Comparison of Storage Stability and In Vitro Digestion of Rice Flour-Based Yogurt Alternatives Made with Lactobacillus rhamnosus Lgg to Milk-Based Yogurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Rice Flour
2.2. Yogurt Alternative (YA) Preparation
2.3. Preparation of Milk Yogurt
2.4. Syneresis During Storage
2.5. Monitoring of Color During Storage
2.6. Monitoring of TA During Storage
2.7. Monitoring of pH During Storage
2.8. Monitoring of Viscosity During Storage
2.9. Enumeration of Streptococcus thermophilus, Lactobacillus bulgaricus and Lactobacillus rhamnosus During Storage
2.10. In Vitro Gastrointestinal Fluid (GIF) Tolerance
2.11. Yeast, Mold, and Coliforms Analysis During Storage
2.12. Statistics
3. Results and Discussion
3.1. Syneresis
3.2. Color
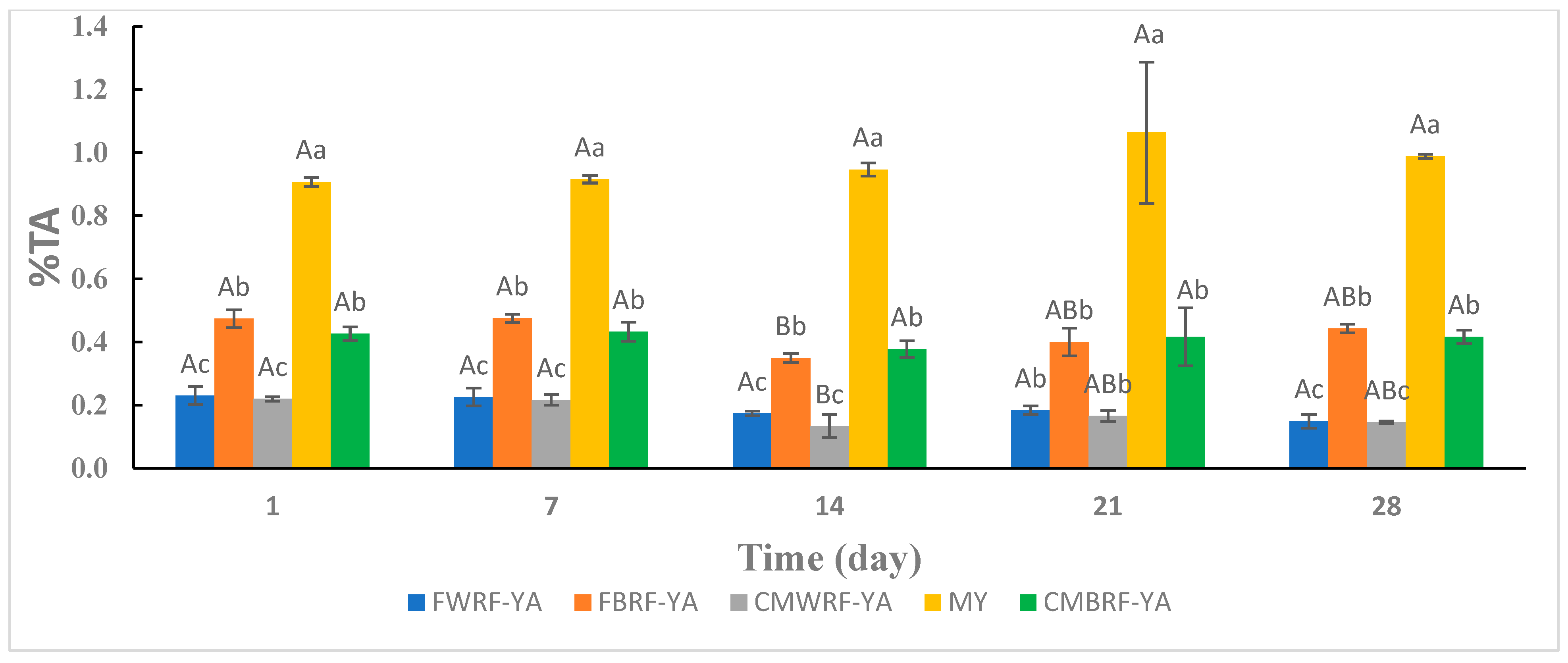
3.3. Titratable Acidity
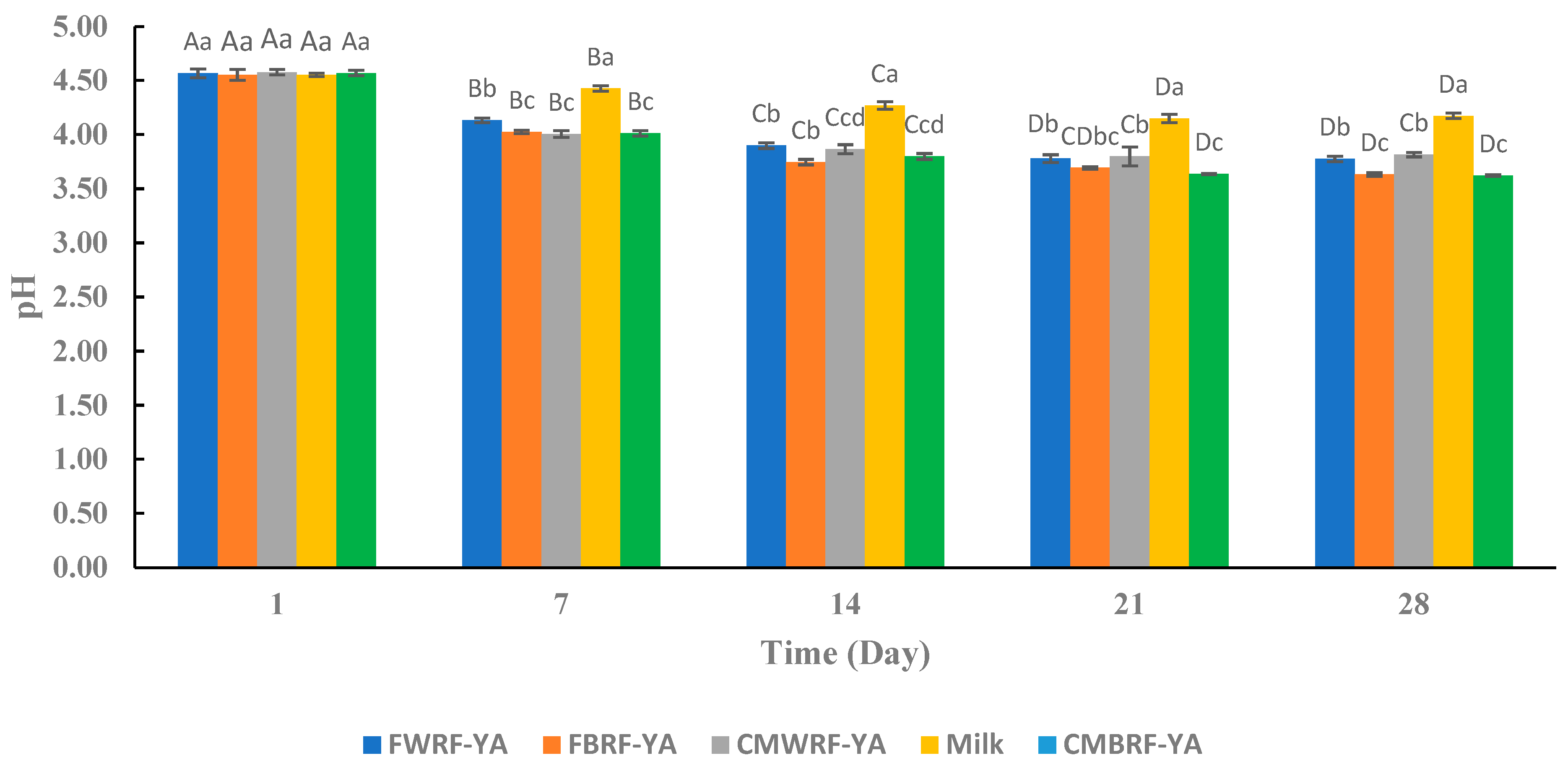
3.4. pH
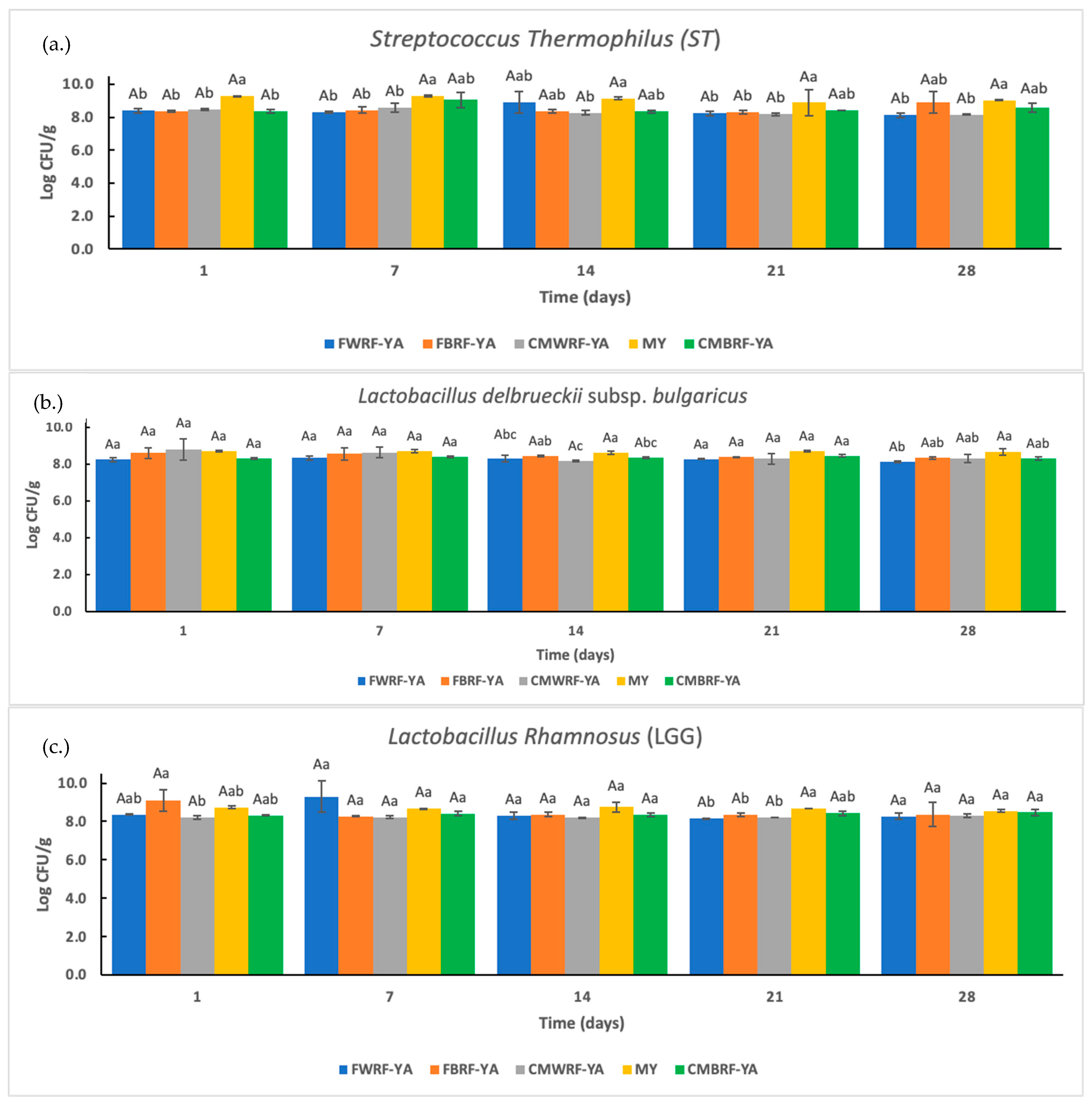
3.5. Viable Cell Counts of ST, LB, and LGG Bacteria
Yeast Mold and Coliform in YAs and MY During Storage
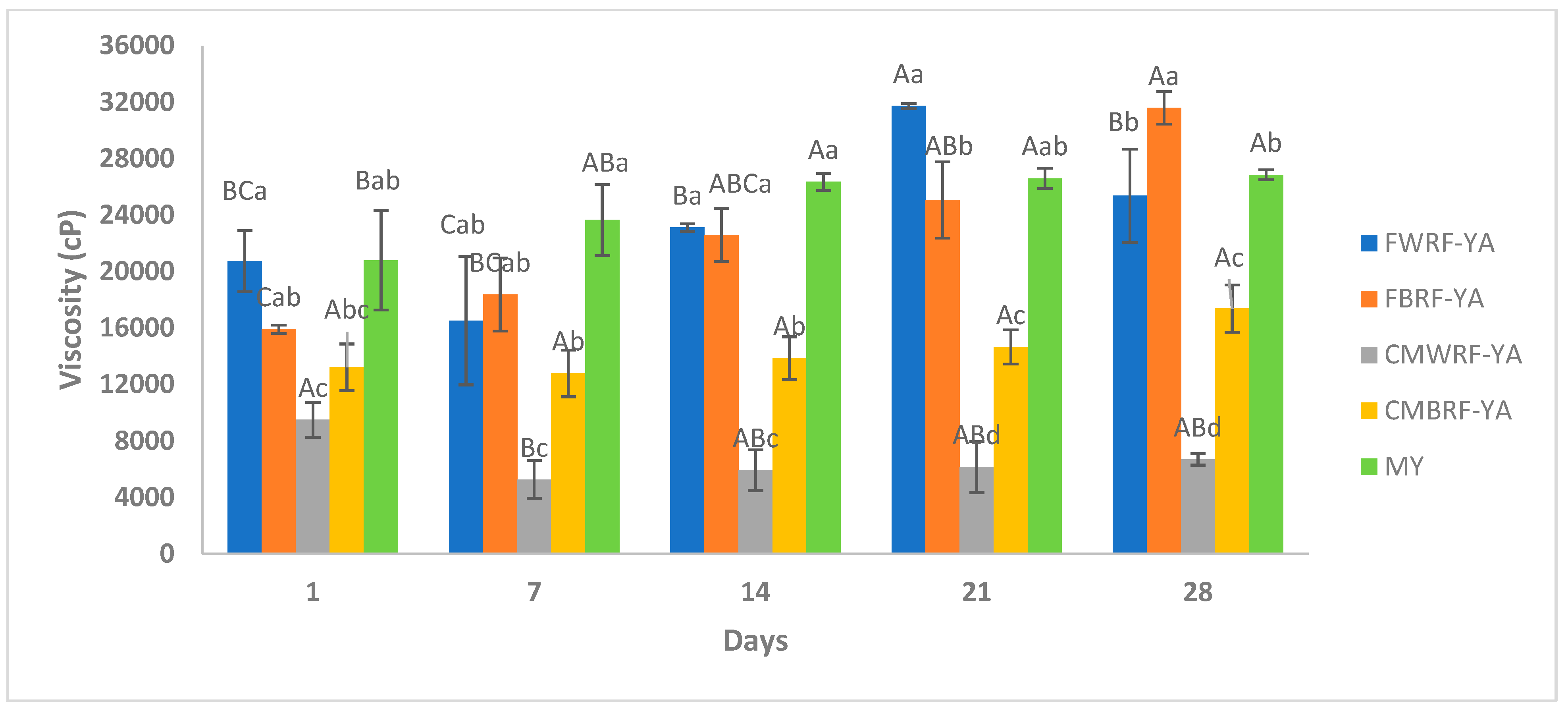
3.6. Viscosity of YAs and MY During Storage
3.7. Gastrointestinal In Vitro Analysis of Lactobacillus rhamnosus (LGG) During Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-based alternatives to yogurt: State-of-the-art and perspectives of new biotechnological challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional properties and health aspects of pulses and their use in plant-based yogurt alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3858–3880. [Google Scholar] [CrossRef]
- Grand View Research. Vegan Yogurt Market Size, Share and Trend Analysis Report by Product (Soy, Almond, Rice) by Distribution Channel (Hypermarket, Supermarket, Convenience Stores, Specialty Stores, Online) by Region, and Segment Forecasts, 2020–2027. 2019. Available online: https://www.grandviewresearch.com/industry-analysis/vegan-yogurt-market (accessed on 26 February 2025).
