Nutritional Values and Biochemical Traits of Rye (Secale cereale L.) Seeds, a Landrace from Matese Mountains (Southern Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Sampling
2.3. Proximate Composition
2.4. Ash Content and Moisture Content
2.5. Amino Acid Composition
2.6. Determination of Trypsin and Chymotrypsin Inhibitory Activities
2.7. Gas Chromatographic Analysis of Fatty Acid Methyl Esters
2.8. Extraction of Free Phenolics
2.9. Determination of Phenolics Content and Antioxidant Evaluation
2.10. Identification and Quantification of Chemical Compounds Using RP-HPLC
2.11. Determination of Minerals Content
2.12. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Values
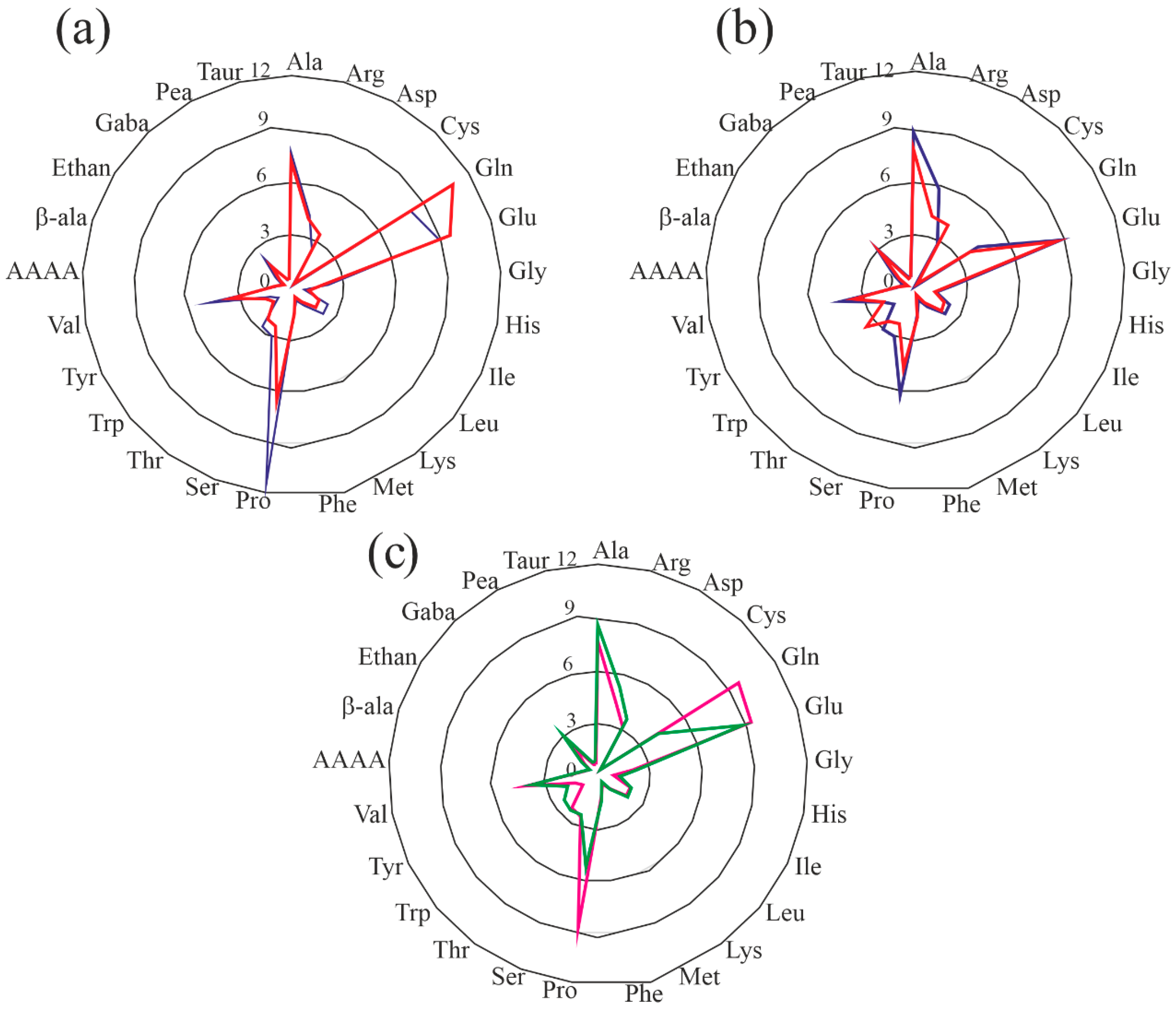
3.2. Amino Acid Content
3.3. Fatty Acid Composition
3.4. Anti-Proteinase Inhibitor Activity
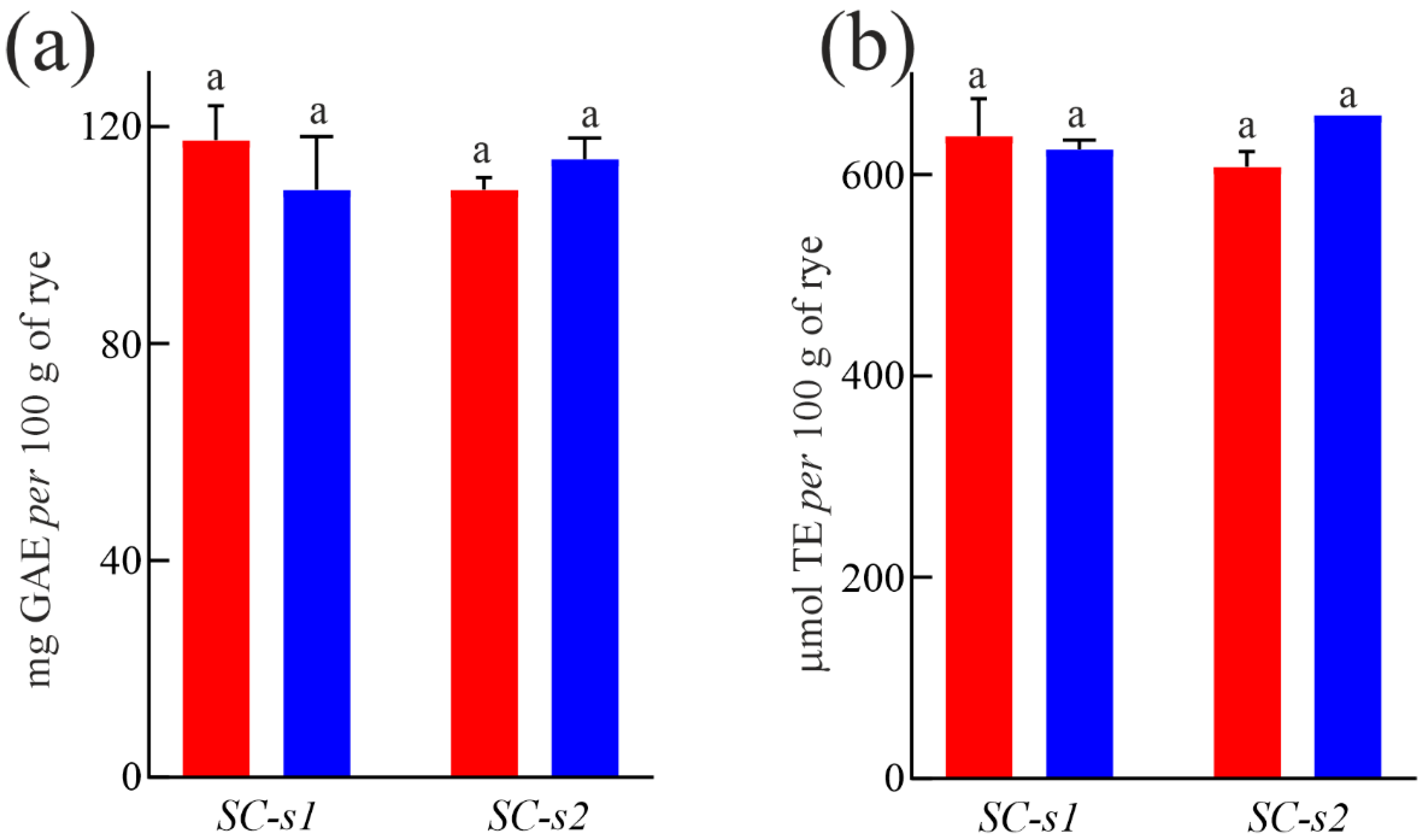
3.5. Total Free Polyphenol Content and Antioxidant Capability of ‘Segale Del Matese’
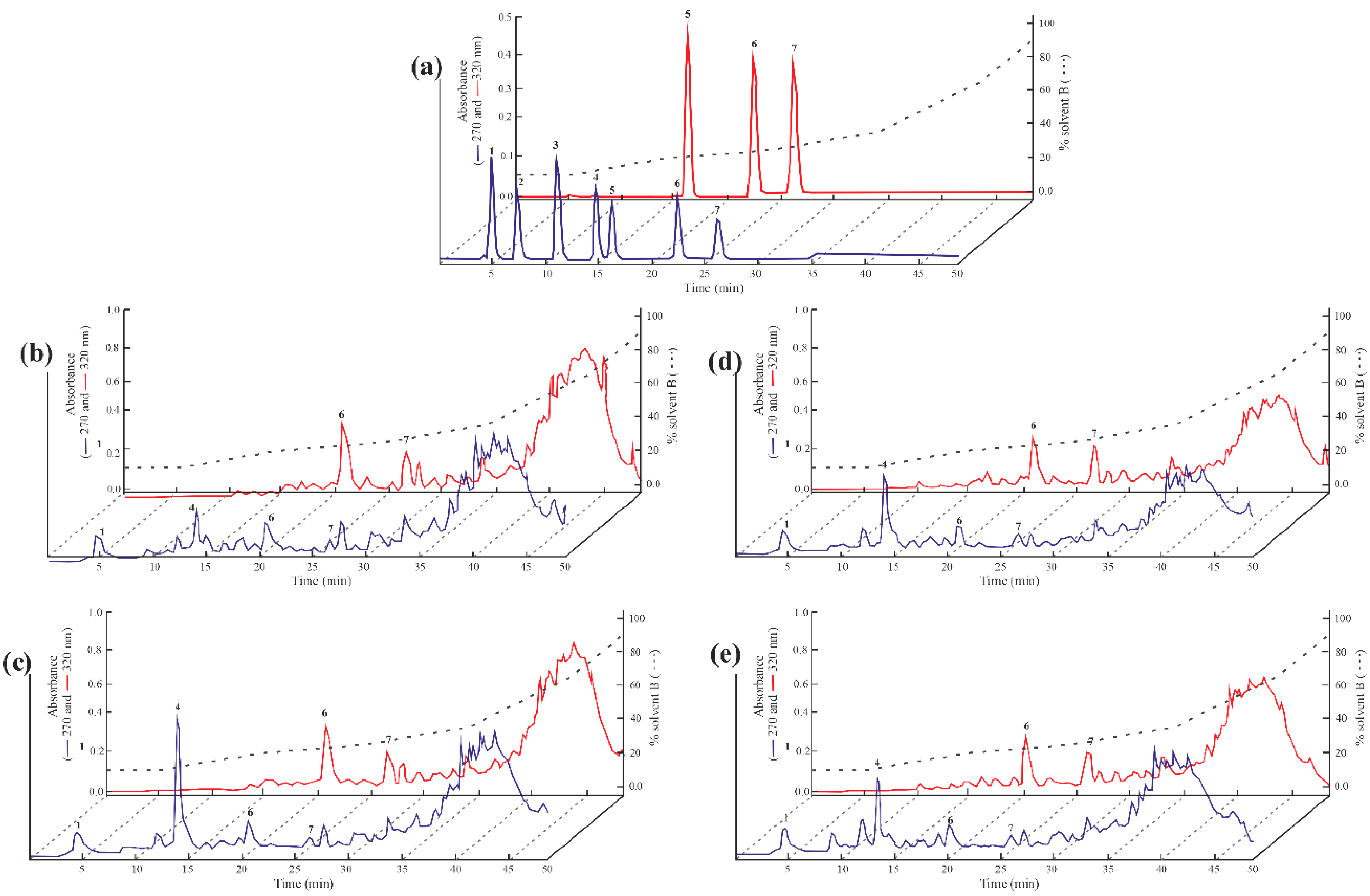
3.6. Tentative Identification and Quantization of the Chemical Compounds
3.7. Minerals Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapirstein, H.D.; Bushuk, W. Rye Grain: Its Genetics, Production, and Utilization. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; pp. 159–167. [Google Scholar]
- Bushuk, W. Rye: Production, Chemistry, and Technology; American Association of Cereal Chemists: St. Paul, MN, USA, 2001. [Google Scholar]
- Hannah Ritchie, H.; Rosado, P.; Roser, M. Rye Production. Available online: https://ourworldindata.org/grapher/rye-production (accessed on 29 October 2024).
