Analysis of Olive Oil Mill Wastewater from Conventionally Farmed Olives: Chemical and Microbiological Safety and Polyphenolic Profile for Possible Use in Food Product Functionalization
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analysis of Micro-Contaminants
2.3. Polyphenolic Profile
2.4. Microbiological Analysis
2.5. Isolation, Typing, and Identification of LAB
2.6. Technological Characterization of LAB
2.7. Statistical Analysis
3. Results and Discussion
3.1. Determination of Micro-Contaminants
3.2. Phenolic Profile of OOMW
3.3. Microbiological Analysis
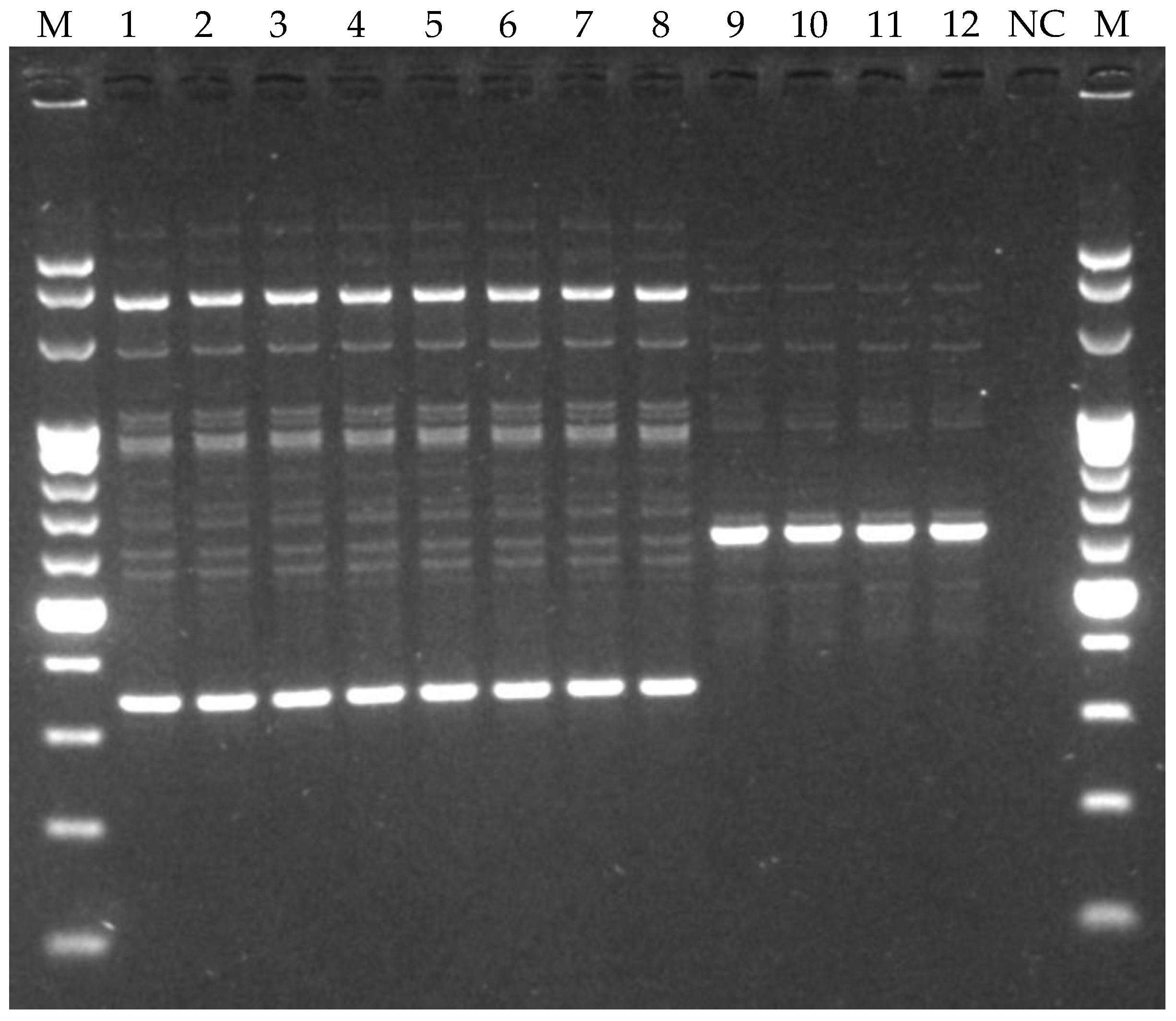
3.4. Phenotypic and Genotypic Characterization of LAB
3.5. Technological Traits of LAB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhat, R. Sustainability challenges in the valorization of agri-food wastes and by-products. In Valorization of Agri-Food Wastes and By-Products; Academic Press: London, UK, 2021; pp. 1–27. [Google Scholar]
- Berenguer, C.V.; Andrade, C.; Pereira, J.A.M.; Perestrelo, R.; Câmara, J.S. Current challenges in the sustainable valorisation of agri-food wastes: A review. Processes 2022, 11, 20. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Gaglio, R.; Barbaccia, P.; Barbera, M.; Restivo, I.; Attanzio, A.; Maniaci, G.; Moschetti, G.; Di Grigoli, A.; Francesca, N.; Tesoriere, L.; et al. The use of winery by-products to enhance the functional aspects of the fresh ovine “Primosale” cheese. Foods 2021, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Nyakang’i, C.O.; Marete, E.; Ebere, R.; Arimi, J.M. Physicochemical properties of avocado seed extract model beverages and baked products incorporated with avocado seed powder. Int. J. Food Sci. 2023, 2023, 6860806. [Google Scholar] [CrossRef]
- Iofrida, N.; Stillitano, T.; Falcone, G.; Gulisano, G.; Nicolò, B.F.; De Luca, A.I. The socio-economic impacts of organic and conventional olive growing in Italy. New Medit 2020, 19, 117–131. [Google Scholar] [CrossRef]
- Maesano, G.; Chinnici, G.; Falcone, G.; Bellia, C.; Raimondo, M.; D’Amico, M. Economic and environmental sustainability of olive production: A case study. Agronomy 2021, 11, 1753. [Google Scholar] [CrossRef]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive mill wastes: From wastes to resources. Environ. Sci. Pollut. Res. 2024, 31, 20853–20880. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Zoubi, H.; Hudaib, B.; Omar, W.; Soleimani, M.; Abu-Romman, S.; Frontistis, Z. Sustainable vs. conventional approach for olive oil wastewater management: A review of the state of the art. Water 2022, 14, 1695. [Google Scholar] [CrossRef]
- Manzanares, P.; Ballesteros, I.; Negro, M.J.; González, A.; Oliva, J.M.; Ballesteros, M. Processing of extracted olive oil pomace residue by hydrothermal or dilute acid pretreatment and enzymatic hydrolysis in a biorefinery context. Renew. Energy 2020, 145, 1235–1245. [Google Scholar] [CrossRef]
