One-Pot Fabrication of Ginger-Waste-Derived Ionic Liquid Electrospun Films: An Efficient Preparation Strategy with Enhanced Antibacterial Functionality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
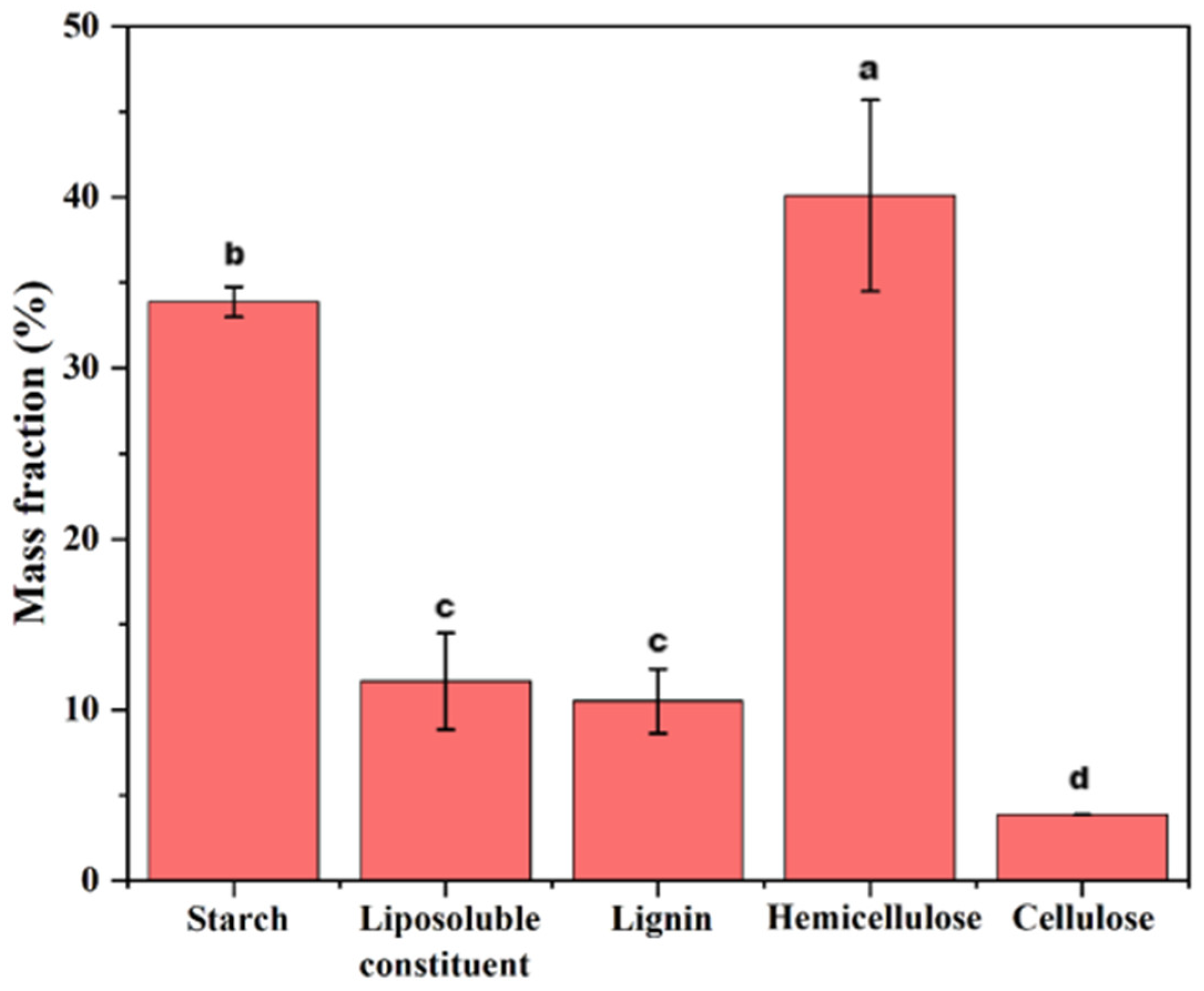
2.2. Biomass Components in Ginger Waste Residue
2.3. Preparation of Electrospinning Solution
2.4. Viscosity and Conductivity
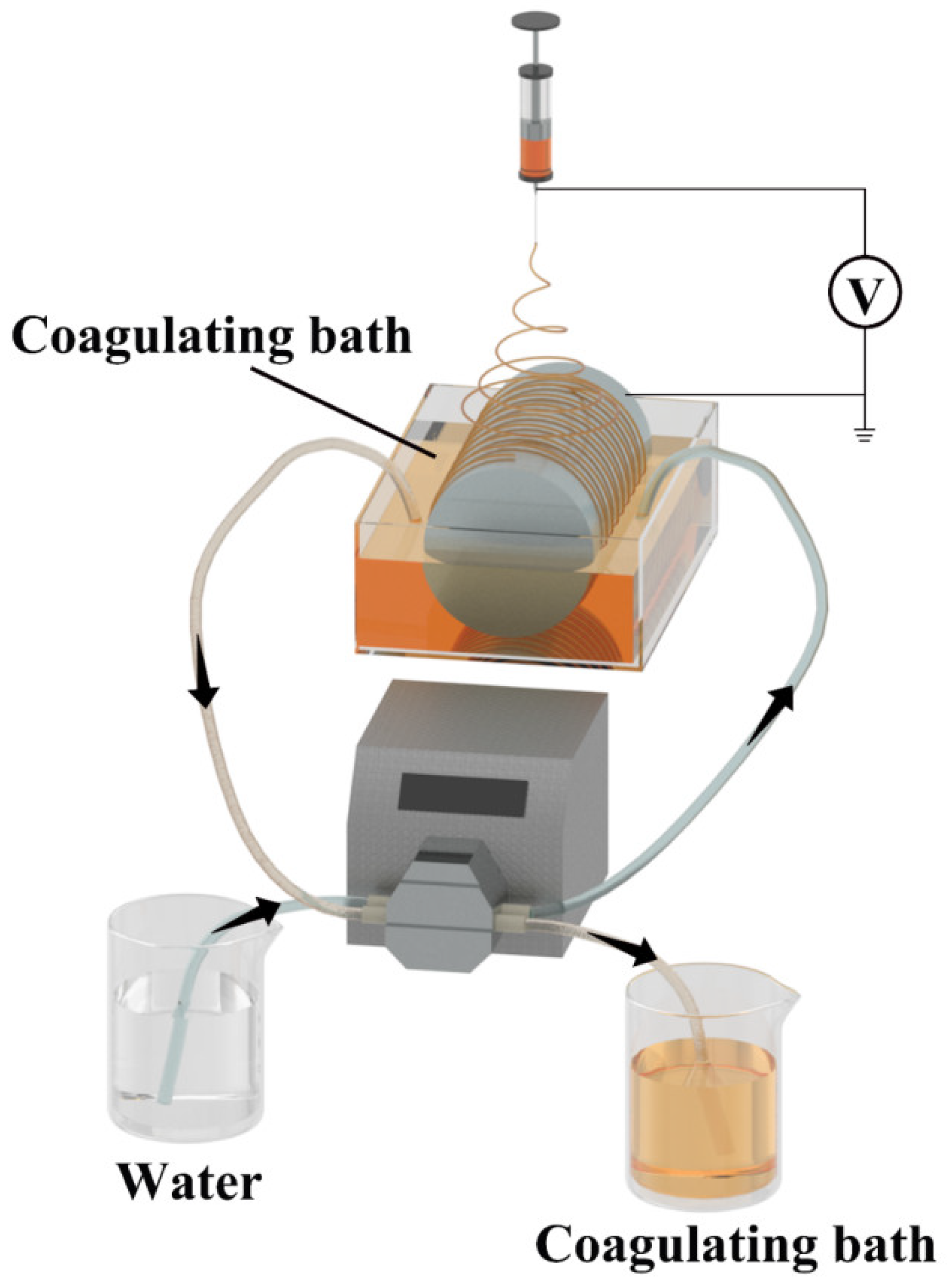
2.5. Electrospinning
2.6. Morphology
2.7. The Elution Effect of [Bmim]Ac
2.8. Crystal Structure Change of Cellulose
2.9. Mechanical Property
2.10. The Determination of Compound Concentration Determination
2.11. Antibacterial Activity
2.12. Strawberry Preservation
2.13. Degradation Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Biomass Components in Ginger Waste Residue
3.2. Elaboration Conditions of Electrospinning Solution
3.3. Viscosity and Conductivity
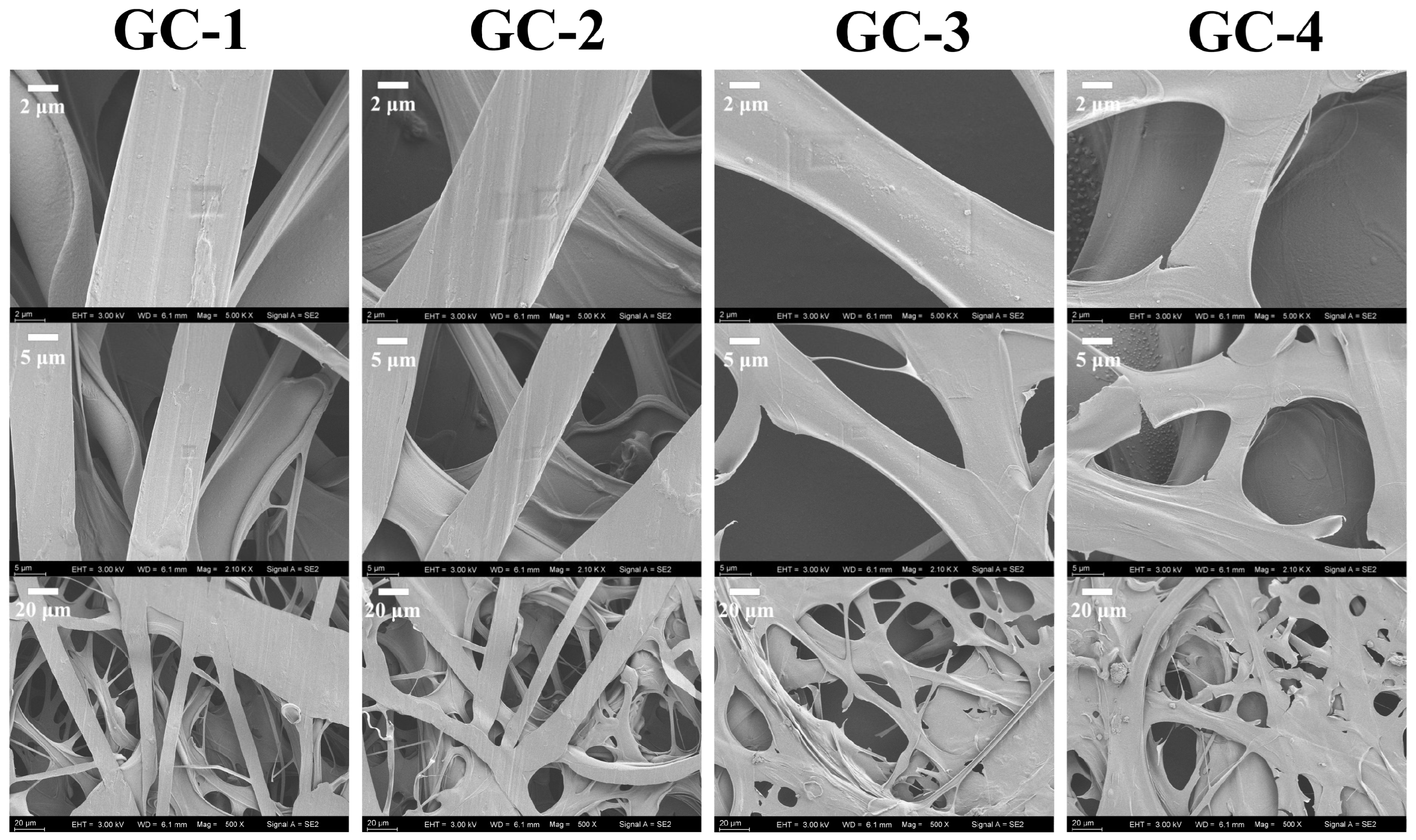
3.4. Morphology
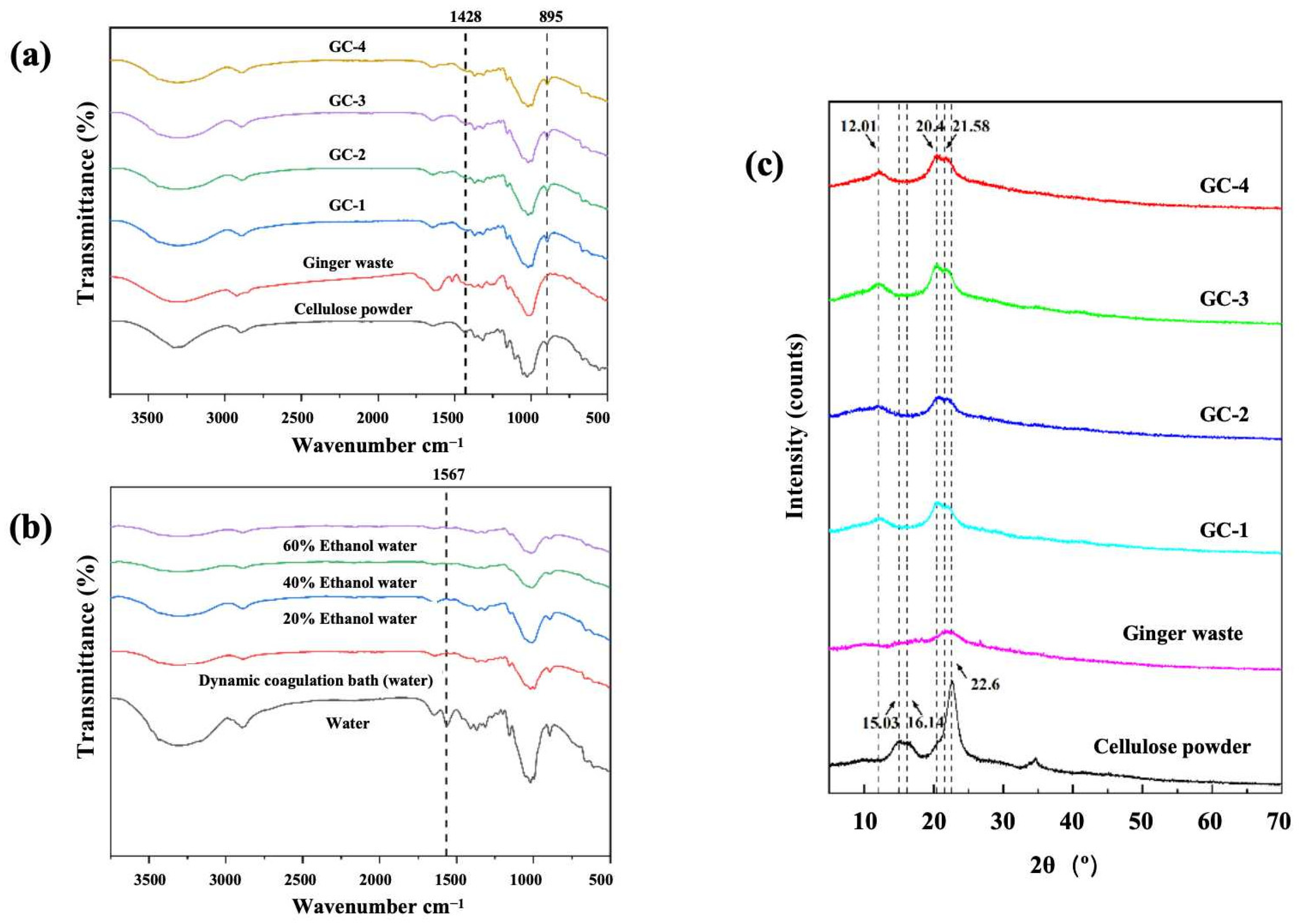
3.5. FTIR
3.6. XRD
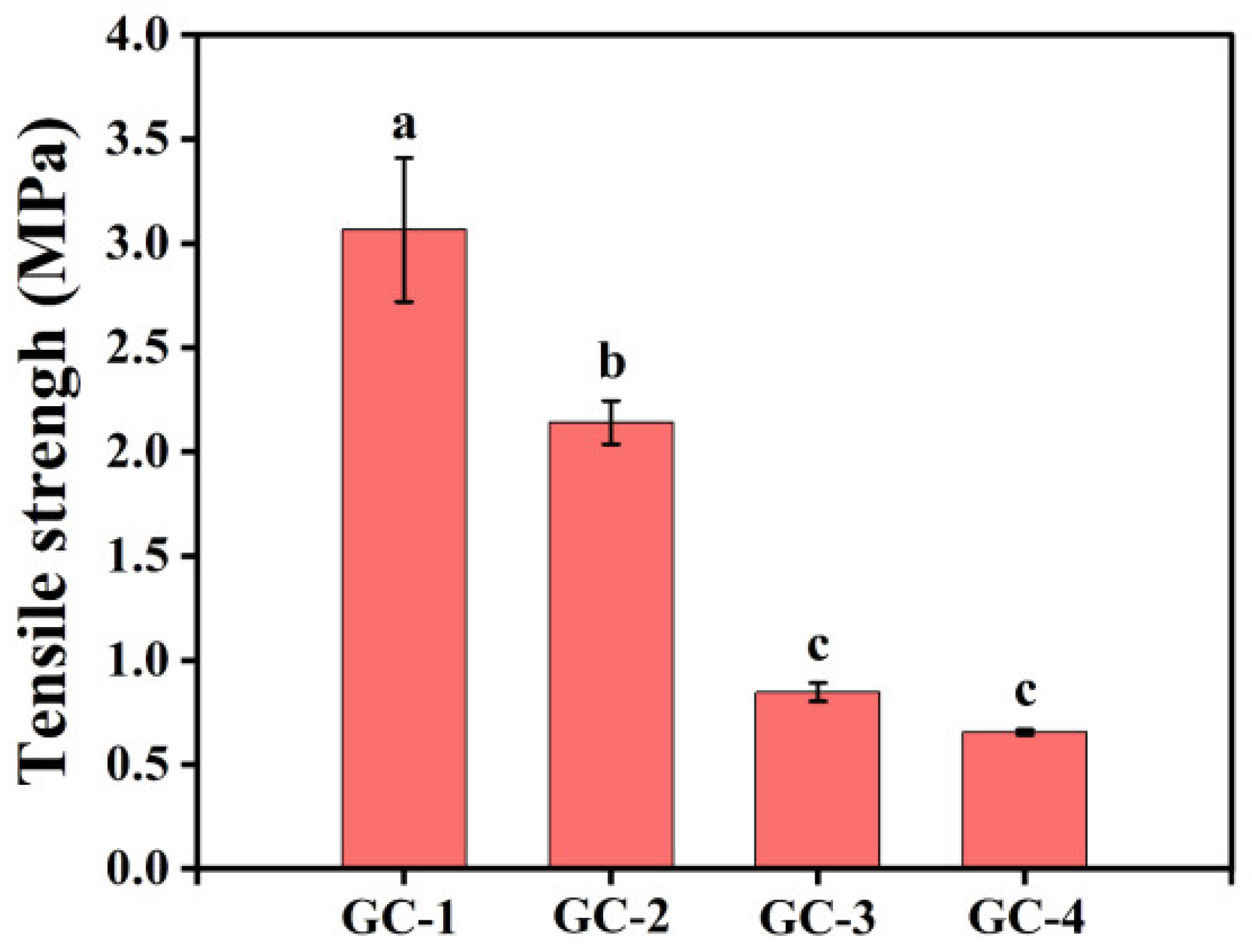
3.7. Mechanical Property
3.8. The Determination of Compound Concentration Determination
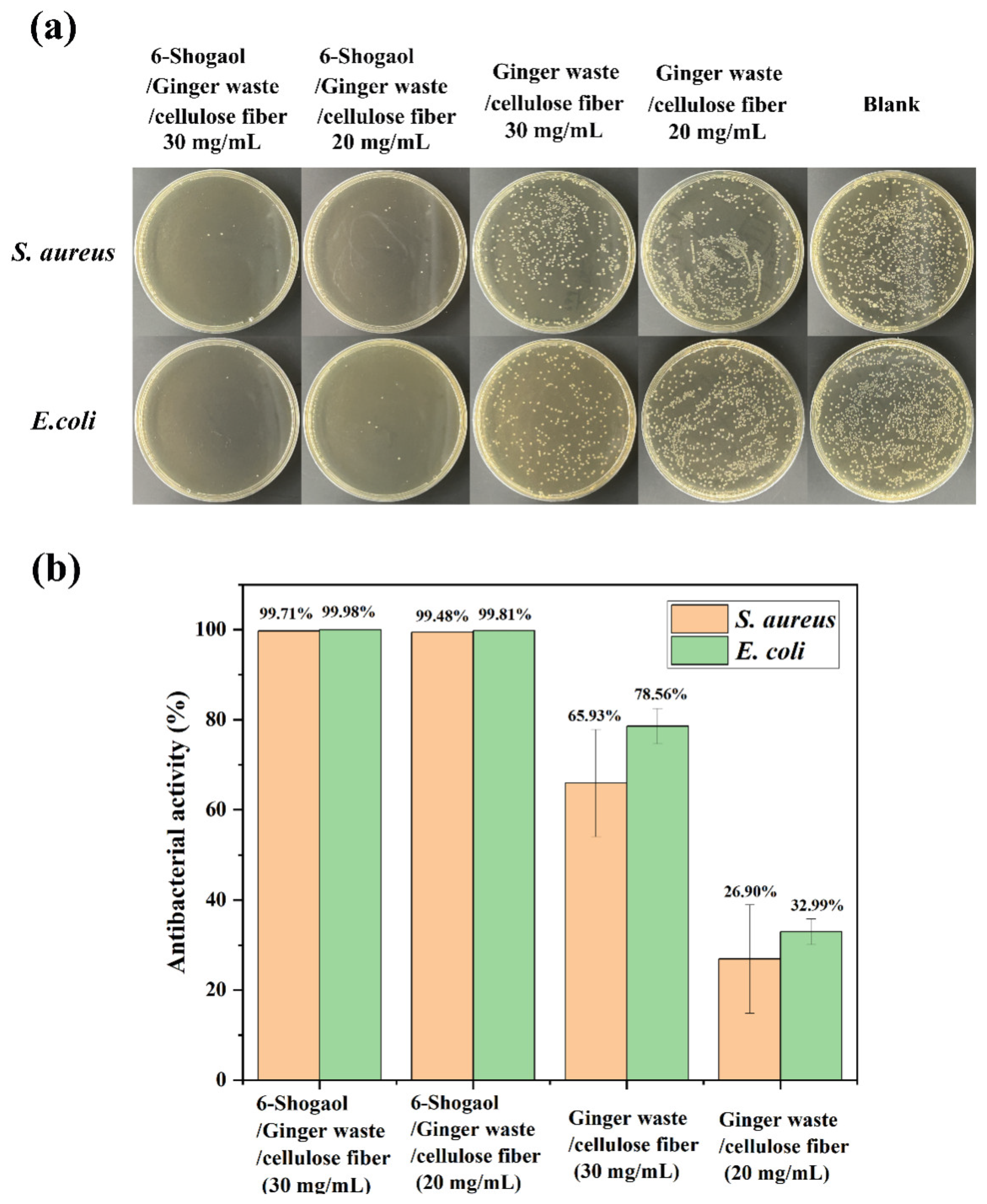
3.9. Antibacterial Activity
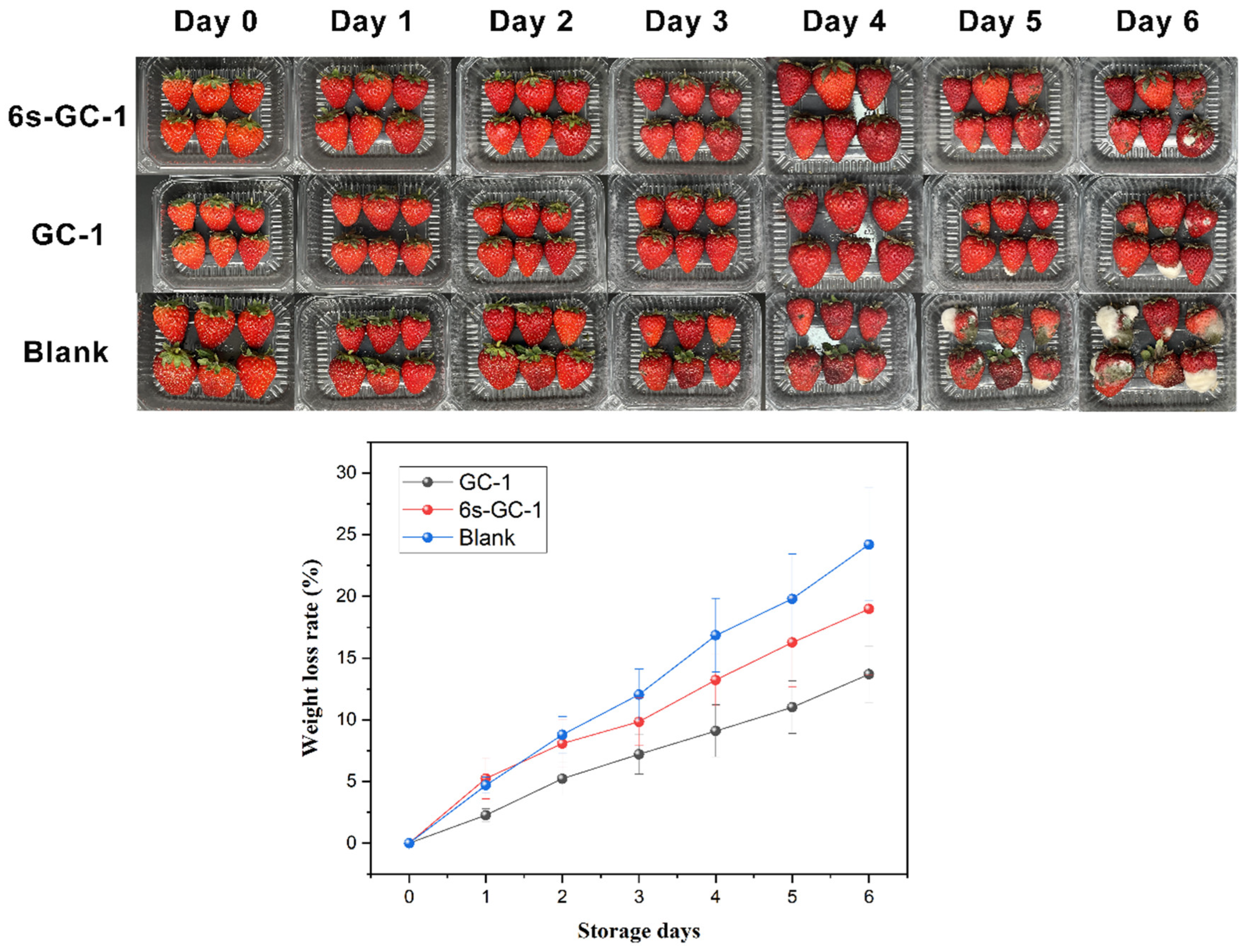
3.10. Application of the Ginger Waste/Cellulose Electrospun Fibers in Food Packaging
