Nutritional Composition, Bioactive Properties, and Sensory Evaluation of Breadsticks Enriched with Carp Meat (Cyprinus carpio, L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Basic Composition
- DM%—dry matter (%);
- LC%—lipid content (%);
- PC%—protein content (%);
- AC%—ash content (%);
- FC%—fiber content (%).
2.3. Content of Micro and Macro Elements
2.4. Antioxidant Activity
2.5. Fatty Acid Profile
2.6. Lipid Value
2.7. Amino Acid Profile
2.8. Available Lysine
2.9. Texture Parameter Analysis
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Basic Composition
3.2. Antioxidant Activity
3.3. Fatty Acids Profile and Lipid Values
3.4. Amino Acid Profile and Loss of Available Lysine
3.5. Texture
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Ash content |
| ANOVA | Analysis of variance |
| AOAC | Association of Official Agricultural Chemists |
| AsV | Anisidine value |
| AV | Acid value |
| DPPH | Radical scavenging activity |
| DHA | Docosahexaenoic acid |
| DM | Dry matter |
| EAA | Essential amino acids |
| EPA | Eicosapentaenoic acid |
| FAMEs | Fatty acid methyl esters |
| FC | Fiber content |
| FRAP | Ferric reducing antioxidant power |
| LC | Lipid content |
| MUFA | Monounsaturated fatty acids |
| NEAA | Non-essential amino acids |
| NFE | Nitrogen-free extract |
| PC | Protein content |
| PUFA | Polyunsaturated fatty acids |
| PV | Peroxide value |
| S | Breadsticks without carp meat |
| S5 | Breadsticks with 5% carp meat |
| S10 | Breadsticks with 10% carp meat |
| S15 | Breadsticks with 15% carp meat |
| S20 | Breadsticks with 20% carp meat |
| S25 | Breadsticks with 25% carp meat |
| S30 | Breadsticks with 30% carp meat |
| SFA | Saturated fatty acids |
| TAA | Total amino acid |
| TEAC | Trolox equivalent antioxidant capacity |
| TOTOX | Total oxidation index |
| TPC | Total polyphenol content |
References
- Gasparre, N.; Rosell, C.M. Snacking: Ingredients, Processing and Safety. In Cereal-Based Foodstuffs: The Backbone of Mediterranean Cuisine; Springer: Berlin/Heidelberg, Germany, 2021; pp. 167–192. [Google Scholar]
- Onyeaka, H.; Nwaiwu, O.; Obileke, K.C.; Miri, T.; Al-Sharify, Z.T. Global Nutritional Challenges of Reformulated Food: A Review. Food Sci. Nutr. 2023, 11, 2483–2499. [Google Scholar] [CrossRef]
- Santosh, O.; Bajwa, H.K.; Bisht, M.S.; Chongtham, N. Antioxidant Activity and Sensory Evaluation of Crispy Salted Snacks Fortified with Bamboo Shoot Rich in Bioactive Compounds. Appl. Food Res. 2021, 1, 100018. [Google Scholar] [CrossRef]
- Clavel-Coibrié, E.; Sales, J.R.; da Silva, A.M.; Barroca, M.J.; Sousa, I.; Raymundo, A. Sarcocornia Perennis: A Salt Substitute in Savory Snacks. Foods 2021, 10, 3110. [Google Scholar] [CrossRef]
- Santosh, O.; Nirmala, C.; Bajwa, H.K.; Bisht, M.S.; Indira, A. Assessment of Nutritional Enhancement through Food-to-Food Fortification: Freeze-Dried Bamboo Shoot Powder as a Natural Mineral Fortifier in Functional Foods. Adv. Bamboo Sci. 2025, 12, 100191. [Google Scholar] [CrossRef]
- Hussein, A.S.; Bahgaat, W.K.; Ibraheim, G.E. Impact of Jameed Fortification on Physicochemical, Antioxidant and Volatile Compounds of Snacks. Egypt. J. Chem. 2022, 65, 1–11. [Google Scholar] [CrossRef]
- Famakinwa, A.; Shuttleworth, A.; Lubisi, S.; Olubi, O.; Oguntibeju, O.; Obilana, A. Chemical and Functional Properties of Snacks Produced from Wheat Flour Fortified with Moringa Oleifera Leaf Powder. Afr. J. Food Agric. Nutr. Dev. 2023, 23, 24467–24486. [Google Scholar] [CrossRef]
- Oprea, O.B.; Sannan, S.; Tolstorebrov, I.; Claussen, I.C.; Gaceu, L. Effects of Fish Protein Hydrolysate on the Nutritional, Rheological, Sensorial, and Textural Characteristics of Bread. Foods 2024, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Khodaveisi, M.; Bahramian, S.; Rokhzadi, A. Incorporation of Common Carp Meat Paste in Bread Formulation as a Strategy to Increase the Usage of Fish Meat in the Human Diet and Investigation of the Physicochemical and Organoleptic Properties of Bread. Hum. Health Halal Metr. 2024, 5, 1–8. [Google Scholar] [CrossRef]
- de Souza, M.L.R.; Gasparino, E.; Goes, E.S.d.R.; Coradini, M.F.; Vieira, V.I.; Oliveira, G.G.; Matiucci, M.A.; de Castro, A.C.V.J.; Siemer, S.; Fernandes, V.R.T.; et al. Fish Carcass Flours from Different Species and Their Incorporation in Tapioca Cookies. Future Foods 2022, 5, 100132. [Google Scholar] [CrossRef]
- Turuk, A.S.; Banerjee, K. Blending Seaweed into Bakery Products. J. Appl. Phycol. 2023, 35, 1893–1909. [Google Scholar] [CrossRef]
- Quitral, V.; Sepúlveda, M.; Gamero-Vega, G.; Jiménez, P. Seaweeds in Bakery and Farinaceous Foods: A Mini-Review. Int. J. Gastron. Food Sci. 2022, 28, 100403. [Google Scholar] [CrossRef]
- Nawaz, A.; Khalifa, I.; Walayat, N.; Lorenzo, J.M.; Irshad, S.; Abdullah; Ahmed, S.; Simirgiotis, M.J.; Ali, M.; Li, E. Whole Fish Powder Snacks: Evaluation of Structural, Textural, Pasting, and Water Distribution Properties. Sustainability 2021, 13, 6010. [Google Scholar] [CrossRef]
- Nedoluzhko, A.V.; Gladysheva-Azgari, M.V.; Shalgimbayeva, G.M.; Volkov, A.A.; Slobodova, N.V.; Tsygankova, S.V.; Boulygina, E.S.; Nguyen, V.Q.; Pham, T.T.; Nguyen, D.T.; et al. Genetic Contribution of Domestic European Common Carp (Cyprinus Carpio Carpio) and Amur Carp (Cyprinus Carpio Haematopterus) to the Wild Vietnamese Carp Population as Revealed by DdRAD Sequencing. Aquaculture 2021, 544, 737049. [Google Scholar] [CrossRef]
- Iqra Nazir, P.B.; Puspendu Bikash Maity, M.A.; Animesh Das, S.S. Significant Role of Fish Nutrients with Special Emphasis to Essential Fatty Acid in Human Nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 2034–2046. [Google Scholar] [CrossRef]
- He, Z.; Wang, J.; Wei, Y.; Yan, X.; Li, Y.; Xie, D.; Nie, G. Optimizing Muscle Quality in Common Carp (Cyprinus Carpio L.): Impacts of Body Size on Nutrient Composition, Texture, and Volatile Profile. Foods 2025, 14, 2794. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A Critical Review on the Health Benefits of Fish Consumption and Its Bioactive Constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar Drugs 2022, 20, 187. [Google Scholar] [CrossRef]
- AOAC Dry Matter Method 945.12. Crude Ash Method 920.153. Crude Protein Method 945.18, 21st ed.; Official Methods of Analysis of the AOAC; AOAC: Gaithersburg, MD, USA, 2019.
- Analytical Techniques in Aquaculture Research. Available online: https://aquaculture.ugent.be/Education/coursematerial/online%20courses/ATA/analysis/NFE.htm (accessed on 23 November 2025).
- McCleary, B.V. Measurement of Dietary Fiber: Which AOAC Official Method of AnalysisSM to Use. J. AOAC Int. 2023, 106, 917–930. [Google Scholar] [CrossRef]
- Cunniff, P.A. Books in Brief. J. AOAC Int. 1997, 80, 127A–128A. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis. Method 991.39; Fatty Acids in Encapsulated Fish Oils and Fish Oil Methyl and Ethyl Esters. Association of Official Analytical Chemists: Rockville, MD, USA, 1984.
- Methods Ce 1b-89; AOAC Official Methods and Recommended Practices of the American Oil Chemists Society, 15th Ed.; Fatty Acid Composition by GLC, Marine Oil. Association of Official Analytical Chemists: Rockville, MD, USA, 2004.