- Vaz, T.; Quina, M.M.; Martins, R.C.; Gomes, J. Olive mill wastewater treatment strategies to obtain quality water for irrigation: A review. Sci. Total Environ. 2024, 931, 172676. [Google Scholar] [CrossRef]
- Paié-Ribeiro, J.; Baptista, F.; Gomes, M.J.; Teixeira, A.; Pinheiro, V.; Outor-Monteiro, D.; Barros, A.N. Exploring the Variability in Phenolic Compounds and Antioxidant Capacity in Olive Oil By-Products: A Path to Sustainable Valorization. Antioxidants 2024, 13, 1470. [Google Scholar] [CrossRef] [PubMed]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Conte, P.; Pulina, S.; Del Caro, A.; Fadda, C.; Urgeghe, P.P.; De Bruno, A.; Difonzo, G.; Caponio, F.; Romeo, R.; Piga, A. Gluten-free breadsticks fortified with phenolic-rich extracts from olive leaves and olive mill wastewater. Foods 2021, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.; Wirkijowska, A.; Teterycz, D.; Łysakowska, P. Innovations in wheat bread: Using food industry by-products for better quality and nutrition. Appl. Sci. 2024, 14, 3976. [Google Scholar] [CrossRef]
- Indelicato, S.; Houmanat, K.; Bongiorno, D.; Ejjilani, A.; Hssaini, L.; Razouk, R.; Hanine, H. Freeze dried pomegranate juices of Moroccan fruits: Main representative phenolic compounds. J. Sci. Food Agric. 2023, 103, 1355–1365. [Google Scholar] [CrossRef]
- Messina, M.C.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological profile and bioactive properties of insect powders used in food and feed formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef]
- Stefańska, I.; Kwiecień, E.; Górzyńska, M.; Sałamaszyńska-Guz, A.; Rzewuska, M. RAPD-PCR-based fingerprinting method as a tool for epidemiological analysis of Trueperella pyogenes infections. Pathogens 2022, 11, 562. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Alfonzo, A.; Ventimiglia, G.; Corona, O.; Di Gerlando, R.; Gaglio, R.; Francesca, N.; Moschetti, G.; Settanni, L. Diversity and technological potential of lactic acid bacteria of wheat flours. Food Microbiol. 2013, 36, 343–354. [Google Scholar] [CrossRef]
- Schillinger, U.; Lücke, F.K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L.; Braga, T.M.; Lopes, M.D.F.S.; Suzzi, G. An investigation of the bacteriocinogenic potential of lactic acid bacteria associated with wheat (Triticum durum) kernels and non-conventional flours. LWT-Food Sci. Technol. 2008, 41, 1173–1182. [Google Scholar] [CrossRef]
- Gaglio, R.; Catania, P.; Orlando, S.; Vallone, M.; Moschetti, G.; Settanni, L. Biodiversity and dairy traits of lactic acid bacteria from foliage of aromatic plants before and after dehydration process monitored by a smart sensors system. FEMS Microbiol. Lett. 2020, 367, fnaa071. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Directive (EU) 2020/2184 of the European Parliament and of the Council. Off. J. Eur. Union 2020, 2019, 1–62. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020L2184&qid=1727688192963 (accessed on 27 September 2024).
