Brazilian Organic Honeydew Reduces In Vitro and In Vivo Periodontal Disease-Related Subgingival Biofilm †
Abstract
1. Introduction
2. Materials and Methods
2.1. Organic Honey Georeferencing, Collection, and Extraction
2.2. Polyphenol Honey Extraction
2.3. Microbial Susceptibility
2.4. Evaluation of Antibiofilm Activity
2.4.1. In Vitro Model of Subgingival Biofilm Multispecies
2.4.2. OHD Treatment on Biofilm
2.4.3. Metabolic Biofilm Activity and DNA–DNA Hybridization (Checkerboard Assay)
2.5. Evaluation of OHD Potential in Periodontal Disease Induced by Ligature and P. gingivalis in Mice
2.5.1. Animals
2.5.2. Periodontal Disease Inducted by Ligature Model Plus P. gingivalis W83
2.5.3. Organic Honeydew (OHD) Treatment
2.5.4. Bone Loss Measure
2.5.5. Evaluation of the Tooth Caries Presence
2.6. Chemical Profile by LC-ESI-IT-MS/MS
2.7. Statistical Analysis
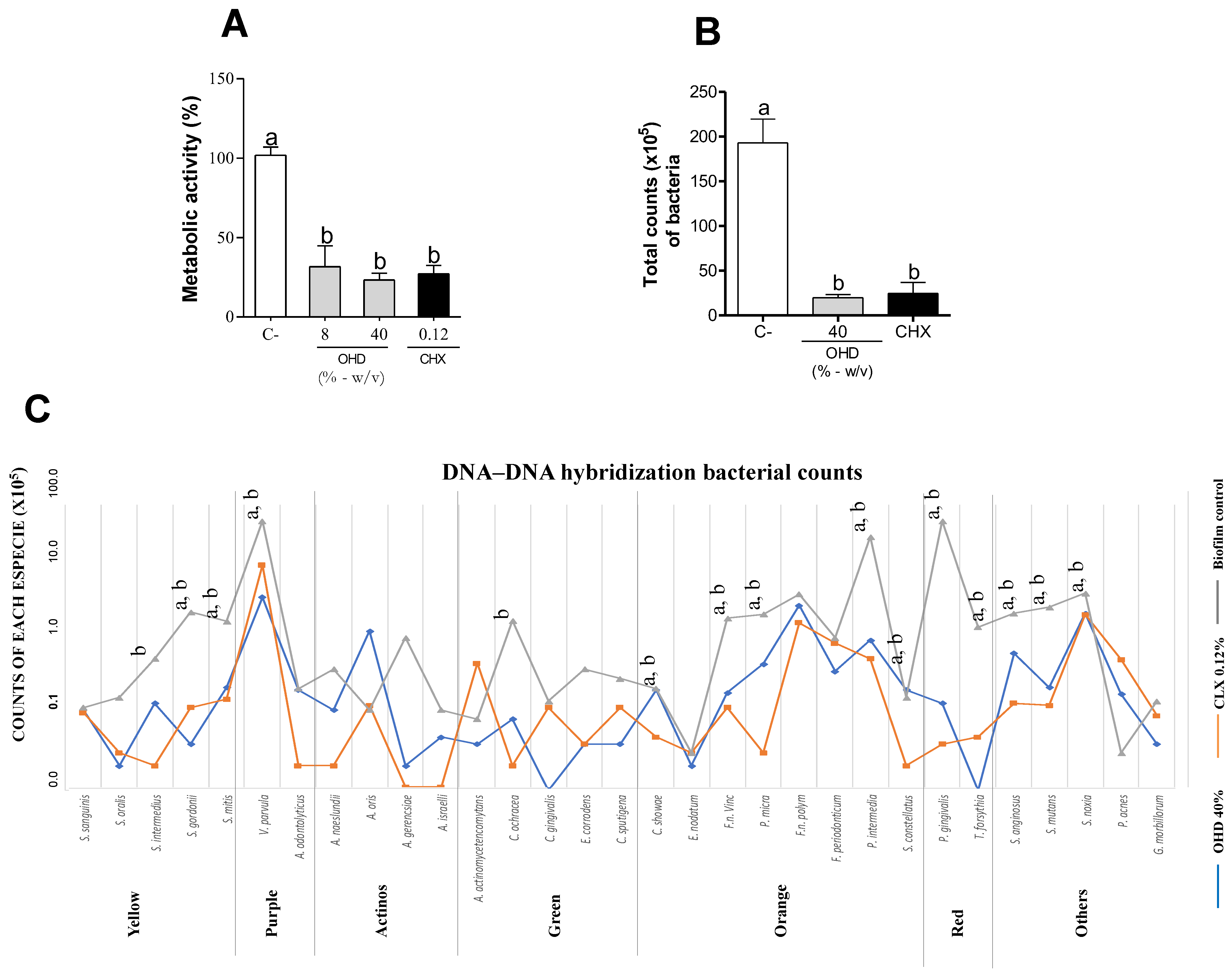
3. Results
3.1. In Vitro Assays
3.2. In Vivo Assays
3.3. Chemical Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Wang, F.; Liu, W.; Geng, Y.; Shi, Y.; Tian, Y.; Zhang, B.; Luo, Y.; Sun, X. New Drug Discovery and Development from Natural Products: Advances and Strategies. Pharmacol. Ther. 2024, 264, 108752. [Google Scholar] [CrossRef] [PubMed]
- Luca, L.; Pauliuc, D.; Oroian, M. Honey Microbiota, Methods for Determining the Microbiological Composition and the Antimicrobial Effect of Honey—A Review. Food Chem. X 2024, 23, 101524. [Google Scholar] [CrossRef]
