Genomic Analysis of Two Histamine-Producing Strains Isolated from Yellowfin Tuna
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains Isolation and Identification
2.2. Phenotypic Characterization
2.3. Determination of Histamine Production
2.4. Genome Sequencing and Assembly
2.5. Gene Function Annotation
2.6. Average Nucleotide Identity (ANI) Analysis
2.7. Comparative Analysis of Homologous Genes of GWT 902 and GWT 904
3. Results and Discussion
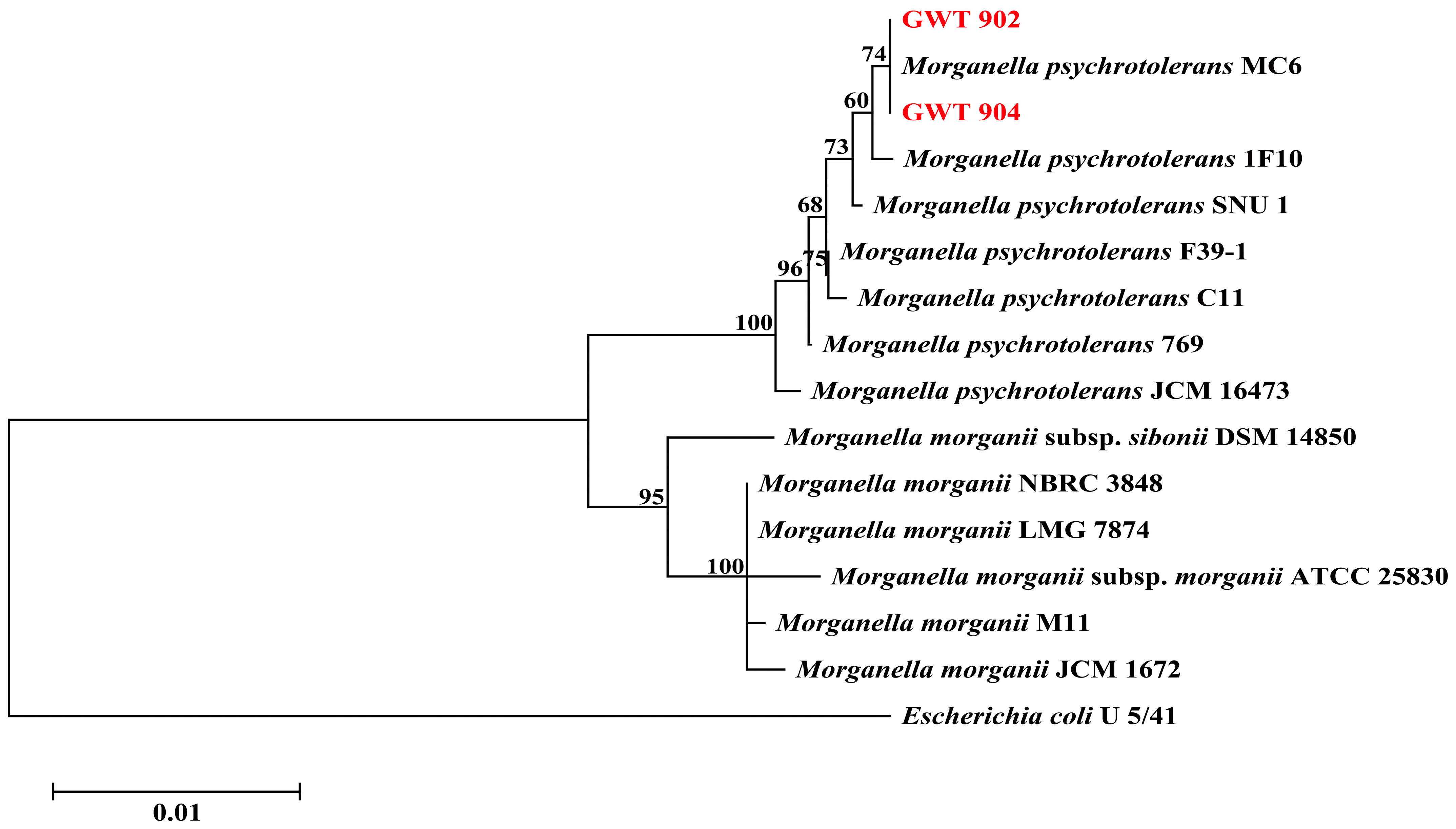
3.1. Strains Identification Analysis
3.2. Histamine Determination
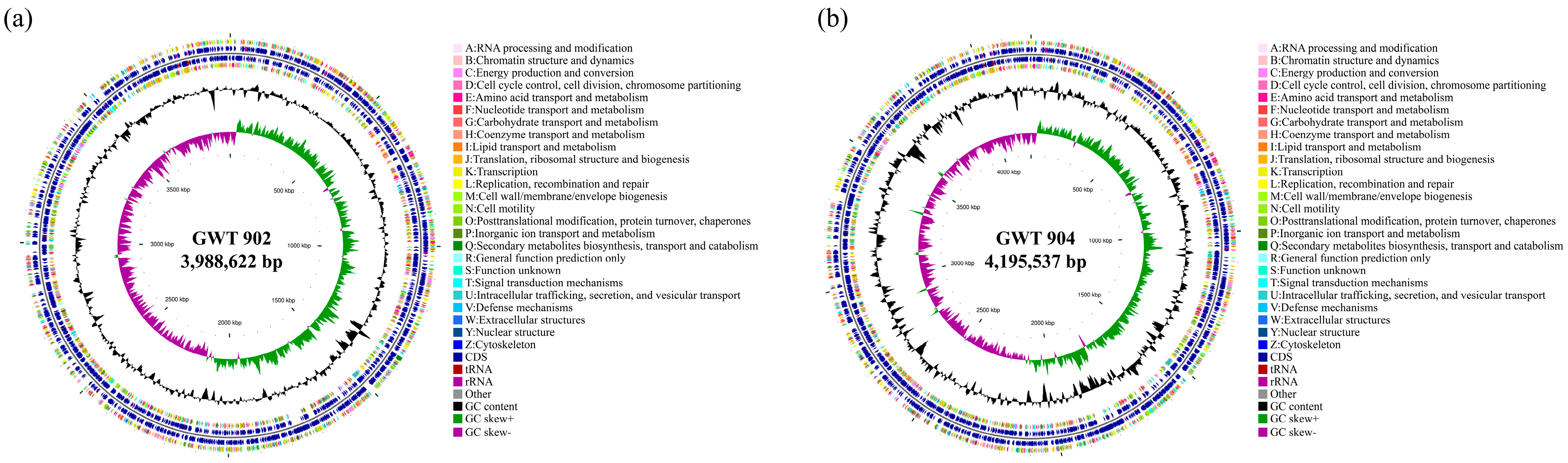
3.3. Genome Features of GWT 902 and GWT 904
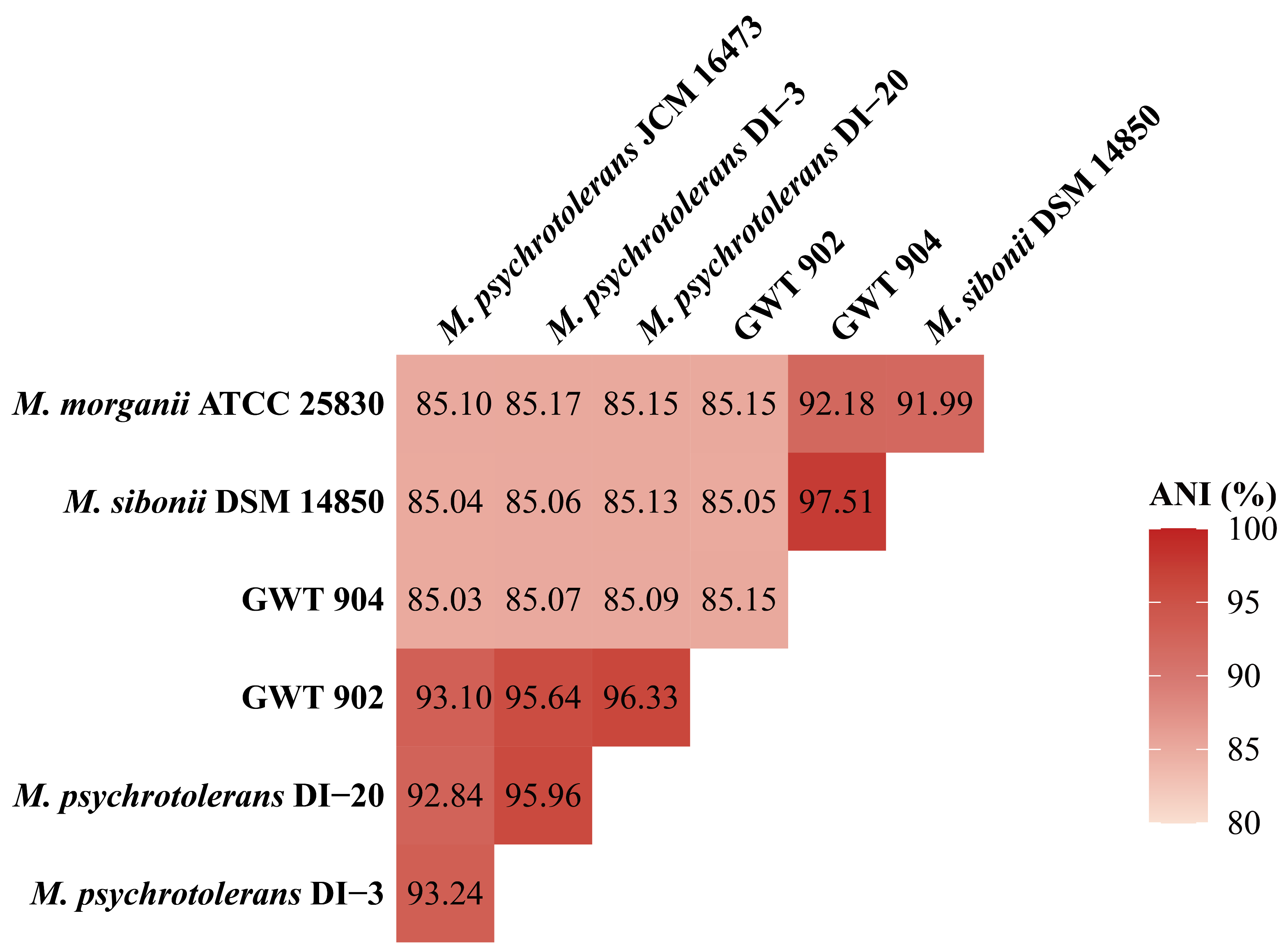
3.4. ANI Analysis
3.5. Functional Annotation of GWT 902 and GWT 904
3.6. Comparative Analysis of Homologous Genes of GWT 902 and GWT 904
3.7. Histamine Metabolism
3.8. Putrescine Metabolism
3.9. Sulfur Metabolism
3.10. Lipase and Protease
3.11. Quorum Sensing System
3.12. Adaptation to Stress
3.13. Drug Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omer, A.K.; Mohammed, R.R.; Ameen, P.S.M.; Abas, Z.A.; Ekici, K. Presence of Biogenic Amines in Food and Their Public Health Implications: A Review. J. Food Prot. 2021, 84, 1539–1548. [Google Scholar] [CrossRef]
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and Other Biogenic Amines in Food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef]
- DeBEER, J.; Bell, J.W.; Nolte, F.; Arcieri, J.; Correa, G. Histamine Limits by Country: A Survey and Review. J. Food Prot. 2021, 84, 1610–1628. [Google Scholar] [CrossRef]
- Landete, J.M.; De las Rivas, B.; Marcobal, A.; Muñoz, R. Updated Molecular Knowledge about Histamine Biosynthesis by Bacteria. Crit. Rev. Food Sci. Nutr. 2008, 48, 697–714. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An Overview of Histamine and Other Biogenic Amines in Fish and Fish Products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef]
- Nevado, D.L.; Delos Santos, S.; Bastian, G.; Deyta, J.; Managuelod, E.; Fortaleza, J.A.; De Jesus, R. Detection, Identification, and Inactivation of Histamine-Forming Bacteria in Seafood: A Mini-Review. J. Food Prot. 2023, 86, 100049. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Creton, E.; Perrin, A.; Girlich, D.; Emeraud, C.; Jousset, A.B.; Duque, M.; Jacquemin, A.; Hopkins, K.; Bogaerts, P.; et al. Spread of Carbapenemase-Producing Morganella Spp from 2013 to 2021: A Comparative Genomic Study. Lancet Microbe 2024, 5, e547–e558. [Google Scholar] [CrossRef]
- Emborg, J.; Dalgaard, P. Growth, Inactivation and Histamine Formation of Morganella psychrotolerans and Morganella morganii —Development and Evaluation of Predictive Models. Int. J. Food Microbiol. 2008, 128, 234–243. [Google Scholar] [CrossRef]
- Wang, D.; Yamaki, S.; Kawai, Y.; Yamazaki, K. Histamine Production Behaviors of a Psychrotolerant Histamine-Producer, Morganella psychrotolerans, in Various Environmental Conditions. Curr. Microbiol. 2020, 77, 460–467. [Google Scholar] [CrossRef]
