Enhanced Thermal and Storage Stability of Glucose Oxidase via Encapsulation in Chitosan-Coated Alginate and Carboxymethyl Cellulose Gel Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SA/CMC Gel Particles
2.3. Preparation of CS/SA/CMC Gel Particles
2.4. Calculating GOD Activity
2.5. Encapsulation Efficiency
2.6. Fourier-Transform Infrared (FTIR) Spectroscopy
2.7. Scanning Electron Microscopy (SEM)
2.8. Swelling Capacity
2.9. Thermal Stability
2.10. In Vitro Enzyme Release Test
2.11. Analysis of Storage Stability
2.12. Statistical Analysis
3. Results and Discussion
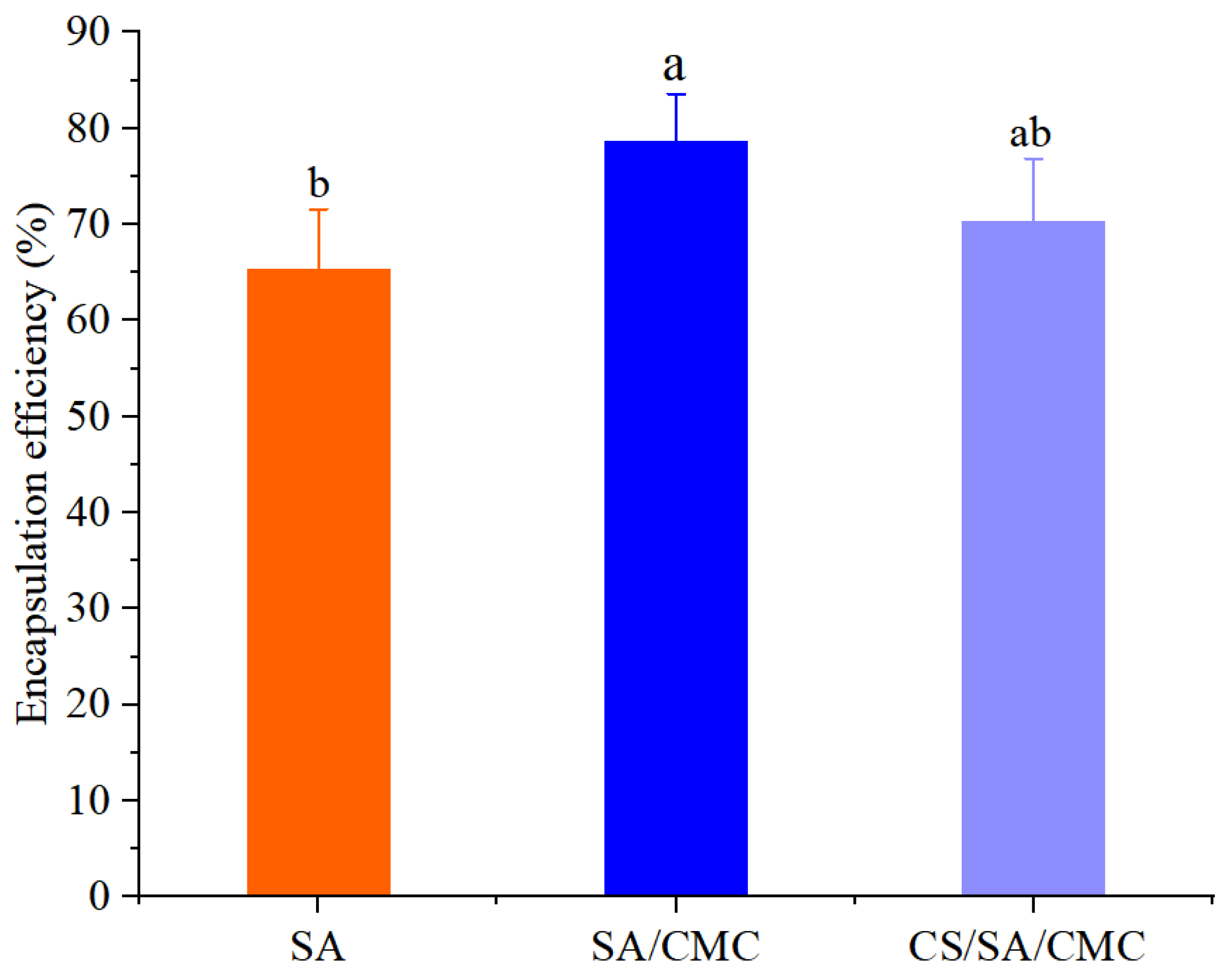
3.1. The EE of GOD Encapsulated in Gel Particles
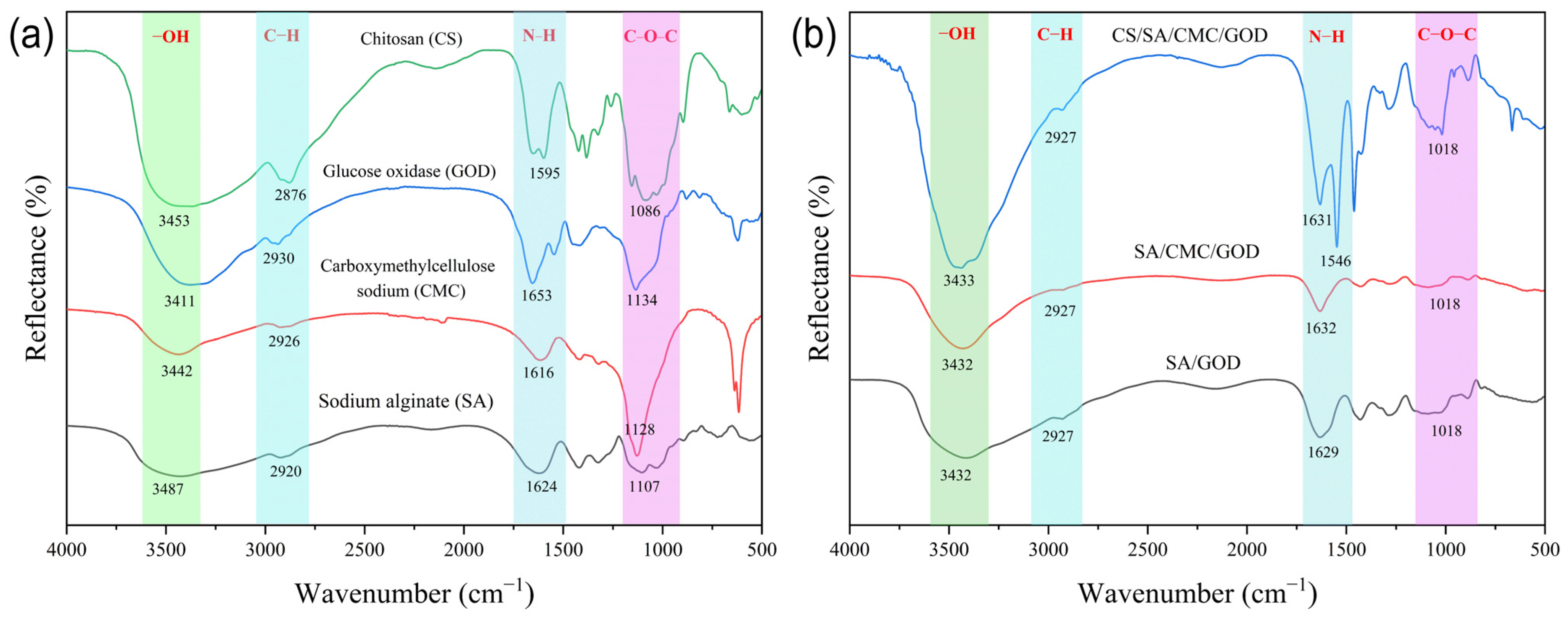
3.2. The Gel Particle Structure Analysis According to FTIR
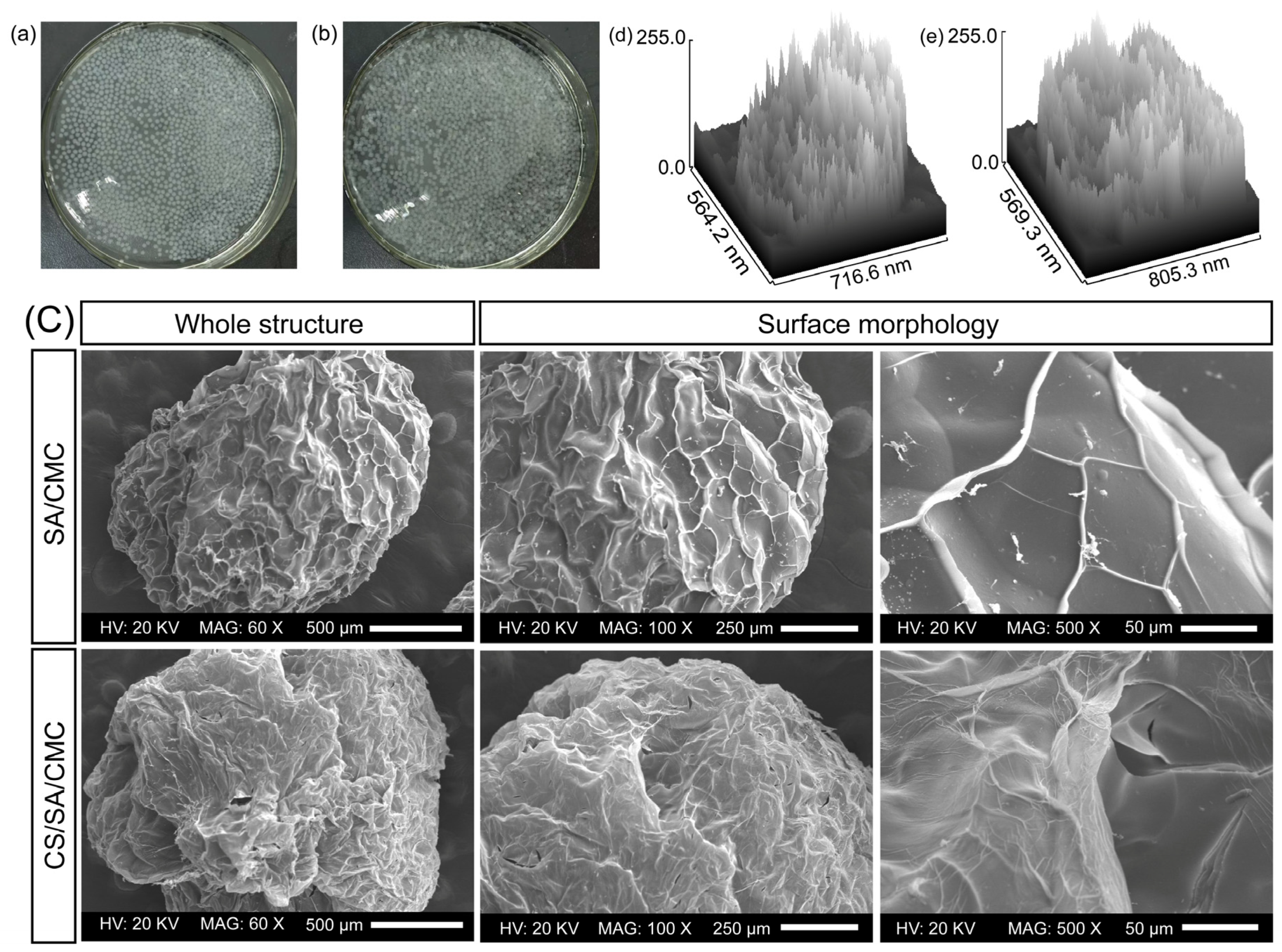
3.3. Appearance and Morphology Analysis of Gel Particles
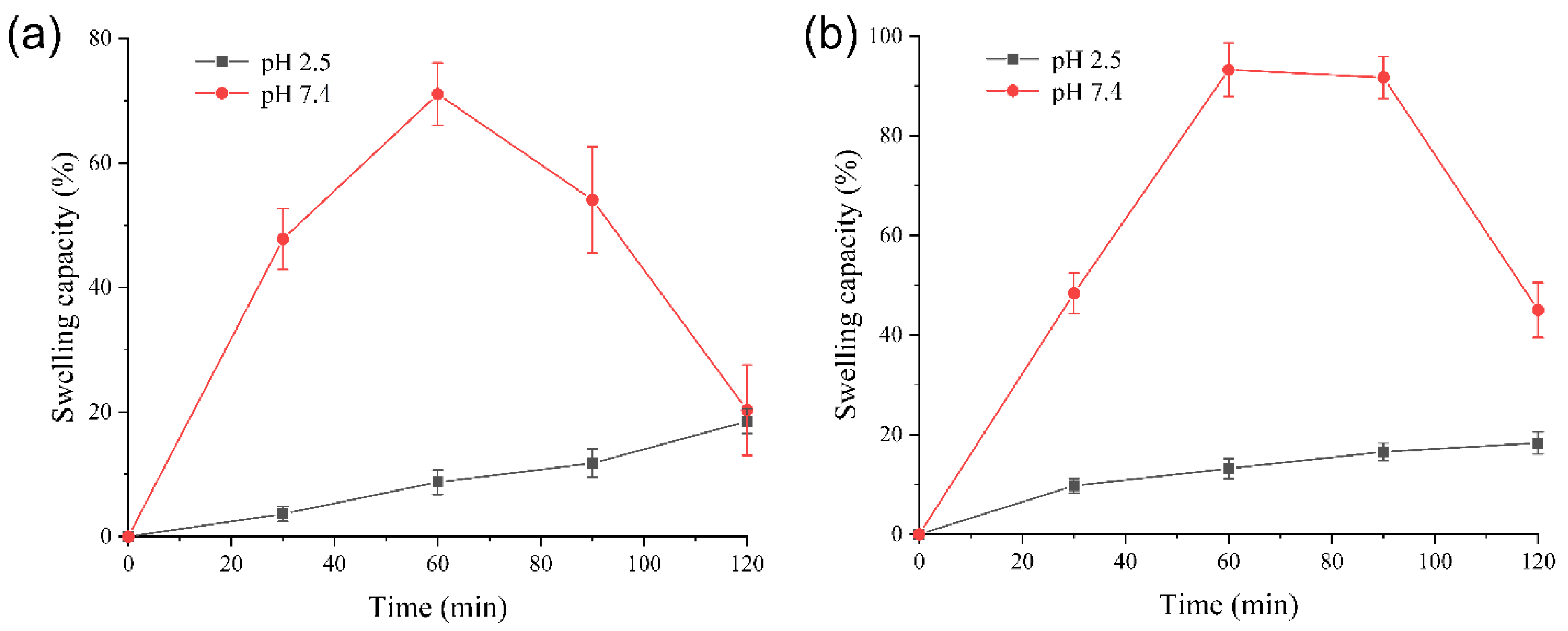
3.4. Swelling Capacity Analysis of Gel Particles
3.5. Thermal Stability Analysis of GOD
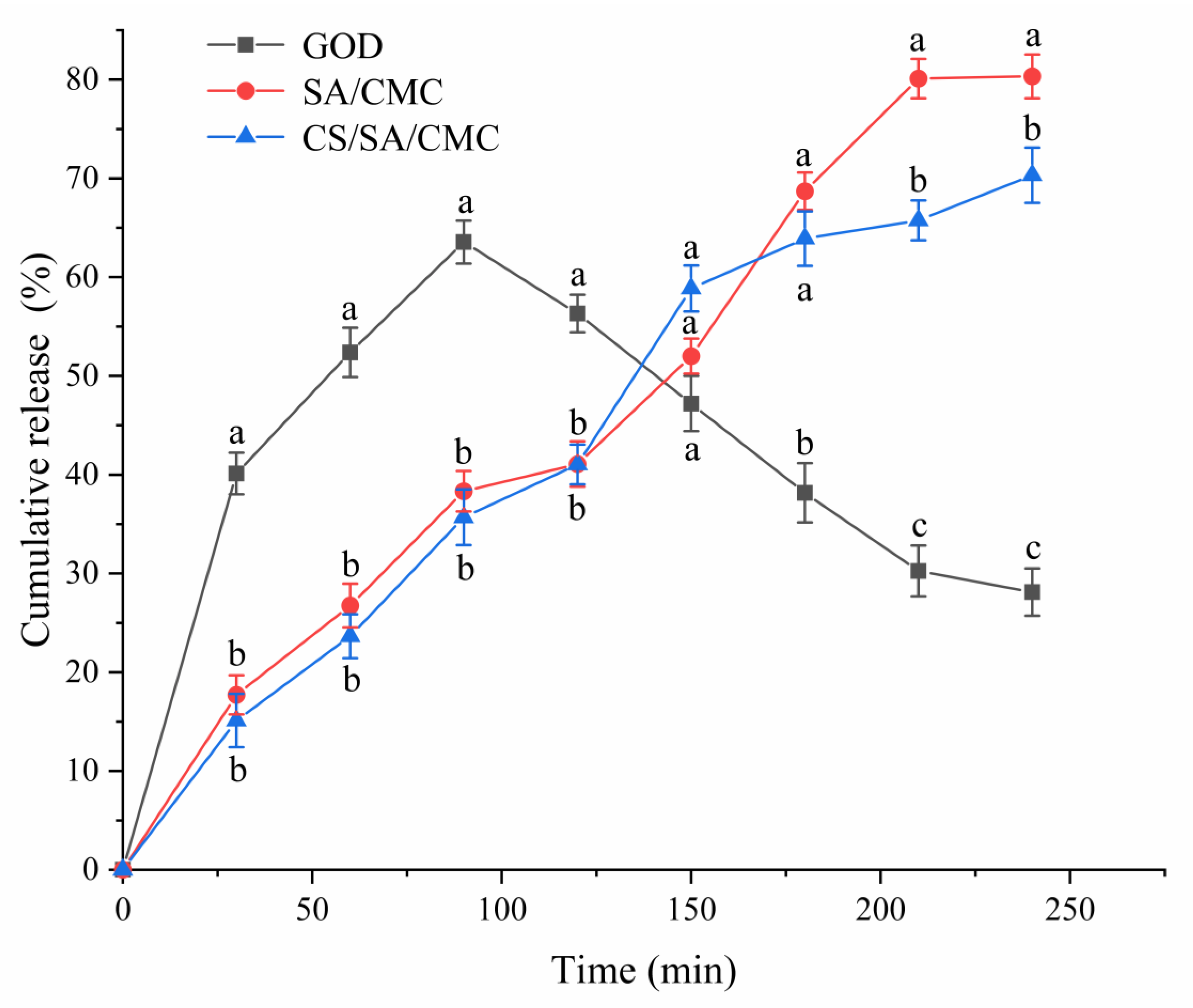
3.6. The Release Characteristics of GOD Gel Particles Under In Vitro Gastrointestinal Conditions
3.7. Storage Stability Analysis of GOD Gel Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, W.B.; Wied, P.; Carraro, F.; Sumby, J.C.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, J.C. Metal-organic framework-based enzyme biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Bonet, A.; Rosell, C.M.; Caballero, P.A.; Gómez, M.; Pérez-Munuera, I.; Lluch, M.A. Glucose oxidase effect on dough rheology and bread quality: A study from macroscopic to molecular level. Food Chem. 2005, 99, 408–415. [Google Scholar] [CrossRef]
- Bauer, A.J.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose oxidase, an enzyme “Ferrari”: Its structure, function, production and properties in the light of various industrial and biotechnological applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Bankar, B.S.; Bule, V.M.; Singhal, S.R.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Kim, H.; Lee, I.; Kwon, Y.; Kim, B.C.; Ha, S.; Lee, J.; Kim, J. Immobilization of glucose oxidase into polyaniline nanofiber matrix for biofuel cell applications. Biosens. Bioelectron. 2011, 26, 3908–3913. [Google Scholar] [CrossRef]
