Composition of Coffee Beans Influenced by Bioprocessing with Selected Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Design
2.2.1. Experiment 1—Screening of Bacterial Strains’ Growth
2.2.2. Experiments 2 and 3—Fermentation Procedure and Analysis
2.3. Coffee Preparation
2.4. Coffee Chemical Analysis
2.5. Statistical Analysis
3. Results and Discussion
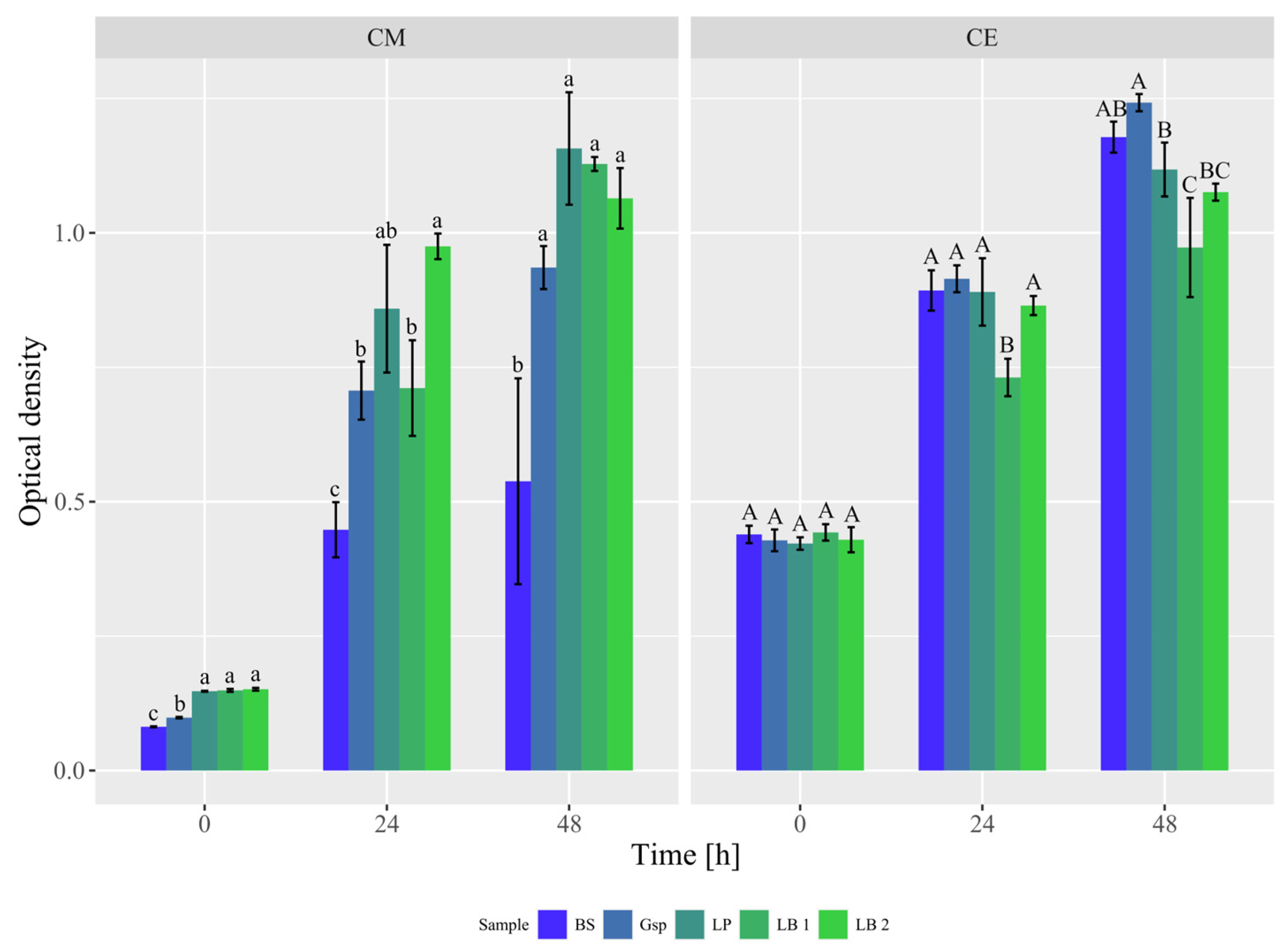
3.1. Experiment 1: Screening of Bacterial Strains’ Growth
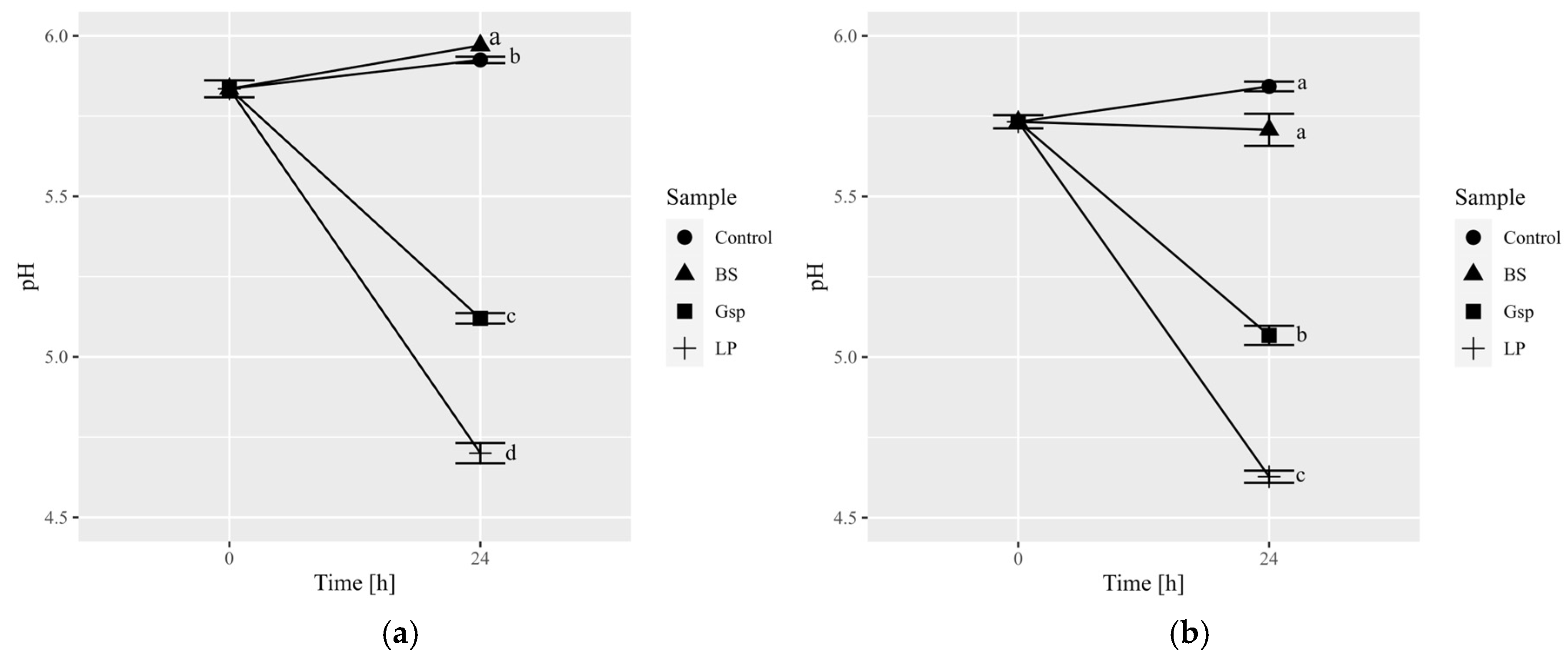
3.2. Experiment 2: Coffee Fermentation with Selected Bacterial Strains
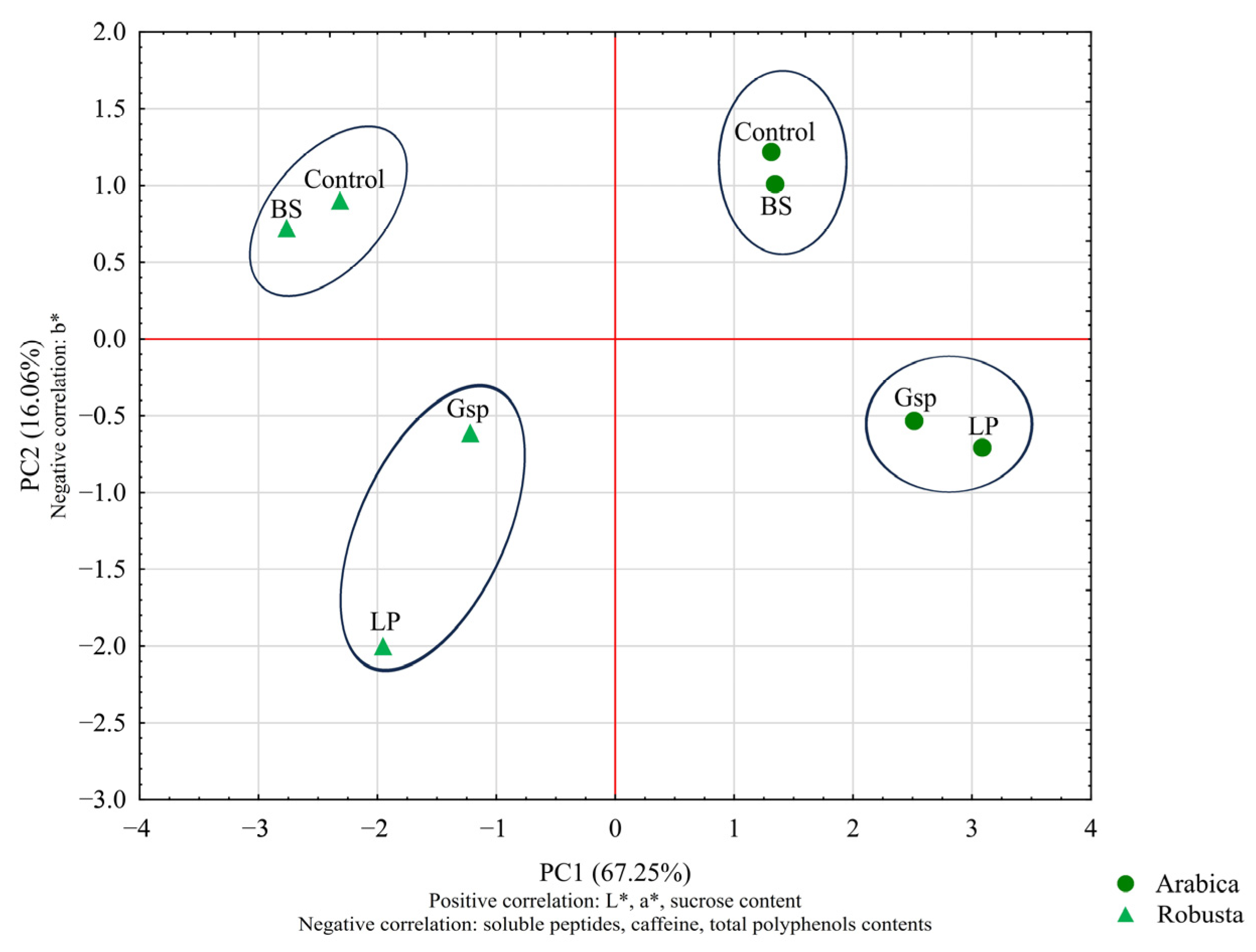
3.2.1. The Influence of Bacterial Strains on Green Coffee Color and Composition
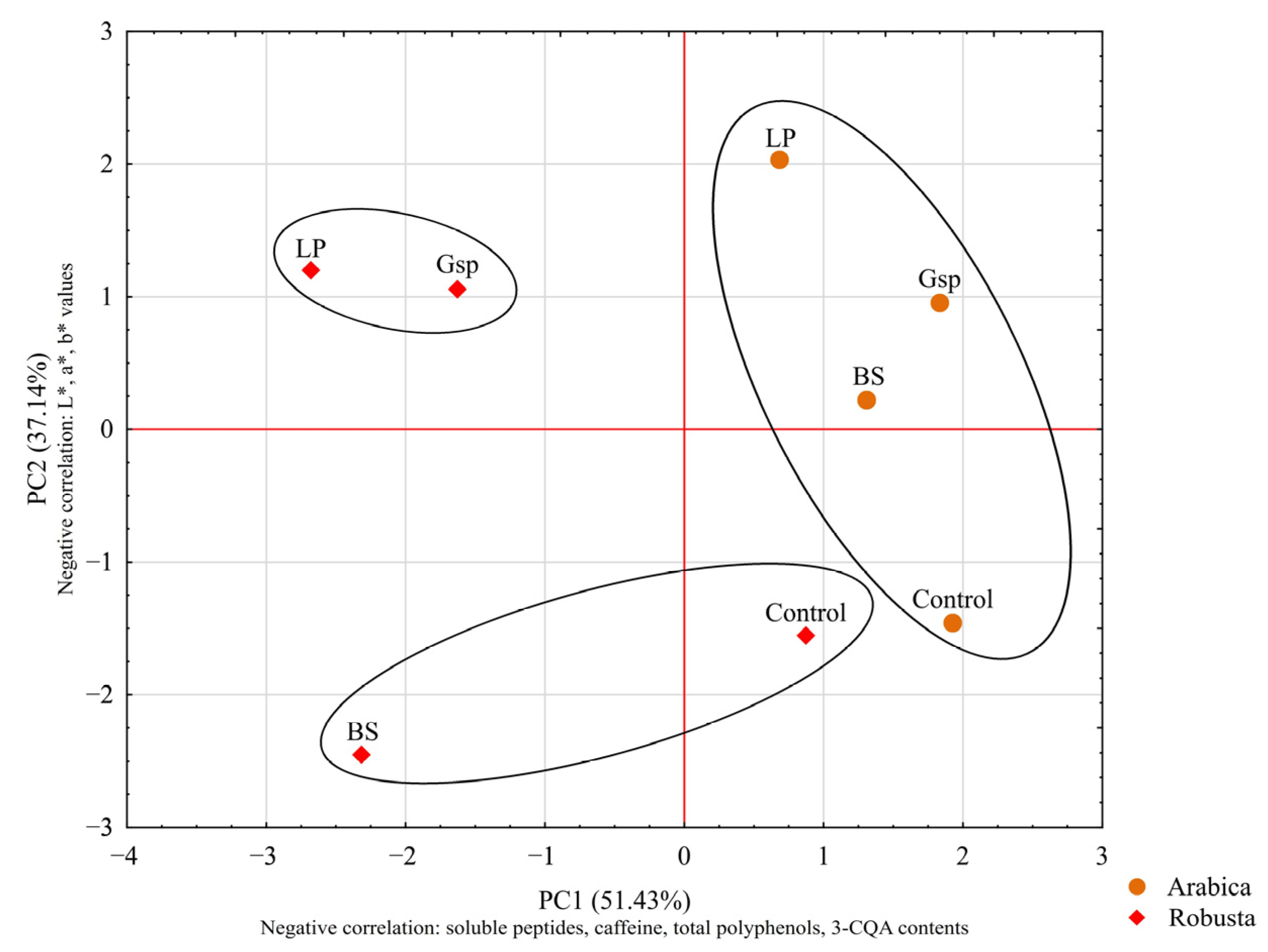
3.2.2. The Influence of Roasting on Fermented Beans’ Color and Composition
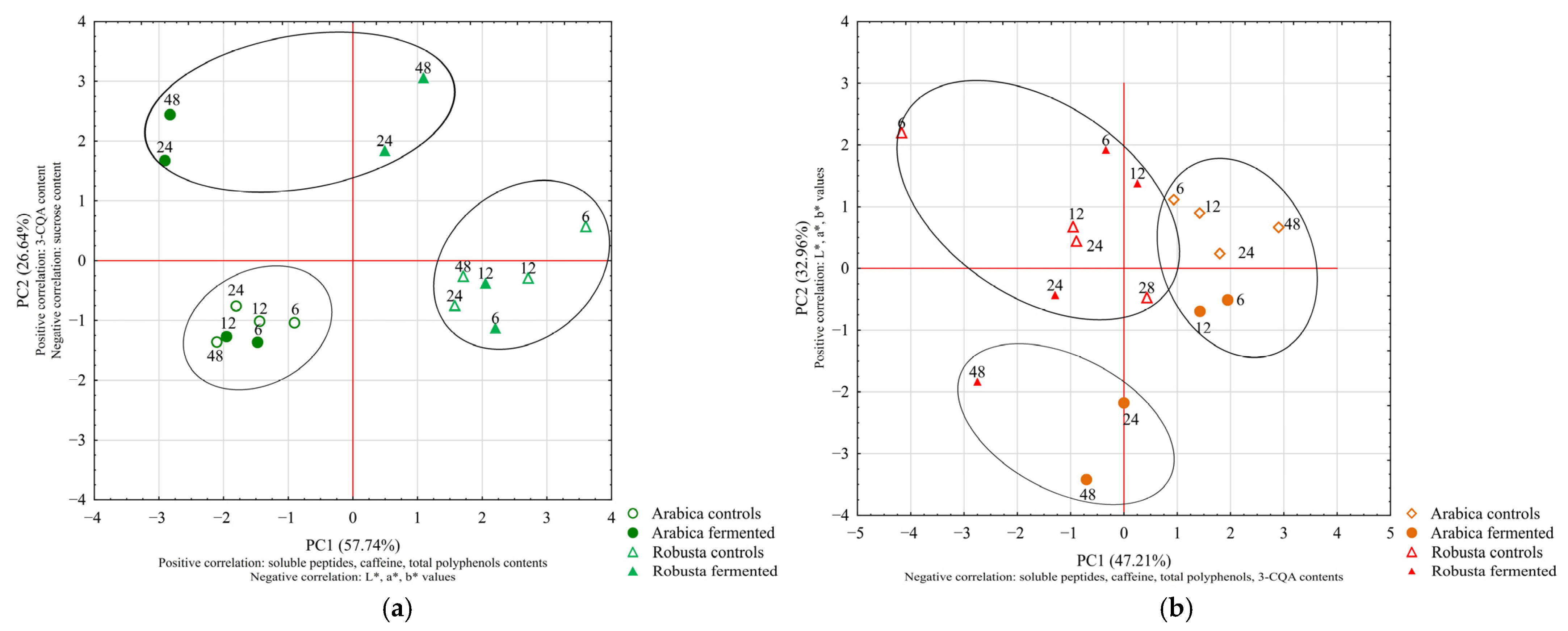
3.3. Experiment 3: Influence of Time on Coffee Fermentation with the Selected Bacterium
3.3.1. The Influence of Fermentation Duration on the Color and Composition of Green and Roasted Coffee Beans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | Lactic acid bacteria |
| CFU | Colony-forming bacteria |
| MRS | de Man, Rogosa and Sharpe medium |
| GY | Glucose and yeast medium |
| TBS | Tryptic soy broth |
| 3-CQA | 3-caffeoylquinic acid |
| OD | Optical density |
| HPLC | High-pressure liquid chromatography |
| ELSD | Evaporative light-scattering detector |
| PDA | Photodiode detector |
| ANOVA | One-way analysis of variance |
| PCA | Principal component analysis |
| dm | Dry matter |
| n.d. | Not detected |
References
- Herawati, D.; Loisanjaya, M.O.; Kamal, R.H.; Adawiyah, D.R.; Andarwulan, N. Profile of Bioactive Compounds, Aromas, and Cup Quality of Excelsa Coffee (Coffea liberica Var Dewevrei) Prepared from Diverse Postharvest Processes. Int. J. Food Sci. 2022, 2022, 2365603. [Google Scholar] [CrossRef]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rębacz-Maron, E.; Gutowska, I. Mineral Composition and Antioxidant Potential of Coffee Beverages Depending on the Brewing Method. Foods 2020, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Cassimiro, D.M.d.J.; Batista, N.N.; Fonseca, H.C.; Oliveira Naves, J.A.; Coelho, J.M.; Bernardes, P.C.; Dias, D.R.; Schwan, R.F. Wet Fermentation of Coffea Canephora by Lactic Acid Bacteria and Yeasts Using the Self-Induced Anaerobic Fermentation (SIAF) Method Enhances the Coffee Quality. Food Microbiol. 2023, 110, 104161. [Google Scholar] [CrossRef]
