Selenium Nanoparticles Derived from Moringa oleifera Lam. Polysaccharides: Construction, Stability, and In Vitro Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of MOLP-SeNPs
2.2.1. Extraction of MOLP
2.2.2. Single-Factor Experiment
2.2.3. Response Surface Methodology (RSM) Optimization
2.3. Interaction Between Moringa Oleifera Polysaccharide and Selenium
2.3.1. Molecular Docking
2.3.2. Quantum Chemistry Calculations
2.4. Structural Characterization and Analysis
2.4.1. Monosaccharide Composition
2.4.2. Dynamic Light Scattering
2.4.3. Fourier Infrared Spectroscopy (FT-IR)
2.4.4. X-Ray Diffraction (XRD)
2.4.5. Fluorescence Spectroscopy (FS)
2.4.6. X-Ray Diffraction Photoelectron Spectroscopy (XPS)
2.4.7. Scanning Electron Microscopy (SEM)
2.5. Stability Measurements of MOLP-SeNPs
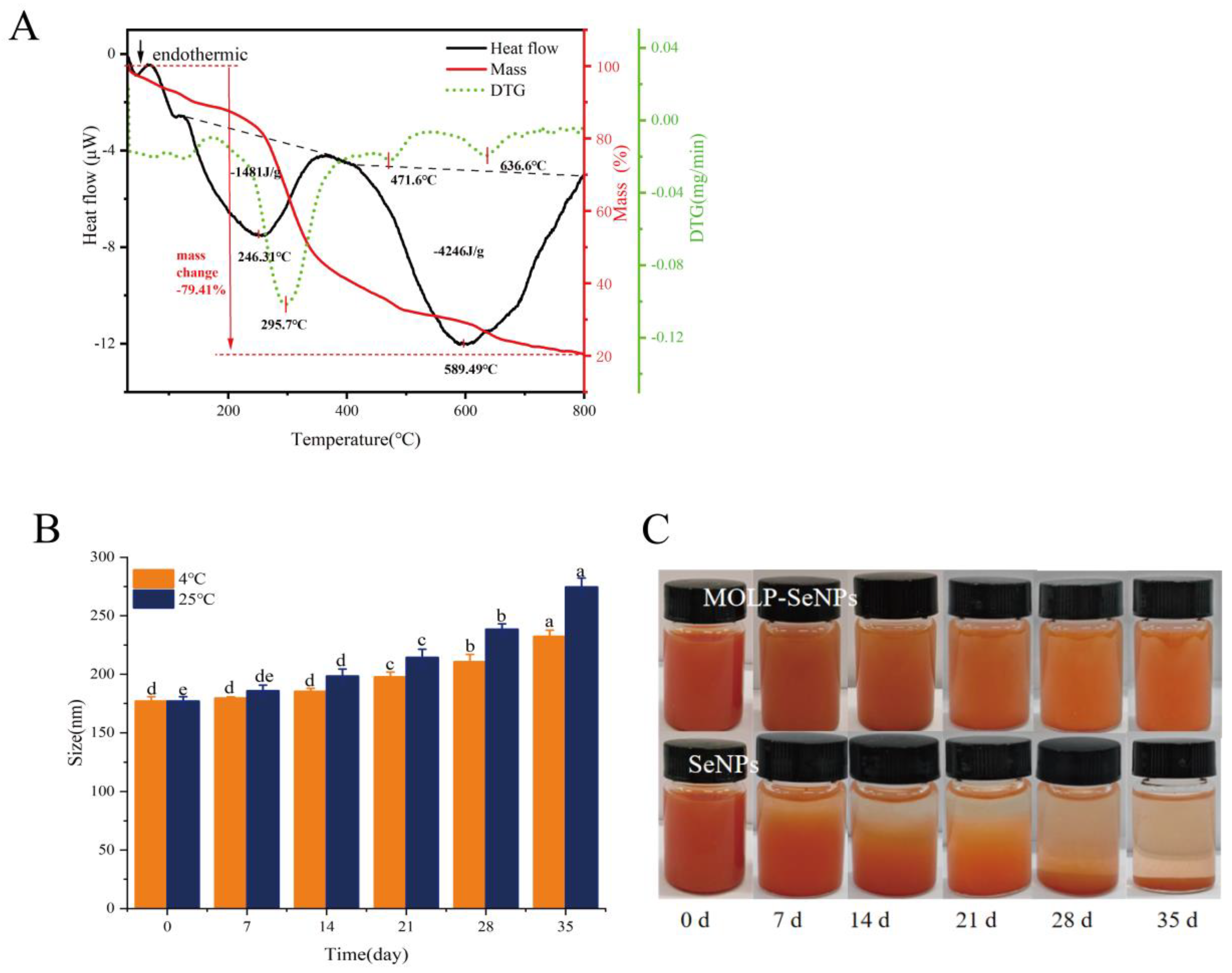
2.5.1. Differential Scanning Calorimetry (DSC) and Thermogravimetry (TG)
2.5.2. Storage Stability
2.6. Gastrointestinal Digestion and Antioxidant Studies
2.6.1. Simulated Oral, Gastric, and Intestinal Digestion in Vitro
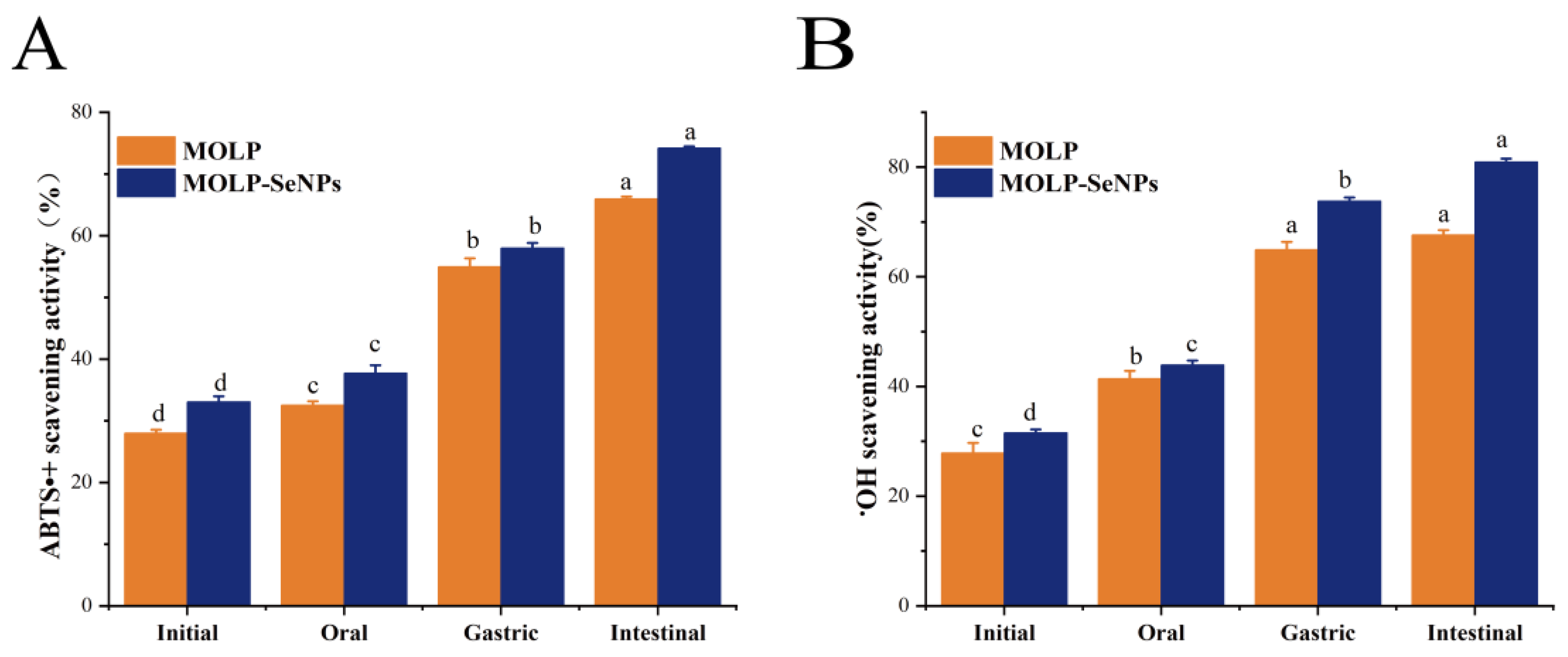
2.6.2. ABTS•+ (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonate) Radical Cation) Clearance Viability
2.6.3. •OH Scavenging Ability
2.6.4. Modeling of H2O2-Induced Oxidative Stress
2.6.5. Cell Viability Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Process Optimization of MOLP-SeNPs
3.1.1. Optimization of Conditions for the Preparation of MOLP-SeNPs in Single-Factor Experiment
3.1.2. Optimization of Preparation Conditions for MOLP-SeNPs Using RSM
3.2. Identification of Monosaccharide Composition and Interaction Between Monosaccharides and Se
3.2.1. Monosaccharide Composition
3.2.2. Molecular Docking
3.2.3. DFT
3.3. Structural Characterization of MOLP-SeNPs
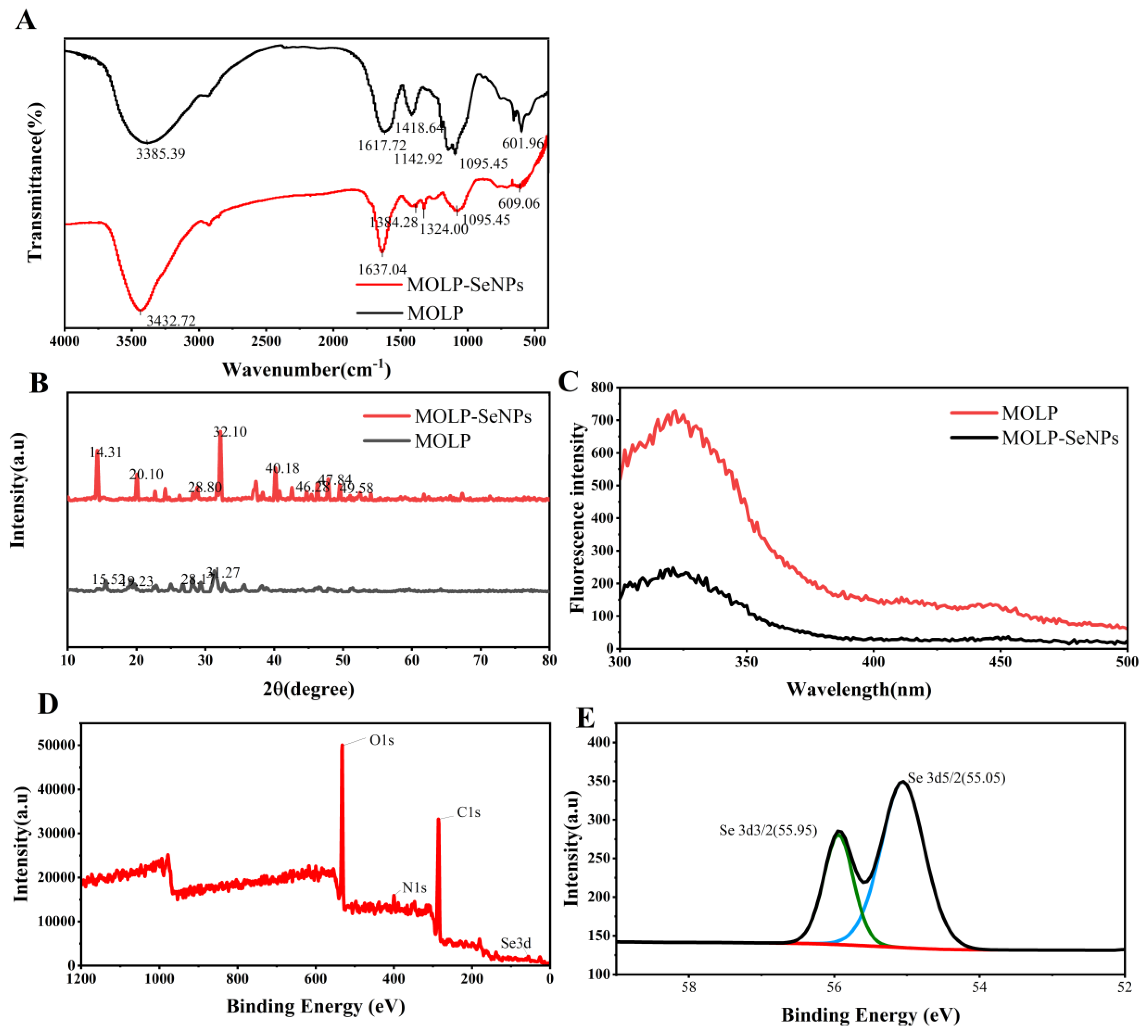
3.3.1. FT-IR
3.3.2. XRD Spectral Analysis
3.3.3. FS
3.3.4. XPS Spectral Analysis
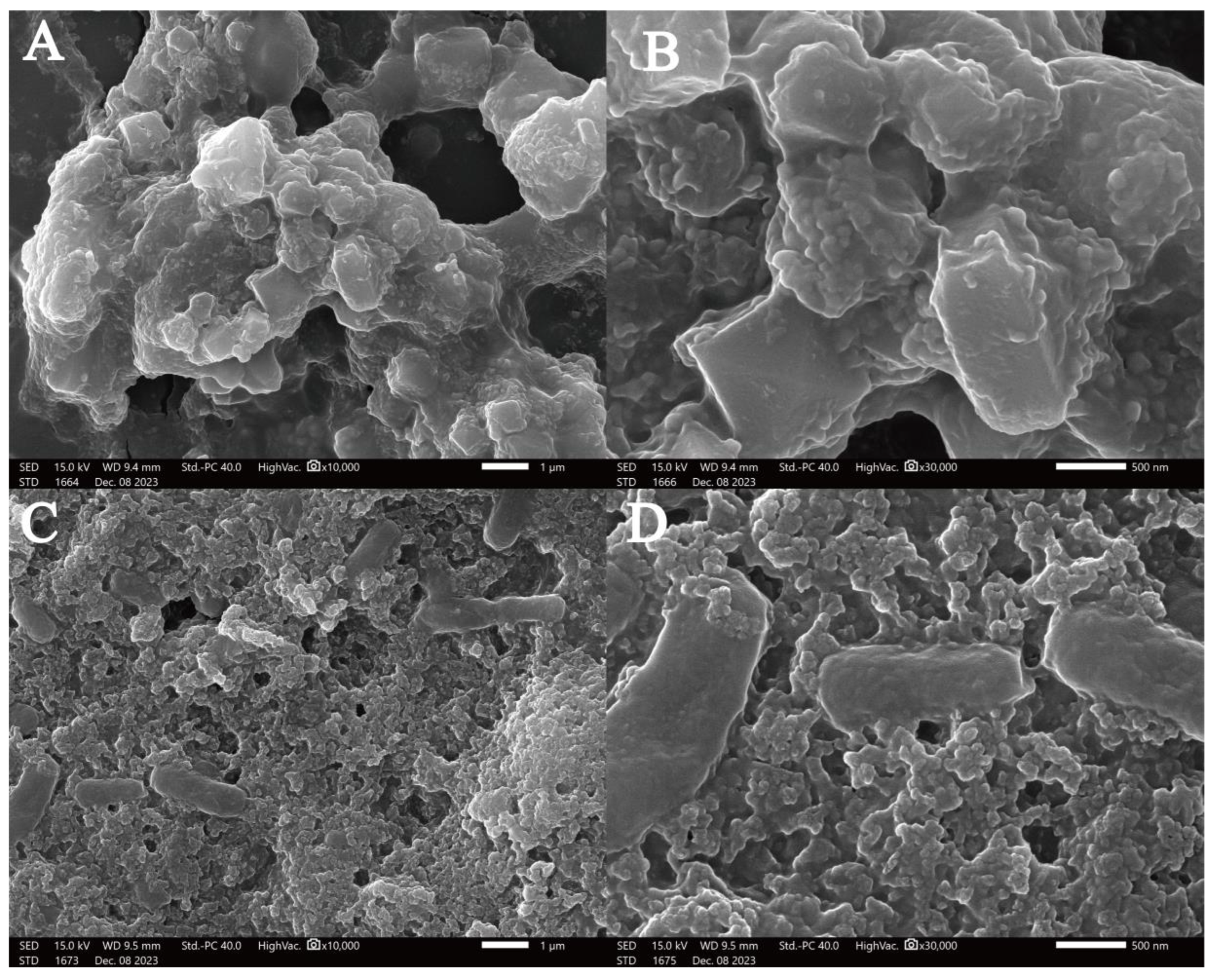
3.3.5. SEM
3.4. Stabilization of Selenium Nanoparticles of Polysaccharides from Chorizo Wood
3.4.1. TG-DSC
3.4.2. Storage Stability Analysis
3.5. Antioxidant Activity of MOLP-SeNPs
3.5.1. In Vitro Analysis of Antioxidant Activity After Simulated Digestion