3.11. Film Degradability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ke, Q.; Ma, K.; Zhang, Y.; Meng, Q.; Huang, X.; Kou, X. Antibacterial Aroma Compounds as Property Modifiers for Electrospun Biopolymer Nanofibers of Proteins and Polysaccharides: A Review. Int. J. Biol. Macromol. 2023, 253, 126563. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Wang, H.; Huang, X.; Zhang, Y.; Meng, Q.; Kou, X. Direct Addition of Vanillin Improved the Physicochemical Properties and Antibacterial Activities of Gelatin/Sodium Carboxymethyl Cellulose Composite Film. Ind. Crops Prod. 2023, 206, 117653. [Google Scholar] [CrossRef]
- Zeng, J.; Ji, Q.; Liu, X.; Yuan, M.; Yuan, M.; Qin, Y. Electrospun Polylactic Acid/Poly (ε-Caprolactone) Fibrous Encapsulated Thymol/MIL-68(Al) as a Food Packaging Material. J. Mater Res. Technol. 2022, 18, 5032–5044. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Zhou, L.; Du, H.; Zhu, Z.; Wen, Y. Electrospun Pullulan/PVA Nanofibers Integrated with Thymol-Loaded Porphyrin Metal−organic Framework for Antibacterial Food Packaging. Carbohydr. Polym. 2021, 270, 118391. [Google Scholar] [CrossRef] [PubMed]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for Food Packaging. Trends Food Sci. Tech. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Alturki, A.M. Rationally Design of Electrospun Polysaccharides Polymeric Nanofiber Webs by Various Tools for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2021, 184, 648–665. [Google Scholar] [CrossRef]
