Antibiofilm Activity of Amomum tsaoko Essential Oil on Staphylococcus aureus and Its Application in Pork Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Bacterial Culture
2.2. Essential Oil Extraction
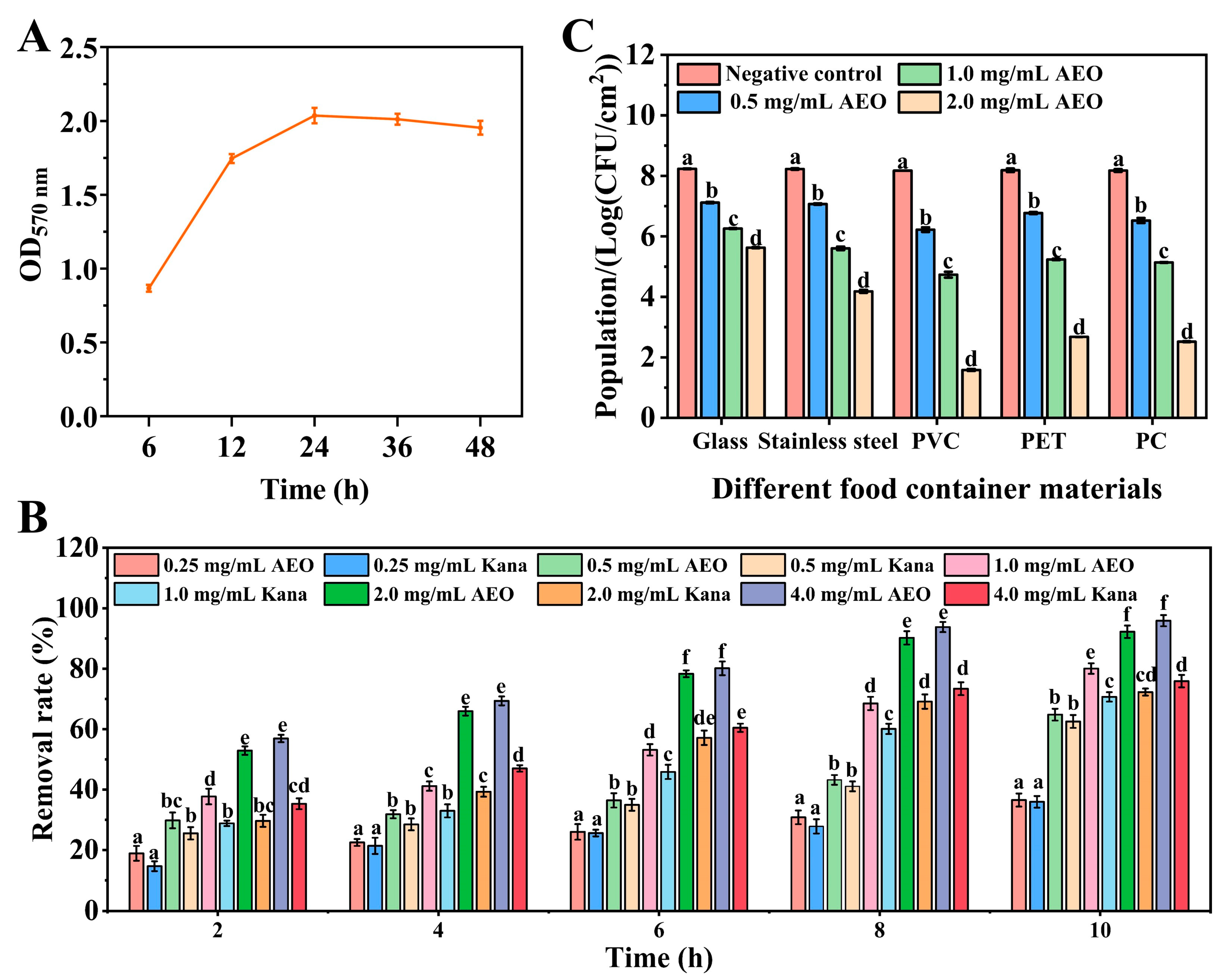
2.3. The Biofilm-Forming Ability of S. aureus
2.4. The Removal Effect of AEO on S. aureus Biofilm
2.5. Effect of AEO on S. aureus Biofilm on the Surface of Different Food Container Materials
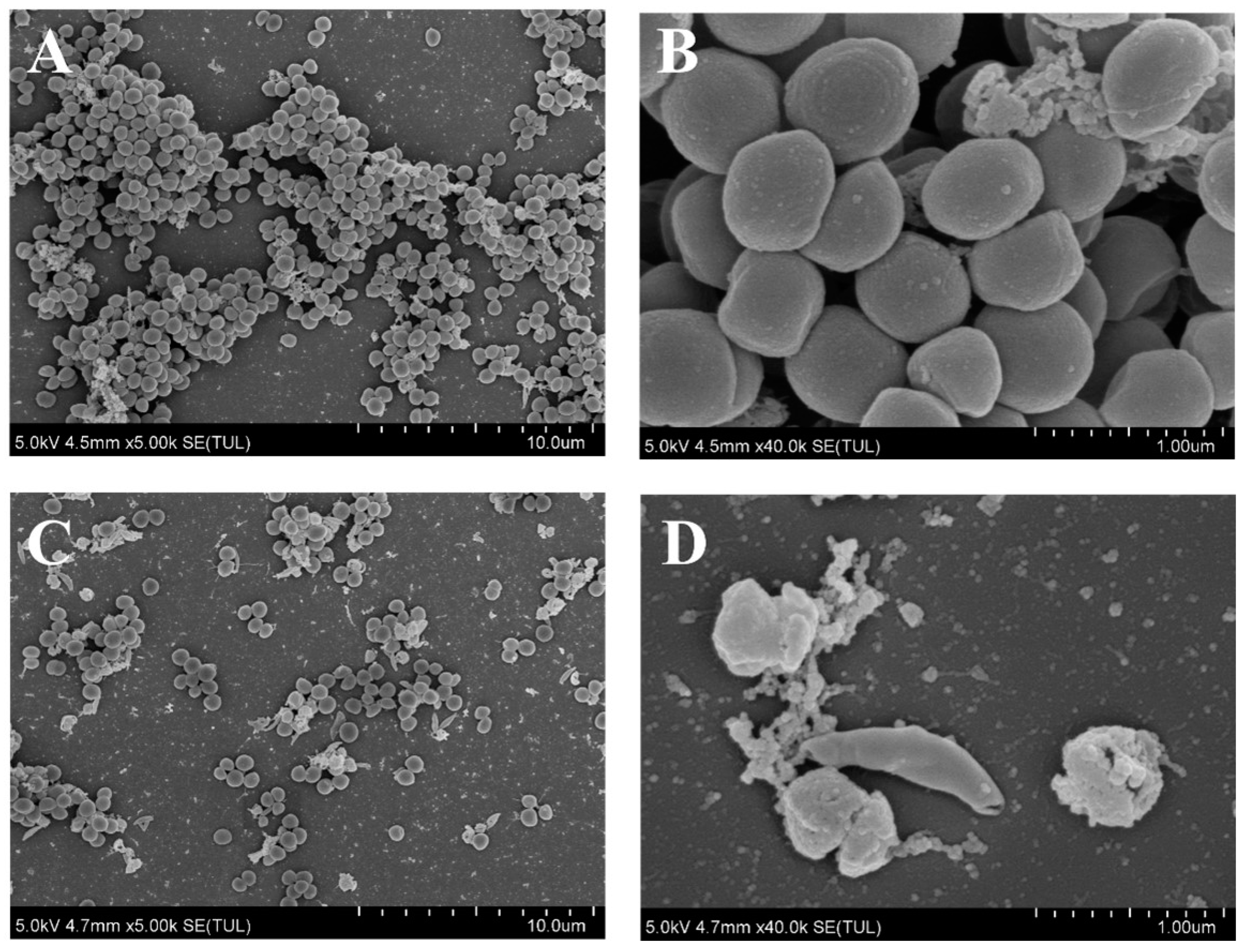
2.6. SEM Analysis of S. aureus Treated with AEO
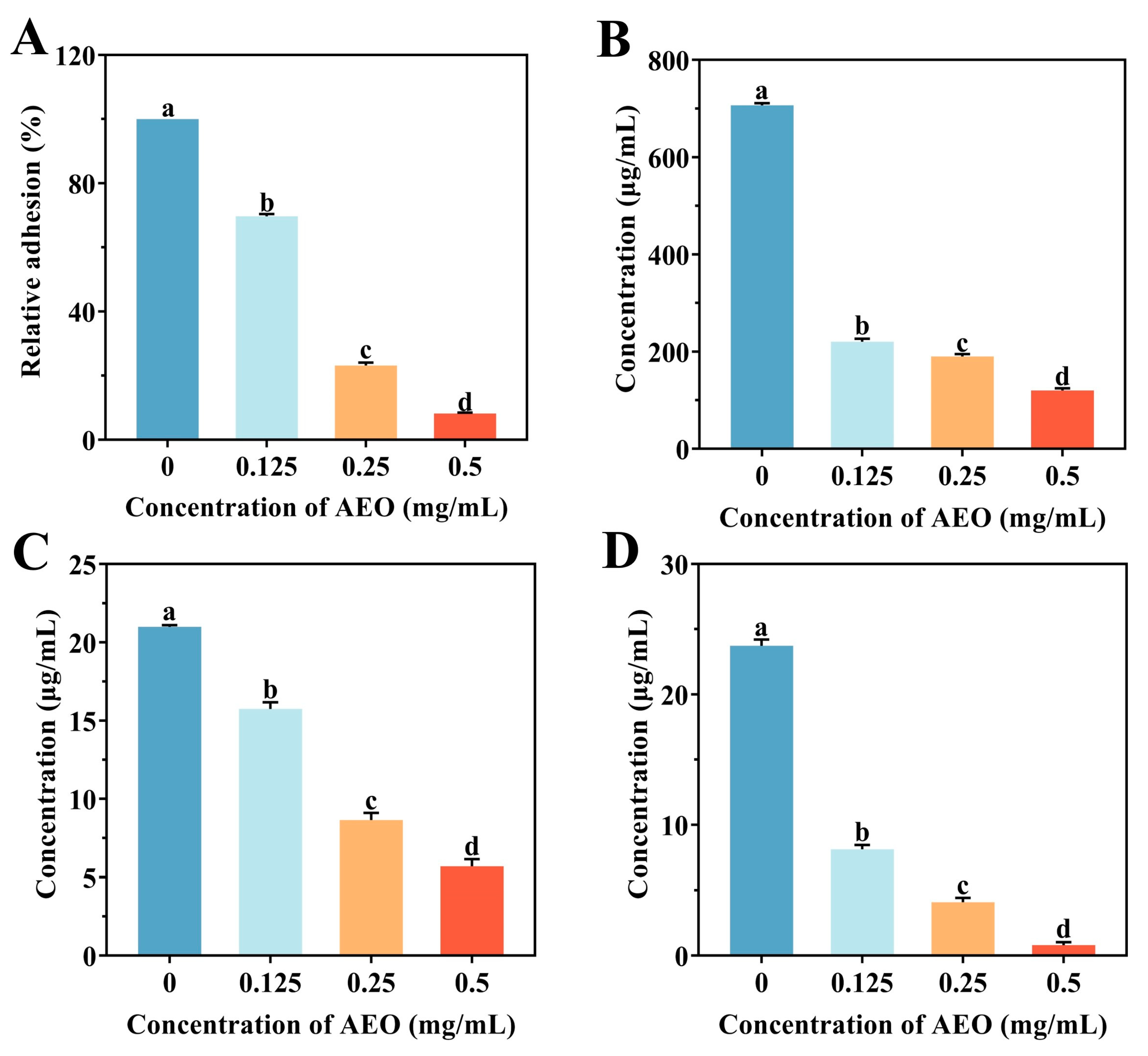
2.7. Impact of AEO on the Initial Adhesion of S. aureus in Biofilm Formation
2.8. Effect of AEO on the Release of EPS
2.9. Effects of AEO on the Relative Expression of Biofilm-Formation-Related Genes in S. aureus
2.10. The Effect of AEO on the Removal of S. aureus Biofilm on Fresh Pork
2.11. Effect of AEO on the Storage Quality of Fresh Pork
2.12. Statistical Analysis
3. Results
3.1. The Removal Effect of AEO on Biofilms of S. aureus
3.2. The Effect of AEO on the Removal of S. aureus Biofilm on Different Food Container Materials
3.3. Effect of AEO on the Micromorphology of S. aureus Biofilm
3.4. Effect of AEO on the Initial Adhesion of S. aureus Biofilm Formation
3.5. Effect of AEO on the Release of EPS
3.6. Effects of AEO on Relative Expression of Biofilm-Formation-Related Genes of S. aureus
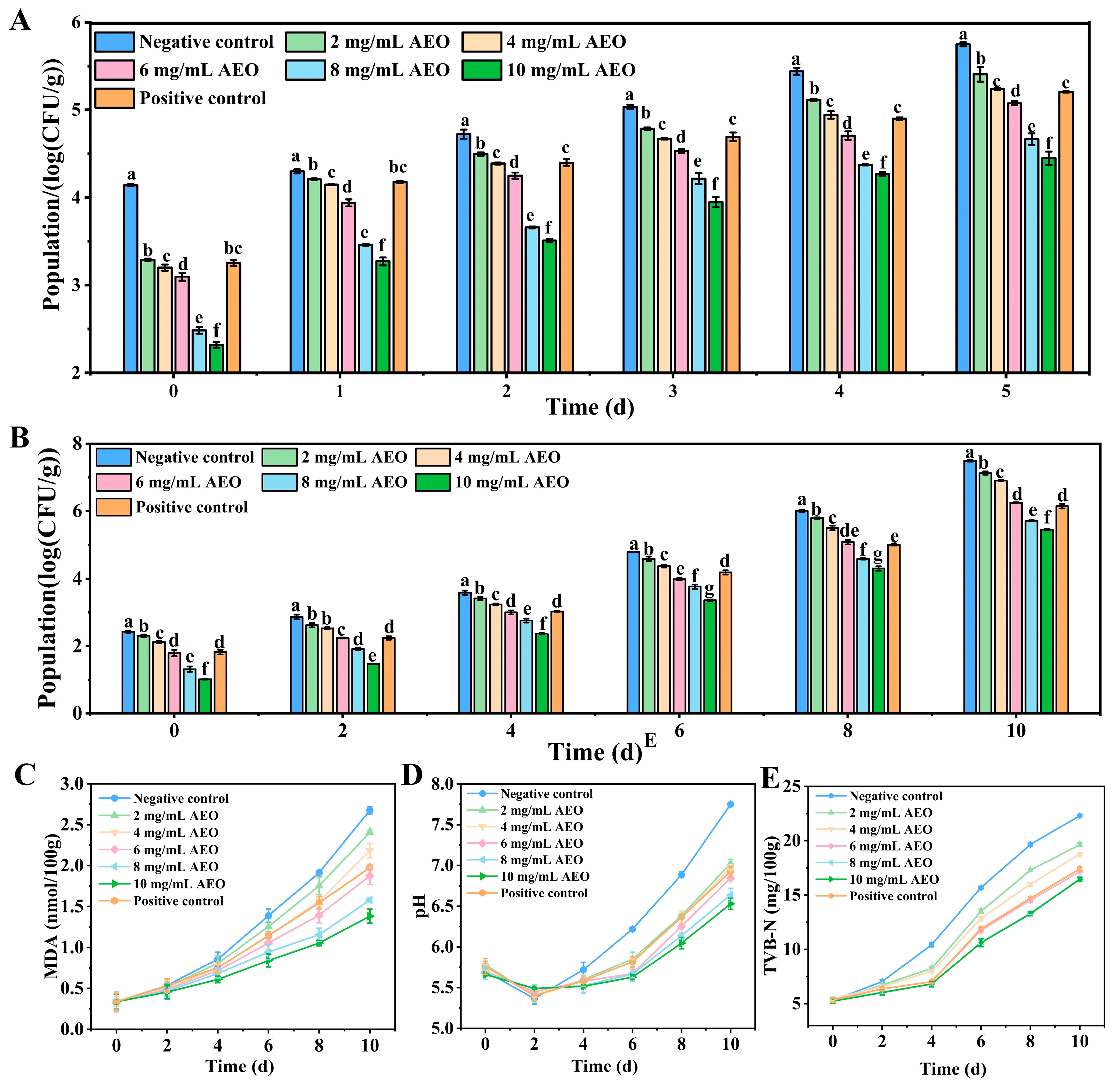
3.7. Effect of AEO on the Removal of S. aureus Biofilm on Fresh Pork
3.8. Effect of AEO on Storage Quality of Fresh Pork
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| S. aureus | Staphylococcus aureus S. aureus |
| AEO | Amomum tsaoko essential oil |
| SEM | Scanning electron microscopy |
| EPS | Extracellular polymeric substances |
| EOs | Essential oils |
| TVC | Total viable count |
| TBARS | Thiobarbituric acid reactive substances |
| TVB-N | Total volatile basic nitrogen |