- Weng, Y.L.; Li, Y.; Chen, X.J.; Song, H.; Zhao, C.X. Encapsulation of enzymes in food industry using spray drying: Recent advances and process scale-ups. Crit. Rev. Food Sci. Nutr. 2023, 64, 7941–7958. [Google Scholar] [CrossRef]
- Zia, M.K.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, N.M. Alginate based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Li, D.D.; Wei, Z.H.; Xue, C.H. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369. [Google Scholar] [CrossRef]
- Pauly, J.; Gröger, H.; Patel, V.A. Design, characterisation and application of alginate-based encapsulated pig liver esterase. J. Biotechnol. 2018, 280, 42–48. [Google Scholar] [CrossRef]
- Chanez, B.; Sylvie, D.; Laurent, P.; Stéphane, D. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118. [Google Scholar]
- Weng, Y.L.; Yang, G.Z.; Li, Y.; Xu, L.T.; Chen, X.J.; Song, H.; Zhao, C.X. Alginate-based materials for enzyme encapsulation. Adv. Colloid Interface Sci. 2023, 318, 102957. [Google Scholar] [CrossRef] [PubMed]
- Dashevsky, A. Protein loss by the microencapsulation of an enzyme (lactase) in alginate beads. Int. J. Pharm. 1998, 161, 1–5. [Google Scholar] [CrossRef]
- Weng, Y.L.; Supun, R.; Zou, D.; Anna, C.; Chen, X.J.; Song, H.; Zhao, C.X. Alginate particles for enzyme immobilization using spray drying. J. Agric. Food Chem. 2022, 70, 7139–7147. [Google Scholar] [CrossRef]
- Singthong, J.; Thongkaew, C. Using hydrocolloids to decrease oil absorption in banana chips. LWT-Food Sci. Technol. 2009, 42, 1199–1203. [Google Scholar] [CrossRef]
- Wang, Y.P.; Milewska, M.; Foster, H.; Chapman, R.; Stenzel, M.H. The Core-Shell structure, not sugar, drives the thermal stabilization of single-enzyme nanoparticles. Biomacromolecules 2021, 22, 4569–4581. [Google Scholar] [CrossRef]
