1. Introduction
With the fast-tracked progress in aquatic product processing and comprehensive utilization in China, the fisheries industry has become one of the three pillar industries [
1]. The high-value utilization of by-products from aquatic product processing has gradually become a key area of focus [
2]. By-products such as fish bones and skins are often regarded as waste in the fishery processing industry, leading to resource wastage and environmental pollution due to their ineffective utilization [
3]. As a result, the development of environmentally friendly and sustainable methods for the processing and high-value utilization of these by-products has become an urgent issue to address [
4]. In recent years, the aquaculture industry of mackerel (
Scomber japonicus) has experienced rapid growth, particularly after the policy lifting in 2016, which resulted in a surge in market demand. However, during the processing of mackerel, large amounts of by-products, such as fish skins, are discarded. These by-products are rich in collagen, making them ideal raw materials for the extraction of collagen peptides [
5]. Collagen peptides, as hydrolysis products, have low molecular weights, high absorption rates, and significant physiological functions and nutritional value. Specifically, collagen peptides derived from aquatic sources have gained attention as an ideal alternative to traditional animal-derived collagen due to their low allergenicity and high safety [
5,
6].
Currently, the preparation methods of fish-derived protein peptides mainly include chemical hydrolysis, microwave-assisted extraction, chemical synthesis, and enzymatic hydrolysis. Chemical hydrolysis is simple and cost-effective but may lead to modifications in amino acids and changes in functionality. Additionally, the process is relatively difficult to control, which limits its effectiveness in obtaining peptides with high biological activity [
7]. While microwave-assisted extraction improves extraction efficiency, the localized high temperatures may cause peptide degradation, affecting their biological activity [
8,
9,
10]. Although chemical synthesis allows for the precise production of target peptides, it is inefficient, costly, and not suitable for large-scale applications [
11]. Therefore, enzymatic hydrolysis, with its mild reaction conditions and higher peptide yields, has gradually become the preferred method for preparing fish-derived protein peptides [
12]. By optimizing enzymatic hydrolysis conditions, such as enzyme concentration, reaction time, and temperature, the yield and activity of peptides can be significantly enhanced [
13].
Furthermore, peptide purification is a key step in improving the quality and activity of fish-derived protein peptides. Traditional purification methods, such as membrane filtration [
14], gel filtration chromatography [
15,
16], and ion-exchange chromatography [
17,
18], are commonly used in the preliminary separation process. However, these methods often fail to provide sufficient purity, and some may involve contamination issues, limiting their effectiveness in large-scale applications [
19]. As a result, reverse-phase high-performance liquid chromatography (HPLC), with its high resolution and excellent operability, has been widely employed for the separation and purification of bioactive peptides [
20]. Additionally, liquid chromatography–tandem mass spectrometry (LC-MS/MS) technology, as an efficient tool for peptide sequence identification and bioactivity assessment, further enhances the depth and precision of peptide research [
21].
Based on the background outlined above, this study focused on the extraction of fish skin peptides from mackerel skin using enzymatic hydrolysis, with peptide yield serving as the optimization indicator. Building on this, the influence of various factors, including enzyme concentration, hydrolysis duration, and the solid-to-liquid ratio, was evaluated. The extraction and enzymatic hydrolysis parameters for mackerel skin peptides (HA-MPPs) were then optimized through Box–Behnken design and response surface methodology (RSM). Following this, HA-MPPs were purified via reverse-phase high-performance liquid chromatography (HPLC), and their bioactivity and peptide sequences were determined using multi-molecular infrared imaging, antioxidant assays, and liquid chromatography–tandem mass spectrometry (LC-MS/MS). This study explored the high-value utilization of mackerel by-products and provides experimental data to support their potential bioactivity and broad applications.