- PN EN ISO 3960:2017; Animal and Vegetable Fats and Oils-Determination of Peroxide Value-Iodometric (Visual) Endpoint Determination (ISO 3960:2017). ISO: Geneva, Switzerland, 2017.
- EN ISO 6885:2016; Animal and Vegetable Fats and Oils-Determination of Anisidine Value (ISO 6885:2016). ISO: Geneva, Switzerland, 2016.
- EN ISO 660:2020; Animal and Vegetable Fats and Oils. Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2020.
- Blackburn, S. Amino Acid Determination. Methods and Techniques; Edward Arnold (Publishers) Ltd.: London, UK, 1968. [Google Scholar]
- Moore, S. On the Determination of Cystine as Cysteic Acid. J. Biol. Chem. 1963, 238, 235–237. [Google Scholar] [CrossRef]
- Landry, J.; Delhaye, S.; Jones, D.G. Determination of Tryptophan in Feedstuffs: Comparison of Two Methods of Hydrolysis Prior to HPLC Analysis. J. Sci. Food Agric. 1992, 58, 439–441. [Google Scholar] [CrossRef]
- Carpenter, K.J. The Estimation of the Available Lysine in Animal-Protein Foods. Biochem. J. 1960, 77, 604–610. [Google Scholar] [CrossRef]
- Booth, V.H. Problems in the Determination of FDNB-available Lysine. J. Sci. Food Agric. 1971, 22, 658–666. [Google Scholar] [CrossRef]
- Levent, H.; Bilgiçli, N. Quality Evaluation of Wheat Germ Cake Prepared with Different Emulsifiers. J. Food Qual. 2013, 36, 334–341. [Google Scholar] [CrossRef]
- Analiza Sensoryczna-Metodologia-Metoda Sprawdzania Wrażliwości Smakowej PN-ISO 3972:1998 /-KATALOG GŁÓWNY Systemu Biblioteczno-Informacyjnego Uniwersytetu Jana Długosza w Częstochowie-BIBLIOTEKA GŁÓWNA-UJD. Available online: https://katalog.bu.ujd.edu.pl/cgi-bin/koha/opac-detail.pl?myhash=0x4C89F3EBDF763E88866CF8C1E7F4F02F&mysearchid=30773&bib=239271 (accessed on 18 June 2024).
- Analiza Sensoryczna-Metodologia-Wprowadzenie i Szkolenie Oceniających w Wykrywaniu i Rozpznawaniu Zapachów PN-ISO 5496:1997 /-KATALOG GŁÓWNY Systemu Biblioteczno-Informacyjnego Uniwersytetu Jana Długosza w Częstochowie-BIBLIOTEKA GŁÓWNA-UJD. Available online: https://www.katalog.bu.ujd.edu.pl/cgi-bin/koha/opac-detail.pl?myhash=0x3D4ABDCAA57B35DAB908F24503DF6620&mysearchid=6742&bib=239275 (accessed on 6 August 2024).
- EN ISO 8589:2010-Sensory Analysis-General Guidance for the Design of Test Rooms (ISO 8589:2007). Available online: https://standards.iteh.ai/catalog/standards/cen/5a430208-46aa-47da-8531-870a268453a3/en-iso-8589-2010?srsltid=AfmBOorEkJ0m7jVr-57f9IsnYiSzcJu9V75bnijRHiGd7VnG4B09oduN (accessed on 9 October 2025).
- Baryłko-Pikielna, N.; Matuszewska, I. Rozdział 10. Metody Sensorycznej Analizy Opisowej. In Sensoryczne Badania Żywności. Podstawy-Metody-Zastosowania; Wydawnictwo Naukowe PTTŻ: Kraków, Poland, 2014; pp. 181–226. [Google Scholar]
- TIBCO Statistica Electronic Manual. Available online: https://www.scribd.com/document/321061529/STATISTICA-Electronic-Manual (accessed on 14 August 2024).
- Kaliniak-Dziura, A.; Skałecki, P.; Florek, M.; Kędzierska-Matysek, M.; Sobczak, P. Chemical Composition and Elements Concentration of Fillet, Spine and Bones of Common Carp (Cyprinus Carpio) in Relation to Nutrient Requirements for Minerals. Animals 2024, 14, 1311. [Google Scholar] [CrossRef]
- Ibraeva, K.; Tabakaev, R.; Yazykov, N.; Rudmin, M.; Dubinin, Y.; Zavorin, A. Flour-Milling Waste as a Potential Energy Source. Study Miner. Part. Fuel 2021, 285, 119240. [Google Scholar] [CrossRef]
- Skałecki, P.; Florek, M.; Pyć, A.; Kaliniak, A.; Staszowska, A. Comparison of Physicochemical Properties, Fatty Acid Composition and Mineral Contents in Common Carp (Cyprinus Carpio L.) Fillet and the Native Traditional Product Carp Ham. Pol. J. Food Nutr. Sci. 2016, 66, 311–319. [Google Scholar] [CrossRef]
- Kłobukowski, J.; Skibniewska, K.; Janowicz, K.; Kłobukowski, F.; Siemianowska, E.; Terech-Majewska, E.; Szarek, J. Selected Parameters of Nutritional and Pro-Health Value in the Common Carp (Cyprinus Carpio L.) Muscle Tissue. J. Food Qual. 2018, 2018, 6082164. [Google Scholar] [CrossRef]
- Ghaffar, F.; Sajjad, R. The Impact of Dry Heat Treatment on Phytic Acid Degradation and Nutritional Quality in Baked and Fried Wheat Products. J. Adv. Nutr. Sci. Technol. 2025, 5, 70–79. [Google Scholar] [CrossRef]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Ion Transporters and Osmoregulation in the Kidney of Teleost Fishes as a Function of Salinity. Front Physiol 2021, 12, 664588. [Google Scholar] [CrossRef]
- Kalman, D.; Hewlings, S.; Madelyn-Adjei, A.; Ebersole, B. Dietary Heme Iron: A Review of Efficacy, Safety and Tolerability. Nutrients 2025, 17, 2132. [Google Scholar] [CrossRef]
- Stiles, L.I.; Ferrao, K.; Mehta, K.J. Role of Zinc in Health and Disease. Clin. Exp. Med. 2024, 24, 38. [Google Scholar] [CrossRef]
- Orata, F.; Sifuna, F. Uptake, Bioaccumulation, Partitioning of Lead (Pb) and Cadmium (Cd) in Aquatic Organisms in Contaminated Environments. In Lead, Mercury and Cadmium in the Aquatic Environment: Worldwide Occurrence, Fate and Toxicity; CRC Press: Boca Raton, FL, USA, 2023; pp. 166–181. [Google Scholar]
- Li, M.; Zhang, B.; Fang, Z. Bioaccumulation of Arsenic, Cadmium, Chromium, Cobalt, Copper, and Zinc in Uroteuthis Edulis from the East China Sea. Toxics 2024, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.; Ng, W.J.; Ooi, A.L.; Lem, F.F.; Chai, T.T. Carp-Derived Antioxidant Peptides and Hydrolysates: Biological Effects and Potential Applications in Health and Food. Antioxidants 2025, 14, 1095. [Google Scholar] [CrossRef]
- Ryu, B.; Shin, K.H.; Kim, S.K. Muscle Protein Hydrolysates and Amino Acid Composition in Fish. Mar Drugs 2021, 19, 377. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Wang, H.; Admassu, H.; Sulieman, A.A.; Wei, F.A. Health Benefits of Bioactive Peptides Produced from Muscle Proteins: Antioxidant, Anti-Cancer, and Anti-Diabetic Activities. Process Biochem. 2022, 116, 116–125. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Xiao, B.; Cui, D.; Lin, Y.; Zeng, J.; Li, J.; Cao, M.J.; Liu, J. Antioxidant Activity of Docosahexaenoic Acid (DHA) and Its Regulatory Roles in Mitochondria. J. Agric. Food Chem. 2021, 69, 1647–1655. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive Peptides from Muscle Sources: Meat and Fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Imran, A.; Nosheen, F.; Fatima, M.; Arshad, M.U.; Afzaal, M.; Ijaz, N.; Noreen, R.; Mehta, S.; Biswas, S.; et al. Functional Roles and Novel Tools for Improving-Oxidative Stability of Polyunsaturated Fatty Acids: A Comprehensive Review. Food Sci. Nutr. 2023, 11, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Ishak, N.H.; Sarbon, N.M. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioproc Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Matos, Â.P.; Matos, A.C.; Moecke, E.H.S. Polyunsaturated Fatty Acids and Nutritional Quality of Five Freshwater Fish Species Cultivated in the Western Region of Santa Catarina, Brazil. Braz. J. Food Technol. 2019, 22, e2018193. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Berköz, M.; Kahraman, T.; Shamsulddin, Z.N.; Krośniak, M. Antioxidant and Anti-Inflammatory Effect of Olive Leaf Extract Treatment in Diabetic Rat Brain. J. Basic. Clin. Physiol. Pharmacol. 2023, 34, 187–196. [Google Scholar] [CrossRef]
- Gnoni, A.; Longo, S.; Damiano, F.; Gnoni, G.V.; Giudetti, A.M. Oleic Acid and Olive Oil Polyphenols Downregulate Fatty Acid and Cholesterol Synthesis in Brain and Liver Cells. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 651–657. [Google Scholar]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, M. The Cardioprotective Effects of Polyunsaturated Fatty Acids Depends on the Balance Between Their Anti- and Pro-Oxidative Properties. Nutrients 2024, 16, 3937. [Google Scholar] [CrossRef]
- Tahir, A.; Siddiq, A. Mechanistic Insights and Clinical Benefits of Omega-3 Fatty Acids in Cardiovascular, Inflammatory, and Neurological Health. Thai J. Pharm. Sci. 2025, 49, 1. [Google Scholar] [CrossRef]
- Ochrem, A.S.; Zychliñska-Buczek, J.; Zapletal, P. Carp (Cyprinus Carpio L.) Lipid Oxidation during Cold Storage. Arch. Pol. Fish. 2015, 23, 101–106. [Google Scholar] [CrossRef]
- Nagabhooshanam, N.; Lakshmi, K.B.; Navya, K.K.; Prasad, P.D.V.; Singh, P.P.; Kumar, K.S.; Rani, T.S.; Anand, D.; Philip, J.M.; Rajaram, A. Analysis of the Oxidative Degradation of Polyunsaturated Fatty Acids in Food and Its Effects on Nutritional Quality. EBSOC 2025, 48, 134–141. Available online: https://research.ebsco.com/c/6bpmpj/viewer/pdf/3emxpc7odj (accessed on 23 November 2025).