- Murty, R.; Bera, K.M.; Walton, I.M.; Whetzel, C.; Prausnitz, R.M.; Walton, S.K. Interrogating encapsulated protein structure within metal-organic frameworks at elevated temperature. J. Am. Chem. Soc. 2023, 145, 7323–7330. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; Iborra-Bernad, C.; García-Segovia, P.; Martínez-Monzó, J. Effect of konjac glucomannan (KGM) and carboxymethylcellulose (CMC) on some physico-chemical and mechanical properties of restructured gilthead sea bream (Sparus aurata) products. Food Bioprocess Technol. 2013, 6, 133–145. [Google Scholar] [CrossRef]
- Mojtaba, R.; Mohammad, H.; Majid, A.; Fatemeh, B.G.; Maryam, E.; Behrooz, J.; Bektas, T.; Ali, M.S.N. Effects of Sodium Alginate and chitosan coating combined with three different essential oils on microbial and chemical attributes of rainbow trout fillets. J. Aquat. Food Prod. Technol. 2020, 29, 253–263. [Google Scholar]
- Ehab, T.; Mansoor, A. Enzyme immobilization in novel alginate-chitosan core-shell microcapsules. Biomaterials 2004, 25, 1937–1945. [Google Scholar]
- Saeedeh, H.; Mehdi, V. Optimization of microbial rennet encapsulation in alginate-chitosan nanoparticles. Food Chem. 2021, 352, 129325. [Google Scholar]
- Li, Q.; Duan, M.; Hou, D.; Chen, X.Q.; Jinglan, S.; Zhou, W. Fabrication and characterization of Ca(II)-alginate-based beads combined with different polysaccharides as vehicles for delivery, release and storage of tea polyphenols. Food Hydrocoll. 2021, 112, 106274. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, W. The neutral protease immobilization: Physical characterization of sodium alginate-chitosan gel beads. Appl. Biochem. Biotechnol. 2022, 194, 2269–2283. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.H.; Yang, M.L.; Wang, Z.Y.; Cheng, S.Z.; Du, M. Preserving the activity of attenuating atherosclerosis of nattokinase in gastrointestinal system by NK/SA/CS microcapsules. Food Biosci. 2024, 59, 103949. [Google Scholar] [CrossRef]