2. Materials and Methods
2.1. Materials
The mackerel skin, a by-product of the Scomber japonicus processing, was provided by Zhongyang Ecological Fishery Co., Ltd., Nantong, China, caught from the East China Sea. The skins were frozen and stored at −20 °C until they were required for the study. The compound protease, composed of alkaline protease and trypsin, with an enzyme activity of 1.5 AU/g, was obtained through fermentation of Bacillus amyloliquefaciens and Bacillus licheniformis, and was supplied by Novozymes Biotechnology Co., Ltd., Beijing, China Concentrated hydrochloric acid was obtained from Kerlings Reagents Co., Ltd., Shanghai, China, and phenol was acquired from McLin Bio-Tech Co., Ltd., Shanghai, China. Reagents such as dithiothreitol, iodoacetamide, and ammonium bicarbonate were purchased from Sigma-Aldrich, St. Louis, MI, USA. Acetonitrile and methanol were sourced from Thermo Fisher Scientific, Waltham, MA, USA. The C18 analytical column (4.6 mm × 250 mm, 5 μm) came from Agilent Technologies, Santa Clara, CA, USA, and the C18 preparative column (19 mm × 150 mm, 5 μm) was provided by Waters Corporation, Milford, MA, USA. The freezing embedding medium (OCT) was purchased from SAKURA, Tokyo, Japan. The ZnSe transmission slides (13 mm × 2 mm), as a component of the ATR imaging system’s transmission attachment, were used for infrared imaging analysis.
2.2. Single-Factor Experimental Design
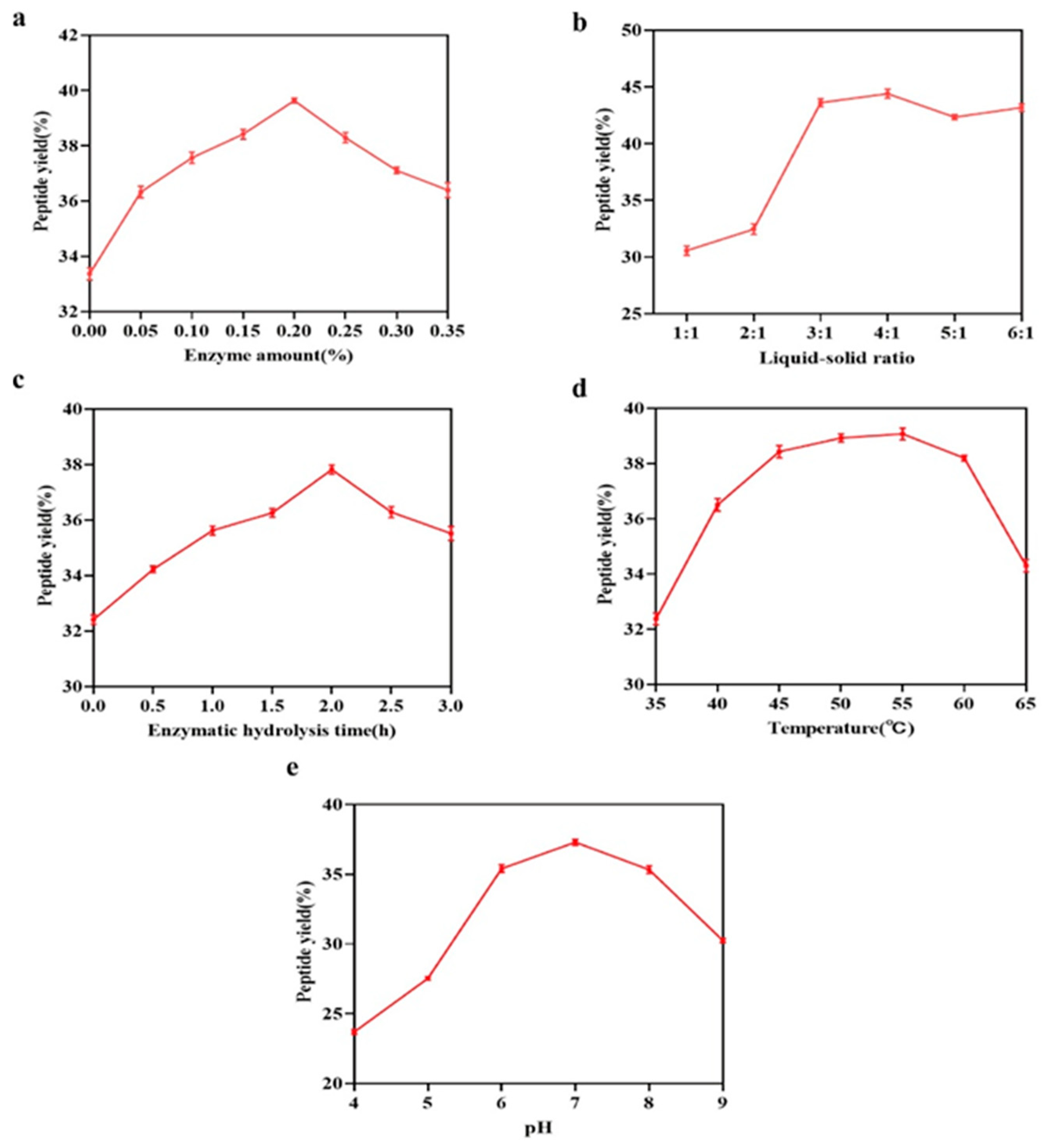
2.2.1. Enzyme Concentration
Mackerel skin was selected as the raw material, and water was added at a liquid-to-solid ratio of 3:1. Compound protease (a mixture of alkaline protease and trypsin) was applied at various concentrations (0, 0.05%, 0.1%, 0.15%, 0.2%, 0.25%, 0.3%, and 0.35%, relative to the quantity of raw material). Enzymatic hydrolysis, with an enzyme activity ratio of 1:1, was conducted at 50 °C for 4 h, followed by enzyme deactivation at 90 °C for 15 min to halt the reaction. Samples were then collected, and the peptide yield was quantified using the trichloroacetic acid (TCA) precipitation method [
22].
