Flaxseed Oilcake: An Ingredient with High Nutritional Value in the Realization of Innovative Food Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
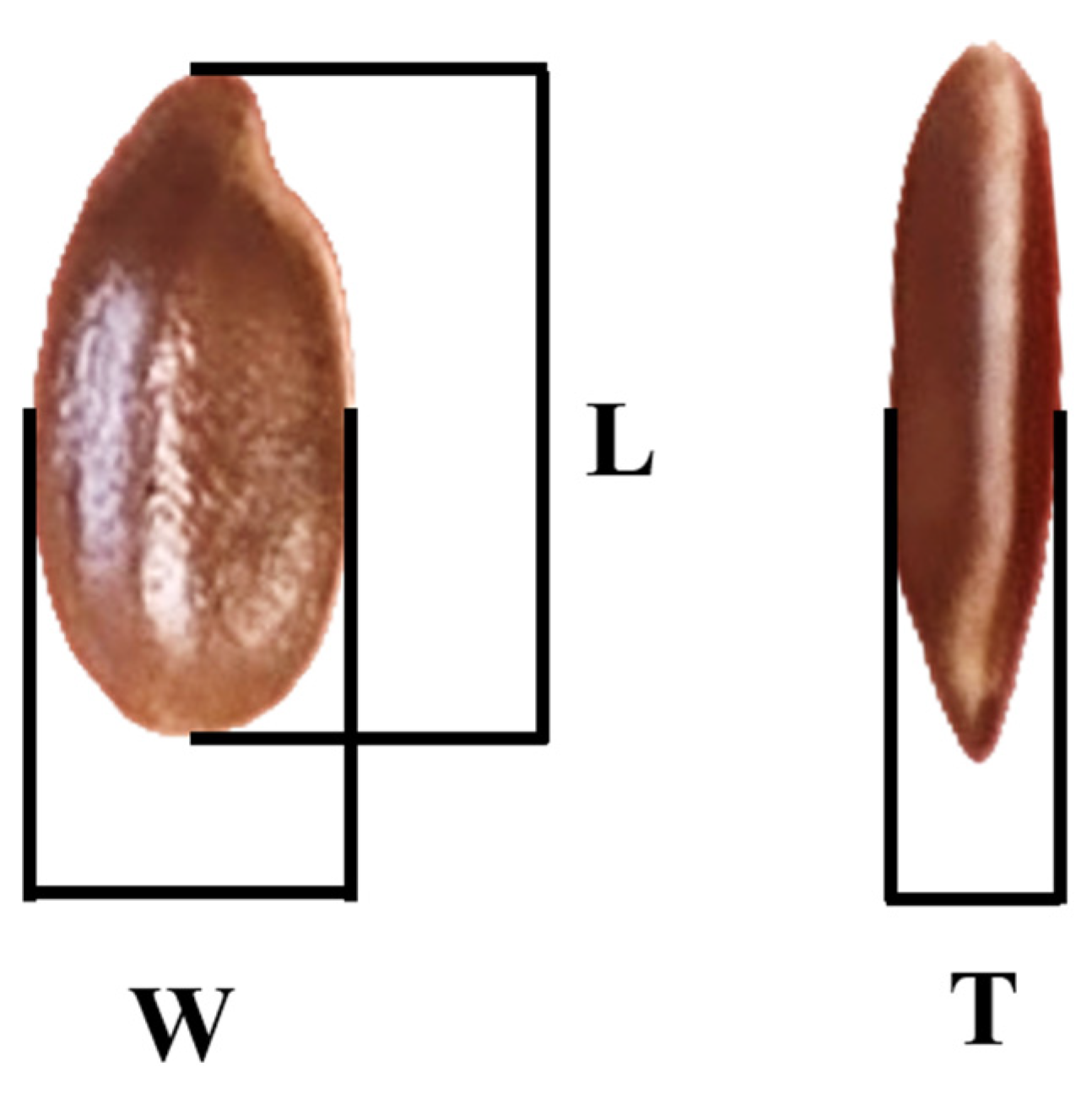
2.2. Physical Properties
2.3. Nutritional Composition of Flax Seeds and Flaxseed Oilcake
2.4. Flaxseed Oilcake Characterization
2.4.1. Safety Assessment
2.4.2. Functional Properties
2.4.3. Color Parameters
2.4.4. FTIR-ATR (Fourier Transformed Infrared Analysis with Attenuated Total Reflectance)
2.5. Fatty Acid Composition of Flax Seeds and Flaxseed Oilcake
2.6. Amino Acid Composition of Flax Seeds and Flaxseed Oilcake
2.7. Mineral Composition of Flax Seeds and Flaxseed Oilcake
2.8. Experimental Design for Determining Total Polyphenolic Content and Antioxidant Activity
2.8.1. Extract Preparation
2.8.2. TPC and AOA
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties
3.2. Nutritional Composition of Flax Seeds and Flaxseed Oilcake
3.3. Flaxseeed Oilcake Characterization: Safety, Functional Properties, and Qualitative Identification of Key Functional Groups
3.3.1. Safety Assessment
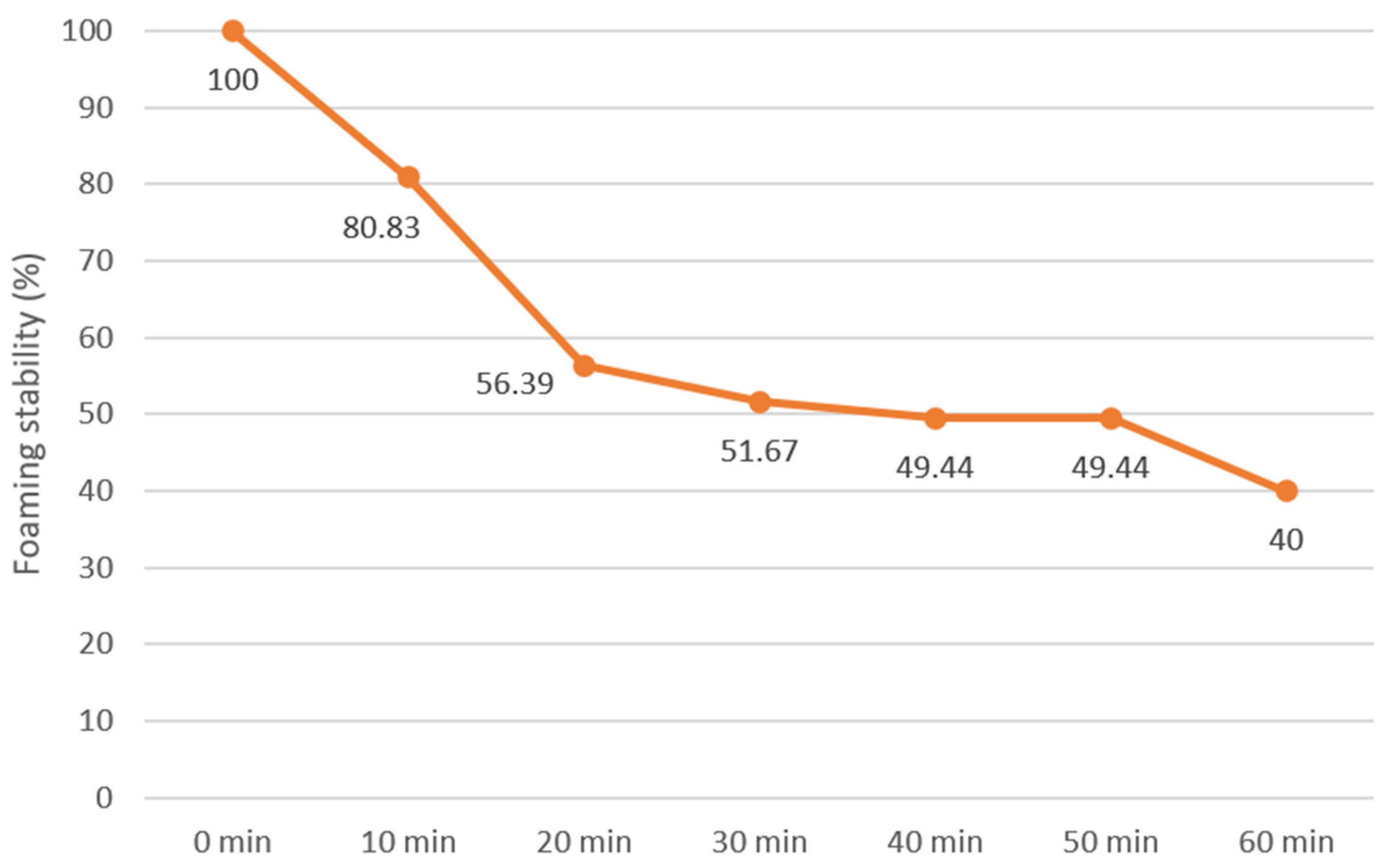
3.3.2. Functional Properties
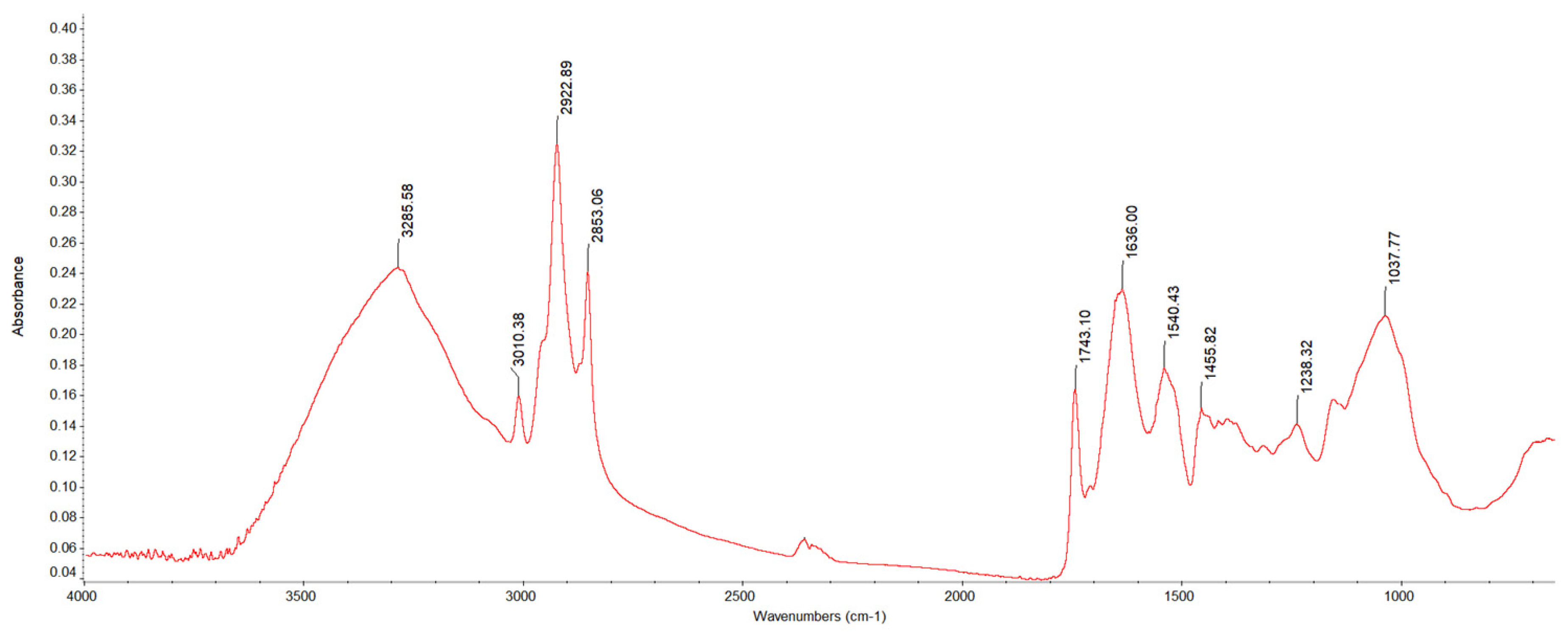
3.3.3. Fourier Transform Infrared-Attenuated Total Reflection (FTIR-ATR)
3.4. Fatty Acid Composition of Flax Seeds and Flaxseed Oilcake
3.5. Amino Acid Composition of Flax Seeds and Flaxseed Oilcake
3.6. Comparison of Mineral Composition Between Flax Seeds and Flaxseed Oilcake
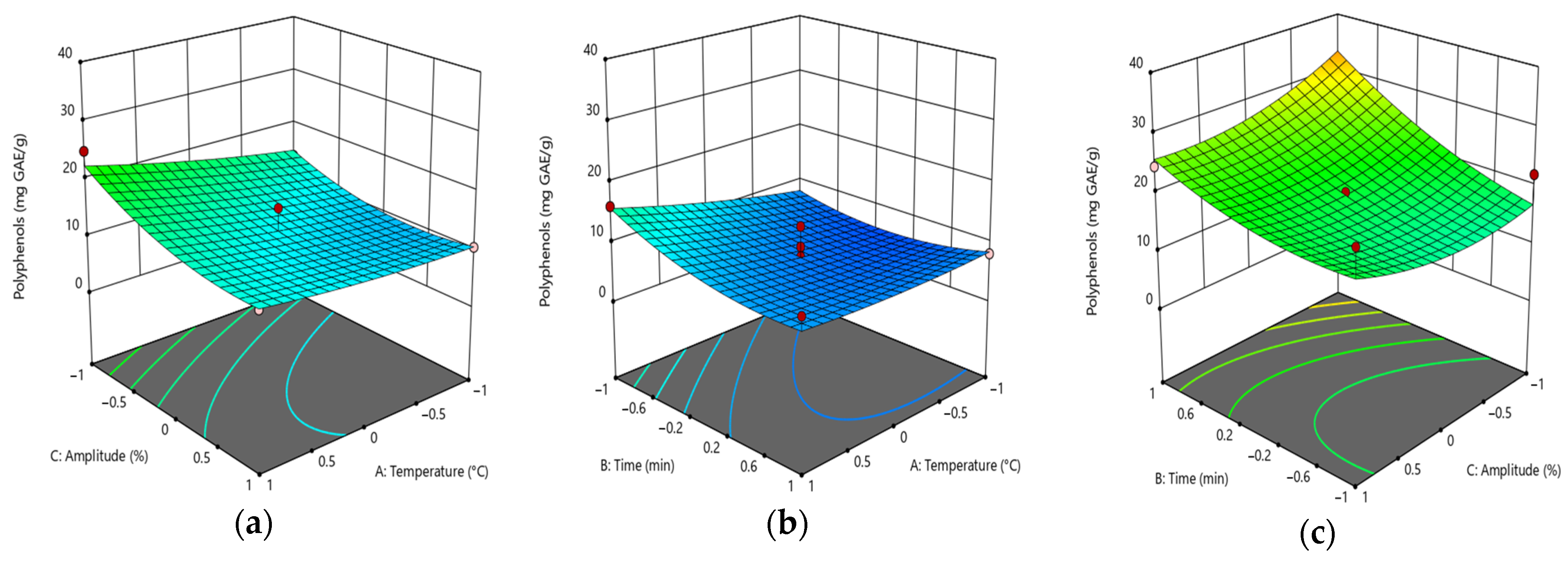
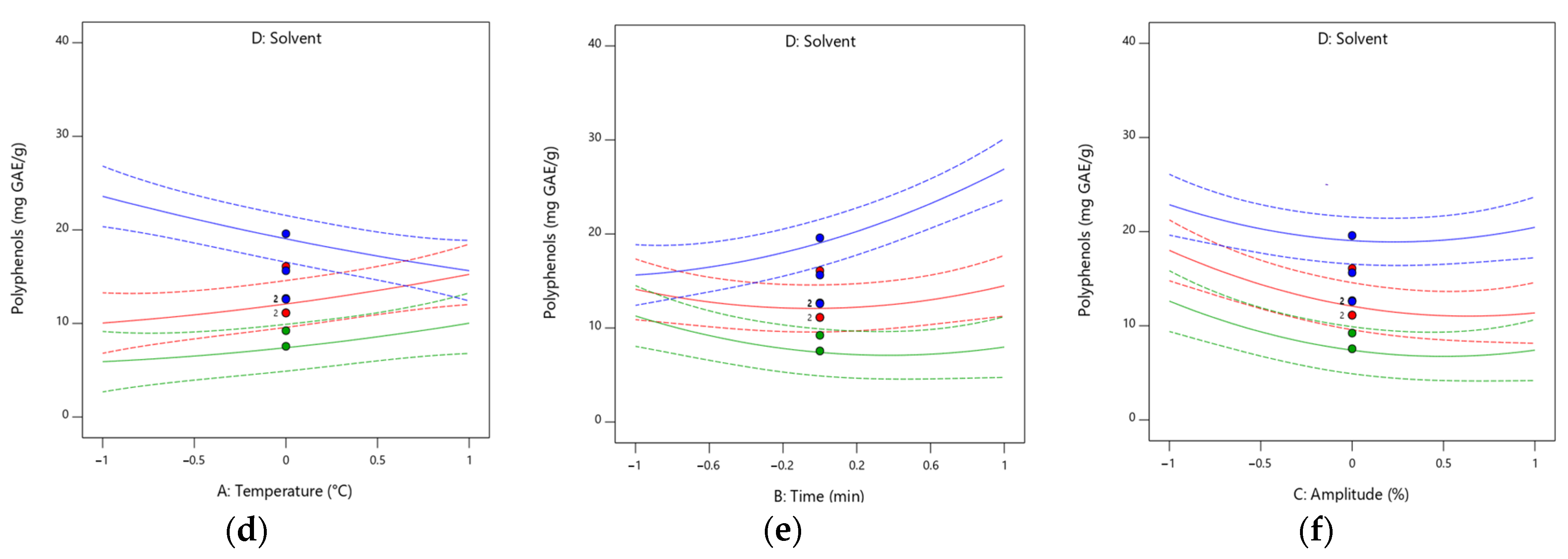
3.7. Model Fitting: Total Polyphenolic Content and Antioxidant Activity
3.8. Phenolic Acids
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drozłowska, E. Valorization of flaxseed oil cake residual from cold-press oil production as a material for preparation of spray-dried functional powders for food applications as emulsion stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Bułkowska, K. Agricultural Wastes and Their By-Products for the Energy Market. Energies 2024, 17, 2099. [Google Scholar] [CrossRef]
- Mihai, A.L.; Negoiță, M.; Horneț, G.-A.; Belc, N. Valorization Potential of Oil Industry By-Products as Sources of Essential Fatty Acids. Processes 2022, 10, 2373. [Google Scholar] [CrossRef]
- Otles, S.; Kartal, C. Food Waste Valorization. In Sustainable Food Systems from Agriculture to Industry: Improving Production and Processing; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 371–399. ISBN 9780128119358. [Google Scholar]
- Mironeasa, S.; Ungureanu-Iuga, M.; Ursachi, V.F.; Mironeasa, C. Seedless grape pomace to increase fiber content in extruded corn snacks. Ukr. Food J. 2024, 13, 657–674. [Google Scholar] [CrossRef]