- Ahmad, I.; Wang, H.; Kamran, M.; Ikram, K.; Hou, F. Simulated Grazing (Clipping) Affected Growth and Nutritional Quality of Barley, Rye, and Wheat in an Arid Climate. J. Plant Growth Regul. 2023, 42, 3017–3031. [Google Scholar]
- Ikram, A.; Saeed, F.; Noor, R.A.; Imran, A.; Afzaal, M.; Rasheed, A.; Islam, F.; Iqbal, A.; Zahoor, T.; Naz, S.; et al. A comprehensive review on biochemical and technological properties of rye (Secale cereale L.). Int. J. Food Prop. 2023, 26, 2212–2228. [Google Scholar]
- Hansen, H.B.; Møller, B.; Andersen, S.B.; Jørgensen, J.R.; Hansen, A. Grain characteristics, chemical composition, and functional properties of rye (Secale cereale L.) as influenced by genotype and harvest year. J. Agric. Food Chem. 2004, 52, 2282–2291. [Google Scholar] [PubMed]
- Schupfer, E.; Pak, S.C.; Wang, S.; Micalos, P.S.; Jeffries, T.; Ooi, S.L.; Golombick, T.; Harris, G.; El-Omar, E. The effects and benefits of arabinoxylans on human gut microbiota—A narrative review. Food Biosci. 2021, 43, 101267. [Google Scholar]
- Choromanska, A.; Kulbacka, J.; Harasym, J.; Dubinska-Magiera, M.; Saczko, J. Anticancer activity of oat β-glucan in combination with electroporation on human cancer cells. Acta Pol. Pharm. 2017, 74, 616–623. [Google Scholar]
- Deleu, L.J.; Lemmens, E.; Redant, L.; Delcour, J.A. The major constituents of rye (Secale cereale L.) flour and their role in the production of rye bread, a food product to which a multitude of health aspects are ascribed. Cereal Chem. 2020, 97, 739–754. [Google Scholar]
- Kulichová, K.; Sokol, J.; Nemeček, P.; Maliarová, M.; Maliar, T.; Havrlentová, M.; Kraic, J. Phenolic compounds and biological activities of rye (Secale cereale L.) grains. Open Chem. 2019, 17, 988–999. [Google Scholar]
- Kaur, P.; Singh Sandhu, K.; Singh Purewal, S.; Kaur, M.; Kumar Singh, S. Rye: A wonder crop with industrially important macromolecules and health benefits. Food Res. Int. 2021, 150 Pt A, 110769. [Google Scholar]
- Rodehutscord, M.; Rückert, C.; Maurer, H.P.; Schenkel, H.; Schipprack, W.; Bach Knudsen, K.E.; Schollenberger, M.; Laux, M.; Eklund, M.; Siegert, W.; et al. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch. Anim. Nutr. 2016, 70, 87–107. [Google Scholar]
- Iversen, K.N.; Jonsson, K.; Landberg, R. The Effect of Rye-Based Foods on Postprandial Plasma Insulin Concentration: The Rye Factor. Front. Nutr. 2022, 9, 868938. [Google Scholar]
- Magnusdottir, O.K.; Landberg, R.; Gunnarsdottir, I.; Cloetens, L.; Åkesson, B.; Rosqvist, F.; Schwab, U.; Herzig, K.H.; Hukkanen, J.; Savolainen, M.J.; et al. Whole grain rye intake, reflected by a biomarker, is associated with favorable blood lipid outcomes in subjects with the metabolic syndrome A randomized study. PLoS ONE 2014, 9, e110827. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Giovannucci, E.; Bergkvist, L.; Wolk, A. Whole grain consumption and risk of colorectal cancer: A population-based cohort of 60,000 women. Br. J. Cancer 2005, 92, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Németh, R.; Tömösközi, S. Rye: Current state and future trends in research and applications. Acta Aliment. 2021, 50, 620–640. [Google Scholar] [CrossRef]