- PPDB: Pesticide Properties Database, University of Hertfordshire. Available online: https://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 28 September 2024).
- European Commission. Commission Implementing Regulation (EU) 2019/1090 of 26 June 2019 Concerning the Non-Renewal of Approval of the Active Substance Dimethoate. Available online: http://data.europa.eu/eli/reg_impl/2019/1090/oj (accessed on 9 October 2023).
- European Commission. Commission Implementing Regulation (EU) 2019/677 of 29 April 2019 Concerning the Non-Renewal of the Approval of the Active Substance Chlorothalonil. Available online: http://data.europa.eu/eli/reg_impl/2019/677/oj (accessed on 9 October 2023).
- European Commission. Commission Implementing Regulation (EU) 2020/18 of 10 January 2020 Concerning the Non-Renewal of the Approval of the Active Substance Chlorpyrifos. Available online: http://data.europa.eu/eli/reg_impl/2020/18/oj (accessed on 27 September 2024).
- European Commission. Commission Implementing Regulation (EU) 2020/2087 of 14 December 2020 Concerning the Non-Renewal of the Approval of the Active Substance Mancozeb. Available online: http://data.europa.eu/eli/reg_impl/2020/2087/oj (accessed on 27 September 2024).
- European Commission. Commission Implementing Regulation (EU) 2019/344 of 28 February 2019 Concerning the Non-Renewal of Approval of the Active Substance Ethoprophos. Available online: http://data.europa.eu/eli/reg_impl/2019/344/oj (accessed on 9 October 2023).
- European Commission. Commission Implementing Regulation (EU) 2021/2081 of 26 November 2021 Concerning the Non-Renewal of Approval of the Active Substance Indoxacarb. Available online: http://data.europa.eu/eli/reg_impl/2021/2081/oj (accessed on 28 September 2024).
- Gensch, L.; Jantke, K.; Rasche, L.; Schneider, U.A. Pesticide risk assessment in European agriculture: Distribution patterns, ban-substitution effects and regulatory implications. Environ. Pollut. 2024, 348, 123836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Chen, W.J.; Wu, S.; Lei, Q.; Zhou, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Environmental occurrence, toxicity concerns, and biodegradation of neonicotinoid insecticides. Environ. Res. 2023, 218, 114953. [Google Scholar] [CrossRef]
- Bhende, R.S.; Jhariya, U.; Srivastava, S.; Bombaywala, S.; Das, S.; Dafale, N.A. Environmental distribution, metabolic fate, and degradation mechanism of chlorpyrifos: Recent and future perspectives. Appl. Biochem. Biotechnol. 2022, 194, 2301–2335. [Google Scholar] [CrossRef]
- European Commission. Guidance on Emergency Authorisations According to Article 53 of Regulation (EC) No 1107/2009 (SANCO/10087/2013 rev. 1). Available online: https://food.ec.europa.eu/system/files/2023-01/pesticides_aas_guidance_wd_emergency_authorisations_article53_post-210301.pdf (accessed on 28 September 2024).