- Stabnikova, O.; Shevchenko, A.; Stabnikov, V.; Paredes-López, O. Utilization of plant processing wastes for enrichment of bakery and confectionery products. Ukr. Food J. 2023, 12, 299–308. [Google Scholar] [CrossRef]
- Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Espírito Santo, L.; Machado, S.; Pardo, J.E.; Oliveira, M.B.P.P. Nutritional and Chemical Characterization of Poppy Seeds, Cold-Pressed Oil, and Cake: Poppy Cake as a High-Fibre and High-Protein Ingredient for Novel Food Production. Foods 2022, 11, 3027. [Google Scholar] [CrossRef]
- Otles, S.; Despoudi, S.; Bucatariu, C.; Kartal, C. Food waste mangement, valorization, and sustainability in the food industry. In Food Waste Recovery; Galanakis, C.M., Ed.; Elsevier Inc.: London, UK, 2015; pp. 3–23. ISBN 978-0-12-800351-0. [Google Scholar]
- Mateos-Aparicio, I.; Matias, A. Food Industry Processing By-Products in Foods; Galanakis, C.M., Ed.; Academic Press: London, UK, 2019; ISBN 9780128164532. [Google Scholar]
- Łopusiewicz, Ł.; Kowalczewski, P.Ł.; Baranowska, H.M.; Masewicz, Ł.; Amarowicz, R.; Krupa-Kozak, U. Effect of Flaxseed Oil Cake Extract on the Microbial Quality, Texture and Shelf Life of Gluten-Free Bread. Foods 2023, 12, 595. [Google Scholar] [CrossRef]
- Man, S.M.; Stan, L.; Păucean, A.; Chiş, M.S.; Mureşan, V.; Socaci, S.A.; Pop, A.; Muste, S. Nutritional, Sensory, Texture Properties and Volatile Compounds Profile of Biscuits with Roasted Flaxseed Flour Partially Substituting for Wheat Flour. Appl. Sci. 2021, 11, 4791. [Google Scholar] [CrossRef]
- Wirkijowska, A.; Zarzycki, P.; Sobota, A.; Nawrocka, A.; Blicharz-Kania, A.; Andrejko, D. The possibility of using by-products from the flaxseed industry for functional bread production. LWT 2020, 118, 108860. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed Bioactive Compounds: Chemical Composition, Functional Properties, Food Applications and Health Benefits-Related Gut Microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef]
- Nowak, W.; Jeziorek, M. The Role of Flaxseed in Improving Human Health. Healthcare 2023, 11, 395. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Safdar, B.; Pang, Z.; Liu, X.; Rashid, M.T.; Jatoi, M.A. Structural and functional properties of raw and defatted flaxseed flour and degradation of cynogenic contents using different processing methods. J. Food Process Eng. 2020, 43, e13406. [Google Scholar] [CrossRef]
- Vichare, S.A.; Morya, S. Exploring waste utilization potential: Nutritional, functional and medicinal properties of oilseed cakes. Front. Food Sci. Technol. 2024, 4, 1441029. [Google Scholar] [CrossRef]