- Arens, U. 7—Authorised EU health claim for rye fibre. In Foods, Nutrients and Food Ingredients with Authorised EU Health Claims: Volume 2; Sadler, M.J., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 129–137. [Google Scholar]
- Sardella, C.; Capo, L.; Adamo, M.; Donna, M.; Ravetto Enri, S.; Vanara, F.; Lonati, M.; Mucciarelli, M.; Blandino, M. The cultivation of rye in marginal Alpine environments: A comparison of the agronomic, technological, health and sanitary traits of local landraces and commercial cultivars. Front. Plant Sci. 2023, 14, 1130543. [Google Scholar] [PubMed]
- Mucciarelli, M.; Massimo Blandino, M.; Capo, L. La Segale in Piemonte—Storia di una Rinascita; Aree Protette Alpi Marittime: Cuneo, Italy, 2022. [Google Scholar]
- Peratoner, G.; Seling, S.; Klotz, C.; Florian, C.; Figl, U.; Schmitt, A.O. Variation of agronomic and qualitative traits and local adaptation of mountain landraces of winter rye (Secale cereale L.) from Val Venosta/Vinschgau (South Tyrol). Genet. Resour. Crop Evol. 2016, 63, 261–273. [Google Scholar] [CrossRef]
- Adamo, M.; Blandino, M.; Capo, L.; Ravetto Enri, S.; Fusconi, A.; Lonati, M.; Mucciarelli, M. A ddRADseq Survey of the Genetic Diversity of Rye (Secale cereale L.) Landraces from the Western Alps Reveals the Progressive Reduction of the Local Gene Pool. Plants 2021, 10, 2415. [Google Scholar] [CrossRef]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and identifying crop landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar]
- Assessorato_Agricoltura_regione_Campania Sécena. Available online: https://agricoltura.regione.campania.it/Tipici/tradizionali/secena.html (accessed on 7 November 2024).
- Landi, N.; Piccolella, S.; Ragucci, S.; Faramarzi, S.; Clemente, A.; Papa, S.; Pacifico, S.; Di Maro, A. Valle Agricola Chickpeas: Nutritional Profile and Metabolomics Traits of a Typical Landrace Legume from Southern Italy. Foods 2021, 10, 583. [Google Scholar] [CrossRef]
- Landi, N.; Ragucci, S.; Formato, M.; Piccolella, S.; Magri, A.; Baiano, S.; Petriccione, M.; Papa, S.; Pedone, P.V.; Pacifico, S.; et al. Wild fennel seeds from Valle Agricola (Southern Italy): Biochemical and antioxidant traits in minced pork meat. J. Funct. Foods 2024, 113, 106010. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000.
- Analytical methods for carbohydrates in foods. In Food Energy—Methods of Analysis and Conversion Factors; FAO Food and Nutrition: Roma, Italy, 2003.