- Pothakos, V.; de Vuyst, L.; Zhang, S.J.; de Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal Shotgun Metagenomics of an Ecuadorian Coffee Fermentation Process Highlights the Predominance of Lactic Acid Bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Várady, M.; Tauchen, J.; Fraňková, A.; Klouček, P.; Popelka, P. Effect of Method of Processing Specialty Coffee Beans (Natural, Washed, Honey, Fermentation, Maceration) on Bioactive and Volatile Compounds. LWT 2022, 172, 114245. [Google Scholar] [CrossRef]
- Yulianti, Y.; Adawiyah, D.R.; Herawati, D.; Indrasti, D.; Andarwulan, N. Detection of Markers in Green Beans and Roasted Beans of Kalosi-Enrekang Arabica Coffee with Different Postharvest Processing Using LC-MS/MS. Int. J. Food Sci. 2023, 2023, 6696808. [Google Scholar] [CrossRef]
- Campos, G.A.F.; Kruizenga, J.G.K.T.; Sagu, S.T.; Schwarz, S.; Homann, T.; Taubert, A.; Rawel, H.M. Effect of the Post-Harvest Processing on Protein Modification in Green Coffee Beans by Phenolic Compounds. Foods 2022, 11, 159. [Google Scholar] [CrossRef]
- Halagarda, M.; Obrok, P. Influence of Post-Harvest Processing on Functional Properties of Coffee (Coffea arabica L.). Molecules 2023, 28, 7386. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, S.J.; Yu, J.S.; Lee, D.Y. Interactive Effect of Post-Harvest Processing Method, Roasting Degree, and Brewing Method on Coffee Metabolite Profiles. Food Chem. 2022, 397, 133749. [Google Scholar] [CrossRef]
- Muzaifa, M.; Hasni, D.; Rahmi, F.; Syarifudin. What Is Kopi Luwak? A Literature Review on Production, Quality and Problems. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 012041. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Coffee Bean Processing: Emerging Methods and Their Effects on Chemical, Biological and Sensory Properties. Food Chem. 2023, 412, 135489. [Google Scholar] [CrossRef]
- Ripper, B.; Barreto, M.S.; Novaes, F.J.M.; de Godoy, M.G.; Freire, D.M.G.; de Rezende, C.M.; Nunes, J.C.; Perrone, D. Comprehensive Composition of Flavor Precursors in Kopi Luwak and Jacu Exotic Green Bioprocessed Coffees. Front. Sustain. Food Syst. 2022, 6, 824929. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Martinez, S.J.; Batista, N.N.; Simão, J.B.P.; Dias, D.R.; Schwan, R.F. Co-Inoculation of Yeasts Starters: A Strategy to Improve Quality of Low Altitude Arabica Coffee. Food Chem. 2021, 361, 130133. [Google Scholar] [CrossRef]