- Ancuța, P.; Sonia, A. Oil Press-Cakes and Meals Valorization through Circular Economy Approaches: A Review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Serra, A.; Conte, G.; Flamini, G.; Angelini, L.G. Effect of the Leavening Agent on the Compositional and Sensorial Characteristics of Bread Fortified with Flaxseed Cake. Appl. Sci. 2020, 10, 5235. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Dmytrów, I.; Mituniewicz-Małek, A.; Kwiatkowski, P.; Kowalczyk, E.; Sienkiewicz, M.; Drozłowska, E. Natural Gum from Flaxseed By-Product as a Potential Stabilizing and Thickening Agent for Acid Whey Fermented Beverages. Appl. Sci. 2022, 12, 10281. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Łopusiewicz, Ł. Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-in-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent. Foods 2021, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Coşkuner, Y.; Karababa, E. Some physical properties of flaxseed (Linum usitatissimum L.). J. Food Eng. 2007, 78, 1067–1073. [Google Scholar] [CrossRef]
- Pradhan, R.C.; Meda, V.; Naik, S.N.; Tabil, L. Physical properties of Canadian grown flaxseed in relation to its processing. Int. J. Food Prop. 2010, 13, 732–743. [Google Scholar] [CrossRef]
- Singh, K.K.; Mridula, D.; Barnwal, P.; Rehal, J. Physical and chemical properties of flaxseed. Int. Agrophysics 2012, 26, 423–426. [Google Scholar] [CrossRef]
- ISO 665:2020; Animal and Vegetable Fats and Oils—Determination of Moisture and Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 2020.
- AOAC Method 935.29; Moisture in Malt. Official Methods of Analysis. 17th ed. Association of Official Analytical Chemists: Arlington, VA, USA, 2003.
- Pojić, M.; Mišan, A.; Sakač, M.; HadnaCrossed D Signev, T.D.; Šarić, B.; Milovanović, I.; HadnaCrossed D Signev, M. Characterization of byproducts originating from hemp oil processing. J. Agric. Food Chem. 2014, 62, 12346–12442. [Google Scholar] [CrossRef]
- AOAC Method 950.48; Protein (crude) in Nuts and Nut Products. Official Methods of Analysis. 17th ed. Association of Official Analytical Chemists: Arlington, VA, USA, 2003.
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An Overview of the Kjeldahl Method of Nitrogen Determination. Part I. Early History, Chemistry of the Procedure, and Titrimetric Finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Sunil, L.; Prakruthi, A.; Prasanth Kumar, P.K.; Gopala Krishna, A.G. Development of Health Foods from Oilseed Cakes. J. Food Process. Technol. 2016, 7, 631–640. [Google Scholar] [CrossRef]
- ISO 659:2009; Animal and Vegetable Fats and Oils—Determination of Oil Content. International Organization for Standardization: Geneva, Switzerland, 2020.