References
- Gunjan; Vidic, J.; Manzano, M.; Raj, V.S.; Pandey, R.P.; Chang, C.M. Comparative meta-analysis of antimicrobial resistance from different food sources along with one health approach in Italy and Thailand. One Health 2023, 16, 100477. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, P.; Fu, X.; Xu, X.; Ruan, F.; Wang, T.; Chang, G.; Wan, Y.; Zhang, Y.; Wang, X. Prevalence and characterization of Staphylococcus aureus in raw eggs and it’s growth and enterotoxin a production in egg contents. LWT 2023, 174, 114379. [Google Scholar] [CrossRef]
- G. Abril, A.; G. Villa, T.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus exotoxins and their detection in the dairy industry and mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Nero, L.A.; Botelho, C.V.; Sovinski, A.I.; Grossi, J.L.; Call, D.R.; Dos Santos Bersot, L. Occurrence and distribution of antibiotic-resistant Staphylococcus aureus in a Brazilian pork production Chain. J. Food Prot. 2022, 85, 973–979. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Azlin-Hasim, S.; Cruz-Romero, M.; Cummins, E.; Kerry, J.P.; Morris, M.A. Antimicrobial effect of benzoic and sorbic acid salts and nano-solubilisates against Staphylococcus aureus, Pseudomonas fluorescens and chicken microbiota biofilms. Food Control 2020, 107, 106786. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Soteyome, T.; Peters, B.M.; Shirtliff, M.E.; Liu, J.; Harro, J.M. Polymicrobial interaction and biofilms between Staphylococcus aureus and Pseudomonas aeruginosa: An underestimated concern in food safety. Curr. Opin. Food Sci. 2019, 26, 57–64. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Chakraborty, R. Formation and development of biofilm an alarming concern in food safety perspectives. Biocatal. Agric. Biotechnol. 2021, 38, 102210. [Google Scholar] [CrossRef]