- Di Maro, A.; Dosi, R.; Ferrara, L.; Rocco, M.; Sepe, J.J.; Ferrari, G.; Parente, A. Free Amino Acid Profile of Malus domestica Borkh Cv. Annurca from the Campania Region and Other Italian Vegetables. Aust. J. Crop Sci. 2011, 5, 154–161. [Google Scholar]
- Moore, S.; Stein, W.H. Chromatographic determination of amino acids by the use of automatic recording equipment. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1963; Volume 6, pp. 819–831. [Google Scholar]
- Landi, N.; Alberico, L.; Clemente, A.; Peddio, S.; Hussain, H.Z.F.; Ragucci, S.; Zucca, P.; Woodrow, P.; Di Maro, A. Nutritional, metabolic and genetic profiling of ‘Cerato’ and ‘Curniciello’ bean landraces from Caserta, Southern Italy. Food Biosci. 2023, 55, 102975. [Google Scholar] [CrossRef]
- Poerio, E.; Gennaro, S.D.; Di Maro, A.; Farisei, F.; Ferranti, P.; Parente, A. Primary Structure and Reactive Site of a Novel Wheat Proteinase Inhibitor of Subtilisin and Chymotrypsin. Biol. Chem. 2003, 384, 295–304. [Google Scholar]
- Landi, N.; Pacifico, S.; Piccolella, S.; Di Giuseppe, A.M.; Mezzacapo, M.C.; Ragucci, S.; Iannuzzi, F.; Zarrelli, A.; Di Maro, A. Valle Agricola lentil, an unknown lentil (Lens culinaris Medik.) seed from Southern Italy as a novel antioxidant and prebiotic source. Food Funct. 2015, 6, 3155–3164. [Google Scholar] [PubMed]
- Irakli, M.N.; Samanidou, V.F.; Biliaderis, C.G.; Papadoyannis, I.N. Development and validation of an HPLC-method for determination of free and bound phenolic acids in cereals after solid-phase extraction. Food Chem. 2012, 134, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Ragucci, S.; Fiorentino, M.; Guida, V.; Maro, A.D. Nutritional values and metabolic profile with and without boiled treatment of ’Gallo Matese’ beans (Phaseolus vulgaris L.), a landrace from Southern Italy. Acta Sci. Pol. Technol. Aliment. 2017, 16, 331–344. [Google Scholar] [PubMed]
- AlimentiNUTrizione—Ricerca Dati. Available online: https://www.alimentinutrizione.it/tabelle-nutrizionali (accessed on 7 November 2024).
- Postles, J.; Curtis, T.Y.; Powers, S.J.; Elmore, J.S.; Mottram, D.S.; Halford, N.G. Changes in Free Amino Acid Concentration in Rye Grain in Response to Nitrogen and Sulfur Availability, and Expression Analysis of Genes Involved in Asparagine Metabolism. Front. Plant Sci. 2016, 7, 917. [Google Scholar]
- Curtis, T.Y.; Powers, S.J.; Balagiannis, D.; Elmore, J.S.; Mottram, D.S.; Parry, M.A.; Rakszegi, M.; Bedö, Z.; Shewry, P.R.; Halford, N.G. Free amino acids and sugars in rye grain: Implications for acrylamide formation. J. Agric. Food Chem. 2010, 58, 1959–1969. [Google Scholar]
- Al-Taher, F.; Nemzer, B. Effect of Germination on Fatty Acid Composition in Cereal Grains. Foods 2023, 12, 3306. [Google Scholar] [CrossRef]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A review on anti-nutritional factors: Unraveling the natural gateways to human health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Lima, A.I.G.; Mota, J.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B. Legume seeds and colorectal cancer revisited: Protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016, 197, 30–38. [Google Scholar]
- Torbica, A.; Belović, M.; Popović, L.; Čakarević, J.; Jovičić, M.; Pavličević, J. Comparative study of nutritional and technological quality aspects of minor cereals. J. Food Sci. Technol. 2021, 58, 311–322. [Google Scholar] [CrossRef] [PubMed]
- WHO. Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization Technical Report Series (935); WHO: Geneva, Switzerland, 2007.