3.5.2. Effect of H2O2 and MOLP-SeNPs on HepG2 Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Yu, K.; Xiao, Q.; Song, B.; Yuan, W.; Long, X.; Cai, D.; Xiong, X.; Zheng, W. Effect of bio-nano-selenium on yield, nutritional quality and selenium content of radish. J. Food Compos. Anal. 2023, 115, 104927. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Bjrnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Guan, H.; Liu, S. Construction of a Cordyceps sinensis exopolysaccharide-conjugated selenium nanoparticles and enhancement of their antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, W.; Han, W.; Li, C.; Zhang, Z.; Hu, B.; Chen, C.; Cui, P.; Luo, S.; Tang, Z.; et al. Structure characterization of Oudemansiella radicata polysaccharide and preparation of selenium nanoparticles to enhance the antioxidant activities. LWT 2021, 146, 111469. [Google Scholar] [CrossRef]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Y.; Jia, H.; Ding, H.; Yuan, Y. Biosynthesis of selenium nanoparticles of Monascus purpureus and their inhibition to Alicyclobacillus acidoterrestris. Food Control 2021, 130, 108366. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Li, Y.; Ma, L.; Lin, Z.; Xu, J.; Guo, Y. Preparation and anti-tumor activity of selenium nanoparticles based on a polysaccharide from Paeonia lactiflora. Int. J. Biol. Macromol. 2023, 232, 123261. [Google Scholar] [CrossRef]
- Lazcano-Ramírez, H.G.; Garza-García, J.J.; Hernández-Díaz, J.A.; León-Morales, J.M.; Macías-Sandoval, A.S.; García-Morales, S. Antifungal activity of selenium nanoparticles obtained by plant-mediated synthesis. Antibiotics 2023, 12, 115. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Y.; Yue, P.; Li, H.; Wu, Y.; Hao, X.; Peng, F. Structure, stability, antioxidant activity, and controlled-release of selenium nanoparticles decorated with lichenan from Usnea longissima. Carbohydr. Polym. 2023, 299, 120219. [Google Scholar] [CrossRef]
- Zhai, C.; Lin, Y.; Mao, C.; Li, X.; Zhang, R.; Liu, J.; Zhang, L. Construction, characterization, antioxidant activity and effects on properties in vitro digestion of selenium nanoparticles decorated with Cyperus esculentus polysaccharides. Food Biosci. 2024, 59, 104062. [Google Scholar] [CrossRef]