- Ghnimi, S.; Budilarto, E.; Kamal-Eldin, A. The New Paradigm for Lipid Oxidation and Insights to Microencapsulation of Omega-3 Fatty Acids. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1206–1218. [Google Scholar] [CrossRef]
- Amaral, R.A.; Pinto, C.A.; Lima, V.; Tavares, J.; Martins, A.P.; Fidalgo, L.G.; Silva, A.M.; Gil, M.M.; Teixeira, P.; Barbosa, J.; et al. Chemical-Based Methodologies to Extend the Shelf Life of Fresh Fish—A Review. Foods 2021, 10, 2300. [Google Scholar] [CrossRef] [PubMed]
- Phadke, G.G.; Rathod, N.B.; Ozogul, F.; Elavarasan, K.; Karthikeyan, M.; Shin, K.H.; Kim, S.K. Exploiting of Secondary Raw Materials from Fish Processing Industry as a Source of Bioactive Peptide-Rich Protein Hydrolysates. Mar Drugs 2021, 19, 480. [Google Scholar] [CrossRef]
- Liu, D.; Mi, J.; Yan, X.; Qin, C.; Wang, J.; Nie, G. Taurine Alleviated the Negative Effects of an Oxidized Lipid Diet on Growth Performance, Antioxidant Properties, and Muscle Quality of the Common Carp (Cyprinus Carpio L.). Aquac. Nutr. 2024, 2024, 5205506. [Google Scholar] [CrossRef] [PubMed]
- Bavisetty, S.C.B.; Benjakul, S.; Olatunde, O.O.; Ali, A.M.M. Bioactive Compounds in Fermented Fish and Meat Products: Health Aspects. In Bioactive Compounds in Fermented Foods; CRC Press: Boca Raton, FL, USA, 2021; pp. 325–362. [Google Scholar]
- Ou, J.; Wang, Y.; Li, Y.; Liu, S.; Kou, X.; Ren, F.; Wang, X.; Zhang, H. Recent Advances in Mining Hypolipidemic Bioactive Compounds from Animal-Derived Foods. Food Funct. 2025, 16, 8627–8646. [Google Scholar] [CrossRef]
- Mahboob, S.; Sultana, S.; Almisned, F.; Profile, S.; Ahmad, Z. Isolation and Characterisation of Collagen from the Waste Material of Two Importnt Freshwater Fish Species. Artic. J. Anim. Plant Sci. 2014, 24, 1802–1810. [Google Scholar]
- Song, D.; Yun, Y.; He, Z.; Mi, J.; Wang, L.; Jin, M.; Zhou, Q.; Nie, G. Fillet Texture, Physicochemical Indexes, Muscle Cellularity and Molecular Expression in Muscle of Yellow River Carp (Cyprinus Carpio Haematopterus) in Response to Dietary Hydroxyproline Supplementation. Aquaculture 2022, 549, 737783. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and Functions of Amino Acids in Fish. Adv. Exp. Med. Biol. 2021, 1285, 133–168. [Google Scholar] [CrossRef]
- Le Bourgot, C.; Liu, X.; Buffière, C.; Hafanaoui, N.; Salis, L.; Pouyet, C.; Dardevet, D.; Rémond, D. Development of a Protein Food Based on Texturized Wheat Proteins, with High Protein Digestibility and Improved Lysine Content. Food Res. Int. 2023, 170, 112978. [Google Scholar] [CrossRef]
- Paoletti, A.; Courtney-Martin, G.; Elango, R. Determining Amino Acid Requirements in Humans. Front. Nutr. 2024, 11, 1400719. [Google Scholar] [CrossRef]
- Sood, S.; Methven, L.; Cheng, Q. Role of Taste Receptors in Salty Taste Perception of Minerals and Amino Acids and Developments in Salt Reduction Strategies: A Review. Crit. Rev. Food Sci. Nutr. 2025, 65, 3444–3458. [Google Scholar] [CrossRef]
- Zello, G.A.; Pencharz, P.B.; Ball, R.O. Dietary Lysine Requirement of Young Adult Males Determined by Oxidation of L-[1-13C]Phenylalanine. Am. J. Physiol.-Endocrinol. Metab. 1993, 264, E677–E685. [Google Scholar] [CrossRef] [PubMed]
- Di Buono, M.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Total Sulfur Amino Acid Requirement in Young Men as Determined by Indicator Amino Acid Oxidation with L-[1-13C]Phenylalanine. Am. J. Clin. Nutr. 2001, 74, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lin, X.; Hanigan, M.D.; Zhao, K.; Hu, Z.; Wang, Y.; Hou, Q.; Wang, Z. Ruminally Protected Isoleucine, Leucine, Methionine, and Threonine Supplementation of Low-Protein Diets Improved the Performance and Nitrogen Efficiency of Dairy Cows. Animals 2025, 15, 1210. [Google Scholar] [CrossRef] [PubMed]