| Segale del Matese | Commercial Rye | ||
|---|---|---|---|
| SC-s1 | SC-s2 | CREA | |
| Crude proteins | 9.40 ± 0.32 a | 9.73 ± 1.67 a | 11.70 |
| Lipids | 1.24 ± 0.01 a | 1.31 ± 0.11 a | 2.00 |
| Ash | 1.77 ± 0.01 a | 1.77 ± 0.01 a | - |
| Moisture | 9.32 ± 2.33 a | 9.32 ± 2.33 a | 9.2 |
| Carbohydrates | 78.27 ± 2.64 a | 78.27 ± 2.93 a | 67.8 |
| Segale del Matese | Commercial rye | ||
|---|---|---|---|
| SC-s1 | SC-s2 | CREA | |
| essential amino acids | |||
| His | 0.19 ± 0.01 a | 0.19 ± 0.04 a | 0.27 |
| Ile | 0.23 ± 0.00 a | 0.24 ± 0.06 a | 0.49 |
| Leu | 0.50 ± 0.02 a | 0.51 ± 0.10 a | 0.78 |
| Lys | 0.33 ± 0.02 a | 0.33 ± 0.06 a | 0.48 |
| Met | 0.13 ± 0.02 a | 0.14 ± 0.02 a | 0.19 |
| Phe | 0.38 ± 0.01 a | 0.39 ± 0.08 a | 0.55 |
| Thr | 0.29 ± 0.00 a | 0.30 ± 0.06 a | 0.43 |
| Trp | n.d. | n.d. | 0.14 |
| Val | 0.29 ± 0.01 a | 0.31 ± 0.05 a | 0.61 |
| non-essential amino acids | |||
| Ala | 0.38 ± 0.01 a | 0.40 ± 0.09 a | 0.49 |
| Arg | 0.38 ± 0.02 a | 0.38 ± 0.08 a | 0.57 |
| Asx | 0.47 ± 0.00 a | 0.45 ± 0.10 a | 0.84 |
| Cys § | 0.41 ± 0.06 a | 0.40 ± 0.04 a | 0.23 |
| Glx | 1.61 ± 0.07 a | 1.45 ± 0.08 b | 2.49 |
| Gly | 0.35 ± 0.01 a | 0.36 ± 0.08 a | 0.56 |
| Pro | 0.79 ± 0.06 a | 0.81 ± 0.28 a | 0.61 |
| Ser | 0.41 ± 0.01 a | 0.43 ± 0.11 a | 0.49 |
| Tyr | 0.16 ± 0.02 a | 0.16 ± 0.00 a | 0.37 |
| Total | 7.39 | 7.23 | 10.61 |
| Fatty Acid | ‘Segale del Matese’ | ||||
|---|---|---|---|---|---|
| SC-s1 | SC-s2 | ||||
| 2023 | 2024 | 2023 | 2024 | ||
| Saturated fatty acids | |||||
| Palmitic | C16 | 10.35 ± 0.35 a | 10.49 ± 0.46 a | 11.11 ± 2.21 a | 10.65 ± 0.01 a |
| Stearic | C18 | 1.12 ± 0.11 a | 1.27 ± 0.03 a | 1.41 ± 0.32 a | 1.18 ± 0.15 a |
| Monounsaturated fatty acids | |||||
| Oleic | C18:1 | 16.95 ± 0.45 a | 17.11 ± 1.47 a | 18.08 ± 2.79 a | 17.97 ± 0.54 a |
| Polyunsaturated fatty acid | |||||
| Linoleic | C18:2 | 59.95 ± 3.41 a | 60.37 ± 3.87 a | 58.96 ± 8.14 a | 60.00 ± 1.72 a |
| Linolenic | C18:3 | 10.94 ± 0.66 a | 10.34 ± 0.96 a | 10.24 ± 1.22 a | 10.40 ± 0.13 a |
| SFA% | 11.47 | 11.76 | 12.52 | 11.82 | |
| MUFA% | 16.95 | 17.11 | 18.08 | 1.18 | |
| PUFA% | 70.90 | 70.72 | 69.20 | 70.40 | |
| Anti-trypsin activity | |||||
| SC-s1 | 2023 | 2024 | SC-s2 | 2023 | 2024 |
| raw | 7.81 ± 0.81 a | 7.86 ± 1.31 a | raw | 9.09 ± 0.06 a | 7.58 ± 1.99 a |