- Emborg, J.; Ahrens, P.; Dalgaard, P. Morganella psychrotolerans—Identification, Histamine Formation and Importance for Histamine Fish Poisoning. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, October 2007. [Google Scholar]
- Zhang, R.; Yang, T.; Zhang, Q.; Liu, D.; Elhadidy, M.; Ding, T. Whole-Genome Sequencing: A Perspective on Sensing Bacterial Risk for Food Safety. Curr. Opin. Food Sci. 2022, 47, 100888. [Google Scholar] [CrossRef]
- Wells, J.M.; Bennik, M.H.J. Genomics of Food-Borne Bacterial Pathogens. Nutr. Res. Rev. 2003, 16, 21. [Google Scholar] [CrossRef]
- Shelburne, S.A.; Kim, J.; Munita, J.M.; Sahasrabhojane, P.; Shields, R.K.; Press, E.G.; Li, X.; Arias, C.A.; Cantarel, B.; Jiang, Y.; et al. Whole-Genome Sequencing Accurately Identifies Resistance to Extended-Spectrum β-Lactams for Major Gram-Negative Bacterial Pathogens. Clin. Infect. Dis. 2017, 65, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, N.; Hess, C.; Hess, M.; Alispahic, M. Sequencing of Five Poultry Strains Elucidates Phylogenetic Relationships and Divergence in Virulence Genes in Morganella morganii. BMC Genom. 2020, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.; Chen, S.; Wu, Y.; Li, C.; Wang, Y. Contamination of Morganella psychrotolerans in Fish Products and Histamine Production Capacity of the Isolated Strains. Food Sci. 2024, 45, 5275–5282. [Google Scholar] [CrossRef]
- Drancourt, M.; Bollet, C.; Carlioz, A.; Martelin, R.; Gayral, J.-P.; Raoult, D. 16S Ribosomal DNA Sequence Analysis of a Large Collection of Environmental and Clinical Unidentifiable Bacterial Isolates. J. Clin. Microbiol. 2000, 38, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Emborg, J.; Dalgaard, P.; Ahrens, P. Morganella psychrotolerans Sp. Nov., a Histamine-Producing Bacterium Isolated from Various Seafoods. Int. J. Syst. Evol. Microbiol. 2006, 56, 2473–2479. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A Self-Training Method for Prediction of Gene Starts in Microbial Genomes. Implications for Finding Sequence Motifs in Regulatory Regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Stothard, P.; Wishart, D.S. Circular Genome Visualization and Exploration Using CGView. Bioinformatics 2005, 21, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, S.; Zheng, J.; Zhang, Y.; Zhou, H.; Zhang, Z.; Cao, X.; Shen, H. Identification of an Aerococcus urinaeequi Isolate by Whole Genome Sequencing and Average Nucleotide Identity Analysis. J. Glob. Antimicrob. Resist. 2022, 29, 353–359. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir-Butler, K.; Bowers, J.C.; Benner, R.A. Prevalence and Characterization of High Histamine-Producing Bacteria in Gulf of Mexico Fish Species. J. Food Prot. 2015, 78, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA–DNA Hybridization Values and Their Relationship to Whole-Genome Sequence Similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, Information, Knowledge and Principle: Back to Metabolism in KEGG. Nucl. Acids Res. 2014, 42, D199–D205. [Google Scholar] [CrossRef]