- Shamekhi, F.; Tamjid, E.; Khajeh, K. Development of chitosan coated calcium-alginate nanocapsules for oral delivery of liraglutide to diabetic patients. Int. J. Biol. Macromol. 2018, 120, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, S.B.; Lin, D.Z.; Wu, Z.M.; Xu, J.; Zhang, J.L.; Miao, Y.; Liu, T.; Chen, T.; Cai, X. Preparation, characterization, and release behavior of doxorubicin hydrochloride from dual cross-linked chitosan/alginate hydrogel beads. ACS Appl. Bio Mater. 2020, 3, 3057–3065. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, L.E.; Fidalgo, C.D.M.; Gomes, A.; Ramalhosa, E. Physicochemical properties and microbial control of chestnuts (Castanea sativa) coated with whey protein isolate, chitosan and alginate during storage. Sci. Hortic. 2020, 263, 109105. [Google Scholar] [CrossRef]
- Shu, X.; Zhu, K. The release behavior of brilliant blue from calcium-alginate gel beads coated by chitosan: The preparation method effect. Eur. J. Pharm. Biopharm. 2002, 53, 193–201. [Google Scholar] [CrossRef]
- Basu, K.S.; Rajendran, A. Studies in the development of nateglinide loaded calcium alginate and chitosan coated calcium alginate beads. Chem. Pharm. Bull. 2008, 56, 1077–1084. [Google Scholar] [CrossRef]
- Seyedehsara, D.M.; Chloe, B.; Noemi, G.; Andrea, C.A.; Ali, S. Chitosan coated alginate beads as probiotic delivery system for New Zealand black footed abalone (Haliotis iris). J. Appl. Polym. Sci. 2022, 139, e52626. [Google Scholar]
- Liu, S.Y.; Fang, Z.X.; Ken, N. Incorporating inulin and chitosan in alginate-based microspheres for targeted delivery and release of quercetin to colon. Food Res. Int. 2022, 160, 111749. [Google Scholar] [CrossRef]
- Linh, T.P.; Erika, B.; Szilárd, K.; Dimitris, C.; Vitaliy, V.K. Electrosprayed mucoadhesive alginate-chitosan microcapsules for gastrointestinal delivery of probiotics. Int. J. Pharm. 2021, 597, 120342. [Google Scholar]