| cooked | 67.72 ± 11.37 abc | 68.32 ± 10.20 abc | cooked | 78.98 ± 2.44 b | 65.95 ± 6.40 c |
| Anti-chymotrypsin activity | |||||
| SC-s1 | 2023 | 2024 | SC-s2 | 2023 | 2024 |
| raw | 19.56 ± 1.36 a | 21.95 ± 4.06 a | raw | 27.93 ± 7.86 a | 31.59 ± 9.14 a |
| cooked | 50.60 ± 2.15 a | 56.78 ± 2.50 a | cooked | 51.51 ± 3.77 a | 58.26 ± 3.60 a |
| Element | SC-s1 | SC-s2 | ||
|---|---|---|---|---|
| 2023 | 2024 | 2023 | 2024 | |
| Fe | 3.20 ± 0.08 a | 3.19 ± 0.13 a | 2.62 ± 0.03 b | 2.62 ± 0.04 b |
| Ca | 35.03 ± 1.36 a | 35.39 ± 1.81 a | 32.33 ± 0.76 a | 31.33 ± 1.02 a |
| Na | 2.12 ± 0.10 a | 2.10 ± 0.06 a | 2.10 ± 0.05 a | 2.10 ± 0.10 a |
| K | 495.33 ± 15.50 a | 516.67 ± 12.58 a | 466.00 ± 3.61 ab | 465.67 ± 12.90 b |
| P | 333.33 ± 10.41 a | 350.00 ± 10.00 a | 332.33 ± 2.52 a | 331.00 ± 7.94 a |
| Zn | 2.82 ± 0.08 a | 2.93 ± 0.08 a | 2.77 ± 0.03 a | 2.75 ± 0.05 a |
| Mg | 110.00 ± 3.61 a | 114.00 ± 4.58 a | 105.00 ± 2.00 a | 104.00 ± 2.00 a |
| Cu | 0.40 ± 0.01 a | 0.43 ± 0.01 a | 0.40 ± 0.003 a | 0.40 ± 0.01 a |
| Se | 36.80 ± 0.26 a | 36.87 ± 0.40 a | 29.80 ± 0.36 b | 29.07 ± 1.78 b |
| Mn | 3.14 ± 0.20 a | 3.14 ± 0.14 a | 2.95 ± 0.15 a | 2.93 ± 0.25 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, N.; Ragucci, S.; Campanile, M.G.; Hussain, H.Z.F.; Papa, S.; Di Maro, A. Nutritional Values and Biochemical Traits of Rye (Secale cereale L.) Seeds, a Landrace from Matese Mountains (Southern Italy). Foods 2025, 14, 1120. https://doi.org/10.3390/foods14071120
Landi N, Ragucci S, Campanile MG, Hussain HZF, Papa S, Di Maro A. Nutritional Values and Biochemical Traits of Rye (Secale cereale L.) Seeds, a Landrace from Matese Mountains (Southern Italy). Foods. 2025; 14(7):1120. https://doi.org/10.3390/foods14071120
Chicago/Turabian StyleLandi, Nicola, Sara Ragucci, Maria Giuseppina Campanile, Hafiza Z. F. Hussain, Stefania Papa, and Antimo Di Maro. 2025. "Nutritional Values and Biochemical Traits of Rye (Secale cereale L.) Seeds, a Landrace from Matese Mountains (Southern Italy)" Foods 14, no. 7: 1120. https://doi.org/10.3390/foods14071120
APA StyleLandi, N., Ragucci, S., Campanile, M. G., Hussain, H. Z. F., Papa, S., & Di Maro, A. (2025). Nutritional Values and Biochemical Traits of Rye (Secale cereale L.) Seeds, a Landrace from Matese Mountains (Southern Italy). Foods, 14(7), 1120. https://doi.org/10.3390/foods14071120