- Palma-Morales, M.; Huertas, J.R.; Rodríguez-Pérez, C. A Comprehensive Review of the Effect of Honey on Human Health. Nutrients 2023, 15, 3056. [Google Scholar] [CrossRef]
- Kassym, L.; Kussainova, A.; Semenova, Y.; McLoone, P. Antimicrobial Effect of Honey Phenolic Compounds against E. Coli—An In Vitro Study. Pharmaceuticals 2024, 17, 560. [Google Scholar] [CrossRef]
- Onyango, L.A.; Liang, J. Manuka Honey as a Non-Antibiotic Alternative against Staphylococcus Spp. and Their Small Colony Variant (SCVs) Phenotypes. Front. Cell. Infect. Microbiol. 2024, 14, 1380289. [Google Scholar] [CrossRef] [PubMed]
- Grabek-Lejko, D.; Worek, M. Honeydew Honey as a Reservoir of Bacteria with Antibacterial and Probiotic Properties. Antibiotics 2024, 13, 855. [Google Scholar] [CrossRef]
- Mara, A.; Mainente, F.; Soursou, V.; Picó, Y.; Perales, I.; Ghorab, A.; Sanna, G.; Borrás-Linares, I.; Zoccatelli, G.; Ciulu, M. New Insights on Quality, Safety, Nutritional, and Nutraceutical Properties of Honeydew Honeys from Italy. Molecules 2025, 30, 410. [Google Scholar] [CrossRef]
- European Community COUNCIL DIRECTIVE 2001/110/EC of 20 December 2001 Relating to Honey. Available online: https://eur-lex.europa.eu/eli/dir/2001/110/oj/eng (accessed on 5 December 2024).