- Xiaolong, J.I.; Jianhang, G.U.O.; Jingyuan, T.I.A.N.; Ke, M.A.; Yanqi, L.I.U. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 52–62. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Tian, J.; Ma, K.; Liu, Y. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao. J. Food Meas. Charact. 2022, 16, 2191–2200. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Wang, M.; Jiang, L.; Ma, X.; Huang, Y.; Liu, T.; Zheng, L.; Li, Y. Construction and anti-pancreatic cancer activity of selenium nanoparticles stabilized by Prunella vulgaris polysaccharide. Int. J. Biol. Macromol. 2024, 278 Pt 3, 134924. [Google Scholar] [CrossRef]
- Chu, W.; Liu, P.; Zhang, Z.; Wu, D.; Li, W.; Chen, W.; Li, Z.; Wang, W.; Yang, Y. Preparation, characterization and cytotoxic activity of selenium nanoparticles stabilized with a heteropolysaccharide isolated from Sanghuangporus vaninii residue. Carbohydr. Polym. 2024, 343, 122468. [Google Scholar] [CrossRef]
- Manjunath, S.H.; Nataraj, P.; Swamy, V.H.; Sugur, K.; Dey, S.K.; Ranganathan, V.; Daniel, S.; Leihang, Z.; Sharon, V.; Chandrashekharappa, S.; et al. Development of Moringa oleifera as functional food targeting NRF2 signaling: Antioxidant and anti-inflammatory activity in experimental model systems. Food Funct. 2023, 14, 4734–4751. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhu, X.; Yang, G.; Zhang, J.; Wang, R.; Shen, Y.; Li, H.; Gatasheh, M.K.; Abbasi, A.M.; Yang, X. Ultrasonic extraction of Moringa oleifera seeds polysaccharides: Optimization, purification, and anti-inflammatory activities. Int. J. Biol. Macromol. 2024, 258 Pt 2, 128833. [Google Scholar] [CrossRef]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 2001, 35, 405–410. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Lin, L.; Zhao, M. Identification of an arabinogalactan with special structure from Moringa Oleifera leaf and exploration of its immunomodulatory activity. Int. J. Biol. Macromol. 2024, 279 Pt 3, 134616. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, M.; Waghmare, R.; Suhag, R.; Gupta, O.P.; Lorenzo, J.M.; Prakash, S.; Radha; Rais, N.; Sampathrajan, V.; et al. Moringa (Moringa oleifera Lam.) polysaccharides: Extraction, characterization, bioactivities, and industrial application. Int. J. Biol. Macromol. 