2.2.2. Liquid-to-Solid Ratio
Mackerel skin was used as the raw material, and a compound enzyme solution (0.2% enzyme concentration with an enzyme activity ratio of 1:1) was applied. A range of liquid-to-solid ratios (1:1, 2:1, 3:1, 4:1, 5:1, and 6:1) was tested. The enzymatic hydrolysis was performed at 50 °C for 4 h, followed by enzyme deactivation at 90 °C for 15 min. Samples were taken for peptide yield analysis.
2.2.3. Hydrolysis Duration
Mackerel skin was employed as the raw material, and 0.2% compound enzyme solution (enzyme activity ratio of 1:1) was added, maintaining a 3:1 ratio of liquid to solid. Enzymatic hydrolysis was conducted at 50 °C for various durations: 0, 0.5, 1, 1.5, 2, 2.5, and 3 h, followed by enzyme deactivation at 90 °C for 15 min to terminate the reaction. The peptide yield was determined by sampling at different time intervals.
2.2.4. Hydrolysis Temperature
Mackerel skin was selected as the starting material, and a 0.2% compound enzyme solution was introduced, maintaining a liquid-to-solid ratio of 3:1. Enzymatic hydrolysis was conducted at different temperatures (35 °C, 40 °C, 45 °C, 50 °C, 55 °C, 60 °C, and 65 °C) for a fixed period of 4 h, followed by enzyme deactivation at 90 °C for 15 min. The peptide yield was assessed from the resulting hydrolysates.
2.2.5. pH of Hydrolysis Reaction
Mackerel skin was utilized as the raw material, with the addition of 0.2% compound enzyme solution (enzyme activity ratio of 1:1) and a liquid-to-solid ratio of 3:1. Enzymatic hydrolysis was performed at varying pH levels (4, 5, 6, 7, 8, and 9) at 50 °C for 4 h, with subsequent enzyme deactivation at 90 °C for 15 min. The peptide yield was quantified by collecting and analyzing samples at different pH conditions.
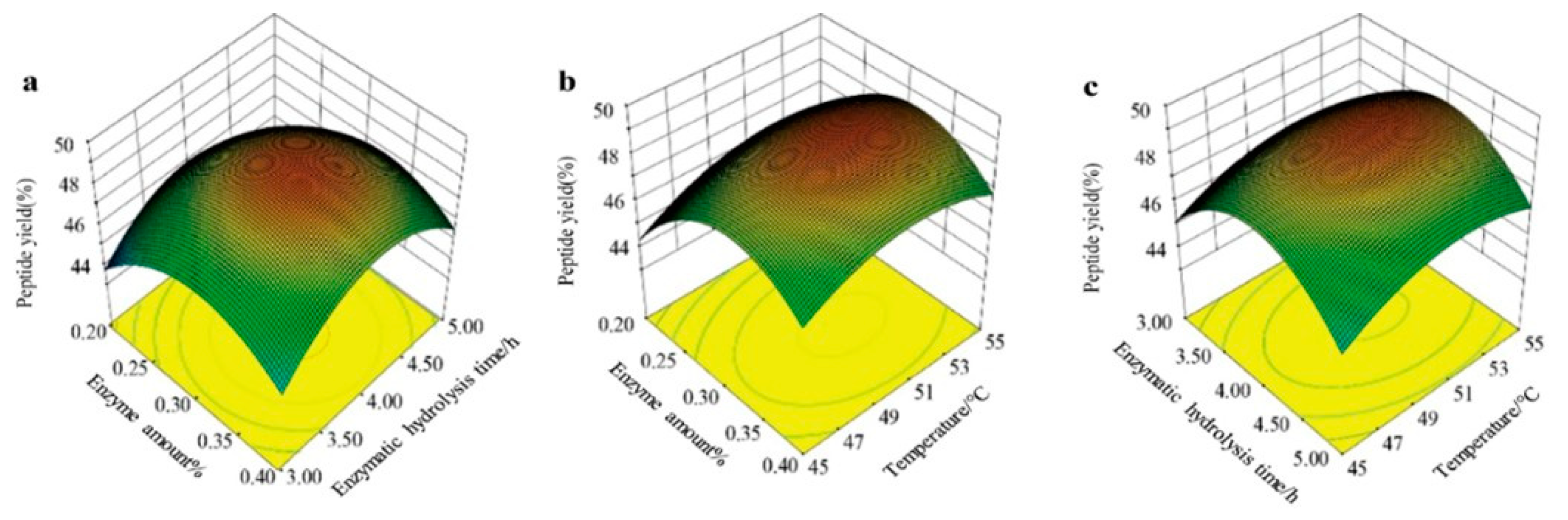
2.3. Response Surface Methodology Optimization
The peptide yield from mackerel skin was used as the response variable. Building on the results of the single-factor experiments, the impacts of enzyme concentration, temperature, and hydrolysis time were assessed. The Box–Behnken design, executed with Design Expert 8.0.6.1 software, was employed to fine-tune the extraction parameters [
23,
24]. The factors and levels for the optimization experiments are shown in