- Thinkohkaew, K.; Jonjaroen, V.; Niamsiri, N.; Panya, A.; Suppavorasatit, I.; Potiyaraj, P. Microencapsulation of probiotics in chitosan-coated alginate/gellan gum: Optimization for viability and stability enhancement. Food Hydrocoll. 2024, 151, 109788. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.H.; Zhang, J.L.; Wang, T.; Zhang, X.D.; Shao, L.; Chen, D.J.; Wang, J. Extending viability of bifidobacterium longum in chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.J.; Alonso, F.N.; Miras, J.; Celma, G.J.M.; Morral, G.; Esquena, J. Triggered protein release from calcium alginate/chitosan gastro-resistant capsules. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 133998. [Google Scholar] [CrossRef]
- Liu, Q.; Rauth, M.A.; Wu, Y.X. Immobilization and bioactivity of glucose oxidase in hydrogel microspheres formulated by an emulsification-internal gelation-adsorption-polyelectrolyte coating method. Int. J. Pharm. 2007, 339, 148–156. [Google Scholar] [CrossRef]
- Yang, S.F.; Feng, M.M.; Xu, J.X.; Deng, Z.L.; Zhang, H.C. Encapsulation, characterization and in vitro releasing of xylanase and glucose oxidase (GOD) into cellulose nanocrystals stabilized three-layer microcapsules. Int. J. Biol. Macromol. 2024, 280, 135515. [Google Scholar] [CrossRef]
- Koczoń, P.; HołajKrzak, T.J.; Palani, K.B.; Bolewski, T.; Dąbrowski, J.; Bartyzel, J.B.; Gruczyńska, S.E. The analytical possibilities of FT-IR spectroscopy powered by vibrating molecules. Int. J. Mol. Sci. 2023, 24, 1013. [Google Scholar] [CrossRef]
- Facin, R.B.; Moret, B.; Baretta, D.; Belfiore, A.L.; Paulino, T.A. Immobilization and controlled release of β-galactosidase from chitosan-grafted hydrogels. Food Chem. 2015, 179, 44–51. [Google Scholar] [CrossRef]
- Ecem, A.; Sibel, K.; Merve, Ö.E.E.; Özer, K. Effect of food matrix and fermentation on angiotensin-converting enzyme inhibitory activity and β-glucan release after in vitro digestion in oat-based products. Food Res. Int. 2023, 165, 112508. [Google Scholar]