- Hemmatian, T.; Seo, K.H.; Yanilmaz, M.; Kim, J. The Bacterial Control of Poly (Lactic Acid) Nanofibers Loaded with Plant-Derived Monoterpenoids via Emulsion Electrospinning. Polymers 2021, 13, 3405. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Seidi, F.; Youssefi Azarfam, M.; Khodadadi Yazdi, M.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J.; et al. Biopolymer-Based Composites for Tissue Engineering Applications: A Basis for Future Opportunities. Compos. Part B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Valachová, K.; El Meligy, M.A.; Šoltés, L. Hyaluronic Acid and Chitosan-Based Electrospun Wound Dressings: Problems and Solutions. Int. J. Biol. Macromol. 2022, 206, 74–91. [Google Scholar] [CrossRef]
- Gao, Y.; Ozel, M.Z.; Dugmore, T.; Sulaeman, A.; Matharu, A.S. A Biorefinery Strategy for Spent Industrial Ginger Waste. J. Hazard. Mater 2021, 401, 123400. [Google Scholar] [CrossRef]
- Kou, X.; Wang, X.; Ji, R.; Liu, L.; Qiao, Y.; Lou, Z.; Ma, C.; Li, S.; Wang, H.; Ho, C.-T. Occurrence, Biological Activity and Metabolism of 6-Shogaol. Food Funct. 2018, 9, 1310–1327. [Google Scholar] [CrossRef]
- Lan, X.; Wang, H.; Bai, J.; Miao, X.; Lin, Q.; Zheng, J.; Ding, S.; Li, X.; Tang, Y. Multidrug-Loaded Electrospun Micro/Nanofibrous Membranes: Fabrication Strategies, Release Behaviors and Applications in Regenerative Medicine. J. Control. Release 2021, 330, 1264–1287. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, H.; Huang, H.; Zhang, Y.; Wang, H.; Li, Y.; Wang, C. Allicin-Loaded Electrospun PVP/PVB Nanofibrous Films with Superior Water Absorption and Water Stability for Antimicrobial Food Packaging. ACS Food Sci. Technol. 2022, 2, 941–950. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; Ghorbani, M.; Mohammadi, M.; Pezeshki, A. Improvement of the Physico-Mechanical Properties of Antibacterial Electrospun Poly Lactic Acid Nanofibers by Incorporation of Guar Gum and Thyme Essential Oil. Colloid Surf. A 2021, 622, 126659. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Bondi, E.; Posati, T.; Sotgiu, G.; Zamboni, R.; Torreggiani, A.; Corticelli, F.; Lotti, N.; Aluigi, A. Effects of the Blending Ratio on the Design of Keratin/Poly(butylene succinate) Nanofibers for Drug Delivery Applications. Biomolecules 2021, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Si, Y.; Zhao, Y.; Ke, Q.; Hu, J. Electrospun Textile Strategies in Tendon to Bone Junction Reconstruction. Adv. Fiber Mater. 2023, 5, 764–790. [Google Scholar] [CrossRef]
- Araldi da Silva, B.; de Sousa Cunha, R.; Valério, A.; De Noni Junior, A.; Hotza, D.; Gómez González, S.Y. Electrospinning of Cellulose Using Ionic Liquids: An Overview on Processing and Applications. Eur. Polym. J. 2021, 147, 110283. [Google Scholar] [CrossRef]