2022, 209 Pt A, 763–778. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Huang, Q. Protective effects of Cordyceps sinensis exopolysaccharide-selenium nanoparticles on H2O2-induced oxidative stress in HepG2 cells. Int. J. Biol. Macromol. 2022, 213, 339–351. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Jiang, H.; Li, L.; Yang, M.; Dai, J.; Tao, L.; Sheng, J.; Tian, Y. Glycated walnut meal peptide-calcium chelates (COS-MMGGED-Ca): Preparation, characterization, and calcium absorption-promoting. Food Chem. 2025, 462, 140975. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Deus, V.L.; Tavano, O.L.; Gloria, M.B.A. In vitro bioaccessibility of amino acids and bioactive amines in 70% cocoa dark chocolate: What you eat and what you get. Food Chem. 2021, 343, 128397. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Phenolic composition and antioxidant activities of 11 celery cultivars. J. Food Sci. 2010, 75, C9–C13. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Liu, Q.; Liu, H.; Yang, C.; Zhao, Q.; Xu, Y.; Wu, W. Structure characterization and antioxidant activity of abalone visceral peptides-selenium in vitro. Food Chem. 2024, 433, 137398. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Chen, Y.; Gao, Y.; Zhang, Z.; Li, S.; Chen, Y. Complex coacervation of zein-chitosan via atmospheric cold plasma treatment: Improvement of encapsulation efficiency and dispersion stability. Food Hydrocoll. 2020, 107, 105943. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, W.; Xue, M.; Wang, H.; Yu, M.; Zhang, P.; Yi, P. Photochromic RAFT reagent helps construct superior photoswitchable fluorescent polymer nanoparticles for rewritable fluorescence patterning and intracellular dual-color imaging. Polym. Chem. 2017, 8, 6520–6526. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Mu, J.; Ho, C.-T.; Su, J.; Zhang, Y.; Lin, X.; Chen, Z.; Li, B.; Xie, Y. Preparation, physicochemical characterization, and anti-proliferation of selenium nanoparticles stabilized by Polyporus umbellatus polysaccharide. Int. J. Biol. Macromol. 2020, 152, 605–615. [Google Scholar] [CrossRef]