- AOAC Method 920.39; Fat (Crude) or Ether Extract in Animal Feed. Official Methods of Analysis. 16th ed. Association of Official Analytical Chemists: Arlington, VA, USA, 1998.
- Jukić, M.; Lukinac, J.; Čuljak, J.; Pavlović, M.; Šubarić, D.; Koceva Komlenić, D. Quality evaluation of biscuits produced from composite blends of pumpkin seed oil press cake and wheat flour. Int. J. Food Sci. Technol. 2019, 54, 602–609. [Google Scholar] [CrossRef]
- Sunil, L.; Appaiah, P.; Prasanth Kumar, P.K.; Gopala Krishna, A.G. Preparation of food supplements from oilseed cakes. J. Food Sci. Technol. 2015, 52, 2998–3005. [Google Scholar] [CrossRef]
- AOAC Method 923.03; Ash of Flour (Direct Method). Official Methods of Analysis. 17th ed. Association of Official Analytical Chemists: Arlington, VA, USA, 2003.
- AOAC Method 985.29; Total Dietary Fiber in Foods, Enzymatic-Gravimetric. Official Methods of Analysis. 17th ed. Association of Official Analytical Chemists: Arlington, VA, USA, 2003.
- Batariuc, A.; Ungureanu-Iuga, M.; Mironeasa, S. Effects of Dry Heat Treatment and Milling on Sorghum Chemical Composition, Functional and Molecular Characteristics. Appl. Sci. 2021, 11, 11881. [Google Scholar] [CrossRef]
- Mihalcea, A.; Amariei, S. Study on Contamination with Some Mycotoxins in Maize and Maize-Derived Foods. Appl. Sci. 2022, 12, 2579. [Google Scholar] [CrossRef]
- Petraru, A.; Amariei, S. Rapeseed—An Important Oleaginous Plant in the Oil Industry and the Resulting Meal a Valuable Source of Bioactive Compounds. Plants 2024, 13, 3085. [Google Scholar] [CrossRef]
- Bhise, S.; Kaur, A. The effect of extrusion conditions on the functional properties of defatted cake of sunflower-maize based expanded snacks. Int. J. Food Ferment. Technol. 2015, 5, 247. [Google Scholar] [CrossRef]
- Omowaye-Taiwo, O.A.; Fagbemi, T.N.; Ogunbusola, E.M.; Badejo, A.A. Effect of germination and fermentation on the proximate composition and functional properties of full-fat and defatted cucumeropsis mannii seed flours. J. Food Sci. Technol. 2015, 52, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sukmanov, V.; Zeng, J. Effect of ultrafine grinding on functional properties of soybean by-products. Ukr. Food J. 2019, 8, 687–698. [Google Scholar] [CrossRef]
- Rani, R.; Badwaik, L.S. Functional Properties of Oilseed Cakes and Defatted Meals of Mustard, Soybean and Flaxseed. Waste Biomass Valorization 2021, 12, 5639–5647. [Google Scholar] [CrossRef]
- Iyenagbe, D.O.; Malomo, S.A.; Idowu, A.O.; Badejo, A.A.; Fagbemi, T.N. Effects of thermal processing on the nutritional and functional properties of defatted conophor nut (Tetracarpidium conophorum) flour and protein isolates. Food Sci. Nutr. 2017, 5, 1170–1178. [Google Scholar] [CrossRef]
- Naczk, M.; Diosady, L.L.; Rubin, L.J. Functional Properties of Canola Meals Produced by a Two-phase Solvent Extraction System. J. Food Sci. 1985, 50, 1685–1688. [Google Scholar] [CrossRef]
- Marasinghe, S.S.K.; Yalegama, C.; Pathirana, D.T.H.; Marikkar, J.M.N. The Physical and Functional Properties of Partially Defatted Coconut Testa Flour. Int. J. Coconut Res. Dev. 2021, 37, 11–22. [Google Scholar]
- North, K.; North, K. Nutritional and functional characterization of defatted seed cake flour of two Sudanese groundnut (Arachis hypogaea) cultivars. Int. Food Res. J. 2012, 19, 629–637. [Google Scholar]
- Jung, H.; Sato, T. Comparison between the Color Properties of Whiteness Index and Yellowness Index on the CIELAB. Text. Color. Finish. 2013, 25, 241–246. [Google Scholar] [CrossRef]
- Chetrariu, A.; Florin Ursachi, V.; Dabija, A. Evaluation of the Fatty Acids and Amino Acids Profiles in Spent Grain From Brewing and Malt Whisky. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2022, 23, 167–177. [Google Scholar]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional characteristics assessment of sunflower seeds, oil and cake. Perspective of using sunflower oilcakes as a functional ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef] [PubMed]
- Chetrariu, A.; Dabija, A. Spent Grain from Malt Whisky: Assessment of the Phenolic Compounds. Molecules 2021, 26, 3236. [Google Scholar] [CrossRef]
- Esmaeilzadeh Kenari, R.; Mohsenzadeh, F.; Amiri, Z.R. Antioxidant activity and total phenolic compounds of dezful sesame cake extracts obtained by classical and ultrasound-assisted extraction methods. Food Sci. Nutr. 2014, 2, 426–435. [Google Scholar] [CrossRef]
- Khan, A.; Saini, C.S. Effect of roasting on physicochemical and functional properties of flaxseed flour. Cogent Eng. 2016, 3, 1145566. [Google Scholar] [CrossRef]
- Selvi, K.Ç.; Pinar, Y.; Yeşiloǧlu, E. Some Physical Properties of Linseed. Biosyst. Eng. 2006, 95, 607–612. [Google Scholar] [CrossRef]
- Sharma, V.; Das, L.; Pradhan, R.C.; Naik, S.N.; Bhatnagar, N.; Kureel, R.S. Physical properties of tung seed: An industrial oil yielding crop. Ind. Crops Prod. 2011, 33, 440–444. [Google Scholar] [CrossRef]
- Adubofuor, J.; Akyereko, Y.G.; Batsa, V.; Apeku, O.-J.D.; Amoah, I.; Diako, C. Nutrient Composition and Physical Properties of Two Orange Seed Varieties. Int. J. Food Sci. 2021, 2021, 6415620. [Google Scholar] [CrossRef]
- Sharma, H.P.; Sharma, S.; Nema, P. Physico-chemical and functional properties of flour prepared from native and roasted whole linseeds. J. Pharmacogn. Phytochem. 2020, 9, 1428–1433. [Google Scholar]
- Shaikh, R.P.; Vidyapeeth, M.K.; Ks, I.G.; Syed, I.; Corresponding, I.; Parbhani, I.; Ks, G.; Sj, S. Studies on physico-chemical and functional properties of flaxseed flour. J. Pharmacogn. Phytochem. 2020, 9, 2309–2312. [Google Scholar]
- Ganguly, S.; Panjagari, N.R.; Raman, R.K. Flaxseed (Linum usitatissimum). In Oilseeds: Health Attributes and Food Applications; Springer: Singapore, 2021; pp. 253–283. [Google Scholar]
- Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Costa, A.S.G.; Machado, S.; Alves, R.C.; Pardo, J.E.; Oliveira, M.B.P.P. Whole or defatted sesame seeds (Sesamum indicum L.)? The effect of cold pressing on oil and cake quality. Foods 2021, 10, 2108. [Google Scholar] [CrossRef] [PubMed]
- Cozea, A.; Ionescu, N.; Popescu, M.; Neagu, M.; Gruia, R. Comparative study concerning the composition of certain oil cakes with phytotherapeutical potential. Rev. Chim. 2016, 67, 422–425. [Google Scholar]
- Ghosh, S.; Khuntia, A.; Mitra, J. Thermodynamic and mathematical background of water activity and its significance from microbiological point of view for food products. In Trends & Prospects in Food Technology, Processing and Perservation; Today and Tomorrow’s Printers and Publishers: New Dehli, India, 2018; pp. 1–12. [Google Scholar]
- Madhusudhan, K.T.; Narendra, S. Effect of heat treatment on the functional properties of linseed meal. J. Agric. Food Chem. 1985, 33, 1222–1226. [Google Scholar] [CrossRef]
- Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J.; Smetana, P.; Kadlec, J.; Filip, V.; Kyselka, J.; Berčíková, M.; Zdráhal, Z.; et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods 2021, 10, 2766. [Google Scholar] [CrossRef]
- Mueller, K.; Eisner, P.; Yoshie-Stark, Y.; Nakada, R.; Kirchhoff, E. Functional properties and chemical composition of fractionated brown and yellow linseed meal (Linum usitatissimum L.). J. Food Eng. 2010, 98, 453–460. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, R.; Gill, B.S. Mineral and amino acid contents of different flaxseed cultivars in relation to its selected functional properties. J. Food Meas. Charact. 2017, 11, 500–511. [Google Scholar] [CrossRef]
- Dev, D.K.; Quensel, E. Preparation and Functional Properties of Linseed Protein Products Containing Differing Levels of Mucilage. J. Food Sci. 1988, 53, 1834–1837. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Jahangir, M.; Jabbar, S.; Deng, Z. A comprehensive review of flaxseed (Linum usitatissimum L.): Health-affecting compounds, mechanism of toxicity, detoxification, anticancer and potential risk. Crit. Rev. Food Sci. Nutr. 2023, 63, 11081–11104. [Google Scholar] [CrossRef]
- Yılmaz, E.; Hüriyet, Z.; Arifoğlu, N.; Emir, D.D. Functional Properties of the Capia Pepper Seed Defatted Press Cakes. Waste Biomass Valorization 2017, 8, 783–791. [Google Scholar] [CrossRef]
- Hasmadi, M.; Noorfarahzilah, M.; Noraidah, H.; Zainol, M.K.; Jahurul, M.H. Functional properties of composite flour: A review. Food Res. 2020, 4, 1820–1831. [Google Scholar] [CrossRef]
- Elsorady, M.E. Characterization and functional properties of proteins isolated from flaxseed cake and sesame cake. Croat. J. Food Sci. Technol. 2020, 12, 77–83. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D.; Shahidi, F. Functional properties and amino-acid composition of solvent-extracted flaxseed meals. Food Chem. 1994, 49, 45–51. [Google Scholar] [CrossRef]
- Coşkuner, Y.; Gökbudak, A. Dimensional specific physical properties of fan palm fruits, seeds and seed coats (Washingtonia robusta). Int. Agrophysics 2016, 30, 301–309. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, O.; Cai, S.; Zhao, L.; Zhao, L. Composition, functional properties, health benefits and applications of oilseed proteins: A systematic review. Food Res. Int. 2023, 171, 113061. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Mehmood, R.; Rehman, T.; Munir, H. Comparative Evaluation of Functional Properties of Some Commonly Used Cereal and Legume Flours and Their Blends. Int. J. Food Allied Sci. 2015, 1, 67. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D. Functional properties of raw and processed canola meal. LWT Food Sci. Technol. 2009, 42, 1119–1124. [Google Scholar] [CrossRef]
- Siano, F.; Moccia, S.; Picariello, G.; Russo, G.L.; Sorrentino, G.; Di Stasio, M.; La Cara, F.; Volpe, M.G. Comparative Study of Chemical, Biochemical Characteristic and ATR-FTIR Analysis of Seeds, Oil and Flour of the Edible Fedora Cultivar Hemp (Cannabis sativa L.). Molecules 2018, 24, 83. [Google Scholar] [CrossRef]
- Andronie, L.; Pop, I.D.; Sobolu, R.; Diaconeasa, Z.; Truţă, A.; Hegeduş, C.; Rotaru, A. Characterization of Flax and Hemp Using Spectrometric Methods. Appl. Sci. 2021, 11, 8341. [Google Scholar] [CrossRef]
- Irnawati; Riyanto, S.; Martono, S.; Rohman, A. Determination of sesame oil, rice bran oil and pumpkin seed oil in ternary mixtures using FTIR spectroscopy and multivariate calibrations. Food Res. 2019, 4, 135–142. [Google Scholar] [CrossRef]
- Multescu, M.; Marinas, I.C.; Susman, I.E.; Belc, N. Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods 2022, 11, 253. [Google Scholar] [CrossRef]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Mao, X.; Zheng, P.; Huang, Z.; Chen, D. Physicochemical Properties Analysis and Secretome of Aspergillus niger in Fermented Rapeseed Meal. PLoS ONE 2016, 11, e0153230. [Google Scholar] [CrossRef]
- Di Lena, G.; Del Pulgar, J.S.; Lucarini, M.; Durazzo, A.; Ondrejíčková, P.; Oancea, F.; Frincu, R.M.; Aguzzi, A.; Nicoli, S.F.; Casini, I.; et al. Valorization potentials of rapeseed meal in a biorefinery perspective: Focus on nutritional and bioactive components. Molecules 2021, 26, 6787. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sun, R.; Zeng, X.; Zhang, J.; Xing, R.; Sun, C.; Chen, Y. Rapid Classification and Quantification of Camellia (Camellia oleifera Abel.) Oil Blended with Rapeseed Oil Using FTIR-ATR Spectroscopy. Molecules 2020, 25, 2036. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.F.; Ting, W. An automated approach for analysis of Fourier Transform Infrared (FTIR) spectra of edible oils. Talanta 2012, 88, 537–543. [Google Scholar] [CrossRef]