- Ahn, Y.; Hu, D.-H.; Hong, J.H.; Lee, S.H.; Kim, H.J.; Kim, H. Effect of Co-Solvent on the Spinnability and Properties of Electrospun Cellulose Nanofiber. Carbohydr. Polym. 2012, 89, 340–345. [Google Scholar] [CrossRef]
- Bazbouz, M.B.; Taylor, M.; Baker, D.; Ries, M.E.; Goswami, P. Dry-Jet Wet Electrospinning of Native Cellulose Microfibers with Macroporous Structures from Ionic Liquids. J. Appl. Polym. Sci. 2019, 136, 47153. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Bonner, J.R.; Gurau, G.; Di Nardo, T.; Rogers, R.D. “Practical” Electrospinning of Biopolymers in Ionic Liquids. ChemSusChem 2017, 10, 106–111. [Google Scholar] [CrossRef]
- Pang, Z.; Wang, P.; Dong, C. Ultrasonic Pretreatment of Cellulose in Ionic Liquid for Efficient Preparation of Cellulose Nanocrystals. Cellulose 2018, 25, 7053–7064. [Google Scholar] [CrossRef]
- Kou, X.; Li, X.; Rahman, M.R.T.; Yan, M.; Huang, H.; Wang, H.; Su, Y. Efficient Dehydration of 6-Gingerol to 6-Shogaol Catalyzed by an Acidic Ionic Liquid under Ultrasound Irradiation. Food Chem. 2017, 215, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ciuzas, D.; Krugly, E.; Sriubaite, S.; Pauliukaityte, I.; Baniukaitiene, O.; Bulota, M.; Martuzevicius, D. Electrospun Cellulose Fibers from Ionic Liquid: Practical Implications toward Robust Morphology. J. Appl. Polym. Sci. 2022, 139, 51525. [Google Scholar] [CrossRef]
