Effect of Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) on Frozen Gelation of Egg Yolk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Yolk Freezing and Thawing Treatments
2.4. Observation of Freezing and Thawing Phenomenon of Egg Yolk
2.5. Rheological Analysis
2.5.1. Mobility Behavior Analysis
2.5.2. Viscoelasticity
2.6. Moisture Distribution (LF-NMR) Analysis
2.7. Protein Conformational Change Analysis
2.7.1. Raman Spectrum Analysis
2.7.2. Fluorescence Spectroscopy Analysis
2.7.3. Hydrophobicity Analysis
2.8. Microstructure
2.9. l* Diffusion Wave Spectrometer Analysis
2.10. Statistical Analysis
3. Results
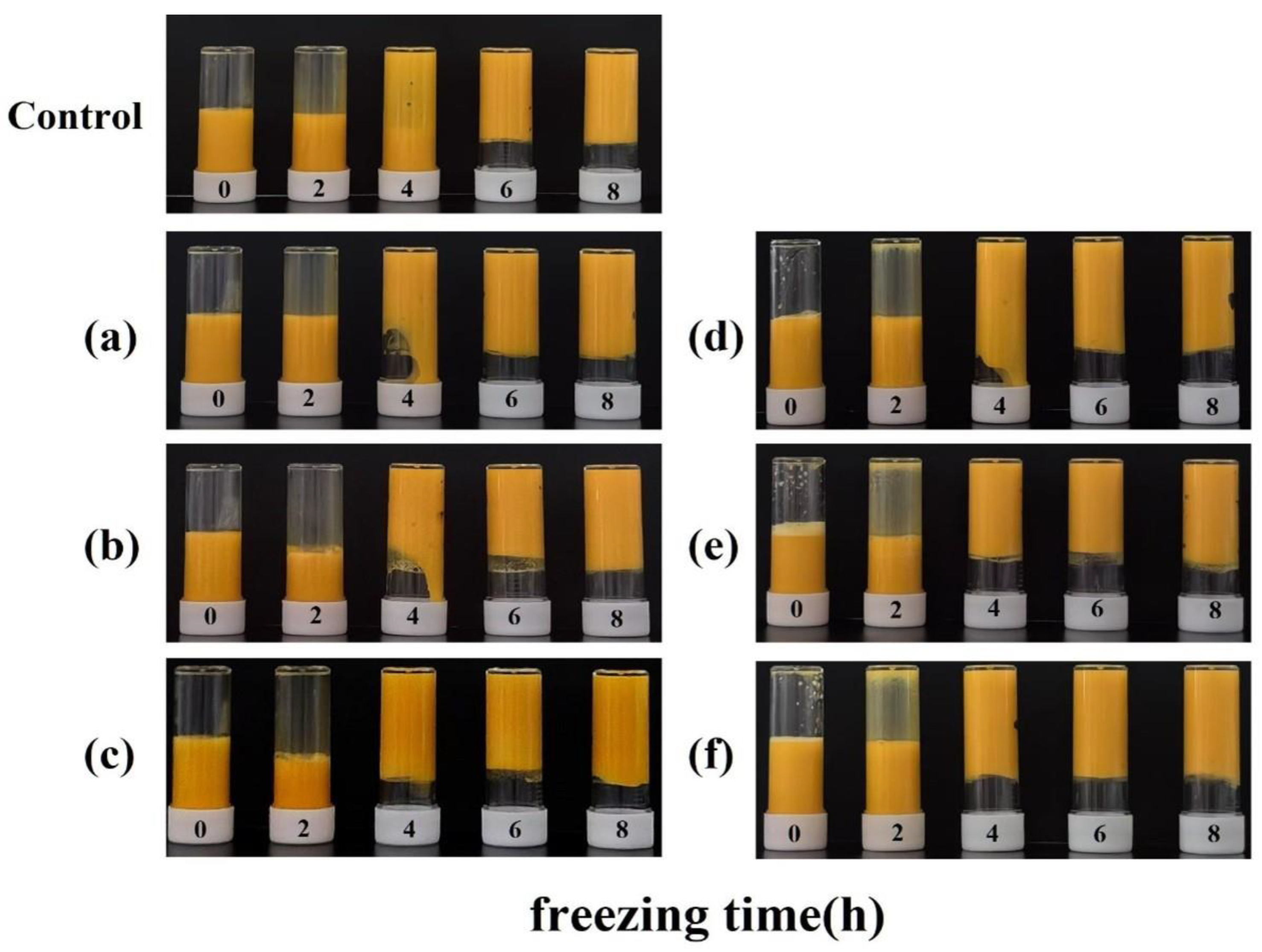
3.1. Freeze–Thawed Egg Yolk Liquid Sample Analysis
3.2. Rheological Analysis
3.2.1. Apparent Viscosity Analysis
3.2.2. Modulus of Elasticity and Loss Modulus Analysis
3.2.3. Tangent Loss Analysis
3.3. Moisture Distribution (LF-NMR) Analysis
3.4. Raman Spectrum Analysis
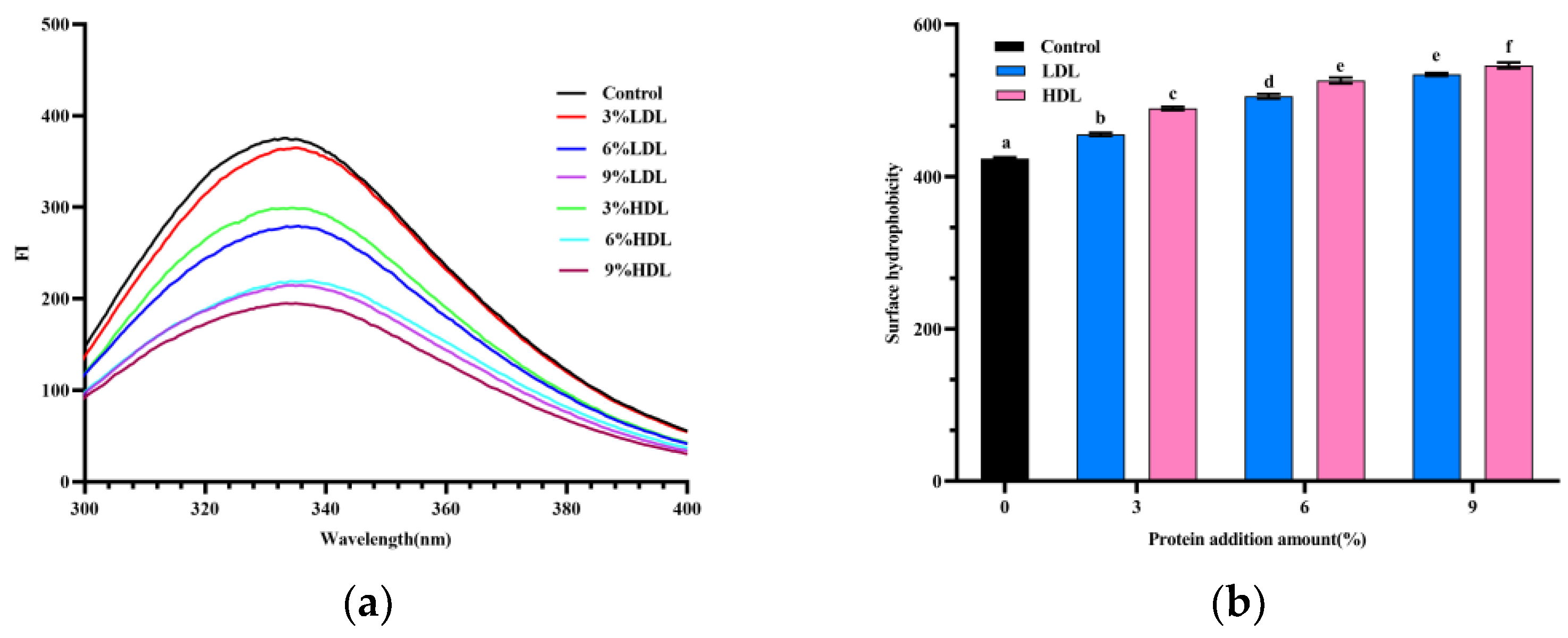
3.5. Fluorescence Spectroscopy Analysis
3.6. Hydrophobicity Analysis
3.7. l* Diffusion Wave Spectrometer Analysis
3.8. Fluorescence Inverted Microscope Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anton, M. Egg Yolk: Structures, Functionalities and Processes. J. Sci. Food Agric. 2013, 93, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, P.; Boulard, C.; Beaumal, V.; Chardot, T.; Anton, M. Protein Components of Low-Density Lipoproteins Purified from Hen Egg Yolk. J. Agric. Food Chem. 2006, 54, 4424–4429. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.A.; Lavers, C.G. Liquid and Frozen Egg: V. viscosity, Baking Quality, and Other Measurements on Frozen Egg Products. Can. J. Res. 1949, 27, 231–240. [Google Scholar] [CrossRef]
- Burley, R.W.; Cook, W.H. Isolation and Composition of Avian Egg Yolk Granules and Their Constituent A- and Β-Lipovitellins. Can. J. Biochem. Physiol. 1961, 39, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Izat, A.L.; Gardner, F.A. Incidence of campylobacter jejuni in processed egg products. Poultry Science 1988, 67, 1431–1435. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, Y.; Wang, R.; Chi, Y. Influence of Antigelation Agents on Frozen Egg Yolk Gelation. J. Food Eng. 2021, 302, 110585. [Google Scholar] [CrossRef]
- Urban, O.M.; Miller, J.N. Relative Merits of Sucrose, Dextrose, and Levulose as Used in the Preservation of Eggs by Freezing1. Ind. Eng. Chem. 1930, 22, 355–356. [Google Scholar] [CrossRef]