Table 1.
2.4. Optimization of Mackerel Skin Peptide Extraction Process
The extraction process of mackerel skin peptides was optimized utilizing the data from preliminary single-factor and response surface trials. Distilled water was added to the fish skin at a fish skin-to-water ratio of 1:3 (
w/
v) and subjected to a constant temperature extraction at 90 °C for 3 h. Subsequently, 2% compound protease was added, and enzymatic hydrolysis was implemented at 55 °C and pH 7.0, with 120 rpm water bath shaking for 2 h. After enzymatic hydrolysis, the enzyme was deactivated at 90 °C for 15 min. After the hydrolysate cooled to room temperature for 20 min, it was centrifuged at 8000 rpm for 15 min at 4 °C. The resulting supernatant was transferred into a 500 mL Erlenmeyer flask, and 1.5% activated carbon was added. The mixture was subjected to decolorization in a 60 °C water bath for 30 min, followed by filtration to obtain mackerel skin protein peptides (HA-MPPs). The peptides were then separated into three molecular weight fractions (10,000–3000 Da, 3000–1000 Da, and <1000 Da) using ultrafiltration [
25]. The fraction with a molecular weight below 1000 Da was chosen as the target peptide, freeze-dried, and stored at −4 °C for subsequent use.
2.5. Amino Acid Analysis of HA-MPPs
The amino acid determination method for HA-MPPs followed the national standard GB5009.124-2016 [
26]. A total of 15 mg of HA-MPP lyophilized powder was weighed into a hydrolysis tube, and 10 mL of 6 mol/L hydrochloric acid was added. After cooling at 4 °C for 5 min, three drops of phenol were added to the mixture for color development. The mixture was then vacuum-sealed and hydrolyzed at 110 °C for 22 h. The solution was then cooled and diluted to 50 mL; 1 mL of the filtrate was absorbed into an evaporating dish and vacuum-dried at 50 °C. The residue was dissolved in 1 mL of distilled water and vacuum-dried again until completely evaporated. The residue was then dissolved in 2 mL of pH 2.2 sodium citrate solution and filtered through a 0.22 μm aqueous-phase membrane. The filtrate was collected in an autosampler vial and stored at −20 °C for amino acid analysis using an amino acid analyzer (model LA-8080).