- Bergamo, G.; Seraglio, S.K.T.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Physicochemical Characteristics of Bracatinga Honeydew Honey and Blossom Honey Produced in the State of Santa Catarina: An Approach to Honey Differentiation. Food Res. Int. 2019, 116, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Sanna, G.; Mara, A.; Borrás-Linares, I.; Mainente, F.; Picó, Y.; Zoccatelli, G.; Lozano-Sánchez, J.; Ciulu, M. Mass Spectrometry Characterization of Honeydew Honey: A Critical Review. Foods 2024, 13, 2229. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Majkut, M.; Kwiecińska-Piróg, J.; Wszelaczyńska, E.; Pobereżny, J.; Gospodarek-Komkowska, E.; Wojtacki, K.; Barczak, T. Antimicrobial Activity of Heat-Treated Polish Honeys. Food Chem. 2020, 343, 128561. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.E.A.; Kabbashi, A.S.; Koko, W.S.; Ansari, M.J.; Adgaba, N.; Al-Ghamdi, A. In Vitro Activity of Some Natural Honeys against Entamoeba Histolytica and Giardia Lamblia Trophozoites. Saudi J. Biol. Sci. 2019, 26, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Deighton, M.; Livanos, G.; Pang, E.C.K.; Mantri, N. Agastache Honey Has Superior Antifungal Activity in Comparison with Important Commercial Honeys. Sci. Rep. 2019, 9, 18197. [Google Scholar] [CrossRef]
- Romário-Silva, D.; Franchin, M.; Alencar, S.M.; Bueno-Silva, B.; de Cássia Orlandi Sardi, J.; da Silva, A.C.B.; Cruz-Vieira, F.; da Silva, P.V.; Rosalen, P.L. Antimicrobial and Antibiofilm Activities of Brazilian Organic Honey against Oral Microorganisms. Braz. J. Microbiol. 2024, 55, 2285–2292. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An Overview of Physicochemical Characteristics and Health-Promoting Properties of Honeydew Honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Miłek, M.; Dżugan, M. The Comparison of the Antioxidant, Antibacterial and Antiviral Potential of Polish Fir Honeydew and Manuka Honeys. Sci. Rep. 2024, 14, 31170. [Google Scholar] [CrossRef]
- Tomczyk, M.; Bocian, A.; Sidor, E.; Miłek, M.; Zaguła, G.; Dżugan, M. The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity. Molecules 2022, 27, 720. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Carmen Seijo, M. Nutritional Value and Antioxidant Activity of Honeys Produced in a European Atlantic Area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Seraglio, S.K.T.; Bergamo, G.; Rocha, G.d.O.; Valese, A.C.; Daguer, H.; Miotto, M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Physicochemical Properties and Biological Activities of Bracatinga Honeydew Honey from Different Geographical Locations. J. Food Sci. Technol. 2021, 58, 3417–3429. [Google Scholar] [CrossRef]
- Kowalski, S. Changes of Antioxidant Activity and Formation of 5-Hydroxymethylfurfural in Honey during Thermal and Microwave Processing. Food Chem. 2013, 141, 1378–1382. [Google Scholar] [CrossRef]
- Vela, L.; De Lorenzo, C.; Pérez, R.A. Antioxidant Capacity of Spanish Honeys and Its Correlation with Polyphenol Content and Other Physicochemical Properties. J. Sci. Food Agric. 2007, 87, 1069–1075. [Google Scholar] [CrossRef]
- Majtan, J.; Majtanova, L.; Bohova, J.; Majtan, V. Honeydew Honey as a Potent Antibacterial Agent in Eradication of Multi-Drug Resistant Stenotrophomonas Maltophilia Isolates from Cancer Patients. Phytother. Res. 2011, 25, 584–587. [Google Scholar] [CrossRef]
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.M.; Pascual-Maté, A.; de la Fuente, D.; de Pablo, A.; Fernández-Muiño, M.A.; Sancho, M.T. Comparison of Methods to Determine Antibacterial Activity of Honeys against Staphylococcus Aureus. NJAS—Wagening. J. Life Sci. 2016, 78, 29–33. [Google Scholar] [CrossRef]
- Lukasiewicz, M.; Kowalski, S.; Makarewicz, M. Antimicrobial an Antioxidant Activity of Selected Polish Herbhoneys. LWT 2015, 64, 547–553. [Google Scholar] [CrossRef]
- Ng, W.J.; Sit, N.W.; Ooi, P.A.C.; Ee, K.Y.; Lim, T.M. The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona Itama) against Antibiotic Resistant Bacteria. Antibiotics 2020, 9, 871. [Google Scholar] [CrossRef]
- Łasica, A.; Golec, P.; Laskus, A.; Zalewska, M.; Gędaj, M.; Popowska, M. Periodontitis: Etiology, Conventional Treatments, and Emerging Bacteriophage and Predatory Bacteria Therapies. Front. Microbiol. 2024, 15, 1469414. [Google Scholar] [CrossRef]
- Wiernik, E.; Renuy, A.; Kab, S.; Steg, P.G.; Goldberg, M.; Zins, M.; Caligiuri, G.; Bouchard, P.; Carra, M.C. Prevalence of Self-Reported Severe Periodontitis: Data from the Population-Based CONSTANCES Cohort. J. Clin. Periodontol. 2024, 51, 884–894. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Afzali, H.; Graves, D.T. An Update on Periodontal Inflammation and Bone Loss. Front. Immunol. 2024, 15, 1385436. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current Concepts in the Pathogenesis of Periodontitis: From Symbiosis to Dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef]
- Duque Duque, A.; Chaparro Padilla, A.; Almeida, M.L.; Marín Jaramillo, R.A.; Romanelli, H.J.; Lafaurie Villamil, G.I. Strategies for the Prevention of Periodontal Disease and Its Impact on General Health: Latin America and the Caribbean Consensus 2024. Braz. Oral Res. 2024, 38, e120. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Tomáas-Barberáan, F.A.; Gil, M.I.; Tomáas-Lorente, F. An HPLc Technique for Flavonoid Analysis in Honey. J. Sci. Food Agric. 1991, 56, 49–56. [Google Scholar] [CrossRef]
- CLSI Standard M11; Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria. 8th ed, Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012.