- Jiao, J.; Yu, J.; Ji, H.; Liu, A. Synthesis of macromolecular Astragalus polysaccharide-nano selenium complex and the inhibitory effects on HepG2 cells. Int. J. Biol. Macromol. 2022, 211, 481–489. [Google Scholar] [CrossRef]
- Li, Z.; Lu, D.; Gao, X. Optimization of mixture proportions by statistical experimental design using response surface method-A review. J. Build. Eng. 2021, 36, 102101. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-de-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef]
- Shu, G.; Wang, Z.; Chen, L.; Zhang, Q.; Xin, N. Enzymolysis technology optimization for production of antioxidant peptides from goat milk casein. Acta Univ. Cibiniensis Ser. E Food Technol. 2017, 21, 51–60. [Google Scholar] [CrossRef]
- Mohamed Husien, H.; Peng, W.; Su, H.; Zhou, R.; Tao, Y.; Huang, J.; Liu, M.; Bo, R.; Li, J. Moringa oleifera leaf polysaccharide alleviates experimental colitis by inhibiting inflammation and maintaining intestinal barrier. Front. Nutr. 2022, 9, 1055791. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Netto, F.M.; Bertoldo-Pacheco, M.T.; Alegría, A.; Cilla, A. Peptide-metal complexes: Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Crit. Rev. Food Sci. Nutr. 2021, 61, 1470–1489. [Google Scholar] [CrossRef]
- Ji, X.; Cheng, Y.; Tian, J.; Zhang, S.; Jing, Y.; Shi, M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54. [Google Scholar] [CrossRef]
- Jiang, W.; He, S.; Su, D.; Ye, M.; Zeng, Q.; Yuan, Y. Synthesis, characterization of tuna polypeptide selenium nanoparticle, and its immunomodulatory and antioxidant effects in vivo. Food Chem. 2022, 383, 132405. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, C.; Saha, S.; Biswas, A.; Kundu, M.; Ghosh, L.; Das, K.P. Structural changes of β-lactoglobulin during thermal unfolding and refolding–an FT-IR and circular dichroism study. Protein J. 2005, 24, 27–35. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, W.; Yang, X.-Q.; Su, D.-X.; He, S.; Nag, A.; Zeng, Q.-Z.; Yuan, Y. Development, characterization and in vitro bile salts binding capacity of selenium nanoparticles stabilized by soybean polypeptides. Food Chem. 2022, 391, 133286. [Google Scholar] [CrossRef]