- Fei, T.; Leyva-Gutierrez, F.M.A.; Wan, Z.; Wang, T. Gelation Inhibiting Additives and Freezing Impact Rheological, Thermal, and Microstructural Properties of Yolk. LWT 2021, 144, 111160. [Google Scholar] [CrossRef]
- Au, C.; Acevedo, N.C.; Horner, H.T.; Wang, T. Determination of the Gelation Mechanism of Freeze–Thawed Hen Egg Yolk. J. Agric. Food Chem. 2015, 63, 10170–10180. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Mann, M. The Chicken Egg Yolk Plasma and Granule Proteomes. Proteomics 2008, 8, 178–191. [Google Scholar] [CrossRef]
- Wakamatu, T.; Sato, Y.; Saito, Y. On Sodium Chloride Action in the Gelation Process of Low Density Lipoprotein (LDL) from Hen Egg Yolk. J. Food Sci. 1983, 48, 507–512. [Google Scholar] [CrossRef]
- Kiosseoglou, V.; Paraskevopoulou, A. Molecular Interactions in Gels Prepared with Egg Yolk and Its Fractions. Food Hydrocoll. 2005, 19, 527–532. [Google Scholar] [CrossRef]
- Kurisaki, J.-I.; Kaminogawa, S.; Yamauchi, K. Studies on Freeze-Thaw Celation of Very Low Density Lipoprotein from Hen’s Egg Yolk. J. Food Sci. 1980, 45, 463–466. [Google Scholar] [CrossRef]
- Chang, C.H.; Powrie, W.D.; Fennema, O. Studies on the Gelation of Egg Yolk and Plasma Upon Freezing and Thawing. J. Food Sci. 1977, 42, 1658–1665. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Y.; Zhang, L.; Zhang, Z.; Chi, Y.; Chi, Y. Changes in Egg Yolk Gelation Behaviour and Mechanisms during Freezing. LWT 2021, 151, 112223. [Google Scholar] [CrossRef]
- Ren, L.; Ma, J.; Xu, W.; Lv, Y.; Tong, Q. Stability of Low Density Lipoprotein Particles Affect the Formation of Off-Flavor in Thermal Egg Yolk. Food Res. Int. 2022, 154, 111029. [Google Scholar] [CrossRef] [PubMed]
- Motta-Romero, H.; Zhang, Z.; Tien Nguyen, A.; Schlegel, V.; Zhang, Y. Isolation of Egg Yolk Granules as Low-Cholesterol Emulsifying Agent in Mayonnaise. J. Food Sci. 2017, 82, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, Y.; Ma, Z.; Du, Q.; Zhao, Y.; Chi, Y. Changes in Gelation, Aggregation and Intermolecular Forces in Frozen-Thawed Egg Yolks during Freezing. Food Hydrocoll. 2020, 108, 105947. [Google Scholar] [CrossRef]
- Au, C.; Wang, T.; Acevedo, N.C. Development of a Low Resolution 1H NMR Spectroscopic Technique for the Study of Matrix Mobility in Fresh and Freeze-Thawed Hen Egg Yolk. Food Chem. 2016, 204, 159–166. [Google Scholar] [CrossRef]