- Miranda, S.L.F.; Damasceno, J.T.; Faveri, M.; Figueiredo, L.; da Silva, H.D.; Alencar, S.M.d.A.; Rosalen, P.L.; Feres, M.; Bueno-Silva, B. Brazilian Red Propolis Reduces Orange-Complex Periodontopathogens Growing in Multispecies Biofilms. Biofouling 2019, 35, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hajishengallis, G. Optimization of the Ligature-Induced Periodontitis Model in Mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef]
- Prates, T.P.; Taira, T.M.; Holanda, M.C.; Bignardi, L.A.; Salvador, S.L.; Zamboni, D.S.; Cunha, F.Q.; Fukada, S.Y. NOD2 Contributes to Porphyromonas gingivalis-Induced Bone Resorption. J. Dent. Res. 2014, 93, 1155–1162. [Google Scholar] [CrossRef]
- Larson, R.M. Merits and Modifications of Scoring Rat Dental Caries by Keyes’ Method. In Animal Models in Cariology; Tanzer, J.M., Ed.; Microbial Abstracts (Special Suppl); IRL Press: Washington, DC, USA, 1981; pp. 195–203. [Google Scholar]
- Soares, J.C.; Rosalen, P.L.; Lazarini, J.G.; Sardi, J.D.C.O.; Massarioli, A.P.; Nani, B.D.; Franchin, M.; De Alencar, S.M. Phenolic Profile and Potential Beneficial Effects of Underutilized Brazilian Native Fruits on Scavenging of ROS and RNS and Anti-Inflammatory and Antimicrobial Properties. Food Funct. 2020, 11, 8905–8917. [Google Scholar] [CrossRef]
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-Bacterial, Anti-Biofilm and Anti-Quorum Sensing Activities of Honey: A Review. J. Ethnopharmacol. 2023, 317, 116830. [Google Scholar] [CrossRef]
- Cremers, N.; Belas, A.; Santos Costa, S.; Couto, I.; de Rooster, H.; Pomba, C. In Vitro Antimicrobial Efficacy of Two Medical Grade Honey Formulations against Common High-Risk Meticillin-Resistant Staphylococci and Pseudomonas Spp. Pathogens. Vet. Dermatol. 2020, 31, 90–96. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Khan, K.A.; Alshehri, A.M.A. Antibacterial Potential of Some Saudi Honeys from Asir Region against Selected Pathogenic Bacteria. Saudi J. Biol. Sci. 2019, 26, 1278–1284. [Google Scholar] [CrossRef]
- Eick, S.; Schäfer, G.; Kwieciński, J.; Atrott, J.; Henle, T.; Pfister, W. Honey—A Potential Agent against Porphyromonas gingivalis: An in Vitro Study. BMC Oral Health 2014, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Safii, S.H.; Tompkins, G.R.; Duncan, W.J. Periodontal Application of Manuka Honey: Antimicrobial and Demineralising Effects In Vitro. Int. J. Dent. 2017, 2017, 9874535. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Qamash, R.A.; Bulkhi, R.A.; Bifari, J.A.; Bakhsh, O.S.; Hawsawi, K.O.; Matuure, E.Y.; Sulaimani, K.A.; Hakim, A.T.; Mujahid, M.S. Biological Studies of the Activity of Manuka Honey against Carbapenem-Resistant Enterobacterales (CRE) Bacteria. Saudi Med. J. 2024, 45, 876–887. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. An Updated Review of Functional Ingredients of Manuka Honey and Their Value-Added Innovations. Food Chem. 2024, 440, 138060. [Google Scholar] [CrossRef] [PubMed]