- Koonin, E.V. Orthologs, Paralogs, and Evolutionary Genomics. Annu. Rev. Genet. 2005, 39, 309–338. [Google Scholar] [CrossRef]
- Medini, D.; Donati, C.; Tettelin, H.; Masignani, V.; Rappuoli, R. The Microbial Pan-Genome. Curr. Opin. Genet. Dev. 2005, 15, 589–594. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Xiao, Y.; Zhang, S.; Sun, Y.; Liu, Z.; Chu, C.; Hu, X.; Yi, J. Biodiversity of Lactic Acid Bacteria in Traditional Fermented Foods in Yunnan Province, China, and Comparative Genomics of Lactobacillus plantarum. Fermentation 2023, 9, 402. [Google Scholar] [CrossRef]
- Carr, V.R.; Shkoporov, A.; Hill, C.; Mullany, P.; Moyes, D.L. Probing the Mobilome: Discoveries in the Dynamic Microbiome. Trends Microbiol. 2021, 29, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Vo, J.L.; Martínez Ortiz, G.C.; Subedi, P.; Keerthikumar, S.; Mathivanan, S.; Paxman, J.J.; Heras, B. Autotransporter Adhesins in Escherichia Coli Pathogenesis. Proteomics 2017, 17, 1600431. [Google Scholar] [CrossRef] [PubMed]
- Lingzhi, L.; Haojie, G.; Dan, G.; Hongmei, M.; Yang, L.; Mengdie, J.; Chengkun, Z.; Xiaohui, Z. The Role of Two-Component Regulatory System in β-Lactam Antibiotics Resistance. Microbiol. Res. 2018, 215, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Stock, A.M. Two-Component Systems. Curr. Biol. 2019, 29, R724–R725. [Google Scholar] [CrossRef]
- Sultan, M.; Arya, R.; Kim, K.K. Roles of Two-Component Systems in Pseudomonas aeruginosa Virulence. Int. J. Mol. Sci. 2021, 22, 12152. [Google Scholar] [CrossRef]
- Vaaler, G.L.; Snell, E.E. Pyridoxal 5’-Phosphate Dependent Histidine Decarboxylase: Overproduction, Purification, Biosynthesis of Soluble Site-Directed Mutant Proteins, and Replacement of Conserved Residues. Biochemistry 1989, 28, 7306–7313. [Google Scholar] [CrossRef]
- Ferrario, C.; Borgo, F.; de las Rivas, B.; Muñoz, R.; Ricci, G.; Fortina, M.G. Sequencing, Characterization, and Gene Expression Analysis of the Histidine Decarboxylase Gene Cluster of Morganella morganii. Curr. Microbiol. 2014, 68, 404–411. [Google Scholar] [CrossRef]
- Ryser, L.T.; Arias-Roth, E.; Perreten, V.; Irmler, S.; Bruggmann, R. Genetic and Phenotypic Diversity of Morganella morganii Isolated From Cheese. Front. Microbiol. 2021, 12, 738492. [Google Scholar] [CrossRef]
- Remenant, B.; Jaffrès, E.; Dousset, X.; Pilet, M.-F.; Zagorec, M. Bacterial Spoilers of Food: Behavior, Fitness and Functional Properties. Food Microbiol. 2015, 45, 45–53. [Google Scholar] [CrossRef]
- Jia, S.; Jia, Z.; An, J.; Ding, Y.; Chang, J.; Wang, Y.; Zhou, X. Insights into the Fish Protein Degradation Induced by the Fish-Borne Spoiler Pseudomonas psychrophila and Shewanella putrefaciens: From Whole Genome Sequencing to Quality Changes. Int. J. Food Microbiol. 2024, 416, 110675. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yan, J.; Xie, J. Applications of Genomics, Metabolomics, Fourier Transform Infrared in the Evaluation of Spoilage Targets of Shewanella putrefaciens from Spoiled Bigeye Tuna. J. Agric. Food Chem. 2023, 71, 9558–9568. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Yang, X.; Dong, R.; Liu, Z.; Zeng, M. Complete Genome Sequence Provides Insights into the Quorum Sensing-Related Spoilage Potential of Shewanella baltica 128 Isolated from Spoiled Shrimp. Genomics 2020, 112, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Akaike, T. Sulfur-Utilizing Cytoprotection and Energy Metabolism. Curr. Opin. Physiol. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Abril, A.G.; Calo-Mata, P.; Villa, T.G.; Böhme, K.; Barros-Velázquez, J.; Sánchez-Pérez, Á.; Pazos, M.; Carrera, M. Comprehensive Shotgun Proteomic Characterization and Virulence Factors of Seafood Spoilage Bacteria. Food Chem. 2024, 448, 139045. [Google Scholar] [CrossRef]
- Yi, Z.; Yan, J.; Ding, Z.; Xie, J. Purification and Characterizations of a Novel Extracellular Protease from Shewanella putrefaciens Isolated from Bigeye Tuna. Food Biosci. 2023, 52, 102384. [Google Scholar] [CrossRef]
- Chandu, D.; Nandi, D. Comparative Genomics and Functional Roles of the ATP-Dependent Proteases Lon and Clp during Cytosolic Protein Degradation. Res. Microbiol. 2004, 155, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, H.; Zhou, Q.; Liao, N. Protein Degradation Control and Regulation of Bacterial Survival and Pathogenicity: The Role of Protein Degradation Systems in Bacteria. Mol. Biol. Rep. 2021, 48, 7575–7585. [Google Scholar] [CrossRef]
- Winzer, K.; Hardie, K.R.; Williams, P. LuxS and Autoinducer-2: Their Contribution to Quorum Sensing and Metabolism in Bacteria. Adv. Appl. Microbiol. 2003, 53, 291–396. [Google Scholar] [CrossRef]
- Hu, Z.; Chin, Y.; Yuan, C.; Ge, Y.; Hang, Y.; Wang, D.; Yao, Q.; Hu, Y. The luxS Deletion Reduces the Spoilage Ability of Shewanella putrefaciens: An Analysis Focusing on Quorum Sensing and Activated Methyl Cycle. Food Microbiol. 2024, 120, 104467. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, M.; Lu, Z. The LuxS/AI-2 System Regulates the Probiotic Activities of Lactic Acid Bacteria. Trends Food Sci. Technol. 2022, 127, 272–279. [Google Scholar] [CrossRef]
- Zhu, Y.; Dou, Q.; Du, L.; Wang, Y. QseB/QseC: A Two-Component System Globally Regulating Bacterial Behaviors. Trends Microbiol. 2023, 31, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Sun, L.; Grenier, D.; Yi, L. The LuxS/AI-2 System of Streptococcus suis. Appl. Microbiol. Biotechnol. 2018, 102, 7231–7238. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; An, Q.; Sa, R.; Zhang, D.; Xu, R. Research on the Role of LuxS/AI-2 Quorum Sensing in Biofilm of Leuconostoc citreum 37 Based on Complete Genome Sequencing. 3 Biotech 2021, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Grenier, D.; Yi, L. Regulatory Mechanisms of the LuxS/AI-2 System and Bacterial Resistance. Antimicrob. Agents Chemother. 2019, 63, e01186-19. [Google Scholar] [CrossRef]