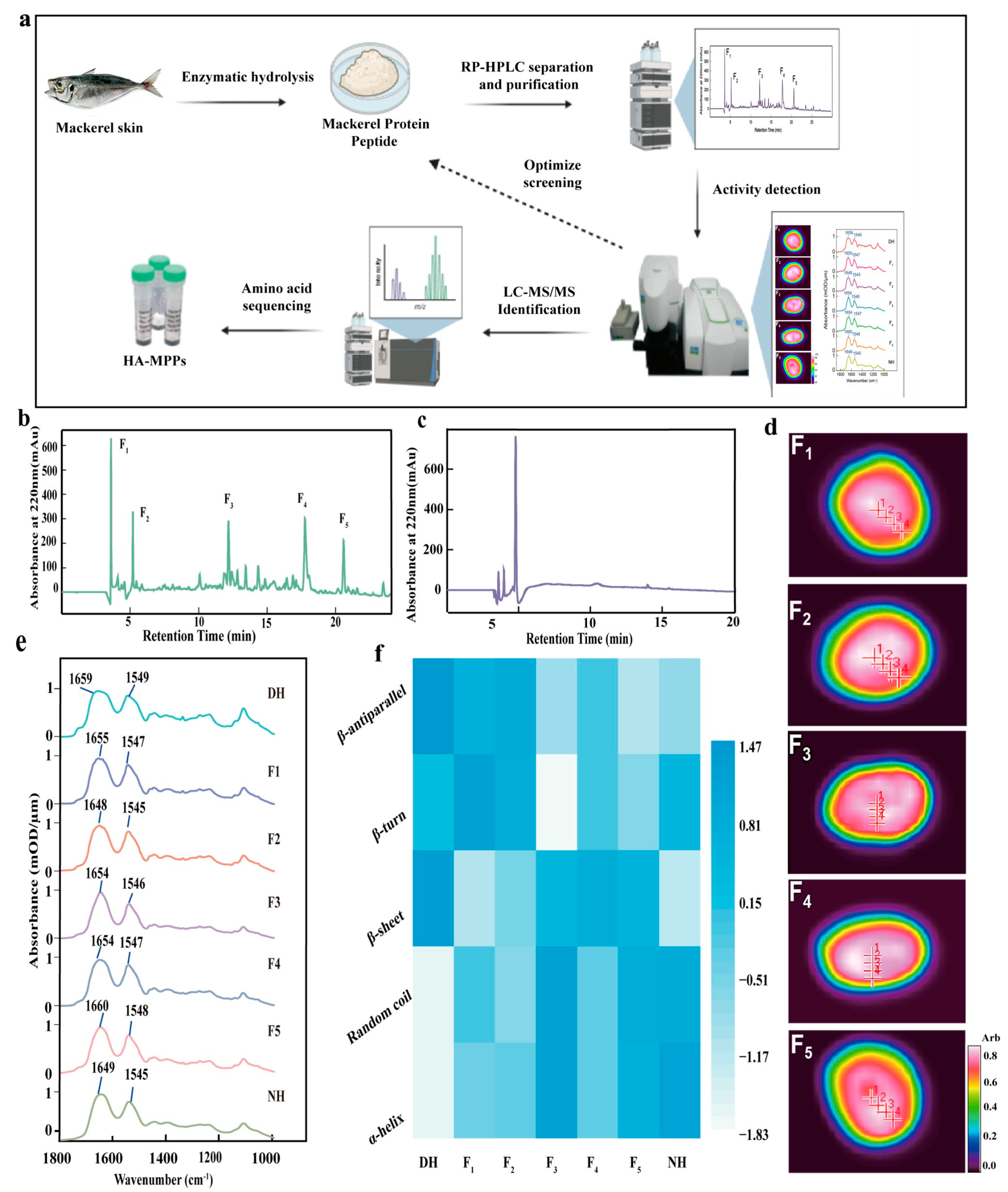
2.6. Purification of HA-MPPs by HPLC
HA-MPPs were prepared at a concentration of 20 mg/mL and passed through a 0.22 μm filter. The resulting solution was purified using a Waters e2695 HPLC system equipped with a UV detector, autosampler, and other modules. The separation was carried out using a SunFire C18 OBD preparative column (19 × 150 mm, 5 μm), with an injection volume of 1 mL. The mobile phase consisted of solvent A (0.05% trifluoroacetic acid, TFA, in water) and solvent B (acetonitrile). A gradient elution was performed at a flow rate of 4 mL/min for 35 min. The elution program was as follows: from 0 to 25 min, solvent A was decreased linearly from 95% to 70%, while solvent B was increased from 5% to 30%; from 25 to 28 min, solvent A was decreased linearly from 70% to 55%, while solvent B was increased from 30% to 45%; from 28 to 35 min, solvent A was increased from 45% to 95%, while solvent B was decreased from 55% to 5%. The eluate was monitored at 220 nm, and fractions were collected based on the chromatographic profile [
27]. These fractions were subsequently freeze-dried for bioactivity assessment.