- Lino, C.; Bongiorno, D.; Pitonzo, R.; Indelicato, S.; Barbera, M.; Di Gregorio, G.; Pane, D.; Avellone, G. Chemical characterization, stability and sensory evaluation of Sicilian extra virgin olive oils: Healthiness evidence at nose reach. Foods 2024, 13, 2149. [Google Scholar] [CrossRef]
- Bongiorno, D.; Di Stefano, V.; Indelicato, S.; Avellone, G.; Ceraulo, L. Bio-phenols determination in olive oils: Recent mass spectrometry approaches. Mass Spectrom. Rev. 2023, 42, 1462–1502. [Google Scholar] [CrossRef]
- Servili, M. The phenolic compounds: A commercial argument in the economic war to come on the quality of olive oil? OCL 2014, 21, D509. [Google Scholar] [CrossRef]
- Celano, R.; Piccinelli, A.L.; Pugliese, A.; Carabetta, S.; Di Sanzo, R.; Rastrelli, L.; Russo, M. Insights into the analysis of phenolic secoiridoids in extra virgin olive oil. J. Agric. Food Chem. 2018, 66, 6053–6063. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in extra virgin olive oil and table olives: Connections between agriculture and processing for health choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Prestes, O.D.; Augusti, P.R.; Moreira, J.C.F. Phenolic compounds and contaminants in olive oil and pomace—A narrative review of their biological and toxic effects. Food Biosci. 2023, 53, 102626. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Capasso, R.; De Martino, A.; Arienzo, M. Recovery and characterization of the metal polymeric organic fraction (polymerin) from olive oil mill wastewaters. J. Agric. Food Chem. 2002, 50, 2846–2855. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Navarro, D.; Maunier, S.; Sigoillot, J.C.; Lorquin, J.; Delattre, M.; Simon, J.L.; Asther, M.; Labat, M. Simple phenolics content in olive oil residues as a function of extraction systems. Food Chem. 2001, 75, 501–507. [Google Scholar] [CrossRef]
- Bouzid, O.; Navarro, D.; Roche, M.; Asther, M.; Haon, M.; Delattre, M.; Lorquin, J.; Labat, M.; Asther, M.; Lesage-Meessen, L. Fungal enzymes as a powerful tool to release simple phenolic compounds from olive oil by-product. Process Biochem. 2005, 40, 1855–1862. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadoura, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- La Scalia, G.; Micale, R.; Cannizzaro, L.; Marra, F.P. A sustainable phenolic compound extraction system from olive oil mill wastewater. J. Clean. Prod. 2017, 142, 3782–3788. [Google Scholar] [CrossRef]
- Miceli, A.; Gaglio, R.; Francesca, N.; Ciminata, A.; Moschetti, G.; Settanni, L. Evolution of shelf life parameters of ready-to-eat escarole (Cichorium endivia var. latifolium) subjected to different cutting operations. Sci. Hortic. 2019, 247, 175–183. [Google Scholar] [CrossRef]