- Doherty, N.; Holden, M.T.G.; Qazi, S.N.; Williams, P.; Winzer, K. Functional Analysis of luxS in Staphylococcus aureus Reveals a Role in Metabolism but Not Quorum Sensing. J. Bacteriol. 2006, 188, 2885–2897. [Google Scholar] [CrossRef]
- Learman, D.R.; Yi, H.; Brown, S.D.; Martin, S.L.; Geesey, G.G.; Stevens, A.M.; Hochella, M.F. Involvement of Shewanella oneidensis MR-1 LuxS in Biofilm Development and Sulfur Metabolism. Appl. Environ. Microbiol. 2009, 75, 1301–1307. [Google Scholar] [CrossRef]
- Jiang, W.; Hou, Y.; Inouye, M. CspA, the Major Cold-Shock Protein of Escherichia coli, Is an RNA Chaperone. J. Biol. Chem. 1997, 272, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Phadtare, S. Recent Developments in Bacterial Cold-Shock Response. Curr. Issues Mol. Biol. 2004, 6, 125–136. [Google Scholar] [CrossRef]
- Ray, S.; Da Costa, R.; Thakur, S.; Nandi, D. Salmonella Typhimurium Encoded Cold Shock Protein E Is Essential for Motility and Biofilm Formation. Microbiology 2020, 166, 460–473. [Google Scholar] [CrossRef]
- Muchaamba, F.; von Ah, U.; Stephan, R.; Stevens, M.J.A.; Tasara, T. Deciphering the Global Roles of Cold Shock Proteins in Listeria monocytogenes Nutrient Metabolism and Stress Tolerance. Front. Microbiol. 2022, 13, 1057754. [Google Scholar] [CrossRef]
- Rocha, E.R.; Smith, C.J. Role of the Alkyl Hydroperoxide Reductase (ahpCF) Gene in Oxidative Stress Defense of the Obligate Anaerobe Bacteroides Fragilis. J. Bacteriol. 1999, 181, 5701–5710. [Google Scholar] [CrossRef]
- Hansen, A.-M.; Gu, Y.; Li, M.; Andrykovitch, M.; Waugh, D.S.; Jin, D.J.; Ji, X. Structural Basis for the Function of Stringent Starvation Protein A as a Transcription Factor. J. Biol. Chem. 2005, 280, 17380–17391. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cui, F.; Ren, L.; Tan, X.; Lv, X.; Li, Q.; Li, J.; Li, T. Complete Genome Analysis Reveals the Quorum Sensing-Related Spoilage Potential of Pseudomonas fluorescens PF08, a Specific Spoilage Organism of Turbot (Scophthalmus Maximus). Front. Microbiol. 2022, 13, 856802. [Google Scholar] [CrossRef]
- Feng, L.; Bi, W.; Chen, S.; Zhu, J.; Liu, X. Regulatory Function of Sigma Factors RpoS/RpoN in Adaptation and Spoilage Potential of Shewanella baltica. Food Microbiol. 2021, 97, 103755. [Google Scholar] [CrossRef]
- Liu, X.; Ji, L.; Wang, X.; Li, J.; Zhu, J.; Sun, A. Role of RpoS in Stress Resistance, Quorum Sensing and Spoilage Potential of Pseudomonas fluorescens. Int. J. Food Microbiol. 2018, 270, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, Y.; Zhu, Y.; Wang, L.; Yuan, L.; Zhu, J.; Sun, A. Involvement of RpoN in Regulating Motility, Biofilm, Resistance, and Spoilage Potential of Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 641844. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Stock, I.; Wiedemann, B. Identification and Natural Antibiotic Susceptibility of Morganella morganii. Diagn. Microbiol. Infect. Dis. 1998, 30, 153–165. [Google Scholar] [CrossRef]