- Ozel, B.; Zhang, Z.; He, L.; McClements, D.J. Digestion of animal-and plant-based proteins encapsulated in κ-carrageenan/protein beads under simulated gastrointestinal conditions. Food Res. Int. 2020, 137, 109662. [Google Scholar] [CrossRef]
- Mai, A.H.T.; Tran, N.V.; Le, M.V.V. Biochemical studies on the immobilized lactase in the combined alginate-carboxymethyl cellulose gel. Biochem. Eng. J. 2013, 74, 81–87. [Google Scholar] [CrossRef]
- Li, J.W.; Wei, X.J.; He, J.M.; Sun, J.; Huang, Y.D. Tailored natural polysaccharides beads as green sorbents for efficient lysozyme adsorption. J. Polym. Environ. 2018, 26, 2803–2812. [Google Scholar] [CrossRef]
- Yousefi, M.; Khanniri, E.; Shadnoush, M.; Khorshidian, N.; Mortazavian, M.A. Development, characterization and in vitro antioxidant activity of chitosan-coated alginate microcapsules entrapping viola odorata linn extract. Int. J. Biol. Macromol. 2020, 163, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.X.; Senay, S.; Chen, B.C.; Rao, J.J. Alginate-based double-network hydrogel improves the viability of encapsulated probiotics during simulated sequential gastrointestinal digestion: Effect of biopolymer type and concentrations. Int. J. Biol. Macromol. 2020, 165, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Frenț, O.D.; Duteanu, N.; Teusdea, A.C.; Ciocan, S.; Vicaș, L.; Jurca, T.; Muresan, M.; Pallag, A.; Ianasi, P.; Marian, E. Preparation and characterization of chitosan-alginate microspheres loaded with quercetin. Polymers 2022, 14, 490. [Google Scholar] [CrossRef]
- Frent, O.D.; Duda-Seiman, D.M.; Vicas, L.G.; Duteanu, N.; Nemes, N.S.; Pascu, B.; Teusdea, A.; Morgovan, C.M.; Muresan, M.E.; Jurca, T.; et al. Study of the influence of the excipients used for the synthesis of microspheres loaded with quercetin: Their characterization and antimicrobial activity. Coatings 2023, 13, 1376. [Google Scholar] [CrossRef]
- Agarwal, T.; Narayana, H.G.S.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef]