- Krugly, E.; Pauliukaityte, I.; Ciuzas, D.; Bulota, M.; Peciulyte, L.; Martuzevicius, D. Cellulose Electrospinning from Ionic Liquids: The Effects of Ionic Liquid Removal on the Fiber Morphology. Carbohydr. Polym. 2022, 285, 119260. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Dai, H.; Huang, H. Enhanced Properties of Tea Residue Cellulose Hydrogels by Addition of Graphene Oxide. J. Mol. Liq. 2017, 244, 110–116. [Google Scholar] [CrossRef]
- Kou, X.; Gao, N.; Xu, X.; Zhu, J.; Ke, Q.; Meng, Q. Preparation, Structural Analysis of Alcohol Aroma Compounds/β-Cyclodextrin Inclusion Complexes and the Application in Strawberry Preservation. Food Chem. 2024, 457, 140160. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The Relevance of Structural Features of Cellulose and Its Interactions to Dissolution, Regeneration, Gelation and Plasticization Phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef]
- Samayam, I.P.; Hanson, B.L.; Langan, P.; Schall, C.A. Ionic Liquid Induced Changes in Cellulose Structure Associated with Enhanced Biomass Hydrolysis. Biomacromolecules 2011, 12, 3091–3098. [Google Scholar] [CrossRef]
- Tiihonen, L.V.; Bernardo, G.; Dalgliesh, R.; Mendes, A.; Parnell, S.R. Influence of the Coagulation Bath on the Nanostructure of Cellulose Films Regenerated from an Ionic Liquid Solution. RSC Adv. 2024, 14, 12888–12896. [Google Scholar] [CrossRef]