- Oh, W.T.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; et al. Morganella psychrotolerans as a Possible Opportunistic Pathogen in Rainbow Trout (Oncorhynchus Mykiss) Fisheries. Aquaculture 2020, 520, 735021. [Google Scholar] [CrossRef]
- Ma, J.; Song, X.; Li, M.; Yu, Z.; Cheng, W.; Yu, Z.; Zhang, W.; Zhang, Y.; Shen, A.; Sun, H.; et al. Global Spread of Carbapenem-Resistant Enterobacteriaceae: Epidemiological Features, Resistance Mechanisms, Detection and Therapy. Microbiol. Res. 2023, 266, 127249. [Google Scholar] [CrossRef]
- Aurilio, C.; Sansone, P.; Barbarisi, M.; Pota, V.; Giaccari, L.G.; Coppolino, F.; Barbarisi, A.; Passavanti, M.B.; Pace, M.C. Mechanisms of Action of Carbapenem Resistance. Antibiotics 2022, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Weston, N.; Sharma, P.; Ricci, V.; Piddock, L.J.V. Regulation of the AcrAB-TolC Efflux Pump in Enterobacteriaceae. Res. Microbiol. 2018, 169, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, A.; Keeney, D.; Bradford, P.A. AcrAB Efflux Pump Plays a Role in Decreased Susceptibility to Tigecycline in Morganella morganii. Antimicrob. Agents Chemother. 2005, 49, 791–793. [Google Scholar] [CrossRef]


| Characteristics | Strains | |
|---|---|---|
| GWT 902 | GWT 904 | |
| 2 °C | + | - |
| 4 °C | + | + |
| 8.5% NaCl | - | + |
| D-galactose | - | + |
| Strains | Histamine Content (mg/L) | |
|---|---|---|
| 20 °C, 60 h | 4 °C, 8 Days | |
| GWT 902 | 4807.79 ± 315.40 a | 4708.17 ± 113.30 a |
| GWT 904 | 5590.27 ± 595.08 a | 3731.57 ± 399.72 b |
| Genome Features | Strains | |
|---|---|---|
| GWT 902 | GWT 904 | |
| Genome size (bp) | 3,990,716 | 4,200,263 |
| DNA G + C (%) | 47.97 | 50.39 |
| Number of CDSs | 3608 | 3946 |
| Number of rRNA genes | 22 | 22 |
| Number of tRNA genes | 77 | 81 |
| Spoilage-Related Pathways | Encoded Protein | Gene | Gene ID | |
|---|---|---|---|---|
| GWT 902 | GWT 904 | |||
| Histamine metabolism | Putative histidine–histamine antiporter | hdcT1 | gene3203 | gene3565 |
| Histidine decarboxylase | hdc | gene3204 | gene3566 | |
| Putative histidine–histamine antiporter | hdcT2 | gene3205 | gene3567 | |
| Histidyl-tRNA synthetase | hisRS | gene3206 | gene3568 | |
| Putrescine metabolism | Arginine decarboxylase | speA | gene2708 | gene2989 |
| Agmatinase | speB | gene2707 | gene2988 | |
| Ornithine decarboxylase | speC | gene1271 | gene1319 | |
| Putrescine importer | puuP | gene1075 gene2433 | gene1138 gene2591 | |
| Spermidine/putrescine transport system ATP-binding protein Spermidine/putrescine ABC transporter permease | potA | gene3252 | gene3623 | |
| potB | gene3251 | gene3622 | ||
| Spermidine/putrescine ABC transporter permease | potC | gene3250 | gene3621 | |
| Spermidine/putrescine ABC transporter substrate-binding protein | potD | gene3249 | gene2899 | |
| Sulfur metabolism | Cysteine synthase | cysM | gene2325 | gene1482 |
| Cysteine synthase | cysK | gene2535 | gene2732 | |
| Sulfate/thiosulfate transport system substrate-binding protein | cysP | gene2329 | gene1478 | |
| Sulfate/thiosulfate transport system permease protein | cysU | gene2328 | gene1479 | |