- Muhu-Din Ahmed, H.G.; Naeem, M.; Faisal, A.; Fatima, N.; Tariq, S.; Owais, M. Enriching the Content of Proteins and Essential Amino Acids in Legumes. In Legumes Biofortification; Springer: Cham, Switzerland, 2023; pp. 417–447. [Google Scholar] [CrossRef]
- Oliveira, M.S.F.; Espinosa, C.D.; Stein, H.H. Heat Damage, Maillard Reactions, and Measurement of Reactive Lysine in Feed Ingredients and Diets. Proc. Ark. Nutr. Conf. 2021, 2021, 7. [Google Scholar]
- Rodrigues, R.C.; Baldassini, W.A.; Tagiariolli, M.A.; Coutinho, M.A.S.; Rovadoscki, G.; Cônsolo, N.R.B.; Ocampos, F.M.M.; Colnago, L.A.; Torrecilhas, J.A.; Pereira, G.L.; et al. Impact of Tenderness, Intramuscular Fat, and Metabolites on the Sensory Quality of Nellore Beef. ACS Food Sci. Technol. 2025, 5, 3890–3901. [Google Scholar] [CrossRef]
- Purlis, E. Browning Development in Bakery Products-A Review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Van Boekel, M.A.J.S. A Review of Maillard Reaction in Food and Implications to Kinetic Modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Assis, L.M.; Zavareze, E.d.R.; Radünz, A.L.; Dias, A.; Gutkosk, L.; Elias, M.C. Nutritional, Technological and Sensory Properties of Biscuits with Replacement of Wheat Flour for Oatmeal or Flour, Parboiled Rice. Alim Nutr. Araraquara 2009, 20, 15–24. [Google Scholar]
- Surówka, K.; Maciejaszek, I. Oddziaływania Białkowo-Polisacharydowe i Ich Praktyczne Wykorzystanie. ŻYWNOŚĆ. Nauka. Technologia. Jakość 2007, 4, 17–32. [Google Scholar]
- Rahaman, A.; Kumari, A.; Zeng, X.A.; Korin, A.; Khalifa, I.; Maqsood, S. Processing of Marine Derived By-Products and Their Applications: A Review. Waste Biomass Valorization 2025, 1–17. [Google Scholar] [CrossRef]
- Areche, F.O.; Dominguez, J.A.J.; Huaman, J.T.; Yapias, R.J.M.; Rivera, T.J.C.; Perales, L.D.M.; Quispe, J.D.D.H.T.; Zea, C.Y.H.; Galarza, C.R.C.; Rodríguez, A.R.; et al. Maximizing the Potential of Marine Resources: A Sustainable Approach to High-Value Product Development from Seafood by-Products and Waste. Curr. Res. Nutr. Food Sci. 2024, 12, 1062. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S.; Makroo, H.A.; Dar, B.N. Role of Pulses to Modulate the Nutritive, Bioactive and Technological Functionality of Cereal-Based Extruded Snacks: A Review. Int. J. Food Sci. Technol. 2022, 57, 3882–3891. [Google Scholar] [CrossRef]
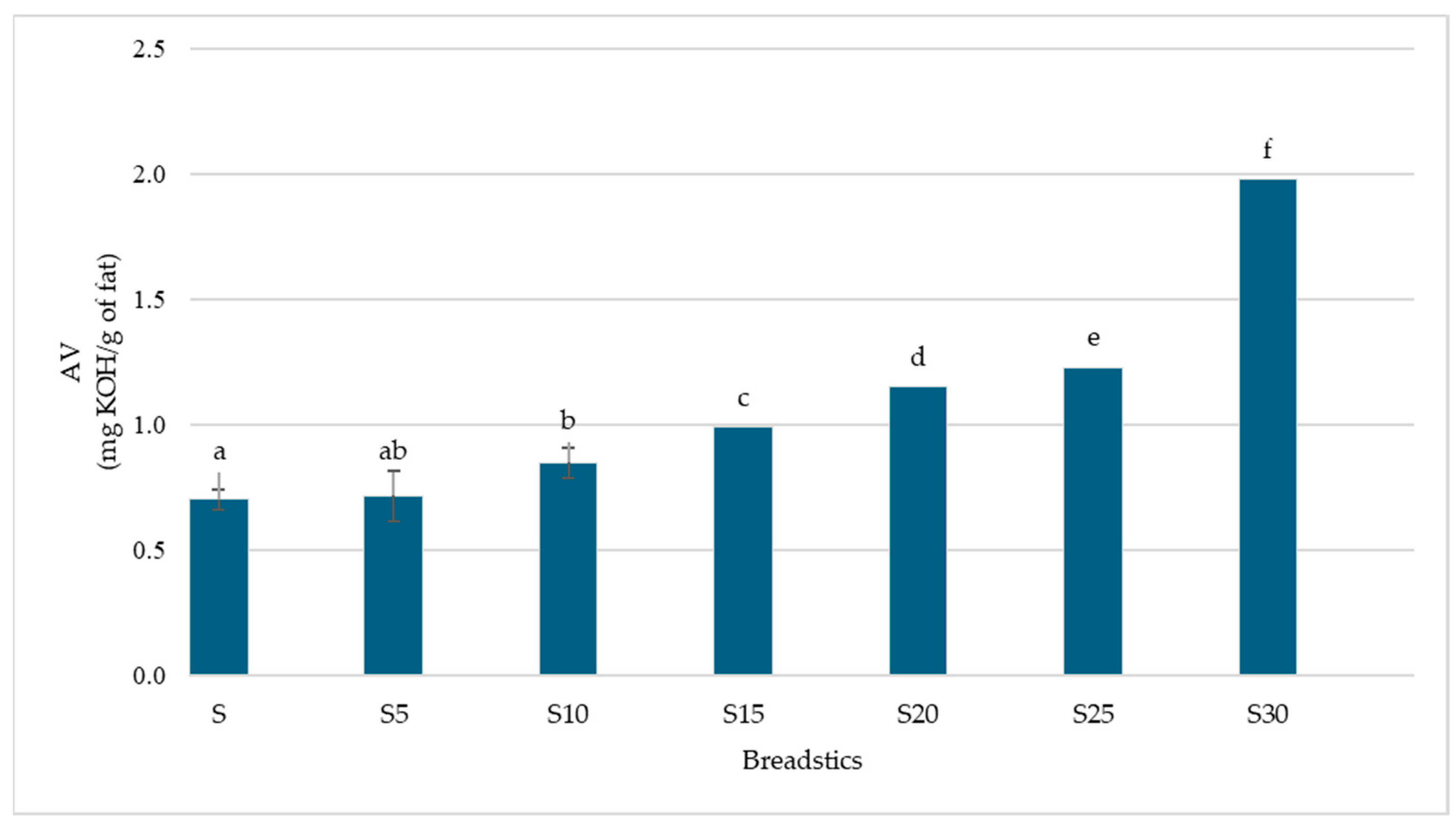
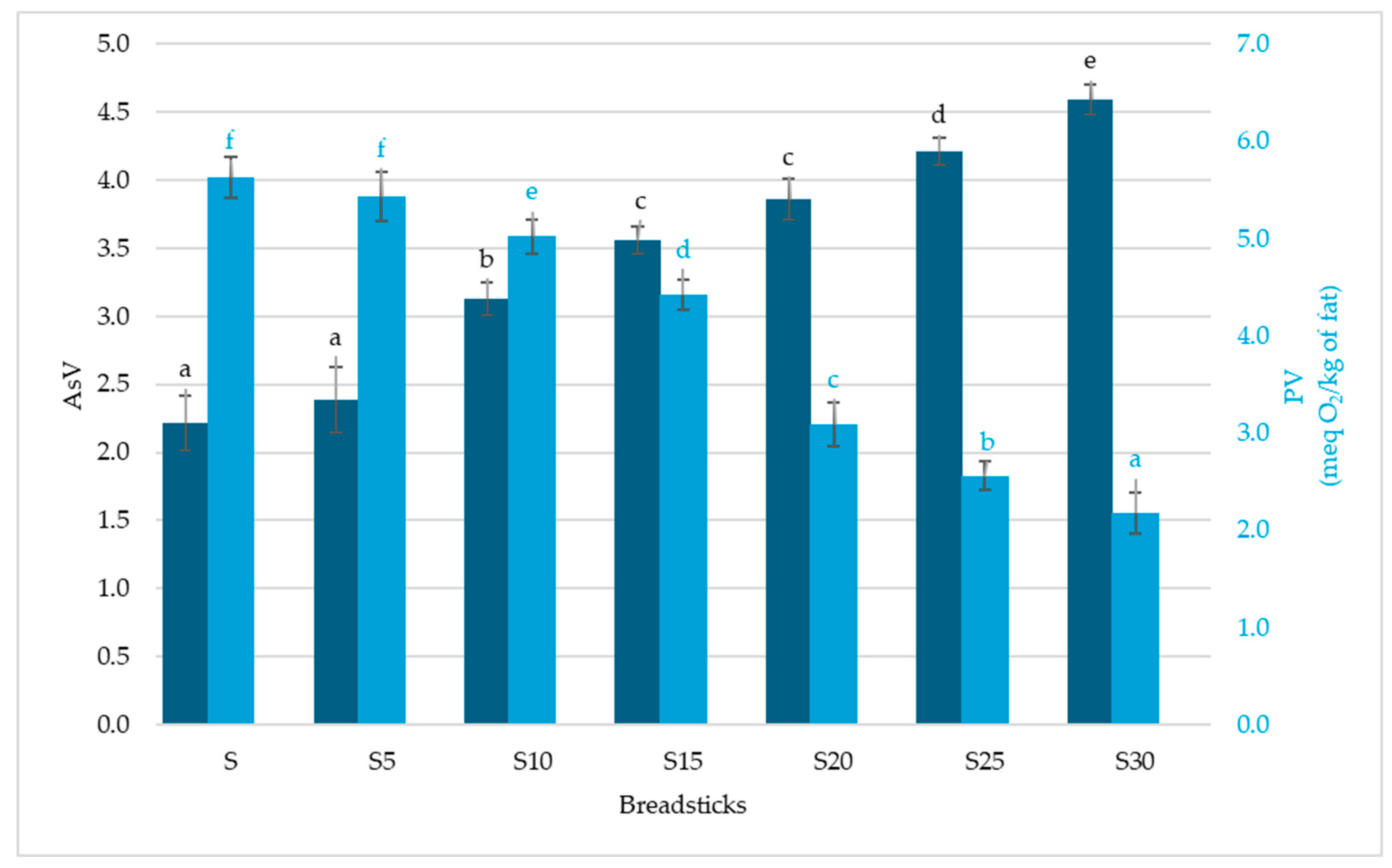
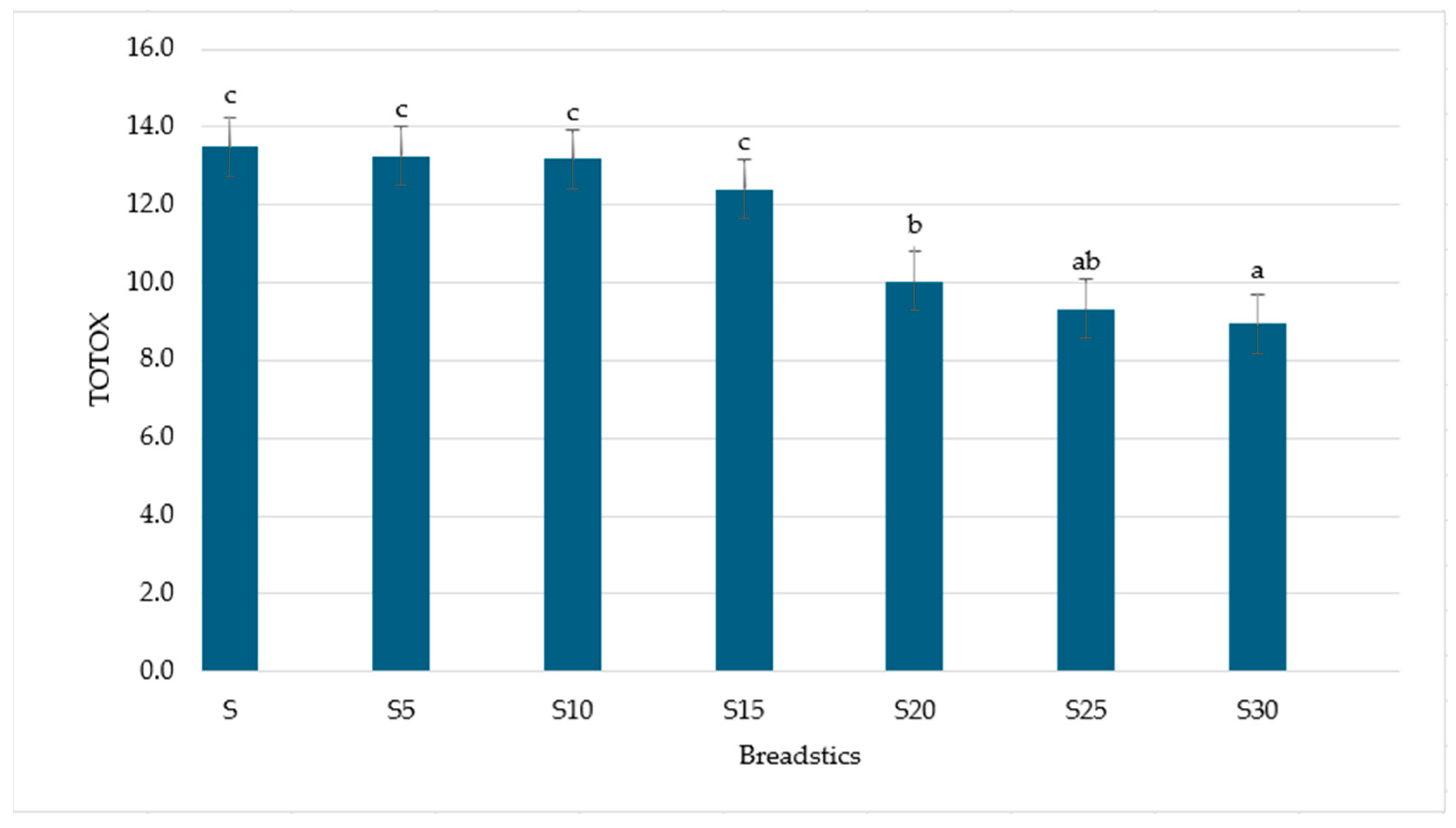

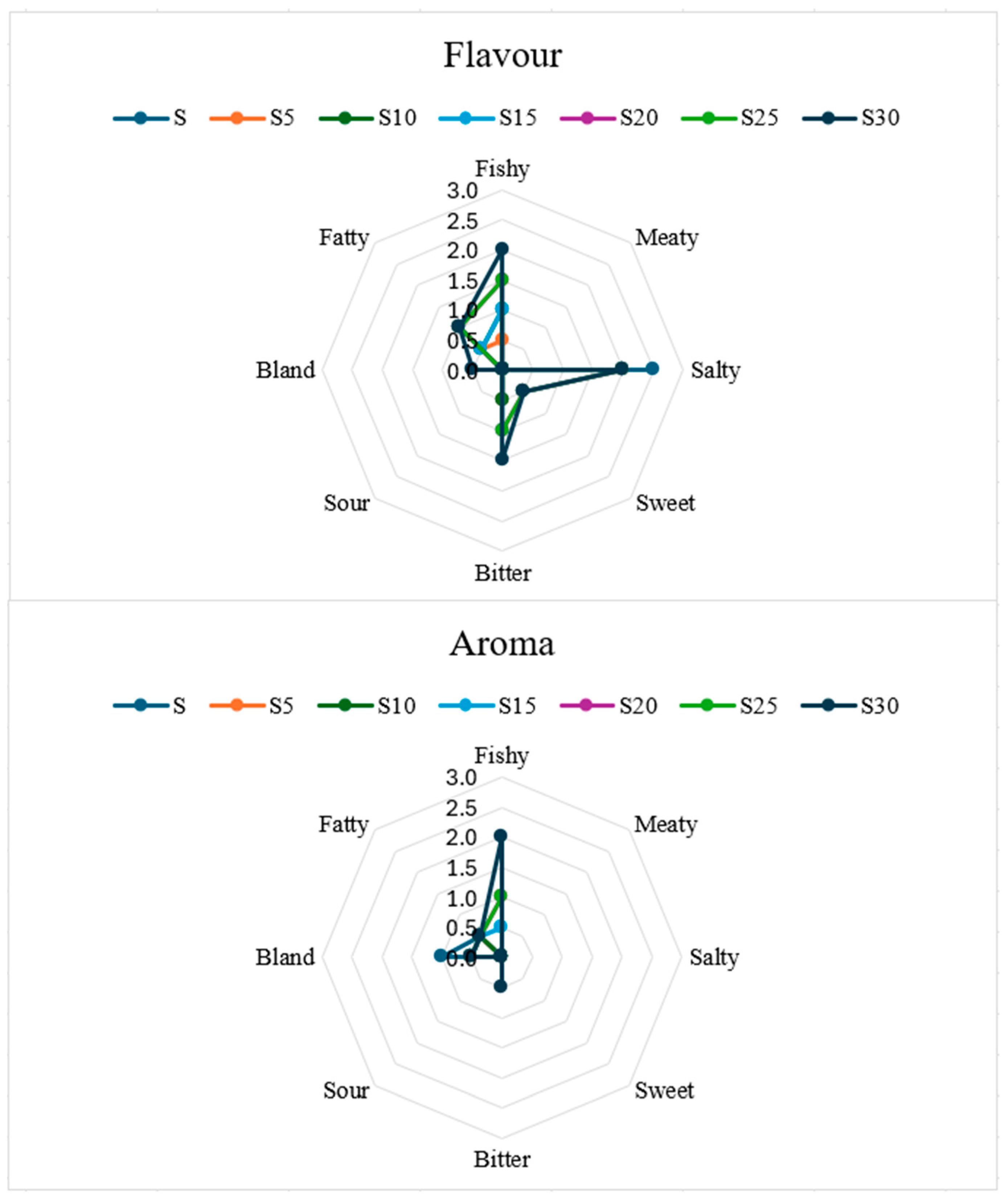
| Breadsticks | |||||||
|---|---|---|---|---|---|---|---|
| S | S5 | S10 | S15 | S20 | S25 | S30 | |
| Flour | 132.5 | 130.0 | 127.5 | 125.0 | 122.5 | 120.0 | 117.5 |
| Oil | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Water | 70.0 | 60.0 | 50.0 | 40.0 | 30.0 | 20.0 | 10.0 |
| Yeast | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Baking powder | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Salt | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Sugar | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Carp meat | 0.0 | 12.5 | 25.0 | 37.5 | 50.0 | 62.5 | 70.0 |
| Carp | Breadsticks | |||||||
|---|---|---|---|---|---|---|---|---|
| Meat | S | S5 | S10 | S15 | S20 | S25 | S30 | |