- Naar, Z.; Adányi, N.; Bata-Vidács, I.; Tömösközi-Farkas, R.; Tömösközi-Farkas, R. Nanoencapsulation technologies for the food and nutraceutical industries. Acta Aliment. 2017, 46, 390–394. [Google Scholar] [CrossRef]
- Sareh, B.; Mahdi, S.J. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar]
- Ning, X.Y.; Zhang, Y.L.; Yuan, T.T.; Li, Q.B.; Tian, J.; Guan, W.S.; Liu, B.; Zhang, W.; Xu, X.X.; Zhang, Y.H. Enhanced thermostability of glucose oxidase through computer-aided molecular design. Int. J. Mol. Sci. 2018, 19, 425. [Google Scholar] [CrossRef]
- Andreas, T.S.; Marie, H.; Jákup, H.J.; Johannes, T.H.; Jorun, H.S. Physical feed quality and starch content causes a biological response in Atlantic salmon (Salmo salar L.). Aquac. Rep. 2021, 21, 100791. [Google Scholar]
- Marie-Claire, B.; Ali, H.; Martina, H.; Richard, H.; Peter, P.; Fabio, S.; David, S.D.V.; Xu, Y.; Angel, E.G. Water determines the structure and dynamics of proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar]
- Weng, Y.L.; Sun, B.; Jin, W.L.; Yan, P.H.; Chen, X.J.; Song, H.; Zhao, C.X. Mechanistic study on phytase stabilization using alginate encapsulation. Food Hydrocoll. 2024, 151, 109837. [Google Scholar] [CrossRef]
- Weng, Y.L.; Supun, R.; Zou, D.; Anna, P.C.; Chen, X.J.; Song, H.; Zhao, C.X. Improved enzyme thermal stability, loading and bioavailability using alginate encapsulation. Food Hydrocoll. 2023, 137, 108385. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.Y.; Guo, R.X.; Ma, Y.F.; Yuan, Y.; Liu, Y.P. Cellulase immobilized by sodium alginate-polyethylene glycol-chitosan for hydrolysis enhancement of microcrystalline cellulose. Process Biochem. 2021, 107, 38–47. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Zhang, X.R.; Guo, Y.; Han, K.G.; Huo, N.R. Preparation and in vitro delivery performance of chitosan-alginate microcapsule for IgG. Food Agric. Immunol. 2016, 28, 1–13. [Google Scholar] [CrossRef]
- Sun, Q.C.; Zhang, Z.P.; Zhang, R.J.; Gao, R.C.; Julian, D.M. Development of functional or medical foods for oral administration of insulin for diabetes treatment: Gastroprotective edible microgels. J. Agric. Food Chem. 2018, 66, 4820–4826. [Google Scholar] [CrossRef]