- da Silva, B.L.; Pereira, P.V.; Bertoli, L.D.; Silveira, D.L.; Batista, N.N.; Pinheiro, P.F.; de Souza Carneiro, J.; Schwan, R.F.; de Assis Silva, S.; Coelho, J.M.; et al. Fermentation of Coffea Canephora Inoculated with Yeasts: Microbiological, Chemical, and Sensory Characteristics. Food Microbiol. 2021, 98, 103786. [Google Scholar] [CrossRef]
- Pereira, G.V.d.M.; de Carvalho Neto, D.P.; Medeiros, A.B.P.; Soccol, V.T.; Neto, E.; Woiciechowski, A.L.; Soccol, C.R. Potential of Lactic Acid Bacteria to Improve the Fermentation and Quality of Coffee during On-Farm Processing. Int. J. Food Sci. Technol. 2016, 51, 1689–1695. [Google Scholar] [CrossRef]
- Brioschi Junior, D.; Guarçoni, R.C.; da Silva, M.d.C.S.; Veloso, T.G.R.; Kasuya, M.C.M.; da Silva Oliveira, E.C.; da Luz, J.M.R.; Moreira, T.R.; Debona, D.G.; Pereira, L.L. Microbial Fermentation Affects Sensorial, Chemical, and Microbial Profile of Coffee under Carbonic Maceration. Food Chem. 2021, 342, 128296. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, S.E.; Kim, E.J.; Seo, S.H.; Son, H.S. Investigation of Metabolite Differences in Green Coffee Beans Fermented with Various Microbes. LWT 2022, 172, 114202. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of Coffee Aroma via the Fermentation of Green Coffee Beans with Rhizopus oligosporus: II. Effects of Different Roast Levels. Food Chem. 2016, 211, 925–936. [Google Scholar] [CrossRef]
- Ngamnok, T.; Nimlamool, W.; Amador-Noguez, D.; Palaga, T.; Meerak, J. Efficiency of Lactiplantibacillus Plantarum JT-PN39 and Paenibacillus Motobuensis JT-A29 for Fermented Coffee Applications and Fermented Coffee Characteristics. Foods 2023, 12, 2894. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Coffee Flavour Modification through Controlled Fermentation of Green Coffee Beans by Lactococcus lactis subsp. cremoris. LWT 2020, 120, 108930. [Google Scholar] [CrossRef]
- Muzaifa, M.; Hasni, D.; Abubakar, Y.; Febriani; Abubakar, A. Enumeration and Characterization of Bacteria from Civet Gastrointestinal Tract. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012002. [Google Scholar] [CrossRef]
- Suhandono, S.; Setiadi, H.; Kristianti, T.; Kusuma, A.B.; Wedaringtyas, A.W.; Djajadi, D.T.; Aryantha, I.N.P. Diversity of Culturable Bacterial in Various Parts of Luwak’s (Paradoxurus Hermaprodithus Javanica) Gastrointestinal Tract. Microbiol. Indones 2016, 10, 65–70. [Google Scholar] [CrossRef]
- Watanabe, H.; Ng, C.H.; Limviphuvadh, V.; Suzuki, S.; Yamada, T. Gluconobacter Dominates the Gut Microbiome of the Asian Palm Civet Paradoxurus hermaphroditus That Produces Kopi Luwak. PeerJ 2020, 8, e9579. [Google Scholar] [CrossRef]