- Wagner, E.M.; Pracser, N.; Thalguter, S.; Fischel, K.; Rammer, N.; Pospisilova, L.; Alispahic, M.; Wagner, M.; Rychli, K. Identification of biofilm hotspots in a meat processing environment: Detection of spoilage bacteria in multi-species biofilms. Int. J. Food Microbiol. 2020, 328, 108668. [Google Scholar] [CrossRef]
- Cha, M.Y.; Ha, J.W. Low-energy X-ray irradiation effectively inactivates major foodborne pathogen biofilms on various food contact surfaces. Food Microbiol. 2022, 106, 104054. [Google Scholar] [CrossRef] [PubMed]
- Abi Assaf, J.; Holden, E.R.; Trampari, E.; Webber, M.A. Common food preservatives impose distinct selective pressures on Salmonella Typhimurium planktonic and biofilm populations. Food Microbiol. 2024, 121, 104517. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Guo, A.; Zhang, X.; Liu, W.; Ruan, Y. Formation of multispecies biofilms and their resistance to disinfectants in food processing environments: A review. J. Food Prot. 2021, 84, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Furquim Dos Santos Cardoso, V.; Amaral Roppa, R.H.; Antunes, C.; Silva Moraes, A.N.; Santi, L.; Konrath, E.L. Efficacy of medicinal plant extracts as dental and periodontal antibiofilm agents: A systematic review of randomized clinical trials. J. Ethnopharmacol. 2021, 281, 114541. [Google Scholar] [CrossRef] [PubMed]
- Kunová, S.; Sendra, E.; Haščík, P.; Vuković, N.L.; Vukić, M.D.; Hsouna, A.B.; Mnif, W.; Kačániová, M. Microbiological quality of deer meat treated with essential oil Litsea cubeba. Animals 2022, 12, 2315. [Google Scholar] [CrossRef]
- Jannat, B.; Mirza Alizadeh, A.; Farshi, P.; Dadgarnejad, M.; Hosseini, H.; Hashempour-Baltork, F.; Jafari, S.M. Anti-biofilm activity of essential oils in fruit and vegetable: A systematic review. Food Control 2023, 152, 109875. [Google Scholar] [CrossRef]