| Dry matter | 24.98 ± 0.22 | 94.24 ± 0.25 d | 94.14 ± 0.18 d | 94.04 ± 0.26 cd | 93.95 ± 0.11 c | 93.76 ± 0.12 b | 93.64 ± 0.26 ab | 93.51 ± 0.12 a |
| Fat | 4.32 ± 0.12 | 18.02 ± 0.08 a | 18.46 ± 0.27 b | 19.25 ± 0.16 c | 20.40 ± 10 d | 21.53 ± 0.15 e | 22.27 ± 0.12 f | 23.16 ± 0.16 g |
| Protein | 18.55 ± 0.09 | 9.73 ± 0.16 a | 11.28 ± 0.20 b | 14.19 ± 0.15 c | 15.59 ± 0.10 d | 17.60 ± 0.12 e | 18.62 ± 0.09 f | 19.52 ± 0.11 g |
| Ash | 0.66 ± 0.06 | 4.18 ± 0.04 g | 4.05 ± 0.03 fs | 3.40 ± 0.11 e | 3.03 ± 0.05 d | 2.55 ± 0.06 c | 2.32 ± 0.05 b | 2.21 ± 0.02 a |
| Fiber | - | 1.97 ± 0.03 d | 1.55 ± 0.01 c | 1.00 ± 0.05 ab | 0.98 ± 0.02 a | 1.06 ± 0.04 b | 1.03 ± 0.03 b | 1.04 ± 0.10 ab |
| NFE | - | 60.34 ± 0.12 g | 58.80 ± 0.15 f | 56.20 ± 0.10 e | 53.95 ± 0.11 d | 51.02 ± 0.10 c | 49.40 ± 0.12 b | 47.58 ± 0.15 a |
| Carp | Breadsticks | |||||||
|---|---|---|---|---|---|---|---|---|
| Meat | S | S5 | S10 | S15 | S20 | S25 | S30 | |
| P [g/kg] | 2.45 ± 0.34 | 3.89 ± 0.11 f | 2.20 ± 0.12 a | 2.15 ± 0.10 a | 2.36 ± 0.05 b | 2.55 ± 0.00 c | 2.76 ± 0.09 d | 2.92 ± 0.10 e |
| K [g/kg] | 3.95 ± 0.19 | 1.60 ± 0.05 a | 1.85 ± 0.05 b | 2.02 ± 0.05 c | 2.16 ± 0.06 d | 2.30 ± 0.04 e | 2.59 ± 0.09 f | 2.72 ± 0.02 g |
| Mg [g/kg] | 1.15 ± 0.09 | 185.20 ± 1.18 a | 196.16 ± 1.19 b | 208.05 ± 2.13 c | 216.20 ± 2.11 d | 228.21 ± 3.16 e | 231.12 ± 2.12 f | 232.11 ± 1.50 f |
| Na [g/kg] | 0.52 ± 0.06 | 5.38 ± 0.15 a | 5.45 ± 0.10 a | 5.25 ± 0.10 a | 5.40 ± 0.10 a | 5.22 ± 0.20 a | 5.36 ± 0.10 a | 5.25 ± 0.10 a |
| Fe [mg/kg] | 7.32 ± 0.22 | 20.24 ± 0.10 a | 21.95 ± 0.22 d | 22.32 ± 0.16 e | 22.02 ± 0.11 d | 21.68 ± 0.05 c | 21.46 ± 0.11 b | 21.55 ± 0.04 b |
| Ca [mg/kg] | 311.20 ± 0.68 | 18.21 ± 0.05 g | 11.63 ± 0.21 a | 12.06 ± 0.10 b | 12.86 ± 0.04 c | 14.86 ± 0.12 d | 15.32 ± 0.06 e | 17.85 ± 0.05 f |
| Ni [mg/kg] | - | - | - | - | - | - | - | - |
| Cr [mg/kg] | 0.04 ± 0.01 | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.02 a | 0.04 ± 0.02 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a |
| Pb [mg/kg] | - | - | - | - | - | - | - | - |
| Cd [mg/kg] | - | - | - | - | - | - | - | - |
| Mn [mg/kg] | 0.76 ± 0.12 | 4.02 ± 0.11 a | 5.12 ± 0.23 d | 5.05 ± 0.23 cd | 4.95 ± 0.15 c | 4.45 ± 0.26 b | 4.52 ± 0.16 b | 4.66 ± 0.10 b |
| Co [mg/kg] | - | - | - | - | - | - | - | - |
| Cu [mg/kg] | - | - | - | - | - | - | - | - |
| Zn [mg/kg] | 3.42 ± 0.33 | 8.20 ± 0.23 a | 8.52 ± 0.21 b | 8.48 ± 0.10 b | 8.45 ± 0.15 b | 8.35 ± 0.05 a | 8.89 ± 0.10 c | 9.24 ± 0.05 d |
| Carp | Breadsticks | |||||||
|---|---|---|---|---|---|---|---|---|
| Meat | S | S5 | S10 | S15 | S20 | S25 | S30 | |
| TEAC [µmol TE/g] | 2.25 ± 0.10 | 1.71 ± 0.18 a | 1.84 ± 0.05 a | 1.94 ± 0.04 a | 2.23 ± 0.06 b | 2.62 ± 0.14 c | 3.12 ± 0.04 d | 3.50 ± 0.26 e |
| FRAP [µmol TE/g] | 28.36 ± 0.60 | 68.7 ± 1.2 a | 75.2 ± 0.9 b | 80.1 ± 0.7 c | 86.3 ± 1.2 d | 89.9 ± 3.6 e | 93.2 ± 0.5 ed | 94.7 ± 2.2 d |
| DPPH [µmol TE/g] | 0.312 ± 0.015 | 0.516 ± 0.031 a | 0.603 ± 0.012 b | 0.636 ± 0.018 c | 0.675 ± 0.015 c | 0.680 ± 0.017 c | 0.722 ± 0.016 d | 0.738 ± 0.021 d |
| TPC [mg GAE/g] | 0.569 ± 0.021 | 0.678 ± 0.009 d | 0.612 ± 0.010 a | 0.615 ± 0.008 a | 0.625 ± 0.012 b | 0.637 ± 0.010 b | 0.659 ± 0.012 c | 0.673 ± 0.028 d |
| Carp | Breadsticks | |||||||
|---|---|---|---|---|---|---|---|---|
| Meat | S | S5 | S10 | S15 | S20 | S25 | S30 | |
| C10:0 | - | 0.01 ± 0.00 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a |
| C12:0 | 0.07 ± 0.02 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.01 a |
| C14:0 | 0.80 ± 0.01 | 0.07 ± 0.03 a | 0.11 ± 0.02 b | 0.14 ± 0.02 c | 0.14 ± 0.02 c | 0.10 ± 0.01 ab | 0.15 ± 0.01 c | 0.15 ± 0.0 c |