- Jauharah, D.J.; Chusniati, S.; Arif, M.A.A.; Tyasningsih, W.; Theresia, A.; Estoepangestie, S. Isolation and Identification of Lactobacillus Sp. Bacteria in Asian Palm Civet (Paradoxurus hermaphroditus) Feces. Eco. Environ. Cons 2020, 26, S267–S270. [Google Scholar]
- Muzaifa, M.; Hasni, D.; Patria, A.; Febriani, A.A. Phenotypic Identification of Lactic Acid Bacteria From Civet (Paradoxorus Hermaphroditus). Int. J. Adv. Sci. Eng. Inf. Technol. 2019, 9, 1681. [Google Scholar] [CrossRef]
- Therdtatha, P.; Jareontanahun, N.; Chaisuwan, W.; Yakul, K.; Paemanee, A.; Manassa, A.; Moukamnerd, C.; Phimolsiripol, Y.; Sommano, S.R.; Seesuriyachan, P. Production of Functional Arabica and Robusta Green Coffee Beans: Optimization of Fermentation with Microbial Cocktails to Improve Antioxidant Activity and Metabolomic Profiles. Biocatal. Agric. Biotechnol. 2023, 53, 102869. [Google Scholar] [CrossRef]
- Dinh, Y.N.; Nguyen, Q.D.; Le, H.P. Investigation of Changes of Antioxidant Properties of Coffee through Fermentation by Using Saccharomyces Cerevisiae and Bacillus Subtilis. J. Tech. Educ. Sci. 2022, 17, 72–79. [Google Scholar] [CrossRef]
- Muzaifa, M.; Hasni, D.; Febriani; Patria, A.; Abubakar, A. Fermentation of Coffee Beans with Inoculation of Bacillus subtilis and Its Impact on Coffee Sensory Quality. IOP Conf. Ser. Earth Environ. Sci. 2019, 364, 012010. [Google Scholar] [CrossRef]
- Wibowo, N.A.; Mangunwardoyo, W.; Yasman, Y.; Santoso, T.J. Effect of Fermentation on Sensory Quality of Liberica Coffee Beans Inoculated with Bacteria from Saliva Arctictis Binturong Raffles, 1821. Biodiversitas J. Biol. Divers. 2021, 22, 3922–3928. [Google Scholar] [CrossRef]
- Pakosz, P.; Wołosiak, R.; Drużyńska, B.; Majewska, E. The Effect of Type and Duration of Digestive Enzyme Treatment on Coffee Bean Composition. Appl. Sci. 2024, 14, 2484. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2023; R Foundation for Statistical Computing: Viena, Austria, 2023. [Google Scholar]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; Rivas, B.d.l.; Felipe, F.L.d.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food Phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K. An Overview of Lactic Acid Bacteria. Int. J. Biosci. 2011, 1, 1–13. [Google Scholar]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus Subtilis: A Universal Cell Factory for Industry, Agriculture, Biomaterials and Medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef]