- Hu, W.; Fitzgerald, M.; Topp, B.; Alam, M.; O’Hare, T.J. A review of biological functions, health benefits, and possible de novo biosynthetic pathway of palmitoleic acid in macadamia nuts. J. Funct. Foods 2019, 62, 103520. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef]
- Gonzalez-Becerra, K.; Barron-Cabrera, E.; Muñoz-Valle, J.F.; Torres-Castillo, N.; Rivera-Valdes, J.J.; Rodriguez-Echevarria, R.; Martinez-Lopez, E. A Balanced Dietary Ratio of n-6:n-3 Polyunsaturated Fatty Acids Exerts an Effect on Total Fatty Acid Profile in RBCs and Inflammatory Markers in Subjects with Obesity. Healthcare 2023, 11, 2333. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Skrede, A.; Mydland, L.T.; Øverland, M. Fractionation of rapeseed meal by milling, sieving and air classification—Effect on crude protein, amino acids and fiber content and digestibility. Anim. Feed. Sci. Technol. 2017, 230, 143–153. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Kim, E.-H. Therapeutic Effects of Amino Acids in Liver Diseases: Current Studies and Future Perspectives. J. Cancer Prev. 2019, 24, 72–78. [Google Scholar] [CrossRef]
- Adu, O.B.; Ogundeko, T.O.; Ogunrinola, O.O.; Saibu, G.M.; Elemo, B.O. The effect of thermal processing on protein quality and free amino acid profile of Terminalia catappa (Indian Almond) seed. J. Food Sci. Technol. 2015, 52, 4637–4641. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.-S.; Bekhit, A.E.-D.A. Utilization of Oilseed Cakes for Human Nutrition and Health Benefits. In Agricultural Biomass Based Potential Materials; Hakeem, K.R., Jawaid, M., Alothman, O.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 191–229. ISBN 9783319138473. [Google Scholar]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Njuguna, D.G.; Wanyoko, J.K.; Kinyanjui, T.; Wachira, F.N. Mineral Elements in the Kenyan Tea Seed Oil Cake. Int. J. Res. Chem. Environ. 2013, 3, 253–261. [Google Scholar]
- Teh, S.S.; El-Din Bekhit, A.; Birch, J. Antioxidative polyphenols from defatted oilseed cakes: Effect of solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef]
- Akl, E.M.; Mohamed, S.S.; Hashem, A.I.; Taha, F.S. Biological activities of phenolic compounds extracted from flaxseed meal. Bull. Natl. Res. Cent. 2020, 44, 27. [Google Scholar] [CrossRef]
- Barthet, V.J.; Klensporf-Pawlik, D.; Przybylski, R. Antioxidant activity of flaxseed meal components. Can. J. Plant Sci. 2014, 94, 593–602. [Google Scholar] [CrossRef]
- Huang, X.; Wang, N.; Ma, Y.; Liu, X.; Guo, H.; Song, L.; Zhao, Q.; Hai, D.; Cheng, Y.; Bai, G.; et al. Flaxseed polyphenols: Effects of varieties on its composition and antioxidant capacity. Food Chem. X 2024, 23, 101597. [Google Scholar] [CrossRef]


| Run | Independent Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Coded | Actual | |||||||
| A | B | C | D | Solvent | Time, min | Amplitude, % | Temperature, °C | |
| 1 | −1 | −1 | 0 | { 1 0 } | water | 10 | 70 | 30 |
| 2 | 1 | −1 | 0 | { 1 0 } | water | 10 | 70 | 50 |
| 3 | −1 | 1 | 0 | { 1 0 } | water | 20 | 70 | 30 |
| 4 | 1 | 1 | 0 | { 1 0 } | water | 20 | 70 | 50 |
| 5 | −1 | 0 | −1 | { 1 0 } | water | 15 | 40 | 30 |
| 6 | 1 | 0 | −1 | { 1 0 } | water | 15 | 40 | 50 |
| 7 | −1 | 0 | 1 | { 1 0 } | water | 15 | 100 | 30 |
| 8 | 1 | 0 | 1 | { 1 0 } | water | 15 | 100 | 50 |
| 9 | 0 | −1 | −1 | { 1 0 } | water | 10 | 40 | 40 |
| 10 | 0 | 1 | −1 | { 1 0 } | water | 20 | 40 | 40 |
| 11 | 0 | −1 | 1 | { 1 0 } | water | 10 | 100 | 40 |
| 12 | 0 | 1 | 1 | { 1 0 } | water | 20 | 100 | 40 |
| 13 | 0 | 0 | 0 | { 1 0 } | water | 15 | 70 | 40 |
| 14 | 0 | 0 | 0 | { 1 0 } | water | 15 | 70 | 40 |
| 15 | 0 | 0 | 0 | { 1 0 } | water | 15 | 70 | 40 |
| 16 | −1 | −1 | 0 | { 0 1 } | ethanol | 10 | 70 | 30 |
| 17 | 1 | −1 | 0 | { 0 1 } | ethanol | 10 | 70 | 50 |
| 18 | −1 | 1 | 0 | { 0 1 } | ethanol | 20 | 70 | 30 |
| 19 | 1 | 1 | 0 | { 0 1 } | ethanol | 20 | 70 | 50 |
| 20 | −1 | 0 | −1 | { 0 1 } | ethanol | 15 | 40 | 30 |
| 21 | 1 | 0 | −1 | { 0 1 } | ethanol | 15 | 40 | 50 |
| 22 | −1 | 0 | 1 | { 0 1 } | ethanol | 15 | 100 | 30 |
| 23 | 1 | 0 | 1 | { 0 1 } | ethanol | 15 | 100 | 50 |
| 24 | 0 | −1 | −1 | { 0 1 } | ethanol | 10 | 40 | 40 |
| 25 | 0 | 1 | −1 | { 0 1 } | ethanol | 20 | 40 | 40 |
| 26 | 0 | −1 | 1 | { 0 1 } | ethanol | 10 | 100 | 40 |
| 27 | 0 | 1 | 1 | { 0 1 } | ethanol | 20 | 100 | 40 |
| 28 | 0 | 0 | 0 | { 0 1 } | ethanol | 15 | 70 | 40 |
| 29 | 0 | 0 | 0 | { 0 1 } | ethanol | 15 | 70 | 40 |
| 30 | 0 | 0 | 0 | { 0 1 } | ethanol | 15 | 70 | 40 |
| 31 | −1 | −1 | 0 | { −1 −1 } | methanol | 10 | 70 | 30 |
| 32 | 1 | −1 | 0 | { −1 −1 } | methanol | 10 | 70 | 50 |
| 33 | −1 | 1 | 0 | { −1 −1 } | methanol | 20 | 70 | 30 |
| 34 | 1 | 1 | 0 | { −1 −1 } | methanol | 20 | 70 | 50 |
| 35 | −1 | 0 | −1 | { −1 −1 } | methanol | 15 | 40 | 30 |
| 36 | 1 | 0 | −1 | { −1 −1 } | methanol | 15 | 40 | 50 |
| 37 | −1 | 0 | 1 | { −1 −1 } | methanol | 15 | 100 | 30 |
| 38 | 1 | 0 | 1 | { −1 −1 } | methanol | 15 | 100 | 50 |
| 39 | 0 | −1 | −1 | { −1 −1 } | methanol | 10 | 40 | 40 |
| 40 | 0 | 1 | −1 | { −1 −1 } | methanol | 20 | 40 | 40 |
| 41 | 0 | −1 | 1 | { −1 −1 } | methanol | 10 | 100 | 40 |
| 42 | 0 | 1 | 1 | { −1 −1 } | methanol | 20 | 100 | 40 |
| 43 | 0 | 0 | 0 | { −1 −1 } | methanol | 15 | 70 | 40 |
| 44 | 0 | 0 | 0 | { −1 −1 } | methanol | 15 | 70 | 40 |
| 45 | 0 | 0 | 0 | { −1 −1 } | methanol | 15 | 70 | 40 |
| Parameters | Interval | Mean Value |
|---|---|---|
| Size and shape | ||
| W, mm | 1.95–2.58 | 2.24 ± 0.20 |
| L, mm | 4.15–5.40 | 4.78 ± 0.21 |
| T, mm | 0.70–1.28 | 1.05 ± 0.08 |
| De, mm | 1.81–2.47 | 2.27 ± 0.07 |
| Ψ, - | 0.43–0.53 | 0.48 ± 0.02 |
| V, mm3 | 2.53–7.35 | 5.56 ± 0.59 |
| S, mm2 | 10.23–19.06 | 16.15 ± 1.04 |
| Ap, mm2 | 6.45–10.59 | 8.76 ± 0.62 |
| Gravimetric properties | ||
| φ, % | 28.34–32.36 | 38.97 ± 3.75 |