- Moreno-Fuquen, R.; Morales, V.; Loaiza, A.; Tenorio, J.C.; Goddard, R. Spectroscopic (FTIR and UV-Vis) Analysis, Supramolecular Studies, XRD Structural Characterization and Theoretical Studies of Two Flavone-Oxime Derivatives. ChemistrySelect 2020, 5, 6365–6372. [Google Scholar] [CrossRef]
- Muhammad, N.; Amjad, A.L.I.; Hussain, I.; Subhani, Q.; Guo, D.-D.; Cui, H.-R.; Zhu, Y. Determination of fluorine and chlorine in standard steel residues and zinc sulfide concentrates by ion chromatography-Matrix interference study. Chin. J. Anal. Chem. 2023, 51, 100147. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, S.; Wu, D.; Xu, Z.; Xu, S.; Chen, H.; Du, M. Hydrophobic peptides from oyster protein hydrolysates show better zinc-chelating ability. Food Biosci. 2021, 41, 100985. [Google Scholar] [CrossRef]
- Muhammad, N.; Hussian, I.; Ali, A.; Hussain, T.; Intisar, A.; Haq, I.U.; Subhani, Q.; Hedar, M.; Zhong, J.-L.; Asif, M. A comprehensive review of liquid chromatography hyphenated to post-column photoinduced fluorescence detection system for determination of analytes. Arab. J. Chem. 2022, 15, 104091. [Google Scholar] [CrossRef]
- Lin, S.; Hu, X.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Yang, S. Preparation, purification and identification of iron-chelating peptides derived from tilapia (Oreochromis niloticus) skin collagen and characterization of the peptide-iron complexes. LWT 2021, 149, 111796. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Sun, H.; Liu, X.; Leng, X. Physicochemical and functional properties of chitosan-stabilized selenium nanoparticles under different processing treatments. Food Chem. 2020, 331, 127378. [Google Scholar] [CrossRef]
- Al-Hagar, O.E.; Abol-Fotouh, D.; Abdelkhalek, E.S.; Elsoud, M.M.A.; Sidkey, N. Bacillus niabensis OAB2: Outstanding bio-factory of selenium nanoparticles. Mater. Chem. Physics. 2021, 273, 125147. [Google Scholar] [CrossRef]
- Canava, B.; Vigneron, J.; Etcheberry, A.; Guillemoles, J.; Lincot, D. High resolution XPS studies of Se chemistry of a Cu (In, Ga) Se2 surface. Appl. Surf. Sci. 2002, 202, 8–14. [Google Scholar] [CrossRef]