- Poisson, L.; Blank, I.; Dunkel, A.; Hofmann, T. The Chemistry of Roasting—Decoding Flavor Formation. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: London, UK, 2017; pp. 273–310. ISBN 978-0-12-803520-7. [Google Scholar]
- Terlabie, N.N.; Sakyi-Dawson, E.; Amoa-Awua, W.K. The Comparative Ability of Four Isolates of Bacillus subtilis to Ferment Soybeans into Dawadawa. Int. J. Food Microbiol. 2006, 106, 145–152. [Google Scholar] [CrossRef]
- Gomes, R.J.; Borges, M.d.F.; Rosa, M.d.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; Finco, A.M.O.; Letti, L.A.J.; Da Silva, B.J.G.; Vandenberghe, L.P.S.; Soccol, C.R. Efficient Coffee Beans Mucilage Layer Removal Using Lactic Acid Fermentation in a Stirred-Tank Bioreactor: Kinetic, Metabolic and Sensorial Studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- Mageshwaran, V.; Inmann, F.I.I.I.; Holmes, L.D. Growth Kinetics of Bacillus subtilis in Lignocellulosic Carbon Sources. Int. J. Microbiol. Res. 2014, 6, 570–574. [Google Scholar]
- Sombolestani, A.S.; Cleenwerck, I.; Cnockaert, M.; Borremans, W.; Wieme, A.D.; De Vuyst, L.; Vandamme, P. Characterization of Novel Gluconobacter Species from Fruits and Fermented Food Products: Gluconobacter cadivus sp. nov., Gluconobacter vitians sp. nov. and Gluconobacter potus sp. nov. Int. J. Syst. Evol. Microbiol. 2021, 71, 004751. [Google Scholar] [CrossRef]
- Ramírez, K.; Pineda-Hidalgo, K.V.; Rochín-Medina, J.J. Fermentation of Spent Coffee Grounds by Bacillus clausii Induces Release of Potentially Bioactive Peptides. LWT 2021, 138, 110685. [Google Scholar] [CrossRef]
- Tsai, C.F.; Jioe, I.P.J. The Analysis of Chlorogenic Acid and Caffeine Content and Its Correlation with Coffee Bean Color under Different Roasting Degree and Sources of Coffee (Coffea arabica Typica). Processes 2021, 9, 2040. [Google Scholar] [CrossRef]
- Liao, Y.C.; Kim, T.; Silva, J.L.; Hu, W.Y.; Chen, B.Y. Effects of Roasting Degrees on Phenolic Compounds and Antioxidant Activity in Coffee Beans from Different Geographic Origins. LWT 2022, 168, 113965. [Google Scholar] [CrossRef]
- Beaufay, F.; Coppine, J.; Hallez, R. When the Metabolism Meets the Cell Cycle in Bacteria. Curr. Opin. Microbiol. 2021, 60, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Rouch, D.A.; Britz, M.L. Biochemistry of Non-Starter Lactic Acid Bacteria Isolate Lactobacillus Casei GCRL163: Production of Metabolites by Stationary-Phase Cultures. Int. Dairy J. 2009, 19, 12–21. [Google Scholar] [CrossRef]
- Navarro Llorens, J.M.; Tormo, A.; Martínez-García, E. Stationary Phase in Gram-Negative Bacteria. FEMS Microbiol. Rev. 2010, 34, 476–495. [Google Scholar] [CrossRef]