- McClements, I.F.; McClements, D.J. Designing healthier plant-based foods: Fortification, digestion, and bioavailability. Food Res. Int. 2023, 169, 112853. [Google Scholar] [CrossRef]
- Dhakal, D.; Younas, T.; Bhusal, R.P.; Devkota, L.; Henry, C.J.; Dhital, S. Design rules of plant-based yoghurt-mimic: Formulation, functionality, sensory profile and nutritional value. Food Hydrocoll. 2023, 142, 108786. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Vegetable milks and their fermented derivative products. Int. J. Food Stud. 2014, 3. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef]
- Pontonio, E.; Raho, S.; Dingeo, C.; Centrone, D.; Carofiglio, V.E.; Rizzello, C.G. Nutritional, functional, and technological characterization of a novel gluten-and yogurt-style snack produced with selected lactic acid bacteria and Leguminosae flours. Front. Microbiol. 2020, 17, 1664. [Google Scholar] [CrossRef]
- Saleh, A.; Mohamed, A.A.; Alamri, M.S.; Hussain, S.; Qasem, A.A.; Ibraheem, M.A. Effect of Different Starches on the Rheological, Sensory and Storage Attributes of Non-fat Set Yogurt. Foods 2020, 9, 61. [Google Scholar] [CrossRef]
- Gong, E.S.; Luo, S.; Li, T.; Liu, C.; Zhang, G.; Chen, J.; Zeng, Z.; Liu, R.H. Phytochemical profiles and antioxidant activity of processed brown rice products. Food Chem. 2017, 232, 67–78. [Google Scholar] [CrossRef]
- Wenefrida, I.; Utomo, H.S.; Linscombe, S.D. Development and Registration of Frontière, a High-Protein Rice Cultivar. J. Plant Regist. 2017, 11, 240–244. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Lactic acid fermentation of cereals and pseudocereals: Ancient nutritional biotechnologies with modern applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef]
- Khalid, K. An overview of lactic acid bacteria. Int. J. Biosci. 2011, 1, 1–13. [Google Scholar]
- Nguyen, P.-T.; Nguyen, T.-T.; Bui, D.-C.; Hong, P.-T.; Hoang, Q.-K.; Nguyen, H.-T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. AIMS Microbiol. 2020, 6, 451. [Google Scholar] [CrossRef]
- Martín, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vázquez, U.; Garault, P.; Cotillard, A.; Pham, H.P.; Chervaux, C.; et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019, 9, 5398. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, H.; Aslim, B.; Yuksekdag, Z. Assessment of anti-biofilm activity and bifidogenic growth stimulator (BGS) effect of lyophilized exopolysaccharides (l-EPSs) from Lactobacilli strains. Int. J. Food Prop. 2017, 20, 362–371. [Google Scholar] [CrossRef]
- Harper, A.R.; Dobson, R.C.J.; Morris, V.K.; Moggré, G. Fermentation of plant based dairy alternatives by lactic acid bacteria. Microb. Biotechnol. 2022, 15, 1404–1421. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Seong, H.; Kim, S.-A.; Song, Y.; Sim, E.Y.; Kang, H.; Han, N.S. Lysine-fortified rice germ yogurt fermented with Lactiplantibacillus plantarum JSA 22 and its beneficial health effects. J. Funct. Foods 2023, 109, 105787. [Google Scholar] [CrossRef]
- Park, Y.H.; Choi, J.S. Fermentation properties of rice-added yogurt using two types of blended lactic acid bacteria as a starter. Korean J. Agric. Sci. 2021, 48, 273–281. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Hisham, S.M.; Alkhalifah, D.H.M. A Sustainable Method: Production of the Fermented Rice Milk Yogurt by Using Three Efficient Lactic Acid Bacteria. Appl. Sci. 2023, 13, 907. [Google Scholar] [CrossRef]
- Salih, Z.A.; Siddeeg, A.; Ammar, A.-F.; Ibrahim, A.I.E.; Ali, A.O. The Physicochemical, Microbiological and Sensory Properties of Yoghurt Processed by Addition of Rice Flour. Ann. Obes. Disord. 2019, 4, 1025. [Google Scholar]
- Wongkhalaung, C.; Boonyaratanakornkit, M. Development of a Yogurt-type Product from Saccharified Rice. Kasetsart J. 2000, 34, 107–116. [Google Scholar]
- Wang, C.; Li, D.; Wang, H.; Guo, M. Formulation and storage properties of symbiotic rice-based yogurt-like product using polymerized whey protein as a gelation agent. CyTA J. Food 2021, 19, 511–520. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Peñas, E.; Martínez-Villaluenga, C.; García-Mora, P.; Frías, J. Development of a multifunctional yogurt-like product from germinated brown rice. LWT 2019, 99, 306–312. [Google Scholar] [CrossRef]