2.7. Bioactivity Evaluation of HA-MPPs Fractions
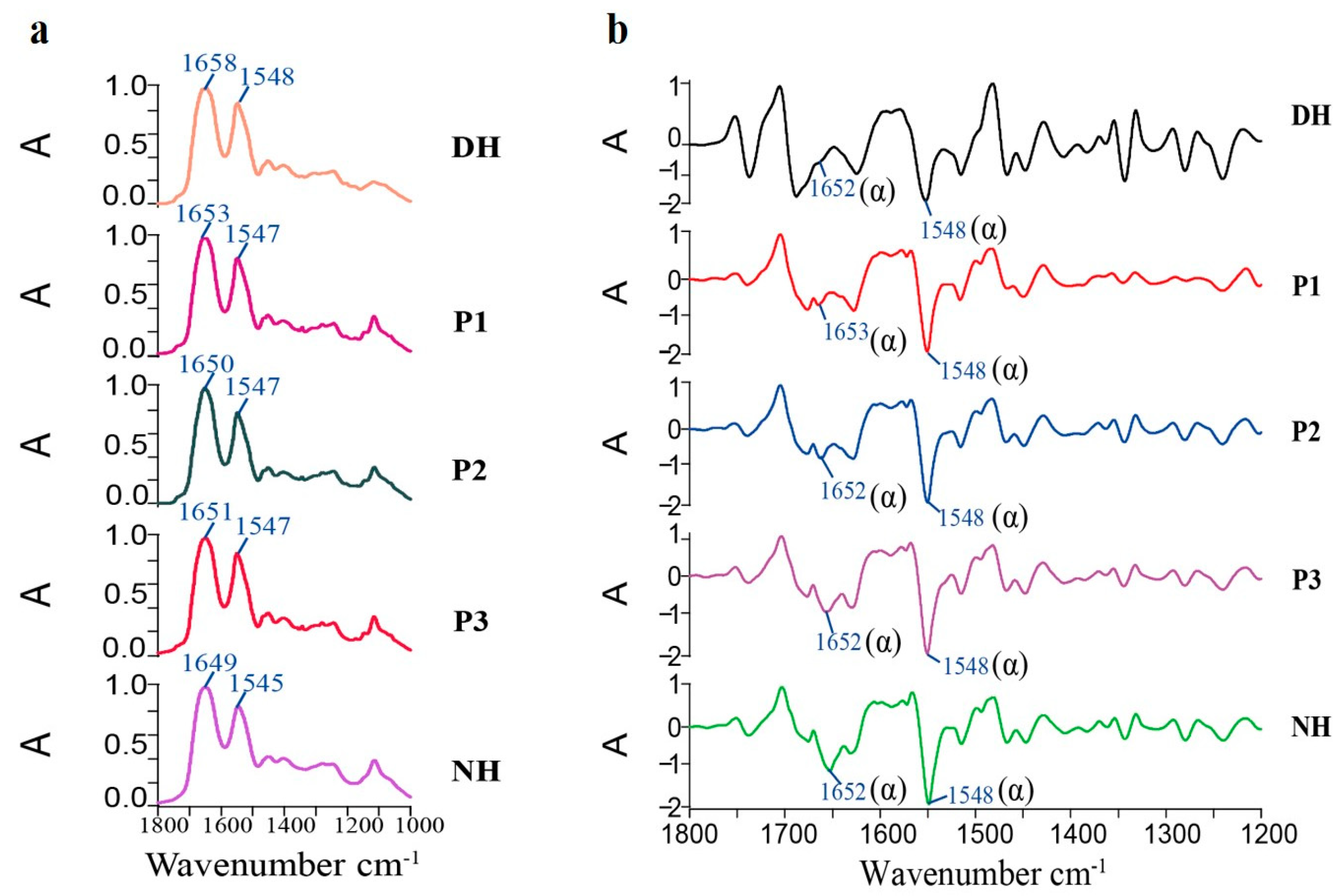
The bioactivity of the collected fractions was assessed using a Spotlight 400 Fourier Transform Infrared (FTIR) Imaging Spectrometer, Thermo Fisher Scientific, Waltham, MA, USA. This technique enabled the identification of the fraction with the most significant repair effect, which was selected for further bioactivity evaluation.