- Atwa, A.D.A.; AbuShahba, R.Y.; Mostafa, M.; Hashem, M.I. Effect of Honey in Preventing Gingivitis and Dental Caries in Patients Undergoing Orthodontic Treatment. Saudi Dent. J. 2014, 26, 108–114. [Google Scholar] [CrossRef]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. A Review of Commonly Used Methodologies for Assessing the Antibacterial Activity of Honey and Honey Products. Antibiotics 2022, 11, 975. [Google Scholar] [CrossRef]
- Szweda, P. Antimicrobial Activity of Honey. In Honey Analysis; InTech: London, UK, 2017. [Google Scholar]
- Hochheim, S.; Pacassa Borges, P.; Boeder, A.M.; Scharf, D.R.; Simionatto, E.L.; Yamanaka, C.N.; Alberton, M.D.; Guedes, A.; de Cordova, C.M.M. A Bioguided Approach for the Screening of Antibacterial Compounds Isolated From the Hydroalcoholic Extract of the Native Brazilian Bee’s Propolis Using Mollicutes as a Model. Front. Microbiol. 2020, 1, 558. [Google Scholar] [CrossRef]
- Almatroudi, A. Investigating Biofilms: Advanced Methods for Comprehending Microbial Behavior and Antibiotic Resistance. Front. Biosci.—Landmark 2024, 29, 133. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-Specific Antibiotic Tolerance and Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Romário-Silva, D.; Alencar, S.M.; Bueno-Silva, B.; Sardi, J.d.C.O.; Franchin, M.; Carvalho, R.D.P.d.; Ferreira, T.E.d.S.A.; Rosalen, P.L. Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges and Solutions. Microorganisms 2022, 10, 2325. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H.M.; Li, M.; Gregory, R.L. Effect of Honey on Streptococcus Mutans Growth and Biofilm Formation. Appl. Environ. Microbiol. 2012, 78, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.; Rosalen, P.L.; Soares, J.C.; Massarioli, A.P.; Campestrini, L.H.; Semarini, R.A.; Ikegaki, M.; Alencar, S.M. Polyphenols in Brazilian Organic Honey and Their Scavenging Capacity against Reactive Oxygen and Nitrogen Species. J. Apic. Res. 2020, 59, 136–145. [Google Scholar] [CrossRef]
- Taleb-Contini, S.H.; Salvador, M.J.; Watanabe, E.; Ito, I.Y.; Rodrigues De Oliveira, D.C. Antimicrobial Activity of Flavonoids and Steroids Isolated from Two Chromolaena Species. Rev. Bras. Cienc. Farm./Braz. J. Pharm. Sci. 2003, 39, 403–408. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.A. Antimicrobial Activity of Ascorbic Acid Alone or in Combination with Lactic Acid on Escherichia Coli O157:H7 in Laboratory Medium and Carrot Juice. Food Control 2011, 22, 801–804. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, M.Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef]
- Lee, G.B.; Kim, Y.; Lee, K.E.; Vinayagam, R.; Singh, M.; Kang, S.G. Anti-Inflammatory Effects of Quercetin, Rutin, and Troxerutin Result From the Inhibition of NO Production and the Reduction of COX-2 Levels in RAW 264.7 Cells Treated with LPS. Appl. Biochem. Biotechnol. 2024, 196, 8431–8452. [Google Scholar] [CrossRef]
- Buzdağlı, Y.; Eyipınar, C.D.; Kacı, F.N.; Tekin, A. Effects of Hesperidin on Anti-Inflammatory and Antioxidant Response in Healthy People: A Meta-Analysis and Meta-Regression. Int. J. Environ. Health Res. 2022, 33, 1390–1405. [Google Scholar] [CrossRef]
- Hao, B.; Yang, Z.; Liu, H.; Liu, Y.; Wang, S. Advances in Flavonoid Research: Sources, Biological Activities, and Developmental Prospectives. Curr. Issues Mol. Biol. 2024, 46, 2884–2925. [Google Scholar] [CrossRef]