- Morris, A.; Boeneke, C.; Prinyawiwatkul, W.; King, J.M. Use of rice flour to produce plant based yogurt alternatives. J. Food Sci. 2024, 89, 7095–7114. [Google Scholar] [CrossRef]
- Morelli, L.; Pellegrino, P. A critical evaluation of the factors affecting the survival and persistence of beneficial bacteria in healthy adults. Benef. Microbes. 2021, 12, 15–25. [Google Scholar] [CrossRef]
- Sridhar, K.; Bouhallab, S.; Croguennec, T.; Renard, D.; Lechevalier, V. Recent trends in design of healthier plant-based alternatives: Nutritional profile, gastrointestinal digestion, and consumer perception. Crit. Rev. Food Sci. Nutr. 2022, 63, 10483–10498. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Y.; Zhang, Y.; Cao, H.; Luo, D.-K.; Yi, C.; Guan, X. Formulation of plant-based yoghurt from soybean and quinoa and evaluation of physicochemical, rheological, sensory and functional properties. Food Biosci. 2022, 49, 10183. [Google Scholar] [CrossRef]
- Pachekrepapol, U.; Kokhuenkhan, Y.; Ongsawat, J. Formulation of yogurt-like product from coconut milk and evaluation of physicochemical, rheological, and sensory properties. Int. J. Gastron. Food Sci. 2021, 25, 100393. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef]
- Achanta, K.; Aryana, K.J.; Boeneke, C.A. Fat free plain set yogurts fortified with various minerals. LWT Food Sci. Technol. 2007, 40, 424–429. [Google Scholar]
- Ranadheera, C.S.; Evans, C.; Adams, M.; Baines, S. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food Res. Int. 2012, 49, 619–625. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V. Syneresis and rheological behaviors of set yogurt containing green tea and green coffee powders. J. Dairy Sci. 2017, 100, 901–907. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, physicochemical and sensorial properties of commercial plant-based yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lucey, J.A. Formation and physical properties of yogurt. Asian Australas. J Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Dabija, A.; Codină, G.G.; Ropciuc, S.; Stroe, S.G. Studies regarding the production of a novel yogurt using some local plant raw materials. J. Food Process. Preserv. 2019, 43, e13826. [Google Scholar] [CrossRef]
- Wong, S.-S.; Wicklund, R.; Bridges, J.; Whaley, J.; Koh, Y.B. Starch swelling behavior and texture development in stirred yogurt. Food Hydrocoll. 2020, 98, 105274. [Google Scholar] [CrossRef]
- Shi, H.; Kraft, J.; Guo, M. Physicochemical and microstructural properties and probiotic survivability of symbiotic almond yogurt alternative using polymerized whey protein as a gelation agent. J. Food Sci. 2020, 85, 3450–3458. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Santiago, C.; Ramos-Solis, L.; Lobato-Calleros, C.; Peña-Valdivia, C.; Vernon-Carter, E.; Alvarez-Ramírez, J. Enrichment of stirred yogurt with soluble dietary fiber from Pachyrhizus erosus L. Urban: Effect on syneresis, microstructure and rheological properties. J. Food Eng. 2010, 101, 229–235. [Google Scholar] [CrossRef]
- Román, L.; Reguilón, M.P.; Gómez, M. Physicochemical characteristics of sauce model systems: Influence of particle size and extruded flour source. J. Food Eng. 2018, 219, 93–100. [Google Scholar] [CrossRef]
- Aleman, R.S.; Morris, A.; Prinyawiwatkul, W.; Moncada, M.; King, J.M. Physiochemical properties of Frontière rice flour and its application in a gluten-free cupcake. Cereal Chem. 2022, 99, 303–315. [Google Scholar] [CrossRef]
- Arocas, A.; Sanz, T.; Fiszman, S. Improving effect of xanthan and locust bean gums on the freeze-thaw stability of white sauces made with different native starches. Food Hydrocoll. 2009, 23, 2478–2484. [Google Scholar] [CrossRef]