- Li, J.-M.; Wang, W.-J.; Chen, H.; Lin, S.-Y.; Mao, X.-Y.; Yu, J.-M.; Chen, L.-L. Characterization, in vitro antioxidant activity and stability of cattle bone collagen peptides-selenium chelate. Food Chem. X 2024, 23, 101789. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins–A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Tang, L.; Luo, X.; Wang, M.; Wang, Z.; Guo, J.; Kong, F.; Bi, Y. Synthesis, characterization, in vitro antioxidant and hypoglycemic activities of selenium nanoparticles decorated with polysaccharides of Gracilaria lemaneiformis. Int. J. Biol. Macromol. 2021, 193, 923–932. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Gong, X.; Li, Z.; Wang, H.; Ma, L.; Tuerhong, M.; Abudukeremu, M.; Ohizumi, Y.; Xu, J.; et al. Preparation, characterization, and antitumor activity of Chaenomeles speciosa polysaccharide-based selenium nanoparticles. Arab. J. Chem. 2022, 15, 103943. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An antioxidant with a critical role in anti-aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Xu, F.; Zhang, Y.; Li, Z.; Ju, X.; Wang, L. Insoluble-bound polyphenols of adlay seed ameliorate H2O2-induced oxidative stress in HepG2 cells via Nrf2 signalling. Food Chem. 2020, 325, 126865. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liang, S.; Ge, L.; Wan, H.; Wu, W.; Fei, J.; Wu, S.; Zhou, B.; Zeng, X. 4,5-di-O-caffeoylquinic acid methyl ester isolated from Lonicera japonica Thunb. targets the Keap1/Nrf2 pathway to attenuate H2O2-induced liver oxidative damage in HepG2 cells. Phytomedicine 2020, 70, 153219. [Google Scholar] [CrossRef]
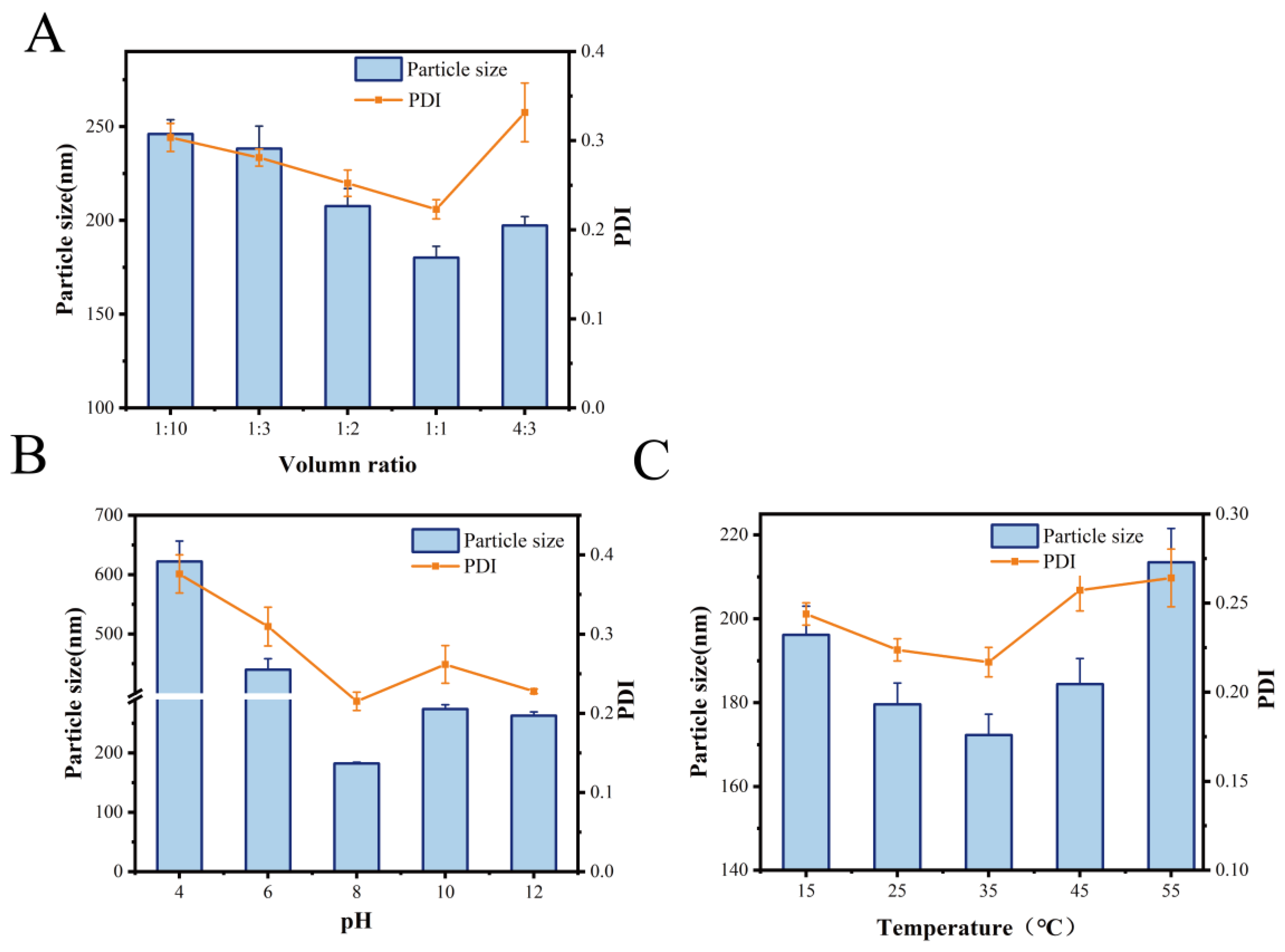
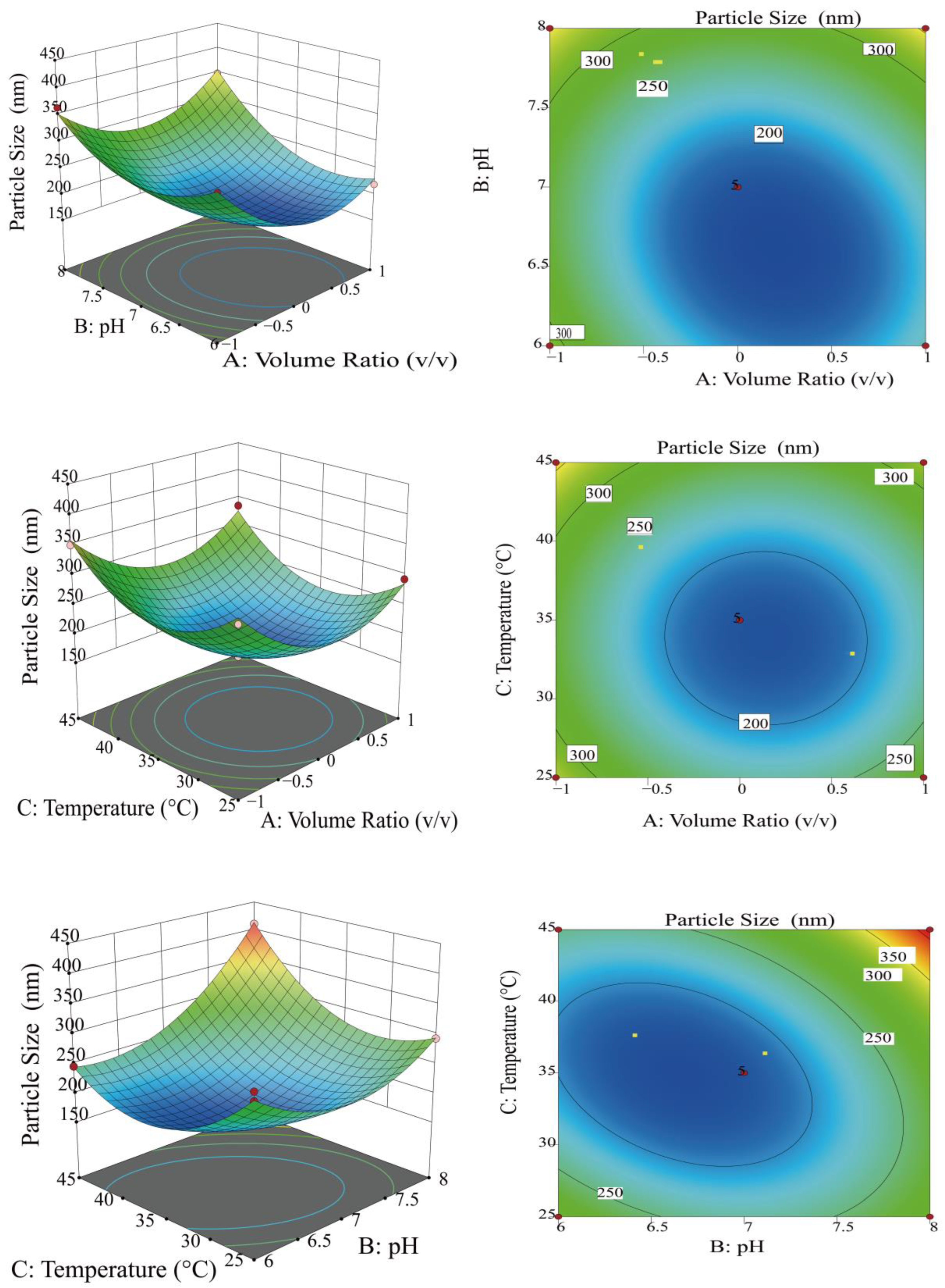


| Factors | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A MOLP: Na2SeO3 (v/v) | 1:2 | 1:1 | 4:3 |
| B pH | 6.0 | 8.0 | 10.0 |
| C Temperature (°C) | 25 | 35 | 45 |
| Number | A Volume Ratio (v/v) | B pH | C Temperature (°C) | Particle Size (nm) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 321.02 ± 12.32 |
| 2 | −1 | 1 | 0 | 363.35 ± 16.17 |
| 3 | −1 | 0 | −1 | 324.38 ± 13.52 |
| 4 | −1 | 0 | 1 | 350.26 ± 14.28 |
| 5 | 0 | −1 | −1 | 302.18 ± 10.27 |
| 6 | 0 | 1 | −1 | 293.75 ± 9.63 |
| 7 | 0 | −1 | 1 | 246.16 ± 12.74 |
| 8 | 0 | 1 | 1 | 410.78 ± 13.62 |
| 9 | 0 | 0 | 0 | 176.73 ± 11.59 |
| 10 | 0 | 0 | 0 | 186.20 ± 10.24 |
| 11 | 0 | 0 | 0 | 202.97 ± 8.47 |
| 12 | 0 | 0 | 0 | 160.32 ± 9.69 |
| 13 | 0 | 0 | 0 | 175.64 ± 12.84 |