- Quan, S.-L.; Kang, S.-G.; Chin, I.-J. Characterization of Cellulose Fibers Electrospun Using Ionic Liquid. Cellulose 2010, 17, 223–230. [Google Scholar] [CrossRef]
- Yang, C.; Chen, W.; Ye, B.; Nie, K. An Overview of 6-Shogaol: New Insights into Its Pharmacological Properties and Potential Therapeutic Activities. Food Funct. 2024, 15, 7252–7270. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Choi, P.; Ham, J.; Park, J.G.; Lee, J. Antibiofilm and Antivirulence Activities of 6-Gingerol and 6-Shogaol Against Candida albicans Due to Hyphal Inhibition. Front. Cell. Infect. Microbiol. 2018, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Liu, Q.; Yang, D.; Guo, P.; Gao, Y.; Mo, R.; Zhang, Y. Characterization of High Amylose Corn Starch-Cinnamaldehyde Inclusion Films for Food Packaging. Food Chem. 2023, 403, 134219. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Nam, H.; Chung, M. Conversion of 6-gingerol to 6-shogaol in ginger (Zingiber officinale) pulp and peel during subcritical water extraction. Food Chem. 2019, 270, 149–155. [Google Scholar] [CrossRef]

| 3 wt% Ginger Waste/[Bmim]Ac (g) | 10 wt% Cellulose Powder/[Bmim]Ac (g) | Polymer Concentration (wt%) | Result | |
|---|---|---|---|---|
| GC-0 | 0 | 10 | 10 | No jet formed |
| GC-1 | 1 | 10 | 9.36 | Jet formed |
| GC-2 | 1 | 9 | 9.3 | Jet formed |
| GC-3 | 1 | 8 | 9.22 | Jet formed |
| GC-4 | 1 | 7 | 9.13 | Jet formed |
| GC-5 | 1 | 4 | 8.6 | No jet formed |