| Sulfate/thiosulfate transport system permease protein | cysW | gene2327 | gene1480 | |
| Sulfate/thiosulfate transport system ATP-binding protein | cysA | gene2326 | gene1481 | |
| Sulfate transport protein | cysZ | gene2534 | gene2731 | |
| Assimilatory sulfite reductase (NADPH) hemoprotein subunit | cysI | - | gene3339 | |
| NADPH-dependent assimilatory sulfite reductase flavoprotein subunit | cysJ | - | gene3340 | |
| 3′(2′), 5′-bisphosphate nucleotidase | cysQ | gene3425 | gene3778 | |
| Serine O-acetyltransferase | cysE | gene3641 | gene3982 | |
| Sulfide: quinone oxidoreductase | sqr | gene0547 | gene0619 | |
| Taurine dioxygenase | tauD | gene3000 | gene3344 | |
| Tetrathionate reductase subunit TtrA | ttrA | gene2301 | gene1518 | |
| Tetrathionate reductase subunit TtrB | ttrB | gene2303 | gene1516 | |
| Tetrathionate reductase subunit TtrC | ttrC | gene2302 | gene1517 | |
| Thiosulfate sulfurtransferase | glpE | gene0143 | gene0136 | |
| Thiosulfate/3-mercaptopyruvate sulfurtransferase | sseA | gene1063 | gene1130 | |
| Lipase | Lipoyl synthase | lipA | gene0940 | gene1011 |
| Lipoyl(octanoyl) transferase | lipB | gene0941 | gene1012 | |
| Lysophospholipase | pldB | gene3457 | gene3807 | |
| Esterase FrsA | frsA | gene0879 | gene0927 | |
| Esterase | ybfF | - | gene1057 | |
| Protease | Serine protease DegQ | degQ | gene2999 | gene3338 |
| Serine protease DegS | degS | gene3001 | gene3345 | |
| Rhomboid protease GluP | gluP | gene1100 | gene1168 | |
| Rhomboid protease GlpG | glpG | gene0142 gene1977 | gene0135 gene2029 | |
| Serine protease inhibitor ecotin | eco | gene2573 | gene2850 | |
| SprT family zinc-dependent metalloprotease | sprT | gene2714 | gene2994 | |
| Metalloprotease PmbA | pmbA | gene3022 | gene3366 | |
| Metalloprotease | rseP | gene0521 | gene0651 | |
| CPBP family intramembrane metalloprotease | - | gene1026 | gene1097 | |
| Metalloprotease TldD | tldD | gene3026 | gene3370 | |
| Cell division protease FtsH | ftsH | gene3403 | gene3754 | |
| ATP-dependent Clp protease ATP-binding subunit ClpB | clpB | gene0913 | gene0960 | |
| ATP-dependent Clp protease adaptor protein ClpS | clpS | gene1137 | gene1210 | |
| ATP-dependent Clp protease ATP-binding subunit ClpA | clpA | gene1138 | gene1211 | |
| ATP-dependent Clp protease ATP-binding subunit ClpX | clpX | gene2889 | gene3161 | |
| ATP-dependent Clp protease, protease subunit | clpP | gene2890 | gene0349 gene3162 | |
| Encoded Protein | Gene | Gene ID | ||
|---|---|---|---|---|
| GWT 902 | GWT 904 | |||
| QS system | S-ribosylhomocysteine lyase | luxS | gene0896 | gene0944 |
| Autoinducer 2 ABC transporter substrate-binding protein | lsrB | gene1867 | gene1923 | |
| (4S)-4-hydroxy-5-phosphonooxypentane-2,3-dione isomerase | lsrG | gene1865 | gene1921 | |
| Autoinducer-2 kinase | lsrK | gene1872 | gene1928 | |
| Autoinducer 2 ABC transporter ATP-binding protein | lsrA | gene1870 | gene1926 | |