- Landete, J.M. Plant and Mammalian Lignans: A Review of Source, Intake, Metabolism, Intestinal Bacteria and Health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Kalinovskii, A.P.; Belozerova, O.A.; Andreev, Y.A.; Kozlov, S.A. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. Int. J. Mol. Sci. 2022, 23, 6031. [Google Scholar] [CrossRef]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef] [PubMed]
- Recklies, K.; Peukert, C.; Kölling-Speer, I.; Speer, K. Differentiation of Honeydew Honeys from Blossom Honeys and According to Their Botanical Origin by Electrical Conductivity and Phenolic and Sugar Spectra. J. Agric. Food Chem. 2021, 69, 1329–1347. [Google Scholar] [CrossRef] [PubMed]
- Pudelka, L.; Sleha, R.; Janovska, S.; Radochova, V.; Bostik, P. Czech Honeydew Honeys—A Potential Source of Local Medical Honey with Strong Antimicrobial Activity. Pharmaceuticals 2024, 17, 840. [Google Scholar] [CrossRef]
- Dżugan, M.; Ciszkowicz, E.; Tomczyk, M.; Miłek, M.; Lecka-Szlachta, K. Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Appl. Sci. 2024, 14, 710. [Google Scholar] [CrossRef]
- Brudzynski, K.; Miotto, D.; Kim, L.; Sjaarda, C.; Maldonado-Alvarez, L.; Fukś, H. Active Macromolecules of Honey Form Colloidal Particles Essential for Honey Antibacterial Activity and Hydrogen Peroxide Production. Sci. Rep. 2017, 7, 7637. [Google Scholar] [CrossRef]
- Romário-Silva, D.; Lazarini, J.G.; Franchin, M.; de Alencar, S.M.; Rosalen, P.L. Brazilian Organic Honey from Atlantic Rainforest Decreases Inflammatory Process in Mice. Vet. Sci. 2022, 9, 268. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Howard, K.C.; Gonzalez, O.A.; Garneau-Tsodikova, S. Porphyromonas gingivalis: Where Do We Stand in Our Battle against This Oral Pathogen? RSC Med. Chem. 2021, 12, 666–704. [Google Scholar] [CrossRef]
- Lamont, R.J.; Hajishengallis, G. Polymicrobial Synergy and Dysbiosis in Inflammatory Disease. Trends Mol. Med. 2015, 21, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.G.; Raveendran, R. Microbial Dysbiosis in Periodontitis. J. Indian Soc. Periodontol. 2013, 17, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Beyond the Red Complex and into More Complexity: The Polymicrobial Synergy and Dysbiosis (PSD) Model of Periodontal Disease Etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bi, L.; Yu, X.; Kawai, T.; Taubman, M.A.; Shen, B.; Han, X. Porphyromonas gingivalis Exacerbates Ligature-Induced, RANKLdependent Alveolar Bone Resorption via Differential Regulation of Toll-like Receptor 2 (TLR2) and TLR4. Infect. Immun. 2014, 82, 4127–4134. [Google Scholar] [CrossRef]
- Nibali, L.; Sun, C.; Akcalı, A.; Meng, X.; Tu, Y.K.; Donos, N. A Retrospective Study on Periodontal Disease Progression in Private Practice. J. Clin. Periodontol. 2017, 44, 290–297. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and Its Virulence Factors in Periodontitis. Adv. Protein Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [CrossRef]
- Mcintosh, M.L.; Hajishengallis, G. Inhibition of Porphyromonas gingivalis-Induced Periodontal Bone Loss by CXCR4 Antagonist Treatment. Mol. Oral. Microbiol. 2012, 27, 449–457. [Google Scholar] [CrossRef]