| Bacterium | Time [h] | |
|---|---|---|
| 0 | 24 | |
| Arabica | ||
| B. subtilis ATCC 6633 | 5.43 ± 0.26 a | 8.12 ± 0.16 a |
| Gluconobacter sp. KKP 3751 | 6.87 ± 0.05 a | 8.29 ± 0.09 b |
| L. plantarum ATCC 4080 | 6.01 ± 0.07 a | 8.56 ± 0.03 b |
| Robusta | ||
| B. subtilis ATCC 6633 | 5.84 ± 0.03 a | 7.92 ± 0.42 a |
| Gluconobacter sp. KKP 3751 | 6.82 ± 0.23 a | 8.37 ± 0.09 b |
| L. plantarum ATCC 4080 | 5.86 ± 0.21 a | 8.75 ± 0.08 b |
| Fructose [g/100 g dm] | Glucose [g/100 g dm] | Sucrose [g/100 g dm] | Soluble Peptides [g/100 g dm] | Caffeine [g/100 g dm] | Total Polyphenols [g/100 g dm] | 3-CQA [g/100 g dm] | |
|---|---|---|---|---|---|---|---|
| Green Arabica | |||||||
| Control | 0.13 ± 0.01 b | 0.12 ± 0.01 b | 3.28 ± 0.06 a | 10.31 ± 0.79 a | 0.66 ± 0.01 a | 1.54 ± 0.01 a | 0.89 ± 0.02 ab |
| B.subtilis ATCC 6633 | 0.14 ± 0.03 b | 0.22 ± 0.04 a | 3.09 ± 0.05 b | 10.73 ± 0.46 a | 0.65 ± 0.01 a | 1.54 ± 0.02 a | 0.89 ± 0.01 a |
| Gluconobacter sp. KKP 3751 | 0.25 ± 0.01 a | 0.16 ± 0.01 a | 2.67 ± 0.03 c | 9.96 ± 0.47 a | 0.64 ± 0.01 b | 1.39 ± 0.09 ab | 0.86 ± 0.01 b |
| L.plantarum ATCC 4080 | traces | traces | 2.89 ± 0.20 abc | 11.21 ± 1.57 a | 0.63 ± 0.02 ab | 1.38 ± 0.01 b | 0.82 ± 0.04 ab |
| Green Robusta | |||||||
| Control | 0.12 ± 0.01 b | 0.11 ± 0.01 c | 2.32 ± 0.04 a | 16.21 ± 0.38 ab | 1.03 ± 0.01 b | 1.90 ± 0.08 ab | 0.88 ± 0.01 b |
| B.subtilis ATCC 6633 | 0.12 ± 0.02 b | 0.19 ± 0.01 a | 2.16 ± 0.18 ab | 17.60 ± 1.89 a | 1.05 ± 0.04 ab | 1.90 ± 0.18 ab | 0.91 ± 0.06 ab |
| Gluconobacter sp. KKP 3751 | 0.21 ± 0.01 a | 0.13 ± 0.01 b | 2.01 ± 0.05 b | 15.23 ± 0.10 b | 1.06 ± 0.02 b | 1.91 ± 0.01 b | 0.88 ± 0.01 b |
| L.plantarum ATCC 4080 | 0.07 ± 0.01 c | traces | 2.44 ± 0.03 a | 14.70 ± 0.77 b | 1.13 ± 0.02 a | 2.07 ± 0.02 a | 1.05 ± 0.02 a |
| Roasted Arabica | |||||||
| Control | n.d. | n.d. | n.d. | 8.69 ± 0.22 b | 0.46 ± 0.03 ab | 1.00 ± 0.07 ab | 0.31 ± 0.02 a |
| B.subtilis ATCC 6633 | n.d. | n.d. | n.d. | 8.82 ± 0.23 b | 0.46 ± 0.01 a | 1.08 ± 0.11 ab | 0.30 ± 0.01 a |
| Gluconobacter sp. KKP 3751 | n.d. | n.d. | n.d. | 9.09 ± 0.71 ab | 0.44 ± 0.01 b | 0.94 ± 0.03 b | 0.29 ± 0.01 a |
| L.plantarum ATCC 4080 | n.d. | n.d. | n.d. | 9.70 ± 0.63 a | 0.46 ± 0.03 ab | 1.04 ± 0.02 a | 0.30 ± 0.01 a |
| Roasted Robusta | |||||||
| Control | n.d. | n.d. | n.d. | 11.86 ± 2.10 ab | 0.60 ± 0.02 b | 1.01 ± 0.03 a | 0.29 ± 0.01 b |
| B.subtilis ATCC 6633 | n.d. | n.d. | n.d. | 14.03 ± 0.47 a | 0.72 ± 0.03 a | 1.29 ± 0.10 a | 0.34 ± 0.01 a |