- Mahadevan, S. Differential Scanning Calorimetric Studies of Native and Freeze-Damaged Very Low Density Lipoproteins in Hen’s Egg Yolk Plasma. J. Biosci. 1987, 11, 299–309. [Google Scholar] [CrossRef]
- Gmach, O.; Golda, J.; Kulozik, U. Freeze-Thaw Stability of Emulsions Made with Native and Enzymatically Modified Egg Yolk Fractions. Food Hydrocoll. 2022, 123, 107109. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Y.; Mo, X.; Duan, S.; Tong, Q. Effects of Freezing and Thawing Treatment on the Rheological and Textural Characteristics and Micro-Structure of Heat-Induced Egg Yolk Gels. J. Food Eng. 2018, 216, 144–150. [Google Scholar] [CrossRef]
- Kocal, J.T.; Nakai, S.; Powrie, W.D. Preparation of Apolipoprotein of Very Low Density Lipoprotein from Egg Yolk Granules. J. Food Sci. 1980, 45, 1761–1767. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Kieckbusch, T.G. Viscoelasticity of Frozen/Thawed Egg Yolk. J. Food Sci. 1997, 62, 548–550. [Google Scholar] [CrossRef]
- Huang, L.; Wang, T.; Han, Z.; Meng, Y.; Lu, X. Effect of Egg Yolk Freezing on Properties of Mayonnaise. Food Hydrocoll. 2016, 56, 311–317. [Google Scholar] [CrossRef]
- Primacella, M.; Wang, T.; Acevedo, N.C. Use of Reconstitued Yolk Systems To Study the Gelation Mechanism of Frozen–Thawed Hen Egg Yolk. J. Agric. Food Chem. 2018, 66, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Regenstein, J.M.; Zhou, P.; Yang, Y. Effects of High Intensity Ultrasound Modification on Physicochemical Property and Water in Myofibrillar Protein Gel. Ultrason. Sonochemistry 2017, 34, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Ganasen, P.; Benjakul, S. Physical Properties and Microstructure of Pidan Yolk as Affected by Different Divalent and Monovalent Cations. LWT-Food Sci. Technol. 2010, 43, 77–85. [Google Scholar] [CrossRef]
- Nara, M.; Okazaki, M.; Kagi, H. Infrared Study of Human Serum Very-Low-Density and Low-Density Lipoproteins. Implication of Esterified Lipid C=O Stretching Bands for Characterizing Lipoproteins. Chem. Phys. Lipids 2002, 117, 1–6. [Google Scholar] [CrossRef]
- Prescott, B.; Renugopalakrishnan, V.; Glimcher, M.J.; Bhushan, A.; Thomas, G.J., Jr. A Raman Spectroscopic Study of Hen Egg Yolk Phosvitin: Structures in Solution and in the Solid State. Biochemistry 1986, 25, 2792–2798. [Google Scholar] [CrossRef]
- Liu, W.-J.; Li, X.-L.; Li, S.-G.; Xu, B.-C.; Zhang, B. Emulsifying and Emulsion Stabilizing Properties of Hydrolysates of High-Density Lipoprotein from Egg Yolk. Food Chem. 2022, 369, 130891. [Google Scholar] [CrossRef] [PubMed]