| Temperature (°C) | Viscosity (mPa·s) | Conductivity (μs/cm) | |
|---|---|---|---|
| GC-1 | 40 | 22,693 ± 136 | 1429 ± 3 |
| GC-2 | 40 | 22,120 ± 408 | 1465 ± 22 |
| GC-3 | 40 | 21,080 ± 312 | 1480 ± 17 |
| GC-4 | 40 | 19,493 ± 264 | 1732 ± 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, X.; Ma, K.; Huang, X.; Wang, H.; Ke, Q. One-Pot Fabrication of Ginger-Waste-Derived Ionic Liquid Electrospun Films: An Efficient Preparation Strategy with Enhanced Antibacterial Functionality. Foods 2025, 14, 1058. https://doi.org/10.3390/foods14061058
Kou X, Ma K, Huang X, Wang H, Ke Q. One-Pot Fabrication of Ginger-Waste-Derived Ionic Liquid Electrospun Films: An Efficient Preparation Strategy with Enhanced Antibacterial Functionality. Foods. 2025; 14(6):1058. https://doi.org/10.3390/foods14061058
Chicago/Turabian StyleKou, Xingran, Kangning Ma, Xin Huang, Hui Wang, and Qinfei Ke. 2025. "One-Pot Fabrication of Ginger-Waste-Derived Ionic Liquid Electrospun Films: An Efficient Preparation Strategy with Enhanced Antibacterial Functionality" Foods 14, no. 6: 1058. https://doi.org/10.3390/foods14061058
APA StyleKou, X., Ma, K., Huang, X., Wang, H., & Ke, Q. (2025). One-Pot Fabrication of Ginger-Waste-Derived Ionic Liquid Electrospun Films: An Efficient Preparation Strategy with Enhanced Antibacterial Functionality. Foods, 14(6), 1058. https://doi.org/10.3390/foods14061058