| C15:0 | 0.67 ± 0.00 | 0.03 ± 0.02 a | 0.03 ± 0.01 a | 0.04 ± 0.00 a | 0.03 ± 0.01 a | 0.02 ± 0.02 a | 0.03 ± 0.01 a | 0.04 ± 0.0 a |
| C16:0 | 14.94 ± 0.53 | 6.29 ± 0.02 a | 6.67 ± 0.04 b | 6.82 ± 0.04 c | 6.85 ± 0.03 cd | 6.87 ± 0.04 de | 6.91 ± 0.03 e | 6.89 ± 0.02 e |
| C16:1 | 2.42 ± 0.24 | 0.27 ± 0.05 a | 0.51 ± 0.03 b | 0.70 ± 0.05 c | 0.74 ± 0.03 c | 0.81 ± 0.03 d | 0.85 ± 0.02 d | 0.92 ± 0.01 e |
| C17:0 | 0.25 ± 0.13 | 0.07 ± 0.02 a | 0.08 ± 0.01 a | 0.09 ± 0.03 a | 0.08 ± 0.02 a | 0.08 ± 0.00 a | 0.08 ± 0.01 a | 0.09 ± 0.04 a |
| C17:1 | 0.25 ± 0.11 | 0.08 ± 0.02 a | 0.10 ± 0.01 a | 0.10 ± 0.03 a | 0.09 ± 0.00 a | 0.10 ± 0.02 a | 0.08 ± 0.01 a | 0.10 ± 0.00 a |
| C18:0 | 5.18 ± 0.10 | 2.91 ± 0.06 c | 3.31 ± 0.03 f | 3.22 ± 0.05 e | 3.06 ± 0.04 d | 3.08 ± 0.02 d | 2.42 ± 0.03 b | 2.19 ± 0.04 a |
| C18:1 ω9 | 44.57 ± 0.89 | 56.78 ± 0.10 d | 55.61 ± 0.12 a | 55.86 ± 0.15 b | 56.32 ± 0.11 c | 56.50 ± 0.20 c | 57.10 ± 0.12 e | 57.32 ± 0.21 e |
| C18:2 ω6 | 17.48 ± 0.23 | 20.52 ± 0.15 d | 20.27 ± 0.12 c | 19.90 ± 0.10 b | 19.94 ± 0.10 b | 19.78 ± 0.10 a | 19.85 ± 0.15 ab | 19.86 ± 0.16 ab |
| C20:0 | 0.27 ± 0.05 | 1.04 ± 0.05 c | 1.01 ± 0.03 c | 1.01 ± 0.04 c | 0.93 ± 0.05 b | 0.89 ± 0.06 ab | 0.85 ± 0.05 a | 0.78 ± 0.10 a |
| C18:3 ω6 | 0.40 ± 0.06 | 0.28 ± 0.04 a | 0.33 ± 0.02 b | 0.28 ± 0.03 a | 0.28 ± 0.02 a | 0.28 ± 0.03 a | 0.27 ± 0.03 a | 0.27 ± 0.02 a |
| C18:3 ω3 | 2.48 ± 0.15 | 8.61 ± 0.12 b | 8.42 ± 0.11 a | 8.39 ± 0.09 a | 8.40 ± 0.12 a | 8.41 ± 0.10 a | 8.38 ± 0.10 a | 8.37 ± 0.11 a |
| C20:1 ω9 | 2.80 ± 0.12 | 1.59 ± 0.12 b | 1.44 ± 0.11 a | 1.71 ± 0.05 c | 1.61 ± 0.08 b | 1.65 ± 0.10 bc | 1.68 ± 0.06 bc | 1.65 ± 0.05 b |
| C18:4 ω3 | 0.14 ± 0.01 | 0.03 ± 0.02 a | 0.04 ± 0.00 a | 0.03 ± 0.01 a | 0.03 ± 0.02 a | 0.03 ± 0.01 a | 0.04 ± 0.01 a | 0.05 ± 0.02 a |
| C20:2 ω6 | 0.49 ± 0.06 | 0.08 ± 0.01 a | 0.11 ± 0.00 b | 0.11 ± 0.01 b | 0.10 ± 0.01 b | 0.12 ± 0.02 b | 0.13 ± 0.02 b | 0.13 ± 0.03 b |
| C22:0 | 0.25 ± 0.02 | 0.55 ± 0.02 c | 0.52 ± 0.01 b | 0.53 ± 0.03 bc | 0.50 ± 0.00 b | 0.50 ± 0.02 b | 0.45 ± 0.02 a | 0.46 ± 0.03 a |
| C22:1 ω9 | 0.25 ± 0.01 | 0.00 ± 0.00 a | 0.51 ± 0.03 f | 0.35 ± 0.01 e | 0.25 ± 0.03 d | 0.20 ± 0.02 c | 0.18 ± 0.02 bc | 0.15 ± 0.04 b |
| C20:4 ω6 | 2.74 ± 0.06 | 0.31 ± 0.04 c | 0.03 ± 0.01 a | 0.05 ± 0.02 a | 0.05 ± 0.01 ab | 0.05 ± 0.00 b | 0.04 ± 0.01 a | 0.05 ± 0.02 ab |
| C23:0 | - | 0.04 ± 0.01 c | 0.04 ± 0.01 c | 0.03 ± 0.00 b | 0.03 ± 0.01 bc | 0.02 ± 0.01 ab | 0.02 ± 0.01 ab | 0.01 ± 0.00 a |
| C20:5 ω3 | 0.35 ± 0.03 | - | 0.03 ± 0.00 a | 0.05 ± 0.00 b | 0.06 ± 0.02 b | 0.05 ± 0.00 b | 0.05 ± 0.01 b | 0.06 ± 0.01 b |
| C24:0 | - | 0.23 ± 0.04 de | 0.37 ± 0.02 f | 0.25 ± 0.02 e | 0.19 ± 0.03 d | 0.15 ± 0.01 c | 0.11 ± 0.02 b | 0.06 ± 0.01 a |
| C24:1 ω9 | 0.11 ± 0.01 | 0.19 ± 0.01 d | 0.33 ± 0.05 e | 0.21 ± 0.03 d | 0.16 ± 0.02 c | 0.14 ± 0.02 c | 0.10 ± 0.01 b | 0.05 ± 0.00 a |
| C22:4 ω6 | 0.21 ± 0.02 | - | 0.04 ± 0.02 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.02 ± 0.00 a |
| C22:5 ω3 | 0.31 ± 0.06 | - | 0.03 ± 0.01 ab | 0.02 ± 0.00 a | 0.04 ± 0.00 bc | 0.04 ± 0.01 bc | 0.05 ± 0.01 c | 0.11 ± 0.01 d |
| C22:6 ω3 | 2.56 ± 0.10 | - | 0.02 ± 0.00 a | 0.05 ± 0.01 b | 0.06 ± 0.01 b | 0.08 ± 0.00 c | 0.13 ± 0.02 d | 0.20 ± 0.03 e |
| SFA | 22.45 ± 0.16 | 11.25 ± 0.12 c | 12.17 ± 0.13 e | 12.15 ± 0.11 e | 11.84 ± 0.09 d | 11.73 ± 0.18 d | 11.04 ± 0.10 b | 10.69 ± 0.16 a |
| MUFA | 50.39 ± 0.23 | 58.92 ± 1.02 a | 58.50 ± 1.12 ab | 58.94 ± 0.93 ab | 59.16 ± 0.86 ab | 59.40 ± 0.63 ab | 59.99 ± 0.78 bc | 60.19 ± 0.67 c |