- Jiao, B.; Wu, B.; Fu, W.; Guo, X.; Zhang, Y.; Yang, J.; Luo, X.; Dai, L.; Wang, Q. Effect of roasting and high-pressure homogenization on texture, rheology, and microstructure of walnut yogurt. Food Chem. X 2023, 20, 101017. [Google Scholar] [CrossRef] [PubMed]
- Almusallam, I.A.; Ahmed, I.A.M.; Babiker, E.E.; Al-Juhaimi, F.Y.; Saleh, A.; Qasem, A.A.; Al Maiman, S.; Osman, M.A.; Ghafoor, K.; Hajji, H.A.; et al. Effect of date palm (Phoenix dactylifera L.) spikelets extract on the physicochemical and microbial properties of set-type yogurt during cold storage. LWT 2021, 148, 111762. [Google Scholar] [CrossRef]
- Plengsaengsri, P.; Krusong, W.; Thompson, A.; Chadseesuwan, U.; Deetae, P. Physico-chemical and microbiological properties of non-dairy yoghurt made from rice derivatives. Int. J. Agric. Technol. 2021, 17, 1027–1040. [Google Scholar]
- Demïr, H.; Simsek, M.; Yıldırım, G. Effect of oat milk pasteurization type on the characteristics of yogurt. LWT 2021, 135, 110271. [Google Scholar] [CrossRef]
- Amani, E.; Eskandari, M.H.; Shekarforoush, S. The effect of proteolytic activity of starter cultures on technologically important properties of yogurt. Food Sci. Nutr. 2017, 5, 525–537. [Google Scholar] [CrossRef]
- Krisnaningsih, A.T.; Radiati, L.E.; Purwadi; Evanuarini, H.; Rosyidi, D. The effect of incubation time to the physicochemical and microbial properties of yoghurt with local taro (Colocasia esculenta (L.) schott) starch as stabilizer. Curr. Res. Nutr. Food Sci. 2019, 7, 547. [Google Scholar] [CrossRef]
- Sabbah, M.; Pramono, Y. The effect of different ratio of bacteria (Lactobacillus Bulgaricus + Streptococcus Thermophilus and Bifidobacterum Longum. atcc15707) on characteristics of yogurt at different storage period. J. Apl. Teknol. Pangan. 2012, 1, 32–38. [Google Scholar]
- García-Pérez, F.J.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.A.; Sendra, E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Ziarno, M.; Cichońska, P. Lactic Acid Bacteria-Fermentable Cereal-and Pseudocereal-Based Beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Ranadheera, C.S.; Fang, Z.; Hutchinson, G.; Ajlouni, S. Protecting the viability of encapsulated Lactobacillus rhamnosus LGG using chocolate as a carrier. Emir. J. Food Agric. 2021, 33, 647–656. [Google Scholar] [CrossRef]
- Frye, C.P.; Kilara, A. Regulations for product standards and labeling. In Dairy Processing and Quality Assurance; Chandan, R., Kilara, A., Shah, N., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; pp. 152–177. [Google Scholar]
- Moh, L.G.; Keilah, L.P.; Etienne, P.T.; Jules-Roger, K. Seasonal microbial conditions of locally made yoghurt (shalom) marketed in some regions of Cameroon. Int. J. Food Sci. 2017, 2017, 5839278. [Google Scholar] [CrossRef]
- Zubairi, S.I.; Ishak, N.; Sani, N.A.; Kasim, Z.M.; Nurzahim, Z. Yogurt drink spoilage profiles: Characterization of physico-chemical properties and coliform potability analysis. Arab J. Chem. 2021, 14, 103340. [Google Scholar] [CrossRef]
- Buehler, A.; Martin, N.; Boor, K.; Wiedmann, M. Evaluation of biopreservatives in Greek yogurt to inhibit yeast and mold spoilage and development of a yogurt spoilage predictive model. J. Dairy Sci. 2018, 101, 10759–10774. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Knøchel, S. Sensitivity of Molds from Spoiled Dairy Products Towards Bioprotective Lactic Acid Bacteria Cultures. Front. Microbiol. 2021, 10, 631730. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Ścibisz, I.; Kozłowska, M. Comprehensive studies on the stability of yogurt-type fermented soy beverages during refrigerated storage using dairy starter cultures. Front. Microbiol. 2023, 14, 1230025. [Google Scholar] [CrossRef]
- Tannock, G.W. A special fondness for lactobacilli. Appl. Environ. Microbiol. 2004, 70, 3189–3194. [Google Scholar] [CrossRef]
- Kashket, E.R. Bioenergetics of lactic acid bacteria: Cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 1987, 3, 233–244. [Google Scholar] [CrossRef]