| 14 | 1 | −1 | 0 | 219.06 ± 11.92 |
| 15 | 1 | 1 | 0 | 348.69 ± 10.03 |
| 16 | 1 | 0 | −1 | 293.35 ± 12.84 |
| 17 | 1 | 0 | 1 | 335.63 ± 13.27 |
| Source | Sum of Squared Deviations | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 94,987.9 | 9 | 10,554.21 | 44.8 | <0.0001 | ** |
| A Volume ratio | 3291.85 | 1 | 3291.85 | 13.97 | 0.0073 | ** |
| B pH | 13,460.3 | 1 | 13,460.3 | 57.13 | 0.0001 | ** |
| C Temperature | 2085.61 | 1 | 2085.61 | 8.85 | 0.0207 | * |
| AB | 1905.32 | 1 | 1905.32 | 8.09 | 0.0249 | * |
| AC | 67.24 | 1 | 67.24 | 0.29 | 0.6097 | |
| BC | 7486.58 | 1 | 7486.58 | 31.78 | 0.0008 | ** |
| A2 | 22,237.17 | 1 | 22,237.17 | 94.38 | <0.0001 | ** |
| B2 | 15,150.44 | 1 | 15,150.44 | 64.3 | <0.0001 | ** |
| C2 | 22,352.07 | 1 | 22,352.07 | 94.87 | <0.0001 | ** |
| residual | 1649.27 | 7 | 235.61 | |||
| lost proposal | 666.89 | 3 | 222.3 | 0.91 | 0.5131 | ns |
| pure error | 982.37 | 4 | 245.59 | |||
| aggregate | 96,637.16 | 16 | ||||
| R2 = 0.9829 Adj R2 = 0.9610 Pre R2 = 0.8737 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, L.; Guan, C.; Wang, Z.; Wang, Y.; Gesang, Q.; Sheng, J.; Dai, J.; Tian, Y. Selenium Nanoparticles Derived from Moringa oleifera Lam. Polysaccharides: Construction, Stability, and In Vitro Antioxidant Activity. Foods 2025, 14, 918. https://doi.org/10.3390/foods14060918
Tao L, Guan C, Wang Z, Wang Y, Gesang Q, Sheng J, Dai J, Tian Y. Selenium Nanoparticles Derived from Moringa oleifera Lam. Polysaccharides: Construction, Stability, and In Vitro Antioxidant Activity. Foods. 2025; 14(6):918. https://doi.org/10.3390/foods14060918
Chicago/Turabian StyleTao, Liang, Chunhua Guan, Zilin Wang, Yue Wang, Quzheng Gesang, Jun Sheng, Jiahe Dai, and Yang Tian. 2025. "Selenium Nanoparticles Derived from Moringa oleifera Lam. Polysaccharides: Construction, Stability, and In Vitro Antioxidant Activity" Foods 14, no. 6: 918. https://doi.org/10.3390/foods14060918
APA StyleTao, L., Guan, C., Wang, Z., Wang, Y., Gesang, Q., Sheng, J., Dai, J., & Tian, Y. (2025). Selenium Nanoparticles Derived from Moringa oleifera Lam. Polysaccharides: Construction, Stability, and In Vitro Antioxidant Activity. Foods, 14(6), 918. https://doi.org/10.3390/foods14060918