| Gluconobacter sp. KKP 3751 | n.d. | n.d. | n.d. | 13.04 ± 0.53 ab | 0.69 ± 0.02 a | 1.17 ± 0.10 a | 0.31 ± 0.02 ab |
| L.plantarum ATCC 4080 | n.d. | n.d. | n.d. | 12.78 ± 0.62 b | 0.69 ± 0.06 ab | 1.25 ± 0.14 a | 0.34 ± 0.03 a |
| Time [h] | Fructose [g/100 g dm] | Glucose [g/100 g dm] | Sucrose [g/100 g dm] | Soluble Peptides [g/100 g dm] | Caffeine [g/100 g dm] | Total Polyphenols [g/100 g dm] | 3-CQA [g/100 g dm] | |
|---|---|---|---|---|---|---|---|---|
| Green Arabica | ||||||||
| 6 | C | 0.18 ± 0.01 a | 0.17 ± 0.01 a | 3.38 ± 0.06 a | 14.14 ± 0.46 a | 0.77 ± 0.01 a | 1.48 ± 0.09 a | 1.12 ± 0.05 a |
| LP | 0.15 ± 0.02 A | 0.15 ± 0.02 A | 3.57 ± 0.36 A | 12.49 ± 0.60 A | 0.78 ± 0.05 A | 1.57 ± 0.26 AB | 1.01 ± 0.10 AB | |
| 12 | C | 0.15 ± 0.04 a | 0.14 ± 0.03 a | 3.11 ± 0.10 b | 13.94 ± 0.22 a | 0.72 ± 0.01 b | 1.39 ± 0.04 a | 1.04 ± 0.05 a |
| LP | 0.08 ± 0.02 B | 0.08 ± 0.02 B | 3.10 ± 0.11 A | 12.60 ± 0.16 A | 0.73 ± 0.03 A | 1.42 ± 0.02 B | 0.93 ± 0.06 B | |
| 24 | C | 0.14 ± 0.01 a | 0.15 ± 0.01 a | 2.70 ± 0.02 c | 11.98 ± 0.28 b | 0.68 ± 0.01 c | 1.34 ± 0.09 a | 1.02 ± 0.05 ab |
| LP | traces | traces | 2.71 ± 0.01 B | 12.94 ± 0.04 A | 0.75 ± 0.01 A | 1.56 ± 0.01 A | 1.06 ± 0.03 AB | |
| 48 | C | 0.13 ± 0.02 a | 0.13 ± 0.01 a | 2.49 ± 0.01 d | 11.26 ± 0.24 b | 0.62 ± 0.01 d | 1.29 ± 0.08 a | 0.91 ± 0.04 b |
| LP | n.d. | traces | 2.24 ± 0.07 C | 12.21 ± 0.78 A | 0.72 ± 0.01 A | 1.58 ± 0.01 A | 1.13 ± 0.05 A | |
| Green Robusta | ||||||||
| 6 | C | 0.13 ± 0.03 a | 0.13 ± 0.02 a | 2.33 ± 0.02 a | 24.42 ± 0.12 a | 1.10 ± 0.01 b | 2.31 ± 0.05 a | 1.26 ± 0.04 a |
| LP | 0.14 ± 0.02 A | 0.13 ± 0.01 A | 2.29 ± 0.17 A | 20.97 ± 0.91 A | 1.11 ± 0.06 A | 1.93 ± 0.17 AB | 0.98 ± 0.07 B | |
| 12 | C | 0.15 ± 0.01 a | 0.15 ± 0.01 a | 2.17 ± 0.02 b | 22.51 ± 0.19 b | 1.13 ± 0.01 a | 2.01 ± 0.03 b | 1.09 ± 0.04 b |
| LP | 0.08 ± 0.01 B | 0.08 ± 0.02 B | 2.14 ± 0.03 A | 17.93 ± 0.71 B | 1.13 ± 0.01 A | 2.11 ± 0.09 AB | 1.02 ± 0.03 B | |
| 24 | C | 0.12 ± 0.01 a | 0.12 ± 0.01 a | 1.99 ± 0.01 c | 17.52 ± 0.06 d | 1.06 ± 0.01 c | 1.80 ± 0.01 c | 1.01 ± 0.04 b |
| LP | 0.05 ± 0.01 C | 0.05 ± 0.01 B | 1.81 ± 0.01 A | 19.46 ± 0.25 A | 1.12 ± 0.01 A | 1.94 ± 0.03 B | 1.04 ± 0.03 B | |
| 48 | C | 0.15 ± 0.01 a | 0.16 ± 0.02 a | 1.98 ± 0.01 c | 18.25 ± 0.18 c | 1.06 ± 0.01 c | 1.93 ± 0.03 b | 1.05 ± 0.05 b |
| LP | traces | traces | 1.44 ± 0.01 A | 17.67 ± 0.12 B | 1.15 ± 0.02 A | 2.14 ± 0.07 A | 1.21 ± 0.04 A | |
| Roasted Arabica | ||||||||