- Gamboa, M.J.; Leong, W.K. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 800–810. [Google Scholar] [CrossRef]
- Sojitra, V.U.; Nadar, S.S.; Rathod, K.V. A magnetic tri-enzyme nanobiocatalyst for fruit juice clarification. Food Chem. 2016, 21, 3296–3305. [Google Scholar] [CrossRef]
- Ismillayli, N.; Hadi, S.; Dharmayani, T.K.N.; Sanjaya, K.R.; Hermanto, D. Characterization of alginate-chitosan membrane as potential edible film. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 012073. [Google Scholar] [CrossRef]
- Asmaa, B.; Zohra, F.S.; Preeti, P.; Soumya, G.; Muhammad, U.; Chia, C.H.; Muhammad, U.; Mika, S. Chitosan nanoparticles as potential nano-sorbent for removal of toxic environmental pollutants. Nanomaterials 2023, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.F.; Xie, J.J.; Du, H.J.; McClements, J.D.; Xiao, H.; Li, L.J. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Ren, J.; Song, C. Enhanced Thermal and Storage Stability of Glucose Oxidase via Encapsulation in Chitosan-Coated Alginate and Carboxymethyl Cellulose Gel Particles. Foods 2025, 14, 664. https://doi.org/10.3390/foods14040664
Guo Z, Ren J, Song C. Enhanced Thermal and Storage Stability of Glucose Oxidase via Encapsulation in Chitosan-Coated Alginate and Carboxymethyl Cellulose Gel Particles. Foods. 2025; 14(4):664. https://doi.org/10.3390/foods14040664
Chicago/Turabian StyleGuo, Zhihao, Jian Ren, and Chunli Song. 2025. "Enhanced Thermal and Storage Stability of Glucose Oxidase via Encapsulation in Chitosan-Coated Alginate and Carboxymethyl Cellulose Gel Particles" Foods 14, no. 4: 664. https://doi.org/10.3390/foods14040664
APA StyleGuo, Z., Ren, J., & Song, C. (2025). Enhanced Thermal and Storage Stability of Glucose Oxidase via Encapsulation in Chitosan-Coated Alginate and Carboxymethyl Cellulose Gel Particles. Foods, 14(4), 664. https://doi.org/10.3390/foods14040664