- Zhang, H.; Yamamoto, E.; Murphy, J.; Locas, A. Microbiological safety of ready-to-eat fresh-cut fruits and vegetables sold on the Canadian retail market. Int. J. Food Microbiol. 2020, 335, 108855. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Ghabbour, N.; Rharrabti, Y. Physicochemical and microbiological characterization of olive mill wastewater (OMW) from different regions of northern Morocco. Environ. Technol. 2020, 41, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, K.; Meziane, M.; Bounedjar, K.; Douma, D.T.; Bensouna, S.; Fellah, M.; El-Miloudi, K. Lactic acid production by immobilization of Lactobacillus sp. isolated from olive mill wastewater. Desalination Water Treat. 2022, 255, 83–93. [Google Scholar] [CrossRef]
- Sahoo, M.; Panigrahi, C.; Aradwad, P. Management strategies emphasizing advanced food processing approaches to mitigate food borne zoonotic pathogens in food systems. Food Front. 2022, 3, 641–665. [Google Scholar] [CrossRef]
- Abril, A.G.; Quintela-Baluja, M.; Villa, T.G.; Calo-Mata, P.; Barros-Velázquez, J.; Carrera, M. Proteomic characterization of virulence factors and related proteins in Enterococcus strains from dairy and fermented food products. Int. J. Mol. Sci. 2022, 23, 10971. [Google Scholar] [CrossRef]
- Ben Braïek, O.; Smaoui, S. Enterococci: Between emerging pathogens and potential probiotics. BioMed Res. Int. 2019, 2019, 5938210. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, S.; Park, Y.S. Molecular typing tools for identifying and characterizing lactic acid bacteria: A review. Food Sci. Biotechnol. 2020, 29, 1301–1318. [Google Scholar] [CrossRef]
- Portilha-Cunha, M.F.; Macedo, A.C.; Malcata, F.X. A review on adventitious lactic acid bacteria from table olives. Foods 2020, 9, 948. [Google Scholar] [CrossRef]
- Rus-Fernández, P.; Fuentes, A. Fermentation starters and bacteriocins as biocontrol strategies for table olives preservation: A mini-review. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.; Magalhães Júnior, A.I.; Soccol, C.R. A review of selection criteria for starter culture development in the food fermentation industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Recent insights in the impact of emerging technologies on lactic acid bacteria: A review. Food Res. Int. 2020, 137, 109544. [Google Scholar] [CrossRef] [PubMed]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of lactic acid bacteria in food preservation and safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Silva Sabo, S.; Vitolo, M.; González, J.M.D.; De Souza Oliveira, R.P. Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Res. Int. 2014, 64, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Zimina, M.; Babich, O.; Prosekov, A.; Sukhikh, S.; Ivanova, S.; Shevchenko, M.; Noskova, S. Overview of global trends in classification, methods of preparation and application of bacteriocins. Antibiotics 2020, 9, 553. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L.; Van Sinderen, D. Characterization of bacteriocin-like inhibitory substances (BLIS) from sourdough lactic acid bacteria and evaluation of their in vitro and in situ activity. J. Appl. Microbiol. 2004, 96, 521–534. [Google Scholar] [CrossRef]

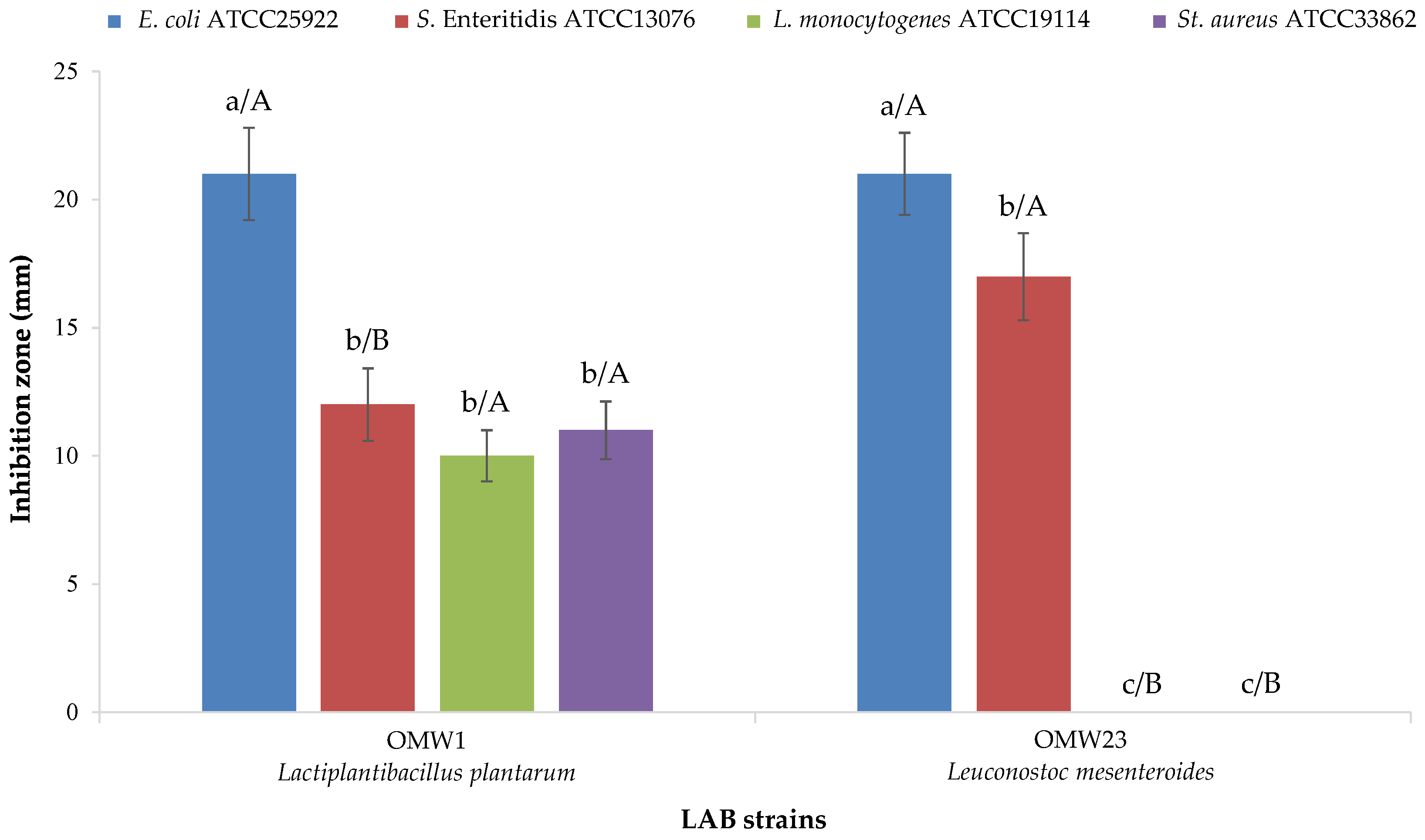
| Active Substances | OOMW-A (μg/L) | OOMW-B (μg/L) | logP (Kow) | Water Solubility | Fat Solubility | EC Regulation 1107/2009 Status | Mode of Action |
|---|---|---|---|---|---|---|---|
| Acetamiprid | n.d | 0.11 | 0.8 | High | Insoluble | Approved | Neonicotinoid insecticide |
| Azoxystrobin | 0.02 | 0.38 | 2.5 | Low | Insoluble | Approved | Fungicide |
| Bromacil | 5.29 | n.d | 1.88 | High | Not Reported | Not Approved | Herbicide |
| Chlorantraniliprole | n.d | 1.19 | 2.86 | Low | Soluble | Approved | insecticide |
| Chlorpyrifos-Ethyl | n.d | 0.14 | 4.7 | Low | Soluble | Not Approved | insecticide |
| Cyproconazol | n.d | 0.71 | 3.09 | Moderate | Soluble | Not Approved | Fungicide |
| Fenpyrazamine | n.d | 13.32 | 3.52 | Low | Soluble | Approved | Fungicide |
| Imidacloprid | 0.17 | 1.25 | 0.57 | High | Insoluble | Not Approved | Neonicotinoid insecticide |
| Mandipropamid | n.d | 10.60 | 3.2 | Low | Insoluble | Approved | Fungicide |
| Metalaxyl | n.d | 4.75 | 1.75 | High | Not Reported | Approved | Fungicide |
| Penthiopyrad | n.d | 29.54 | 4.62 | Low | Insoluble | Approved | Fungicide |
| Prometryn | n.d | 0.12 | 3.34 | Low | Not Reported | Not Approved | Herbicide |
| Pyraclostrobin | n.d | 0.34 | 3.99 | Low | Soluble | Approved | Fungicide |
| Simazine | 0.21 | n.d | 2.3 | Low | Not Reported | Not Approved | Herbicide |
| Tebuconazole | n.d | 2.1 | 3.7 | Low | Likely to be Soluble | Approved | Fungicide |
| Terbuthylazine | n.d | 0.17 | 3.4 | Low | Soluble | Approved | Herbicide |
| Terbuthylazin-Desethyl | n.d | 0.34 | 2.3 | Moderate | Not Reported | Not Reported | metabolite |
| Tetraconazole | n.d | 0.68 | 3.5 | Moderate | Soluble | Approved | Fungicide |
| ∑pesticide | 5.7 | 65.8 |
| Active Substances | Samples | p Value | |
|---|---|---|---|
| OOMW-A (μg/mL) | OOMW-B (μg/mL) | ||
| Caffeic acid | 40.1 ± 2.4 a | 25.3 ± 1.4 b | 0.001 |
| Hydroxycinnamic acid | 92.2 ± 3.7 | 88.5 ± 3.9 | 0.298 |
| Hydroxytyrosol | 143.0 ± 5.1 | 148.0 ± 4.3 | 0.266 |