| pb, kg/m3 | 682.88–698.39 | 605.19 ± 5.10 |
| pt, kg/m3 | 792.22–1320.91 | 994.94 ± 62.40 |
| M, g | 0.0040–0.0090 | 0.0070 ± 0.001 |
| Variables | W | L | T | De | Ψ | V | S | Ap | M |
|---|---|---|---|---|---|---|---|---|---|
| W | 1 | ||||||||
| L | 0.458 | 1 | |||||||
| T | 0.075 | −0.061 | 1 | ||||||
| De | 0.669 | 0.595 | 0.678 | 1 | |||||
| Ψ | 0.057 | −0.665 | 0.706 | 0.203 | 1 | ||||
| V | 0.609 | 0.306 | 0.825 | 0.944 | 0.502 | 1 | |||
| S | 0.670 | 0.598 | 0.674 | 0.999 | 0.198 | 0.945 | 1 | ||
| Ap | 0.839 | 0.867 | 0.002 | 0.736 | −0.376 | 0.526 | 0.739 | 1 | |
| M | 0.103 | 0.127 | 0.137 | 0.197 | 0.021 | 0.178 | 0.197 | 0.136 | 1 |
| Parameter | Seeds | Oilcake |
|---|---|---|
| Total dietary fibers, % | 26.10 ± 0.04 a | 24.90 ± 0.69 b |
| Proteins, % | 19.03 ± 0.01 b | 34.67 ± 0.17 a |
| Ash, % | 3.80 ± 0.06 b | 4.79 ± 0.04 a |
| Lipids, % | 27.74 ± 0.48 a | 11.61 ± 0.40 b |
| Moisture, % | 5.70 ± 0.02 b | 8.54 ± 0.11 a |
| Remaining carbohydrates, % | 36.66 ± 0.52 a | 15.47 ± 0.80 b |
| Energy value, kcal/100 g | 524.57 ± 2.21 a | 354.87 ± 2.69 b |
| Incidence of Mycotoxins | ||||
|---|---|---|---|---|
| Properties | Limit of Detection (LOD), µg/Kg | Limit of Quantification (LOQ), µg/Kg | Results, µg/Kg | Maximum Limit 2006/576/EC, µg/Kg |
| Zearalenone | 10 | 15 | 44.01 ± 5.08 | 2000 |
| Ochratoxin A | 0.5 | 1.5 | 22.19 ± 3.60 | 50 |
| Aflatoxin B1 | 0.3 | 0.7 | <LOQ | 10 |
| Deoxynivalenol | 0.011 | 0.042 | <LOD | 0.9 |
| Functional characteristics | ||||
| Oil-holding capacity (g/g) | 1.19 ± 0.04 | |||
| Water-holding capacity (g/g) | 4.14 ± 0.18 | |||
| Emulsion capacity (%) | 26.90 ± 1.68 | |||
| Emulsion stability (%) | 100.00 ± 0.00 | |||
| Foaming capacity (%) | 8.41 ± 0.56 | |||
| Bulk density (g/mL) | 0.6262 ± 0.001 | |||
| Least gelatinization concentration (%) | 4.00 ± 0.00 | |||
| Wettability | Good: upon contact with water, the powder underwent a gradual wetting process. Some of the powder was dispersed in the water and the rest was deposited at the bottom of the Berzelius beaker. The powder settled to the bottom of the beaker after a short interval. Following a duration of half an hour, the powder particles fully settled to the bottom of the Berzelius beaker. Through the application of vortexing, the sample was dispersed throughout the liquid. | |||
| Color parameters | ||||
| L* | 52.59 ± 0.02 | |||
| a* | 5.05 ± 0.03 | |||
| b* | 12.72 ± 0.02 | |||
| Fatty Acids | Seed, % | Oilcake, % | |
|---|---|---|---|
| Caprylic acid (C8:0) | SFA | 0.32 ± 0.01 b | 1.01 ± 0.04 a |
| Capric acid (C10:0) | SFA | - | 0.37 ± 0.02 a |
| Lauric acid (C12:0) | SFA | 0.35 ± 0.01 b | 0.40 ± 0.01 a |
| Tridecanoic acid (C13:0) | SFA | - | 0.23 ± 0.00 a |
| Myristic acid (C14:0) | SFA | 0.46 ± 0.02 b | 0.61 ± 0.01 a |
| Myristoleic acid (C14:1, n-5) | MUFA | 0.19 ± 0.01 b | 0.31 ± 0.01 a |
| Pentadecanoic acid (C15:0) | SFA | 11.86 ± 0.04 a | 4.98 ± 0.14 b |
| cis-10-pentadecanoic acid (C15:1, n-5) | MUFA | - | 4.27 ± 0.04 a |
| Palmitic acid (C16:0) | SFA | 6.79 ± 0.03 a | 0.92 ± 0.03 b |
| Palmitoleic acid (C16:1, n-7) | MUFA | 0.19 ± 0.01 b | 0.68 ± 0.01 a |
| Heptadecanoic acid (C17:0) | SFA | 7.80 ± 0.14 a | 1.31 ± 0.07 b |
| cis-10 heptadecanoic acid | MUFA | 4.34 ± 0.10 b | 8.06 ± 0.16 a |
| Stearic acid (C18:0) | SFA | 1.65 ± 0.08 a | 1.16 ± 0.01 b |
| Oleici acid+ elaidic acid (C18:1, cis + trans, n-9) | MUFA | 20.64 ± 0.07 b | 34.29 ± 0.05 a |
| Linoleic acid + Linolelaidic acid (C18:2, cis + trans, n-6) | PUFA | 18.03 ± 0.19 b | 22.91 ± 0.16 a |
| γ-Linolenic acid (C18:3, n-6) | PUFA | 1.05 ± 0.06 b | 1.43 ± 0.01 a |
| α-Linolenic acid (C18:3, n-3) | PUFA | 2.46 ± 0.02 a | 1.65 ± 0.02 b |
| Arachidonic acid (C20:0) | SFA | 8.50 ± 0.06 a | - |
| Gondoic acid (C20:1, n-9) | MUFA | 1.90 ± 0.01 a | 1.08 ± 0.03 b |
| cis-11,14-eicosadienoic acid + cis-8,11,14-eicosatrienoic acid (C20:2, n-6) | PUFA | 0.43 ± 0.01 b | 0.87 ± 0.01 a |
| cis-11,14,17-eicosatrienoic acid (C20:3, n-3) | PUFA | 0.25 ± 0.01 b | 0.34 ± 0.0 a |
| Arachidonic acid (C20:4, n-6) | PUFA | 0.28 ± 0.01 a | 0.33 ± 0.01 a |
| cis-5,8,11,14,17-eicosapentenoic acid (C20:5, n-3) | PUFA | 0.15 ± 0.00 b | 0.35 ± 0.01 a |
| Heneicosanoic acid (C21:0) | SFA | 0.52 ± 0.00 b | 0.69 ± 0.01 a |
| Eicosadienoic acid (C22:0) | SFA | 0.15 ± 0.00 b | 0.23 ± 0.01 a |
| Erucic acid (C22:1, n-9) | MUFA | 4.89 ± 0.01 b | 5.98 ± 0.04 a |
| cis-4,7,10,13, 16, 19-docosahexanoic acid (C22:2, n-6) | PUFA | 0.18 ± 0.00 a | 0.30 ± 0.30 a |
| cis-4,7,10,13,16,19-docosa-hexanoic+ nervonic acid (C22:6, n-3 + C24:1, n-9) | PUFA | 2.45 ± 0.04 b | 5.03 ± 0.03 a |
| Tricosanoic acid (C23:0) | SFA | 4.05 ± 0.03 a | - |
| Lignoceric acid (C24:0) | SFA | 0.14 ± 0.00 a | 0.21 ± 0.00 a |
| C18:2 w-6/C18:3 w-3 | 7.33 ± 0.17 b | 13.90 ± 0.09 a | |
| C18:1 w-9/C18:2 w-6 | 1.14 ± 0.02 b | 1.50 ± 0.01 a | |
| ΣSFAs (%) | 42.58 ± 0.00 a | 12.11 ± 0.16 b | |
| ΣUFAs (%) | 57.40 ± 0.26 b | 87.90 ± 0.25 a | |
| ΣMUFAs (%) | 32.15 ± 0.02 b | 54.68 ± 0.09 a | |
| ΣPUFAs (%) | 25.27 ± 0.24 b | 33.21 ± 0.16 a | |
| ΣSFAs/ΣUFAs | 0.74 ± 0.003 a | 0.14 ± 0.002 b | |
| ΣPUFAs/ΣMUFAs | 0.74 ± 0.01 a | 0.61 ± 0.00 b |
| Amino Acids | Seed | Oilcake | ||
|---|---|---|---|---|
| nmol/g | % | nmol/g | % | |
| Glycine | 1264.60 ± 14.68 a | 6.84 | 543.70 ± 0.32 b | 1.56 |
| Alanine | 1049.26 ± 2.66 a | 5.68 | 998.97 ± 17.55 b | 2.87 |
| α-aminobutiric acid | - | - | 563.09 ± 15.72 a | 1.62 |
| Valine * | 635.45 ± 8.47 b | 3.44 | 6140.77 ± 0.07 a | 17.64 |
| Leucine * | 494.13 ± 2.54 a | 2.67 | 548.76 ± 49.43 a | 1.58 |
| Isoleucine * | 480.28 ± 10.95 b | 2.60 | 523.94 ± 2.25 a | 1.50 |
| Threonine * | - | - | 673.63 ± 3.04 a | 1.93 |
| Serine | 657.92 ± 3.01 a | 3.56 | 674.10 ± 35.38 a | 1.94 |
| Proline | - | - | 571.57 ± 4.24 a | 1.64 |