| 6 | C | n.d. | n.d. | n.d. | 9.69 ± 0.04 b | 0.62 ± 0.01 a | 1.38 ± 0.03 a | 0.36 ± 0.01 a |
| LP | n.d. | n.d. | n.d. | 10.28 ± 0.19 A | 0.53 ± 0.01 A | 1.06 ± 0.01 B | 0.25 ± 0.02 C | |
| 12 | C | n.d. | n.d. | n.d. | 9.96 ± 0.03 a | 0.55 ± 0.01 b | 1.20 ± 0.01 b | 0.35 ± 0.01 a |
| LP | n.d. | n.d. | n.d. | 10.78 ± 0.63 A | 0.55 ± 0.03 A | 1.13 ± 0.09 B | 0.25 ± 0.01 BC | |
| 24 | C | n.d. | n.d. | n.d. | 9.16 ± 0.12 c | 0.55 ± 0.01 b | 1.24 ± 0.02 b | 0.31 ± 0.01 b |
| LP | n.d. | n.d. | n.d. | 9.77 ± 1.17 A | 0.55 ± 0.02 A | 1.38 ± 0.07 A | 0.30 ± 0.01 A | |
| 48 | C | n.d. | n.d. | n.d. | 9.30 ± 0.09 c | 0.46 ± 0.01 c | 0.96 ± 0.05 c | 0.29 ± 0.01 b |
| LP | n.d. | n.d. | n.d. | 10.63 ± 0.34 A | 0.53 ± 0.03 A | 1.38 ± 0.12 AB | 0.28 ± 0.02 AB | |
| Roasted Robusta | ||||||||
| 6 | C | n.d. | n.d. | n.d. | 14.57 ± 0.21 b | 1.01 ± 0.01 a | 2.18 ± 0.05 a | 0.59 ± 0.02 a |
| LP | n.d. | n.d. | n.d. | 14.08 ± 0.89 A | 0.91 ± 0.02 AB | 1.50 ± 0.08 AB | 0.32 ± 0.04 AB | |
| 12 | C | n.d. | n.d. | n.d. | 14.42 ± 0.11 b | 0.90 ± 0.01 b | 1.48 ± 0.06 b | 0.32 ± 0.01 b |
| LP | n.d. | n.d. | n.d. | 13.25 ± 0.18 A | 0.86 ± 0.05 AB | 1.35 ± 0.06 B | 0.29 ± 0.01 B | |
| 24 | C | n.d. | n.d. | n.d. | 16.60 ± 0.02 a | 0.77 ± 0.01 c | 1.39 ± 0.04 b | 0.29 ± 0.01 b |
| LP | n.d. | n.d. | n.d. | 12.98 ± 1.02 A | 0.88 ± 0.01 B | 1.50 ± 0.01 B | 0.32 ± 0.02 AB | |
| 48 | C | n.d. | n.d. | n.d. | 11.85 ± 0.07 c | 0.64 ± 0.01 d | 1.21 ± 0.02 c | 0.26 ± 0.01 c |
| LP | n.d. | n.d. | n.d. | 13.10 ± 1.19 A | 0.92 ± 0.01 A | 1.73 ± 0.01 A | 0.34 ± 0.02 A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakosz, P.; Bzducha-Wróbel, A.; Drużyńska, B.; Majewska, E.; Wołosiak, R. Composition of Coffee Beans Influenced by Bioprocessing with Selected Bacteria. Foods 2025, 14, 1143. https://doi.org/10.3390/foods14071143
Pakosz P, Bzducha-Wróbel A, Drużyńska B, Majewska E, Wołosiak R. Composition of Coffee Beans Influenced by Bioprocessing with Selected Bacteria. Foods. 2025; 14(7):1143. https://doi.org/10.3390/foods14071143
Chicago/Turabian StylePakosz, Paulina, Anna Bzducha-Wróbel, Beata Drużyńska, Ewa Majewska, and Rafał Wołosiak. 2025. "Composition of Coffee Beans Influenced by Bioprocessing with Selected Bacteria" Foods 14, no. 7: 1143. https://doi.org/10.3390/foods14071143
APA StylePakosz, P., Bzducha-Wróbel, A., Drużyńska, B., Majewska, E., & Wołosiak, R. (2025). Composition of Coffee Beans Influenced by Bioprocessing with Selected Bacteria. Foods, 14(7), 1143. https://doi.org/10.3390/foods14071143