- Kassem, A.; Henning, P.; Lundberg, P.; Souza, P.P.C.; Lindholm, C.; Lerner, U.H. Porphyromonas gingivalis Stimulates Bone Resorption by Enhancing RANKL (Receptor Activator of NF-κB Ligand) through Activation of Toll-like Receptor 2 in Osteoblasts. J. Biol. Chem. 2015, 290, 20147–20158. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. Effect of Hydrogen Peroxide on Antibacterial Activities of Canadian Honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef]
- Bang, L.M.; Buntting, C.; Molan, P. Peroxide Production in Honey & Its Implications. J. Altern. Complement. Med. 2003, 9, 267–273. [Google Scholar]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-Examining the Role of Hydrogen Peroxide in Bacteriostatic and Bactericidal Activities of Honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef]

| Honey Samples and Internal Control | P. gingivalis W83 |
|---|---|
| MIC (%/µg/mL)|MBC (%/µg/mL) | |
| OHD | 4.0/40,000|6.0/60,000 |
| Metronidazole | 0.0000039/0.039|0.0000078/0.078 |
| Compound | RT (min) | m/z | Fragmentation | Molecular Formula |
|---|---|---|---|---|
| Apigenidin isomer (I) | 5.5 | 256.07 | (MS2) 256.05; 255.00; 257.05; 253.8 | C15H11O4 |
| Matairesinol | 6.4 | 359.14 | (MS2) 341.14; 323.08; 274.97; 342.12 | C20H22O6 |
| Quercetin | 12.7 | 303.05 | (MS2) 284.98; 285.93; 303.03; 301.06 | C15H10O7 |
| Apigenidin isomer (II) | 18.2 | 256.07 | (MS2) 256.04; 254.94; 257.04; 238.88 | C15H11O4 |
| Hesperidin | 24.2 | 611.18 | (MS2) 302.87; 465.08; 303.89; 575.22 | C27H30O16 |
| 5,6,7,3′,4′ pentahydroxyisoflavone | 13.5 | 303.02 | (MS2) 284.97; 285.94; 303.04; 257.81 | C15H10O7 |
| Anhydrosecoisolariciresinol | 25.1 | 345.16 | (MS2) 327.09; 200.78; 164.73; 136.76 | C20H24O5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romário-Silva, D.; Franchin, M.; Bueno-Silva, B.; Saliba, A.S.M.C.; Sardi, J.O.; Alves-Ferreira, T.; Lazarini, J.G.; Cunha, G.A.; de Alencar, S.M.; Rosalen, P.L. Brazilian Organic Honeydew Reduces In Vitro and In Vivo Periodontal Disease-Related Subgingival Biofilm. Foods 2025, 14, 997. https://doi.org/10.3390/foods14060997
Romário-Silva D, Franchin M, Bueno-Silva B, Saliba ASMC, Sardi JO, Alves-Ferreira T, Lazarini JG, Cunha GA, de Alencar SM, Rosalen PL. Brazilian Organic Honeydew Reduces In Vitro and In Vivo Periodontal Disease-Related Subgingival Biofilm. Foods. 2025; 14(6):997. https://doi.org/10.3390/foods14060997
Chicago/Turabian StyleRomário-Silva, Diego, Marcelo Franchin, Bruno Bueno-Silva, Ana Sofia Martelli Chaib Saliba, Janaína Orlandi Sardi, Thayna Alves-Ferreira, Josy Goldoni Lazarini, Gustavo Aparecido Cunha, Severino Matias de Alencar, and Pedro Luiz Rosalen. 2025. "Brazilian Organic Honeydew Reduces In Vitro and In Vivo Periodontal Disease-Related Subgingival Biofilm" Foods 14, no. 6: 997. https://doi.org/10.3390/foods14060997
APA StyleRomário-Silva, D., Franchin, M., Bueno-Silva, B., Saliba, A. S. M. C., Sardi, J. O., Alves-Ferreira, T., Lazarini, J. G., Cunha, G. A., de Alencar, S. M., & Rosalen, P. L. (2025). Brazilian Organic Honeydew Reduces In Vitro and In Vivo Periodontal Disease-Related Subgingival Biofilm. Foods, 14(6), 997. https://doi.org/10.3390/foods14060997







