± 1.77 a |
| Fat (%) | 9.52 ± 0.14 d | 14.01 ± 0.14 a | 9.71 ± 0.07 c | 3.05 ± 0.10 b |
| Carbohydrates (%) | 78.15 ± 0.75 a | 59.07 ± 0.59 c | 62.00 ± 0.52 b | 58.88 ± 0.28 c |
| Energy (kcal/100 g DW) | 439.84 ± 6.69 a | 456.85 ± 7.83 a | 434.91 ± 7.96 a | 445.56 ± 10.76 a |
| TDF (g/100 g DW) | 13.85 ± 0.55 c | 26.19 ± 0.05 b | 26.99 ± 0.09 b | 30.51 ± 0.02 a |
| P (mg/100 g DW) | 237.52 ± 2.04 b | 636.00 ± 0.74 a | 655.00 ± 1.17 a | 658.00 ± 1.80 a |
| P (%DRI/100 g DW) | 33.93 ± 0.04 b | 90.86 ± 0.14 a | 93.57 ± 0.17 a | 94.00 ± 0.80 a |
| Mg (mg/100 g DW) | ND c | 122.00 ± 0.14 b | 116.00 ± 0.31 b | 125.00 ± 0.20 a |
| Mg (%DRI/100 g DW) | ND c | 34.86 ± 0.04 b | 33.14 ± 0.01 b | 35.71 ± 0.02 a |
| Na (mg/100 g DW) | 735.00 ± 1.15 a | 754.00 ± 0.92 a | 746.00 ± 1.42 a | 754.00 ± 2.39 a |
| Na (%DRI/100 g DW) | 49.00 ± 0.15 a | 50.27 ± 0.22 a | 49.73 ± 0.42 a | 50.27 ± 0.39 a |
| K (mg/100 g DW) | 171.83 ± 1.58 c | 577.00 ± 0.22 b | 570.00 ± 0.17 b | 588.00 ± 0.84 a |
| K (%DRI/100 g DW) | 4.91 ± 0.08 c | 577.00 ± 0.22 b | 570.00 ± 0.17 b | 588.00 ± 0.84 a |
| TPC (mg GAE/100 gDW) | 54.70 ± 19.40 b | 16.49 ± 2.10 a | 16.29 ± 1.40 a | 16.80 ± 1.70 a |
| ABTS (g TE/100 gDW) | 4.93 ± 0.96 c | 13.35 ± 2.63 b | 18.59 ± 1.32 a | 20.10 ± 0.55 a |
| Water Activity (aw) | 0.76 ± 0.07 a | 0.64 ± 0.05 ab | 0.68 ± 0.03 ab | 0.60 ± 0.04 b |
| L* | 86.97 ± 4.05 a | 54.81 ± 1.89 b | 61.08 ± 1.34 b | 56.45 ± 2.35 b |
| a* | 5.39 ± 0.33 a | 11.52 ± 1.97 b | 11.38 ± 0.86 b | 11.01 ± 1.28 b |
| b* | 20.73 ± 0.25 bc | 23.15 ± 0.91 ab | 24.57 ± 1.50 a | 19.60 ± 0.86 c |
| Diameter (cm) | 5.57 ± 0.27 a | 5.47 ± 0.15 a | 5.99 ± 0.21 a | 5.80 ± 0.22 a |
| Thickness (cm) | 0.85 ± 0.09 a | 0.61 ± 0.13 ab | 0.75 ± 0.12 ab | 0.55 ± 0.06 b |
| Weight (g) | 10.63 ± 0.60 a | 8.753 ± 0.87 ab | 10.12 ± 2.39 ab | 7.12 ± 0.33 b |
| Density (g/cm3) | 0.44 ± 0.05 a | 0.37 ± 0.06 ab | 0.36 ± 0.07 ab | 0.27 ± 0.01 b |
| Hardness (N) | 68.40 ± 10.94 a | 108.20 ± 27.20 a | 94.60 ± 28.60 a | 115.24 ± 11.70 a |
| Fracturability (mm) | 2.18 ± 0.50 b | 2.25 ± 1.17 b | 6.57 ± 1.06 a | 3.15 ± 0.23 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brassesco, M.E.; Paupério, A.; Pereira, C.D.; Ferreira, J.P.; Pintado, M. Peptide-Rich Yeast Fractions from Brewer’s Spent Yeast: A Scalable Fractionation Approach and Their Functional Application in Bakery Products. Foods 2025, 14, 1144. https://doi.org/10.3390/foods14071144
Brassesco ME, Paupério A, Pereira CD, Ferreira JP, Pintado M. Peptide-Rich Yeast Fractions from Brewer’s Spent Yeast: A Scalable Fractionation Approach and Their Functional Application in Bakery Products. Foods. 2025; 14(7):1144. https://doi.org/10.3390/foods14071144
Chicago/Turabian StyleBrassesco, María Emilia, Ana Paupério, Carlos D. Pereira, João Paulo Ferreira, and Manuela Pintado. 2025. "Peptide-Rich Yeast Fractions from Brewer’s Spent Yeast: A Scalable Fractionation Approach and Their Functional Application in Bakery Products" Foods 14, no. 7: 1144. https://doi.org/10.3390/foods14071144
APA StyleBrassesco, M. E., Paupério, A., Pereira, C. D., Ferreira, J. P., & Pintado, M. (2025). Peptide-Rich Yeast Fractions from Brewer’s Spent Yeast: A Scalable Fractionation Approach and Their Functional Application in Bakery Products. Foods, 14(7), 1144. https://doi.org/10.3390/foods14071144