| PUFA | 27.16 ± 0.21 | 29.83 ± 0.26 c | 29.33 ± 0.22 b | 28.91 ± 0.19 ab | 28.99 ± 0.15 ab | 28.87 ± 0.23 a | 28.97 ± 0.30 ab | 29.12 ± 0.21 b |
| ω3/ω6 | 0.27 | 0.41 | 0.41 | 0.42 | 0.42 | 0.42 | 0.43 | 0.43 |
| DHA/EPA | 7.31 | - | 0.68 | 0.94 | 1.00 | 1.60 | 2.60 | 3.33 |
| Carp Meat | Breadsticks | |||||||
|---|---|---|---|---|---|---|---|---|
| S | S5 | S10 | S15 | S20 | S25 | S30 | ||
| Alanine * | 1110.0 ± 12.0 | 440.0 ± 25.0 c | 430.0 ± 15.0 bc | 400.0 ± 10.0 a | 425.0 ± 11.0 bc | 400.0 ± 15.0 ab | 415.0 ± 11.0 b | 430.0 ± 20.0 bc |
| Arginine * | 1026.0 ± 32.0 | 730.0 ± 23.0 a | 810.0 ± 13.0 b | 850.0 ± 20.0 c | 890.0 ± 10.0 f | 962.0 ± 21.0 e | 1084.0 ± 21.0 f | 1106.0 ± 32.0 f |
| Cysteine * | 275.0 ± 19.0 | 251.0 ± 12.0 c | 235.0 ± 10.0 a | 240.0 ± 10.0 ab | 250.0 ± 10.0 bc | 265.0 ± 10.0 d | 280.0 ± 15.0 de | 290.0 ± 11.0 e |
| Cystine | 188.0 ± 15.0 | 240.0 ± 29.0 ab | 240.0 ± 10.0 a | 244.0 ± 12.0 a | 251.0 ± 11.0 b | 263.0 ± 15.0 bc | 277.0 ± 12.0 cd | 290.2 ± 21.0 d |
| Phenylalanine | 855.0 ± 22.0 | 350.0 ± 31.0 a | 442.0 ± 12.00 b | 580.0 ± 32.0 c | 602.0 ± 16.0 c | 699.0 ± 12.0 d | 786.0 ± 19.0 e | 822.0 ± 26.0 f |
| Glycine * | 732.0 ± 10.0 | 240.0 ± 21.0 a | 265.0 ± 13.0 b | 288.0 ± 20.0 c | 312.0 ± 16.0 d | 366.0 ± 14.0 e | 402.0 ± 10.0 f | 430.0 ± 26.0 g |
| Histidine | 841.0 ± 23.0 | 652.0 ± 34.0 b | 632.0 ± 20.0 ab | 613.0 ± 21.0 a | 698.0 ± 12.0 c | 745.0 ± 18.0 d | 803.0 ± 0.11 e | 820.0 ± 12.0 f |
| Hydroxyproline * | 243.0 ± 11.0 | 120.0 ± 14.0 a | 110.0 ± 11.0 a | 120.0 ± 15.0 a | 126.0 ± 10.0 a | 156.0 ± 11.0 b | 195.0 ± 13.0 c | 201.0 ± 16.0 c |
| Isoleucine | 1203.0 ± 30.0 | 640.0 ± 26.0 a | 653.0 ± 19.0 a | 660.0 ± 23.0 a | 698.0 ± 13.0 b | 746.0 ± 13.0 c | 829.0 ± 19.0 d | 881.0 ± 29.0 e |
| Aspartic acid * | 1451.0 ± 35.0 | 820.0 ± 36.0 a | 974.0 ± 21.0 b | 1033.0 ± 23.0 c | 1096.0 ± 23.0 d | 1129.0 ± 0.16 e | 1185.0 ± 0.21f | 1200.0 ± 32.0 f |
| Glutamic acid * | 2982.0 ± 23.0 | 1581.0 ± 21.0 a | 1650.0 ± 23.0 b | 1870.0 ± 14.0 e | 1762.0 ± 26.0 d | 1687.0 ± 25.0 c | 1555.0 ± 29.0 a | 1582.0 ± 38.0 a |
| Leucine | 1835.0 ± 18.0 | 623.0 ± 29.0 a | 721.0 ± 19.0 b | 940.0 ± 13.0 c | 946.0 ± 10.0 c | 957.0 ± 13.0 c | 953.0 ± 18.0 c | 980.0 ± 24.0 d |
| Lysine | 1778.0 ± 13.0 | 143.0 ± 18.0 a | 213.0 ± 11.0 b | 325.0 ± 19.0 c | 523.0 ± 21.0 d | 798.0 ± 19.0 e | 886.0 ± 26.0 f | 980.0 ± 36.0 g |
| Methionine | 726.0 ± 28.0 | 110.0 ± 11.0 a | 123.0 ± 15.0 ab | 131.0 ± 10.0 b | 136.0 ± 10.0 b | 156.0 ± 11.0 c | 168.0 ± 16.0 c | 172.0 ± 21.0 c |
| Proline * | 830.0 ± 13.0 | 1016.0 ± 28.0 a | 1010.0 ± 15.0 a | 1005.0 ± 10.0 a | 995.0 ± 21.0 ab | 986.0 ± 09.0 b | 968.0 ± 13.0 c | 970.0 ± 15.0 c |
| Serine * | 869.0 ± 21.0 | 580.0 ± 21.0 a | 650.0 ± 25.0 b | 700.0 ± 28.0 c | 786.0 ± 18.0 d | 821.0 ± 20.0 e | 902.0 ± 10.0 f | 920.0 ± 10.0 g |
| Taurine (total) * | - | - | - | - | - | - | - | - |
| Threonine | 836.0 ± 23.0 | 330.0 ± 21.0 a | 356.0 ± 19.0 b | 370.0 ± 21.0 b | 423.0 ± 13.0 c | 516.0 ± 20.0 d | 602.0 ± 0.20 e | 616.0 ± 11.0 e |
| Tryptophan | 292.0 ± 16.0 | 302.0 ± 32.0 a | 301.0 ± 21.0 a | 300.0 ± 29.0 a | 303.0 ± 21.0 a | 305.0 ± 16.0 a | 307.0 ± 10.0 a | 310.0 ± 10.0 a |
| Tyrosine * | 896.0 ± 10.0 | 500.0 ± 20.0 a | 620.0 ± 25.0 b | 651.0 ± 21.0 b | 720.0 ± 10.0 c | 790.0 ± 25.0 d | 815.0 ± 21.0 d | 914.0 ± 22.0 e |
| Valine | 1322.0 ± 19.0 | 651.0 ± 23.0 a | 698.0 ± 23.0 b | 720.0 ± 26.0 b | 752.0 ± 28.0 c | 777.0 ± 20.0 c | 826.0 ± 18.0 d | 890.0 ± 23.0 d |