| Asparagine | 675.17 ± 23.83 b | 3.65 | 1206.59 ± 16.49 a | 3.47 |
| Thioproline | 489.28 ± 0.80 b | 2.65 | 686.43 ± 5.65 a | 1.97 |
| Aspartic acid | 672.51 ± 22.95 b | 3.64 | 3498.83 ± 258.64 a | 10.05 |
| Methionine * | 533.01 ± 10.39 b | 2.88 | 573.86 ± 11.70 a | 1.65 |
| 3/4-Hidroxiproline | 699.44 ± 9.26 a | 3.78 | 692.77 ± 30.29 a | 1.99 |
| Phenylalanine * | 436.78 ± 23.96 a | 2.36 | - | - |
| Glutamic acid | 988.98 ± 20.40 b | 5.35 | 2762.27 ± 105.61 a | 7.93 |
| Glutamine | 4415.02 ± 72.75 b | 23.89 | 8484.73 ± 313.81 a | 24.37 |
| Ornithine | 543.93 ± 0.57 a | 2.94 | 566.32 ± 26.46 a | 1.63 |
| Glycylproline | 447.08 ± 3.25 a | 2.42 | 460.41 ± 0.52 a | 1.32 |
| Hidroxylysine | 518.35 ± 0.12 a | 2.80 | 553.12 ± 42.19 a | 1.59 |
| Proline-Hydroxyproline | - | - | 453.36 ± 10.67 a | 1.30 |
| Histidine * | 719.46 ± 12.55 a | 3.89 | 786.66 ± 0.16 a | 2.26 |
| Lysine * | 475.92 ± 7.50 b | 2.57 | 503.29 ± 0.69 a | 1.45 |
| Tyrosine | 434.65 ± 0.34 a | 2.35 | 446.57 ± 14.88 a | 1.28 |
| Tryptophan * | 530.36 ± 3.60 a | 2.87 | 532.56 ± 1.85 a | 1.53 |
| Cystathionine | 656.81 ± 0.18 a | 3.55 | 663.97 ± 9.10 a | 1.91 |
| Cystine | 665.76 ± 14.77 a | 3.60 | 703.88 ± 0.12 a | 2.02 |
| Total AA | 18,484.16 | 34,814.15 | ||
| Essential AA % | 76.71 | 70.46 | ||
| Non essential AA % | 23.29 | 29.54 | ||
| Mineral Elements | Seed | Oilcake |
|---|---|---|
| Beryllium (Be), mg/Kg | 20.50 ± 0.50 b | 38.90 ± 1.90 a |
| Lithium (Li), mg/Kg | 2.20 ± 0.10 a | 2.10 ± 0.10 a |
| Molybdenum (Mo), mg/Kg | 0.30 ± 0.00 a | 0.50 ± 0.02 a |
| Magnesium (Mg), mg/Kg | 4694.90 ± 290.50 a | - |
| Calcium (Ca), mg/Kg | 974.40 ± 90.00 b | 2006.20 ± 112.00 a |
| Titan (Ti), mg/Kg | 12.00 ± 0.70 b | 23.50 ± 1.20 a |
| Chromium (Cr), mg/Kg | 71.50 ± 6.10 b | 147.00 ± 4.50 a |
| Manganese (Mn), mg/Kg | 63.80 ± 3.30 b | 173.40 ± 2.20 a |
| Cesium (Ce), mg/Kg | 359.30 ± 27.00 b | 9025.50 ± 763.00 a |
| Iron (Fe-II), mg/Kg | 4.50 ± 0.30 b | 7.60 ± 0.20 a |
| Iron (Fe-III), mg/Kg | 2.40 ± 0.59 a | 2.10 ± 0.10 a |
| Cobalt (Co), mg/Kg | 6.10 ± 0.10 a | 2.60 ± 0.10 b |
| Nickel (Ni), mg/Kg | 18.30 ± 1.70 b | 26.50 ± 1.10 a |
| Copper (Cu), mg/Kg | 50.40 ± 3.30 b | 82.60 ± 2.90 a |
| Zinc (Zn), mg/Kg | 74.70 ± 4.70 b | 107.60 ± 4.10 a |
| Selenium (Se), mg/Kg | 596.40 ± 13.10 b | 1319.60 ± 33.40 a |
| Thallium (Tl), mg/Kg | 1111.9 ± 65.00 a | 839.40 ± 68.10 a |
| Total, mg/Kg | 8063.60 | 13,805.10 |
| Parameters | Run | TPC, mg GAE/g | AA_DPPH, % | |
|---|---|---|---|---|
| Temperature (°C) | 30 | 1 | 11.55 | 50.07 |
| 7 | 10.43 | 36.70 | ||
| 35 | 25.00 | 42.00 | ||
| 40 | 9 | 16.57 | 57.62 | |
| 11 | 16.00 | 50.56 | ||
| 12 | 11.09 | 44.96 | ||
| 40 | 31.94 | 44.87 | ||
| 44 | 15.64 | 44.33 | ||
| 50 | 17 | 16.04 | 47.16 | |
| 23 | 8.65 | 44.96 | ||
| 38 | 17.00 | 71.07 | ||
| Time (min) | 10 | 24 | 13.27 | 33.59 |
| 39 | 22.00 | 44.32 | ||
| 41 | 24.49 | 70.71 | ||
| 15 | 13 | 11.13 | 44.04 | |
| 14 | 16.09 | 36.35 | ||
| 21 | 13.15 | 37.89 | ||
| 29 | 7.56 | 27.00 | ||
| 43 | 12.59 | 44.25 | ||
| 30 | 18 | 8.00 | 51.01 | |
| 25 | 14.00 | 46.97 | ||
| 42 | 24.48 | 73.33 | ||
| Amplitude (%) | 40 | 10 | 22.00 | 53.92 |
| 20 | 11.43 | 39.31 | ||
| 36 | 22.62 | 40.71 | ||
| 70 | 2 | 16.99 | 57.54 | |
| 3 | 14.00 | 57.62 | ||
| 15 | 11.13 | 39.31 | ||
| 16 | 6.90 | 43.15 | ||
| 31 | 15.22 | 55.00 | ||
| 34 | 22.60 | 65.60 | ||
| 100 | 8 | 13.20 | 39.00 | |
| 22 | 6.06 | 40.31 | ||
| 37 | 26.00 | 62.61 | ||
| Solvent | Water | 4 | 15.53 | 60.03 |
| 5 | 13.82 | 47.37 | ||
| 6 | 24.88 | 52.63 | ||
| Ethanol | 19 | 11.00 | 57.19 | |
| 26 | 13.16 | 30.47 | ||
| 27 | 3.14 | 54.75 | ||
| 28 | 9.23 | 30.00 | ||
| 30 | 12.67 | 30.00 | ||
| Methanol | 32 | 11.00 | 60.61 | |
| 33 | 38.75 | 71.66 | ||
| 45 | 19.59 | 44.00 | ||
| Variables | TPC | DPPH |
|---|---|---|
| R2 | 0.8823 | 0.9433 |
| Adjusted R2 | 0.8082 | 0.9076 |
| F value | 11.91 | 26.43 |
| p value | <0.0001 | <0.0001 |
| Lack of Fit | 0.5130 | 0.1361 |
| Constant | +12.85 | +37.70 |
| A | +0.23 | +1.57 * |
| B | +1.39 * | +3.38 *** |
| C | −2.37 *** | +3.26 *** |
| D1 | −0.76 *** | +0.15 *** |
| D2 | −5.44 *** | −7.45 *** |
| A·B | −1.83 * | −1.21 |
| A·C | −1.17 | +1.07 |
| A·D1 | +2.37 *** | +0.6138 |
| A·D2 | +1.83 *** | +0.1117 |
| B·C | −2.59 ** | +0.9228 |
| B·D1 | −1.20 *** | −3.29 ** |
| B·D2 | −3.04 *** | +3.56 ** |
| C·D1 | −0.9446 | −8.30 *** |
| C·D2 | −0.2308 | −1.67 *** |
| A2 | +0.5628 | +7.20 *** |
| B2 | +2.22 * | +11.49 *** |
| C2 | +2.61 ** | +1.32 |
| Phenolic Acids | Results, mg/Kg |
|---|---|
| 4-Hydroxybenzoic acid | 17.22 ± 0.68 |
| Vanillic acid | 2599.00 ± 149.18 |
| Caffeic acid | 62.51 ± 1.37 |
| Chlorogenic acid | 2415.81 ± 235.41 |
| p-cumaric acid | 14.71 ± 1.54 |
| Rosmarinic acid | 61.14 ± 5.46 |
| Myricetin | 968.39 ± 32.95 |
| Luteolin | 24.68 ± 2.21 |
| Quercetin | 335.02 ± 8.98 |
| Kaempferol | 16.49 ± 0.54 |
| Total | 6514.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petraru, A.; Amariei, S.; Senila, L. Flaxseed Oilcake: An Ingredient with High Nutritional Value in the Realization of Innovative Food Products. Foods 2025, 14, 1087. https://doi.org/10.3390/foods14071087
Petraru A, Amariei S, Senila L. Flaxseed Oilcake: An Ingredient with High Nutritional Value in the Realization of Innovative Food Products. Foods. 2025; 14(7):1087. https://doi.org/10.3390/foods14071087
Chicago/Turabian StylePetraru, Ancuța, Sonia Amariei, and Lăcrimioara Senila. 2025. "Flaxseed Oilcake: An Ingredient with High Nutritional Value in the Realization of Innovative Food Products" Foods 14, no. 7: 1087. https://doi.org/10.3390/foods14071087
APA StylePetraru, A., Amariei, S., & Senila, L. (2025). Flaxseed Oilcake: An Ingredient with High Nutritional Value in the Realization of Innovative Food Products. Foods, 14(7), 1087. https://doi.org/10.3390/foods14071087