- Jacobsen, C.N.; Nielsen, V.R.; Hayford, A.E.; Møller, P.L.; Michaelsen, K.F.; Pærregaard, A.; Sandström, B.; Tvede, M.; Jakobsen, M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999, 65, 4949–4956. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, T.; Shah, N.P. Probiotics—From Metchnikoff to bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Ashwar, B.A.; Gani, A.; Wani, I.A.; Shah, A.; Masoodi, F.A.; Saxena, D.C. Production of resistant starch from rice by dual autoclaving-retrogradation treatment: Invitro digestibility, thermal and structural characterization. Food Hydrocoll. 2016, 56, 108–117. [Google Scholar] [CrossRef]
- Wu, K.; Gunaratne, A.; Gan, R.; Bao, J.; Corke, H.; Jiang, F. Relationships between cooking properties and physicochemical properties in brown and white rice. Starch Starke 2018, 70, 1700167. [Google Scholar] [CrossRef]
| Syneresis (%) | |||||
|---|---|---|---|---|---|
| Treatment | Storage Period (day) | ||||
| 1 | 7 | 14 | 21 | 28 | |
| FWRF-YA | 0.07 ± 0.01 Db | 0.27 ± 0.02 Db | 5.13 ± 0.15 Cb | 10.60 ± 0.35 Bb | 16.79 ± 0.18 Ab |
| FBRF-YA | 0.00 ± 0.00 Bb | 0.00 ± 0.00 Bb | 0.00 ± 0.00 Bc | 0.00 ± 0.00 Bc | 0.42 ± 0.04 Ac |
| CMWRF-YA | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ad |
| MY | 35.79 ± 0.22 Aa | 37.11 ± 0.51 Aa | 37.54 ± 0.48 Aa | 36.07 ± 3.2 Aa | 38.24 ± 0.30 Aa |
| CMBRF-YA | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ad |
| D (10) (µm) | D (50) (µm) | D (90) (µm) | MV (µm) | |
|---|---|---|---|---|
| CMBRF | 63.87 ± 10.87 A | 204.10 ± 58.77 AB | 371.87 ± 47.37 B | 230.10 ± 47.47 BC |
| CMWRF | 56.18 ± 1.05 A | 262.67 ± 76.89 AB | 417.40 ± 1.66 B | 295.33 ± 54.93 AB |
| FBRF | 47.51 ± 4.64 A | 304.70 ± 6.01 A | 1315 ± 224.34 A | 403.40 ± 53.58 A |
| FWRF | 20.96 ± 2.53 B | 132.53 ± 24.98 B | 235.30 ± 7.95 B | 131.37 ± 10.70 C |
| Color Parameters | Treatment | Storage Period (day) | ||||
|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | ||
| L* | FWRF-YA | 73.03 ± 0.57 BCb | 72.57 ± 0.40 Cbc | 73.38 ± 0.24 ABCc | 74.00 ± 0.70 ABc | 74.29 ± 0.20 Ab |
| FBRF-YA | 74.87 ± 1.44 Ab | 74.40 ± 1.28 Ab | 75.47 ± 0.08 Ab | 75.97 ± 0.59 Ab | 74.16 ± 0.05 Ab | |
| CMWRF-YA | 62.63 ± 0.15 ABd | 62.27 ± 0.68 Bd | 62.68 ± 0.19 ABe | 63.00 ± 0.30 ABe | 63.58 ± 0.20 Ad | |
| MY | 91.90 ± 0.72 Aa | 91.67 ± 0.90 Aa | 93.20 ± 0.17 Aa | 93.20 ± 0.36 Aa | 91.30 ± 1.04 Aa | |
| CMBRF-YA | 70.77 ± 0.72 Ac | 70.90 ± 0.46 Ac | 69.20 ± 0.10 Ad | 70.37 ± 1.21 Ad | 69.33 ± 0.06 Ac | |
| a* | FWRF-YA | −2.60 ± 0.00 Cb | −2.50 ± 0.00 Cb | −2.10 ± 0.06 Ab | −2.23 ± 0.12 ABb | −2.30 ± 0.00 Bc |
| FBRF-YA | −2.17 ± 0.06 Aab | −2.10 ± 0.10 Aab | −2.16 ± 0.06 Ab | −2.13 ± 0.12 Ab | −2.07 ± 0.12 Ab | |
| CMWRF-YA | −2.07 ± 0.06 Aab | −2.13 ± 0.12 ABab | −2.17 ± 0.04 ABb | −2.20 ± 0.10 ABb | −2.33 ± 0.06 Bc | |
| MY | −1.70 ± 0.46 Aa | −1.70 ± 0.46 Aa | −1.60 ± 0.10 Aa | −1.70 ± 0.10 Aa | −1.57 ± 0.06 Aa | |
| CMBRF-YA | −2.47 ± 0.12 Ab | −2.40 ± 0.17 Ab | −2.16 ± 0.05 Ab | −2.23 ± 0.15 Ab | −2.20 ± 0.00 Abc | |
| b* | FWRF-YA | −1.73 ± 0.15 Bd | −1.70 ± 0.10 Bd | −0.80 ± 0.10 Ad | −0.73 ± 0.15 Ac | −0.86 ± 0.05 Ac |
| FBRF-YA | 3.70 ± 0.66 Ab | 3.70 ± 0.66 Ab | 2.90 ± 0.00 Ab | 2.90 ± 0.10 Ab | 2.90 ± 0.10 Ab | |
| CMWRF-YA | −2.50 ± 0.10 Ad | −2.47 ± 0.06 Ad | −2.20 ± 0.00 Ae | −2.37 ± 0.31 Ad | −2.37 ± 0.2 Ad | |
| MY | 7.53 ± 0.25 Ba | 7.47 ± 0.15 Ba | 8.07 ± 0.03 Aa | 8.23 ± 0.06 Aa | 8.03 ± 0.06 Aa | |
| CMBRF-YA | 2.27 ± 0.06 Bc | 2.23 ± 0.06 Bc | 2.70 ± 0.10 Ac | 2.67 ± 0.23 Ab | 2.82 ± 0.07 Ab | |
| Treatment | Storage Period (day) | ||||
|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | |
| Molds (log CFU/g) | |||||
| FWRF-YA | N.D. | N.D. | N.D. | 1.69 ± 0.09 Ba | 2.33 ± 0.35 Aa |
| FBRF-YA | N.D. | N.D. | N.D. | 1.46 ± 0.15 Ba | 2.42 ± 0.24 Aa |
| CMWRF-YA | N.D. | N.D. | N.D. | 1.59 ± 0.11 Ba | 2.40 ± 0.46 Aa |
| MY | N.D. | N.D. | N.D. | N.D. | N.D. |
| CMBRF-YA | N.D. | N.D. | N.D. | 1.39 ± 0.27 Ba | 2.31 ± 0.15 Aa |
| Yeast (log CFU/g) | |||||
| FWRF-YA | N.D. | N.D. | N.D. | N.D. | N.D. |
| FBRF-YA | N.D. | N.D. | N.D. | N.D. | N.D. |
| CMWRF-YA | N.D. | N.D. | N.D. | N.D. | N.D. |
| MY | N.D. | N.D. | N.D. | N.D. | N.D. |