| Autoinducer 2 ABC transporter permease | lsrC | gene1869 | gene1925 | |
| Autoinducer 2 ABC transporter permease | lsrD | gene1868 | gene1924 | |
| 3-hydroxy-5-phosphonooxypentane-2,4-dione thiolase | lsrF | gene1866 | gene1922 | |
| Transcriptional regulator | lsrR | gene1871 | gene1927 | |
| Adaptation to stress | Cold shock protein | cspA | gene0938 gene1136 gene1162 gene1463 gene1584 gene2292 gene2461 | gene1009 gene1209 gene1242 gene1529 gene1715 gene2356 gene2413 gene2660 gene2667 |
| Sodium/proton antiporter NhaB | nhaB | gene1725 | gene2229 | |
| Na+/H+ antiporter | nhaK | gene0324 gene0815 | gene0389 gene1176 | |
| Na+/H+ antiporter | nhaA | gene0427 | gene0497 gene1033 | |
| Trk system potassium transporter | trkA | gene3527 | gene3874 | |
| Trk system potassium transporter | trkH | gene0346 | gene0414 | |
| Magnesium/cobalt transporter CorA | corA | gene3461 | gene3811 | |
| Magnesium/cobalt transporter CorC | corC | gene0968 | gene1037 | |
| Glutathione-regulated potassium-efflux system protein KefB | kefB | gene0238 | gene0245 | |
| Glutathione-regulated potassium-efflux system ancillary protein KefG | kefG | gene0237 | grnr0244 | |
| RNA polymerase sigma factor | rpoS | gene0561 | gene0633 | |
| Sigma-54 RNA polymerase factor sigma-54 | rpoN | gene3015 | gene3359 | |
| NADH-dependent peroxiredoxin subunit C | ahpC | gene3253 | gene3625 | |
| NADH-dependent peroxiredoxin subunit F | ahpF | gene3254 | gene3626 | |
| Stringent starvation protein A | sspA | gene2994 | gene3333 | |
| Stringent starvation protein B | sspB | gene2993 | gene3332 | |
| Drug Class | Number of Genes | |
|---|---|---|
| GWT 902 | GWT 904 | |
| Tetracycline antibiotic | 58 | 61 |
| Fluoroquinolone antibiotic | 52 | 50 |
| Penam | 34 | 43 |
| Cephalosporin | 33 | 41 |
| Peptide antibiotic | 36 | 38 |
| Macrolide antibiotic | 36 | 37 |
| Disinfecting agents and antiseptics | 28 | 33 |
| Cephamycin | 25 | 31 |
| Phenicol antibiotic | 33 | 31 |
| Carbapenem | 26 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, D.; Chen, S.; Yu, G.; Ma, Z.; Wei, Y.; Li, C.; Wang, Y.; Shen, C.; Zhao, Y. Genomic Analysis of Two Histamine-Producing Strains Isolated from Yellowfin Tuna. Foods 2025, 14, 1532. https://doi.org/10.3390/foods14091532
Wang Y, Wang D, Chen S, Yu G, Ma Z, Wei Y, Li C, Wang Y, Shen C, Zhao Y. Genomic Analysis of Two Histamine-Producing Strains Isolated from Yellowfin Tuna. Foods. 2025; 14(9):1532. https://doi.org/10.3390/foods14091532
Chicago/Turabian StyleWang, Yazhe, Di Wang, Shengjun Chen, Gang Yu, Zhenhua Ma, Ya Wei, Chunsheng Li, Yueqi Wang, Chaoming Shen, and Yongqiang Zhao. 2025. "Genomic Analysis of Two Histamine-Producing Strains Isolated from Yellowfin Tuna" Foods 14, no. 9: 1532. https://doi.org/10.3390/foods14091532
APA StyleWang, Y., Wang, D., Chen, S., Yu, G., Ma, Z., Wei, Y., Li, C., Wang, Y., Shen, C., & Zhao, Y. (2025). Genomic Analysis of Two Histamine-Producing Strains Isolated from Yellowfin Tuna. Foods, 14(9), 1532. https://doi.org/10.3390/foods14091532