2.8. Purity Determination of HA-MPPs
The purity of the bioactive peptide fractions was analyzed using an analytical HPLC system [
28]. The fractions were prepared at a concentration of 10 mg/mL and passed through a 0.22 μm filter. Purification was carried out using a C18 analytical column (250 × 4.6 mm), and 20 μL of each sample was injected. The mobile phase consisted of solvent A (0.05% TFA in water) and solvent B (acetonitrile). A gradient elution was conducted at a flow rate of 0.833 mL/min for 40 min with the following conditions: 0–5 min, 80% solvent A; 5–30 min, 80–55% solvent A; 30–35 min, 55–70% solvent A; 35–40 min, 70–95% solvent A. The eluate was monitored at 220 nm, and fractions were collected based on the chromatographic data. The fractions collected were then freeze-dried for subsequent analysis.
2.9. LC-MS/MS Analysis of the Amino Acid Sequence of High-Activity HA-MPPs
LC-MS/MS was employed to further separate and identify the component with the highest bioactivity. Dithiothreitol (DTT) was utilized to reduce disulfide bonds within the peptides, facilitating complete denaturation and enabling the separation of peptide chains. Following reduction, iodoacetamide (IAM) was introduced to alkylate the cysteine residues, thereby preventing the reformation of disulfide bonds and stabilizing the peptide structure. The sample was subsequently buffered with ammonium bicarbonate (NH
4HCO
3) to maintain an optimal pH environment for enzymatic digestion, ensuring compatibility with LC-MS/MS analysis. After LC analysis, the component was introduced into a Q-Exactive Plus mass spectrometer for sequencing. The EASY-nLC 1200 system coupled with a C18 micro-column (75 μm × 15 cm, 3 μm) was used for the analysis [
29]. The chromatographic conditions were set as follows: mobile phase A was a 0.1% formic acid aqueous solution, while mobile phase B consisted of an 80% acetonitrile aqueous solution containing 0.1% formic acid. The elution gradient was configured as follows: 0–3 min, 2–6% B; 3–42 min, 6–20% B; 42–47 min, 22–35% B; 47–48 min, 35–100% B; 48–60 min, 100% B. Amino acid sequence analysis was carried out using the PEAKS Studio 10.0 search engine.
2.10. Synthesis of High-Activity Mackerel Protein Peptides
The peptides identified through mass spectrometry were produced by Gil Biochemical Co., Ltd., Shanghai, China. The purity and molecular weight of the produced peptides were assessed using RP-HPLC with an AU-2000 LC system (4.6 mm × 250 mm, 5 μm) and analyzed by mass spectrometry (Agilent 61258B, Santa Clara, CA, USA.).
2.11. Permeability Assessment of HA-MPPs
The biological activity of HA-MPPs was characterized using infrared ATR imaging. The damaged hair samples treated with P1, P2, and P3 in
Section 2.11 were analyzed via ATR imaging. The samples were embedded in OCT, processed into frozen sections, and subjected to ATR imaging to obtain protein optical imaging and infrared absorption maps of the hair cross-sections. These results were compared with those of normal hair samples. The parameters for spectral acquisition were configured as follows: a wavenumber range from 4000 to 750 cm
−1, a resolution of 4 cm
−1, a pixel size of 6.25 μm, and 16 scans for each pixel.