- Abdessemed, M.; Bouacida, S.; Turki, M.; Ben Haj Koubaier, H.; Omrani, S.; Allouache, R.; Bouzouita, N.; Karoui, R.; Snoussi, A. Chemical characterization and biological activities evaluation of Myrtus communis L. essential Oil extraction by-product towards circular economy and sustainability. Foods 2024, 13, 2211. [Google Scholar] [CrossRef] [PubMed]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. GOST 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Melo Coutinho, H.D.; et al. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Hou, F.; Chen, X.; Yi, F.; Song, L.; Zhan, S.; Han, X.; Zhang, L.; Li, F.; Wang, X.; Liu, Z. Antibacterial and antibiofilm properties of cinnamon essential oil on Pseudomonas tolaasii and application of potato starch/CEO active pads in preservation of mushroom (Agaricus bisporus). Food Control 2024, 165, 110705. [Google Scholar] [CrossRef]
- Hazitai, A.; Fei, H.-L.; Zhu, C.-Y.; Li, R.-X.; Zhang, L.-X.; Fan, P.-F. Cardamom (Amomum tsaoko) agroforest is important habitat for skywalker hoolock gibbon (Hoolock tianxing) in Mt. Gaoligong, Yunnan, China. Glob. Ecol. Conserv. 2024, 54, e03129. [Google Scholar] [CrossRef]
- Thinh, B.B.; Chac, L.D.; Hanh, D.H.; Korneeva, A.A.; Hung, N.; Igoli, J.O. Effect of extraction method on yield, chemical composition and antimicrobial activity of essential oil from the fruits of Amomum villosum var. xanthioides. J. Essent. Oil Bear. Plants 2022, 25, 28–37. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, M.; Han, Y.; Gao, L.; Chen, J.; Chen, W.; Li, Y. Study on antibacterial activity of Amomum tsao-ko extracts against bacillus subtilis and Listeria monocytogenes and preliminary investigation of its antibacterial mchanism. IOP Conf. Ser. Earth Environ. Sci. 2020, 512, 012080. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Qin, Z.; Wang, Y.; Zhao, P.; Gao, H. Insights into the composition and antibacterial activity of Amomum tsao-ko essential oils from different regions based on GC-MS and GC-IMS. Foods 2022, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, W.; Wan, S.; Zhou, J.; Qin, Z.; Gao, H. Antibacterial activity of Amomum tsaoko essential oil and its interaction with Staphylococcus aureus. LWT 2024, 191, 115700. [Google Scholar] [CrossRef]
- Andersen, J.B.; Rybtke, M.; Tolker-Nielsen, T. The dynamics of biofilm development and dispersal should be taken into account when quantifying biofilm via the crystal violet microtiter plate assay. Biofilm 2024, 8, 100207. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition mechanism of cardamom essential oil on methicillin-resistant Staphylococcus aureus biofilm. LWT 2020, 122, 109057. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Abdel-Samie, M.A.; Cui, H.; Lin, L. Unraveling the inhibitory mechanism of clove essential oil against Listeria monocytogenes biofilm and applying it to vegetable surfaces. LWT 2020, 134, 110210. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Feng, L.; Wu, Y.; Zhu, J. Extracellular matrix affects mature biofilm and stress resistance of psychrotrophic spoilage Pseudomonas at cold temperature. Food Microbiol. 2023, 112, 104214. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yang, Y.; Hou, W.; Zheng, Y. Inhibitory effects of lactobionic acid on biofilm formation and virulence of Staphylococcus aureus. Foods 2024, 13, 2781. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Nguyen, T.H.; Otto, M. The staphylococcal exopolysaccharide PIA-Biosynthesis and role in biofilm formation, colonization, and infection. Comput. Struct. Biotechnol. J. 2020, 18, 3324–3334. [Google Scholar] [CrossRef]