| CMBRF-YA | N.D. | N.D. | N.D. | N.D. | N.D. |
| Coliforms (log CFU/g) | |||||
| FWRF-YA | N.D. | N.D. | N.D. | N.D. | N.D. |
| FBRF-YA | N.D. | N.D. | N.D. | N.D. | N.D. |
| CMWRF-YA | N.D. | N.D. | N.D. | N.D. | N.D. |
| MY | N.D. | N.D. | N.D. | N.D. | N.D. |
| CMBRF-YA | N.D. | N.D. | N.D. | N.D. | N.D. |
| Storage Day | Time (min) | Viable Cell Counts (log CFU/g) | ||||
|---|---|---|---|---|---|---|
| FWRF-YA | FBRF-YA | CMWRF-YA | MY | CMBRF-YA | ||
| 1 | 0 | 8.37 ± 0.04 Aa | 8.77 ± 0.68 Aa | 8.23 ± 0.14 Aa | 8.71 ± 0.11 Aa | 8.32 ± 0.04 Aa |
| 1 | 1 | 8.20 ± 0.10 Ab | 8.17 ± 0.13 Ab | 8.15± 0.07 Ab | 8.63 ± 0.01 Aa | 8.30 ± 0.03 Ab |
| 1 | 90 | 8.18 ± 0.09 Ab | 8.17 ± 0.06 Ab | 8.16 ± 0.05 Ab | 8.61 ± 0.06 Aa | 8.12 ± 0.08 Ab |
| 1 | 180 | 8.20 ± 0.07 Ab | 8.19 ± 0.04 Ab | 8.12 ± 0.03 Ab | 8.57 ± 0.04 Aa | 8.01 ± 0.16 Ab |
| 14 | 0 | 8.21 ± 0.16 Ab | 8.33 ± 0.16 Ab | 8.17 ± 0.06 Ab | 9.19 ± 0.30 Aa | 8.33 ± 0.10 Ab |
| 14 | 1 | 8.16 ± 0.11 Ab | 8.30 ± 0.08 Aab | 8.14 ± 0.13 Ab | 8.61 ± 0.03 Ba | 8.38 ± 0.16 Aab |
| 14 | 90 | 8.07 ± 0.09 Ab | 7.78 ± 0.07 Ab | 7.92 ± 0.11 Ab | 8.49 ± 0.02 Ba | 8.08 ± 0.06 Ab |
| 14 | 180 | 8.09 ± 0.07 Aa | 7.30 ± 0.49 Aa | 7.96 ± 0.03 Aa | 8.52 ± 0.15 Ba | 7.85 ± 0.46 Aa |
| 28 | 0 | 8.18 ± 0.10 Aa | 8.01 ± 0.81 Aa | 8.25 ± 0.03 Aa | 8.52 ± 0.04 Aa | 8.37 ± 0.04 Aa |
| 28 | 1 | 8.23 ± 0.06 Aa | 8.47 ± 0.24 Aa | 8.17 ± 0.05 Aa | 8.54 ± 0.11 Aa | 8.41 ± 0.05 Aa |
| 28 | 90 | 8.02 ± 0.24 Aab | 7.89 ± 0.09 Aab | 7.79 ± 0.20 Ab | 8.49 ± 0.13 Aa | 8.15 ± 0.08 Bab |
| 28 | 180 | 7.53 ± 0.64 Aa | 7.68 ± 0.22 Aa | 7.90 ± 0.22 Aa | 8.57 ± 0.10 Aa | 7.91 ± 0.05 Ca |
| Storage Day | Time (min) | Viable Cell Counts (log CFU/g) | ||||
|---|---|---|---|---|---|---|
| FWRF-YA | FBRF-YA | CMWRF-YA | MY | CMBRF-YA | ||
| 1 | 0 | 8.37 ± 0.04 Aa | 8.77 ± 0.68 Aa | 8.23 ± 0.14 Aa | 8.71 ± 0.11 Aa | 8.32 ± 0.04 Aa |
| 1 | 1 | 8.08 ± 0.03 ABa | 7.00 ± 0.03 Bb | 7.98 ± 0.20 Aa | 7.05 ± 0.17 Bb | 8.10 ± 0.05 Aa |
| 1 | 120 | 7.79 ± 0.07 ABa | 5.92 ± 0.11 BCb | 7.28 ± 0.52 Aa | 6.81 ± 0.04 Bab | 4.88 ± 0.14 Bc |
| 1 | 240 | 7.44 ± 0.36 Ba | 4.87 ± 0.04 Cb | 7.04 ± 0.50 Aa | 6.55 ± 0.21 Ba | 5.03 ± 0.07 Bb |
| 14 | 0 | 8.21 ± 0.16 Ab | 8.33 ± 0.16 Ab | 8.17 ± 0.06 Ab | 9.19 ± 0.30 Aa | 8.33 ± 0.10 Ab |
| 14 | 1 | 7.97 ± 0.18 Aab | 6.08 ± 0.06 Bc | 7.92 ± 0.04 ABab | 6.90 ± 0.05 Bbc | 8.48 ± 0.73 Aa |
| 14 | 120 | 7.10 ± 0.11 Ba | 3.89 ± 0.07 Cc | 7.43 ± 0.16 BCa | 6.28 ± 0.09 Cab | 4.23 ± 1.18 Bbc |
| 14 | 240 | 6.81 ± 0.30 Ba | 3.95 ± 0.08 Cb | 7.16 ± 0.26 Ca | 5.94 ± 0.23 Cab | 4.15 ± 1.06 Bb |
| 28 | 0 | 8.18 ± 0.10 Aa | 8.01 ± 0.81 Aa | 8.25 ± 0.03 Aa | 8.52 ± 0.04 Aa | 8.37 ± 0.04 Aa |
| 28 | 1 | 7.20 ± 0.79 Aa | 5.63 ± 0.28 ABab | 7.40 ± 0.28 Aa | 6.99 ± 0.20 Ba | 3.92 ± 0.88 Bb |
| 28 | 120 | 6.96 ± 0.90 Aa | 4.97 ± 1.87 ABa | 7.23 ± 0.40 Aa | 6.25 ± 0.38 BCa | 3.65 ± 0.33 Ba |
| 28 | 240 | 7.18 ± 0.50 Aa | 3.63 ± 0.26 Bc | 7.13 ± 0.48 Aa | 5.42 ± 0.26 Cb | 3.09 ± 0.50 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, A.; Boeneke, C.; King, J.M. Comparison of Storage Stability and In Vitro Digestion of Rice Flour-Based Yogurt Alternatives Made with Lactobacillus rhamnosus Lgg to Milk-Based Yogurt. Foods 2025, 14, 1129. https://doi.org/10.3390/foods14071129
Morris A, Boeneke C, King JM. Comparison of Storage Stability and In Vitro Digestion of Rice Flour-Based Yogurt Alternatives Made with Lactobacillus rhamnosus Lgg to Milk-Based Yogurt. Foods. 2025; 14(7):1129. https://doi.org/10.3390/foods14071129
Chicago/Turabian StyleMorris, Anita, Charles Boeneke, and Joan M. King. 2025. "Comparison of Storage Stability and In Vitro Digestion of Rice Flour-Based Yogurt Alternatives Made with Lactobacillus rhamnosus Lgg to Milk-Based Yogurt" Foods 14, no. 7: 1129. https://doi.org/10.3390/foods14071129
APA StyleMorris, A., Boeneke, C., & King, J. M. (2025). Comparison of Storage Stability and In Vitro Digestion of Rice Flour-Based Yogurt Alternatives Made with Lactobacillus rhamnosus Lgg to Milk-Based Yogurt. Foods, 14(7), 1129. https://doi.org/10.3390/foods14071129