2.12. HA-MPP Antioxidant Activity Assay
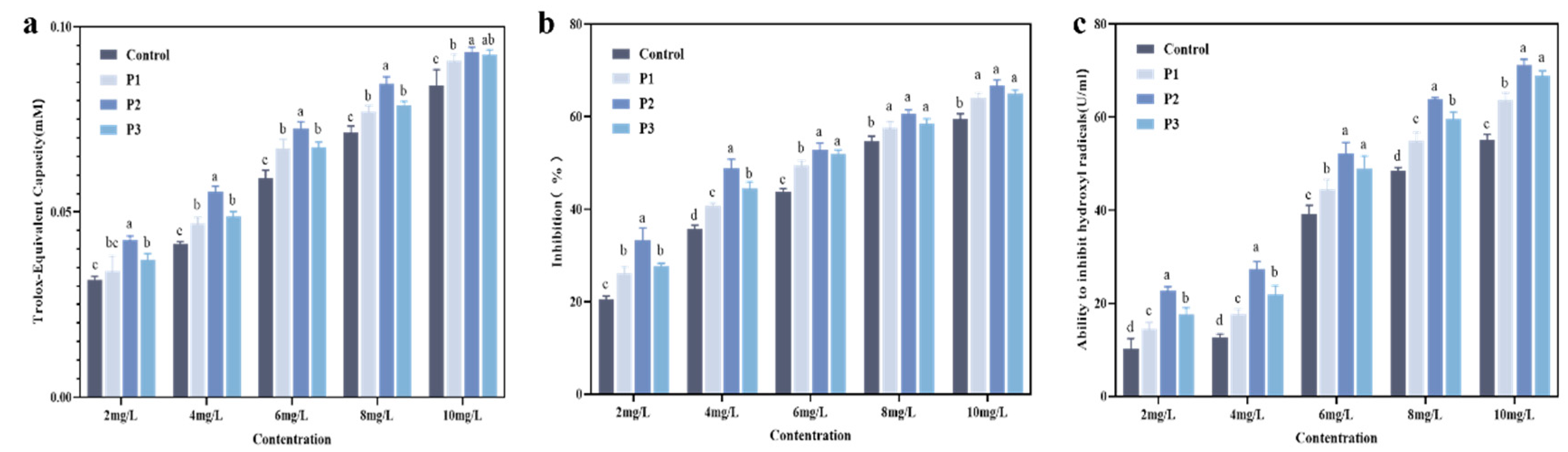
2.12.1. Total Antioxidant Activity Test
The overall antioxidant activity was measured using the ABTS approach with an ABTS assay kit [
30]. According to the kit instructions, a working solution was prepared. The antioxidant capacity of a Trolox standard solution was first measured to generate a standard curve. Then, the working solution was incubated with different types of HA-MPPs (crude peptides, P1, P2, P3) at room temperature for 6 min, and the absorbance was measured at 414 nm [
31].
2.12.2. Total Superoxide Dismutase (SOD) Activity Assay
The total SOD activity was measured using an SOD Assay Kit, Yuan Ye Biotechnology Co., Ltd., Shanghai, China. Following the kit instructions, the working solution was prepared and incubated with different types of HA-MPPs (crude peptides, P1, P2, P3) at 37 °C for 30 min. The absorbance was then measured at 560 nm [
32].
2.12.3. Hydroxyl Radical (OH-) Scavenging Activity Assay
The scavenging ability of hydroxyl radicals was assessed using a hydroxyl radical scavenging activity assay kit. A working solution was prepared according to the kit instructions, and the solution was pre-warmed at 37 °C for 3 min. Different types of HA-MPPs (crude peptides, P1, P2, P3) were then mixed with the working solution and incubated at 37 °C for 20 min. The absorbance was measured at 550 nm at room temperature.
2.13. Data Processing
GraphPad Prism 9.5.1 and SPSS 27.0 software were used for the creation of relevant data charts and statistical analysis, including variance analysis.
4. Conclusions
Based on the experimental results, this study proposes an innovative method for extracting mackerel protein peptides from mackerel by-products (fish skin) using targeted enzymatic hydrolysis. Through single-factor experiments and response surface optimization, the optimal extraction parameters were determined: enzyme concentration of 0.22%, hydrolysis time of 2.03 h, hydrolysis temperature of 55.05 °C, and a solid-to-liquid ratio of 1:3, resulting in a peptide yield of 59.66%. Subsequently, multi-stage separation and purification techniques, including ultrafiltration and HPLC, were employed to successfully isolate HA-MPPs. Further mass spectrometry sequencing identified the main peptide fragments of HA-MPPs, such as P1, P2, and P3. Through infrared imaging, the purified HA-MPPs demonstrated significant bio-repair activity, effectively penetrating the hair cuticle to repair damaged keratin and enhance the strength and resilience of hair fibers. Further antioxidant assessments revealed that HA-MPPs exhibited strong antioxidant activity, helping to reduce oxidative hair damage and maintain hair health.
In conclusion, this study not only provided a new strategy for the high-value utilization of mackerel by-products but also demonstrated the potential of HA-MPPs in hair repair. By combining targeted enzyme extraction with bioactivity screening, a marine protein peptide with good biological activity was successfully developed. This research lays a theoretical foundation for the future development of hair care medical products and offers a new direction for the high-value utilization of fish by-products.