- Maggio, F.; Rossi, C.; Serio, A.; Chaves-Lopez, C.; Casaccia, M.; Paparella, A. Anti-biofilm mechanisms of action of essential oils by targeting genes involved in quorum sensing, motility, adhesion, and virulence: A review. Int. J. Food Microbiol. 2025, 426, 110874. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Fernandes, M.J.; Fernandes, M.H.; Teixeira, M.P.; Alfaia, C.M.; Serrano, C.; Patarata, L.; Fraqueza, M.J. Salt reduction strategies for dry cured meat products: The use of KCl and microencapsulated spices and aromatic plant extracts. Meat Sci. 2025, 221, 109719. [Google Scholar] [CrossRef]
- Campana, R.; Casettari, L.; Fagioli, L.; Cespi, M.; Bonacucina, G.; Baffone, W. Activity of essential oil-based microemulsions against Staphylococcus aureus biofilms developed on stainless steel surface in different culture media and growth conditions. Int. J. Food Microbiol. 2017, 241, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Erhabor, R.C.; Erhabor, J.O.; Nkadimeng, S.M.; McGaw, L.J. In vitro antimicrobial, antibiofilm and antioxidant activities of six South African plants with efficacy against selected foodborne pathogens. S. Afr. J. Bot. 2022, 146, 643–652. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Abdel-Samie, M.A.; Surendhiran, D.; Lin, L. Anti-Listeria monocytogenes biofilm mechanism of cold nitrogen plasma. Innov. Food Sci. Emerg. Technol. 2021, 67, 102571. [Google Scholar] [CrossRef]
- Wu, X.; Ma, G.L.; Chen, H.W.; Zhao, Z.Y.; Zhu, Z.P.; Xiong, J.; Yang, G.X.; Hu, J.F. Antibacterial and antibiofilm efficacy of the preferred fractions and compounds from Euphorbia humifusa (herba euphorbiae humifusae) against Staphylococcus aureus. J. Ethnopharmacol. 2023, 306, 116177. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, P.; Chen, X.; Hu, W.; Abdel-Samie, M.A.; Li, C.; Cui, H. Inhibition of Staphylococcus aureus biofilms by poly-L-aspartic acid nanoparticles loaded with Litsea cubeba essential oil. Int. J. Biol. Macromol. 2023, 242, 124904. [Google Scholar] [CrossRef]
- Frassinetti, S.; Falleni, A.; Del Carratore, R. Effect of itraconazole on Staphylococcus aureus biofilm and extracellular vesicles formation. Microb. Pathog. 2020, 147, 104267. [Google Scholar] [CrossRef]