- Anton, M.; Martinet, V.; Dalgalarrondo, M.; Beaumal, V.; David-Briand, E.; Rabesona, H. Chemical and Structural Characterisation of Low-Density Lipoproteins Purified from Hen Egg Yolk. Food Chem. 2003, 83, 175–183. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, Q.; Wang, T.; Xue, J.; Luo, Y. Effects of Different Polysaccharides on the Formation of Egg Yolk LDL Complex Nanogels for Nutrient Delivery. Carbohydr. Polym. 2016, 153, 336–344. [Google Scholar] [CrossRef]
- Ramondo, A.; Marulo, S.; Sorrentino, A.; Masi, P.; Di Pierro, P. Modification of Physicochemical and Functional Properties of Pumpkin Seeds Protein Isolate (PsPI) by High-Intensity Ultrasound: Effect of Treatment Time. ACS Food Sci. Technol. 2024, 4, 40–48. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Su, Y.; Chang, C.; Yang, Y. Freeze-Thaw Treatment Assists Isolation of IgY from Chicken Egg Yolk. Food Chem. 2021, 364, 130225. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Ma, Z.; Wang, R.; Chi, Y. A Comprehensive Review on Freeze-Induced Deterioration of Frozen Egg Yolks: Freezing Behaviors, Gelation Mechanisms, and Control Techniques. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70019. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Kumar, T.K.S.; Gopal Ganesh Jayaraman, G.; Yang, P.-W.; Chang, M.-M.; Trivedi, V.D.; Wang, S.-L.; Hwang, K.-C.; Chang, D.-K.; Yu, C.; et al. Proline Inhibits Aggregation during Protein Refolding. Protein Sci. 2000, 9, 344–352. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Lin, S.; Liu, K.; Guo, F.; Bao, Z. Effect of Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) on Frozen Gelation of Egg Yolk. Foods 2025, 14, 522. https://doi.org/10.3390/foods14030522
Yuan J, Lin S, Liu K, Guo F, Bao Z. Effect of Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) on Frozen Gelation of Egg Yolk. Foods. 2025; 14(3):522. https://doi.org/10.3390/foods14030522
Chicago/Turabian StyleYuan, Junze, Songyi Lin, Kun Liu, Fujun Guo, and Zhijie Bao. 2025. "Effect of Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) on Frozen Gelation of Egg Yolk" Foods 14, no. 3: 522. https://doi.org/10.3390/foods14030522
APA StyleYuan, J., Lin, S., Liu, K., Guo, F., & Bao, Z. (2025). Effect of Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) on Frozen Gelation of Egg Yolk. Foods, 14(3), 522. https://doi.org/10.3390/foods14030522