| Cumaric acid | 71.6 ± 3.2 | 77.9 ± 4.0 | 0.100 |
| Ferulic acid | 20.9 ± 1.2 a | 12.7 ± 0.6 b | 0.001 |
| Oleaceinic acid | 8.1 ± 0.5 | 8.2 ± 0.5 | 0.815 |
| Luteonin | 9.9 ± 0.4 b | 10.8 ± 0.3 a | 0.040 |
| Apigenin | 6.0 ± 0.3 | 6.1 ± 0.3 | 0.707 |
| Σpolyphenols | 391.8 | 377.5 | |
| Microorganisms | Samples | p Value | |
|---|---|---|---|
| OOMW-A | OOMW-B | ||
| TMM | 1.85 ± 0.34 | 1.7 ± 0.29 | 0.683 |
| Mesophilic coccus LAB | 2.40 ± 0.23 | 2.33 ± 0.19 | 0.491 |
| Thermophilic coccus LAB | 2.35 ± 0.43 | 2.23 ± 0.39 | 0.799 |
| Mesophilic rod LAB | 2.32 ± 0.23 | 2.43 ± 0.32 | 0.654 |
| Thermophilic rod LAB | 1.70 ± 0.54 | 2.43 ± 0.49 | 0.271 |
| Enterobacteriaceae | <1 | <1 | n.e. |
| Enterococci | <1 | <1 | n.e. |
| CPS | <1 | <1 | n.e. |
| L. monocytogenes | <1 | <1 | n.e. |
| E. coli | <1 | <1 | n.e. |
| Salmonella spp. | <1 | <1 | n.e. |
| Pseudomonads | <1 | <1 | n.e. |
| Aerobic spore-forming bacteria | <1 | <1 | n.e. |
| Yeasts | <1 | <1 | n.e. |
| Molds | <1 | <1 | n.e. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciurba, L.; Indelicato, S.; Gaglio, R.; Barbera, M.; Marra, F.P.; Bongiorno, D.; Davino, S.; Piazzese, D.; Settanni, L.; Avellone, G. Analysis of Olive Oil Mill Wastewater from Conventionally Farmed Olives: Chemical and Microbiological Safety and Polyphenolic Profile for Possible Use in Food Product Functionalization. Foods 2025, 14, 449. https://doi.org/10.3390/foods14030449
Sciurba L, Indelicato S, Gaglio R, Barbera M, Marra FP, Bongiorno D, Davino S, Piazzese D, Settanni L, Avellone G. Analysis of Olive Oil Mill Wastewater from Conventionally Farmed Olives: Chemical and Microbiological Safety and Polyphenolic Profile for Possible Use in Food Product Functionalization. Foods. 2025; 14(3):449. https://doi.org/10.3390/foods14030449
Chicago/Turabian StyleSciurba, Lino, Serena Indelicato, Raimondo Gaglio, Marcella Barbera, Francesco Paolo Marra, David Bongiorno, Salvatore Davino, Daniela Piazzese, Luca Settanni, and Giuseppe Avellone. 2025. "Analysis of Olive Oil Mill Wastewater from Conventionally Farmed Olives: Chemical and Microbiological Safety and Polyphenolic Profile for Possible Use in Food Product Functionalization" Foods 14, no. 3: 449. https://doi.org/10.3390/foods14030449
APA StyleSciurba, L., Indelicato, S., Gaglio, R., Barbera, M., Marra, F. P., Bongiorno, D., Davino, S., Piazzese, D., Settanni, L., & Avellone, G. (2025). Analysis of Olive Oil Mill Wastewater from Conventionally Farmed Olives: Chemical and Microbiological Safety and Polyphenolic Profile for Possible Use in Food Product Functionalization. Foods, 14(3), 449. https://doi.org/10.3390/foods14030449