- Banerji, R.; Mahamune, A.; Saroj, S.D. Aqueous extracts of spices inhibit biofilm in Listeria monocytogenes by down regulating release of eDNA. LWT 2022, 154, 112566. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, X.; Tian, L.; Wang, N.; Li, Y.; Kushmaro, A.; Marks, R.; Sun, Q. Antibiofilm activity of 3,3′-diindolylmethane on Staphylococcus aureus and its disinfection on common food-contact surfaces. Food Sci. Hum. Wellness 2022, 11, 1222–1232. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Ye, Y.; Cui, H.; Lin, L. Inhibition mechanism of cyclo (L-Phe-L-Pro) on early stage Staphylococcus aureus biofilm and its application on food contact surface. Food Biosci. 2022, 49, 101968. [Google Scholar] [CrossRef]
- Liu, M.; Wu, X.; Li, J.; Liu, L.; Zhang, R.; Shao, D.; Du, X. The specific anti-biofilm effect of gallic acid on Staphylococcus aureus by regulating the expression of the ica operon. Food Control 2017, 73, 613–618. [Google Scholar] [CrossRef]
- Formosa-Dague, C.; Feuillie, C.; Beaussart, A.; Derclaye, S.; Kucharíková, S.; Lasa, I.; Van Dijck, P.; Dufrêne, Y.F. Sticky matrix: Adhesion mechanism of the Staphylococcal Polysaccharide intercellular adhesin. ACS Nano 2016, 10, 3443–3452. [Google Scholar] [CrossRef] [PubMed]
- Gless, B.H.; Bojer, M.S.; Peng, P.; Baldry, M.; Ingmer, H.; Olsen, C.A. Identification of autoinducing thiodepsipeptides from staphylococci enabled by native chemical ligation. Nat. Chem. 2019, 11, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Tao, J.; Yan, S.; Zhou, C.; Liu, Q.; Zhu, H.; Wen, Z. Total flavonoids from Potentilla kleiniana Wight et Arn inhibits biofilm formation and virulence factors production in methicillin-resistant Staphylococcus aureus (MRSA). J. Ethnopharmacol. 2021, 279, 114383. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, X.; Yang, L.; Li, Q.; Ma, L.; Bai, T.; Zheng, X.; Wang, X. Anti Staphylococcus aureus activity of shikonin @ chitosan nanoemulsion and its effect on the storage quality of pork. Food Control 2024, 158, 110203. [Google Scholar] [CrossRef]