| TAA | 20,290.0 ± 26.0 | 10,319.0 ± 19.0 a | 11,343.0 ± 15.0 b | 12,040.0 ± 21.0 c | 12,694.0 ± 12.0 d | 14,126.0 ± 22.0 e | 14,238.0 ± 15.0 f | 14,804.0 ± 32.0 g |
| EAA | 9876.0 ± 23.0 | 4041.0 ± 12.0 a | 4379.0 ± 16.0 b | 4883.0 ± 13.0 c | 5332.0 ± 10.0 d | 6562.0 ± 16.0 f | 6437.0 ± 14.0 e | 6761.0 ± 20.0 g |
| NEAA * | 10,414.0 ± 30.0 | 6278.0 ± 22.0 a | 6964.0 ± 10.0 b | 7157.0 ± 16.0 c | 7362.0 ± 31.0 d | 7562.0 ± 12.0 e | 7801.0 ± 21.0 f | 8043.0 ± 21.0 g |
| Breadsticks | |||||||
|---|---|---|---|---|---|---|---|
| S | S5 | S10 | S15 | S20 | S25 | S30 | |
| Before baking [mg/100 g] | 113.0 ± 10.0 a | 126.0 ± 12.0 b | 230.0 ± 20.0 c | 415.0 ± 21.0 d | 688.0 ± 22.0 e | 781.0 ± 28.0 f | 887.0 ± 25.0 g |
| After baking [mg/100 g] | 68.0 ± 3.0 a | 95.0 ± 5.0 b | 182.0 ± 14.0 c | 334.0 ± 16.0 d | 557.0 ± 18.0 e | 648.0 ± 16.0 f | 734.0 ± 20.0 g |
| Losses of available lysine [%] | 40.0 ± 6.0 d | 24.4 ± 3.0 c | 21.0 ± 2.8 b | 19.6 ± 2.0 b | 19.0 ± 3.0 ab | 17.0 ± 2.2 a | 17.3 ± 2.0 a |
| Breadsticks | |||||||
|---|---|---|---|---|---|---|---|
| S | S5 | S10 | S15 | S20 | S25 | S30 | |
| Hardness [N] | 6.03 ± 0.14 a | 3.53 ± 0.05 b | 3.06 ± 0.19 c | 3.03 ± 0.10 c | 2.98 ± 0.06 cd | 2.94 ± 0.08 d | 2.82 ± 0.02 e |
| Crispness [mm] | 0.479 ± 0.027 a | 0.356 ± 0.015 b | 0.339 ± 0.026 c | 0.316 ± 0.016 d | 0.295 ± 0.010 e | 0.276 ± 0.009 f | 0.252 ± 0.012 g |
| Breadsticks | |||||||
|---|---|---|---|---|---|---|---|
| S | S5 | S10 | S15 | S20 | S25 | S30 | |
| Appearance | 5.0 ± 0.0 b | 5.0 ± 0.0 b | 5.0 ± 0.0 b | 5.0 ± 0.0 b | 5.0 ± 0.0 b | 4.5.0 ± 0.0 a | 4.5.0 ± 0.5 ab |
| Color | 5.0 ± 0.0 b | 4.8 ± 0.3 b | 4.8 ± 0.3 b | 5.0 ± 0.0 b | 5.0 ± 0.5 ab | 4.5 ± 0.0 a | 4.5 ± 0.0 a |
| Aroma | 4.3 ± 0.0 b | 4.8 ± 0.2 c | 5.0 ± 0.0 d | 4.5 ± 0.5 cd | 4.0 ± 0.0 a | 4.3 ± 0.2 bc | 4.0 ± 0.0 a |
| Flavor | 4.3 ± 0.2 bc | 4.5 ± 0.0 c | 5.0 ± 0.0 d | 5.0 ± 0.0 d | 4.5 ± 0.0 c | 4.0 ± 0.2 a | 4.3 ± 0.0 b |
| Texture | 5.0 ± 0.0 c | 5.0 ± 0.0 c | 5.0 ± 0.0 c | 4.8 ± 0.3 bc | 4.5 ± 0.0 b | 4.2 ± 0.2 a | 4.0 ± 0.0 a |
| Overall acceptability | 8.0 ± 0.5 cd | 8.5 ± 0.5 d | 8.7 ± 0.2 d | 8.8 ± 0.0 d | 7.5 ± 0.5 bc | 7.0 ± 0.0 ab | 6.8 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarczyk, G.; Bienkiewicz, G.; Felisiak, K.; Biernacka, P.; Krzywiński, T.; Bury, M.; Podsiadło, C.; López Arroyos, E. Nutritional Composition, Bioactive Properties, and Sensory Evaluation of Breadsticks Enriched with Carp Meat (Cyprinus carpio, L.). Foods 2025, 14, 4066. https://doi.org/10.3390/foods14234066
Tokarczyk G, Bienkiewicz G, Felisiak K, Biernacka P, Krzywiński T, Bury M, Podsiadło C, López Arroyos E. Nutritional Composition, Bioactive Properties, and Sensory Evaluation of Breadsticks Enriched with Carp Meat (Cyprinus carpio, L.). Foods. 2025; 14(23):4066. https://doi.org/10.3390/foods14234066
Chicago/Turabian StyleTokarczyk, Grzegorz, Grzegorz Bienkiewicz, Katarzyna Felisiak, Patrycja Biernacka, Tomasz Krzywiński, Marek Bury, Cezary Podsiadło, and Eire López Arroyos. 2025. "Nutritional Composition, Bioactive Properties, and Sensory Evaluation of Breadsticks Enriched with Carp Meat (Cyprinus carpio, L.)" Foods 14, no. 23: 4066. https://doi.org/10.3390/foods14234066
APA StyleTokarczyk, G., Bienkiewicz, G., Felisiak, K., Biernacka, P., Krzywiński, T., Bury, M., Podsiadło, C., & López Arroyos, E. (2025). Nutritional Composition, Bioactive Properties, and Sensory Evaluation of Breadsticks Enriched with Carp Meat (Cyprinus carpio, L.). Foods, 14(23), 4066. https://doi.org/10.3390/foods14234066