| Gene | Sequence (5’-3’) |
|---|---|
| icaA | Forward: AGTGCAGTTGTCGATGTTGGCTAC Reverse: ACACATGGCAAGCGGTTCATACTT |
| icaB | Forward: TTTGAAACACATACCCACGATTTGC Reverse: TTGGAGTTCGGAGTGACTGCTT |
| agrA | Forward: CTGATAATCCTTATGAGGTGCTTGA Reverse: AGGTAAGTTCACTGTGACTCGTA |
| cidA | Forward: CTTGGGTAGAAGACGGTGCAAACTT Reverse: AGCGTAATTTCGGAAGCAACATCCA |
| cidB | Forward: AGCCGCAGTAGGTATCGAAGTGT Reverse: CTAGTGCTTTAGCTGTGCCAAATGC |
| cidC | Forward: GGTACAATGGGTTGCGGTCTTCC Reverse: ACCTTTACCACCTGCTGCCTCA |
| 16S rRNA | Forward: TGTTCTCAGTTCGGATTGTA Reverse: ATACGGCTACCTTGTTACG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Guo, J.; Chen, Q.; Wan, S.; Qin, Z.; Gao, H. Antibiofilm Activity of Amomum tsaoko Essential Oil on Staphylococcus aureus and Its Application in Pork Preservation. Foods 2025, 14, 662. https://doi.org/10.3390/foods14040662
Yan Z, Guo J, Chen Q, Wan S, Qin Z, Gao H. Antibiofilm Activity of Amomum tsaoko Essential Oil on Staphylococcus aureus and Its Application in Pork Preservation. Foods. 2025; 14(4):662. https://doi.org/10.3390/foods14040662
Chicago/Turabian StyleYan, Zhifeng, Junrui Guo, Qiming Chen, Sibao Wan, Zhen Qin, and Haiyan Gao. 2025. "Antibiofilm Activity of Amomum tsaoko Essential Oil on Staphylococcus aureus and Its Application in Pork Preservation" Foods 14, no. 4: 662. https://doi.org/10.3390/foods14040662
APA StyleYan, Z., Guo, J., Chen, Q., Wan, S., Qin, Z., & Gao, H. (2025). Antibiofilm Activity of Amomum tsaoko Essential Oil on Staphylococcus aureus and Its Application in Pork Preservation. Foods, 14(4), 662. https://doi.org/10.3390/foods14040662





