Preharvest Application of Oxalic Acid to ‘Calabacita’ Fresh Figs: Effects on Physicochemical and Antioxidant Profile During Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Methodology
2.3. Statistical Analysis
3. Results
3.1. Physiological Parameters
3.2. Physicochemical Parameters
3.3. Profiles of Individual Sugars and Organic Acids
3.4. Enzymatic and Non-Enzymatic Antioxidant Activities
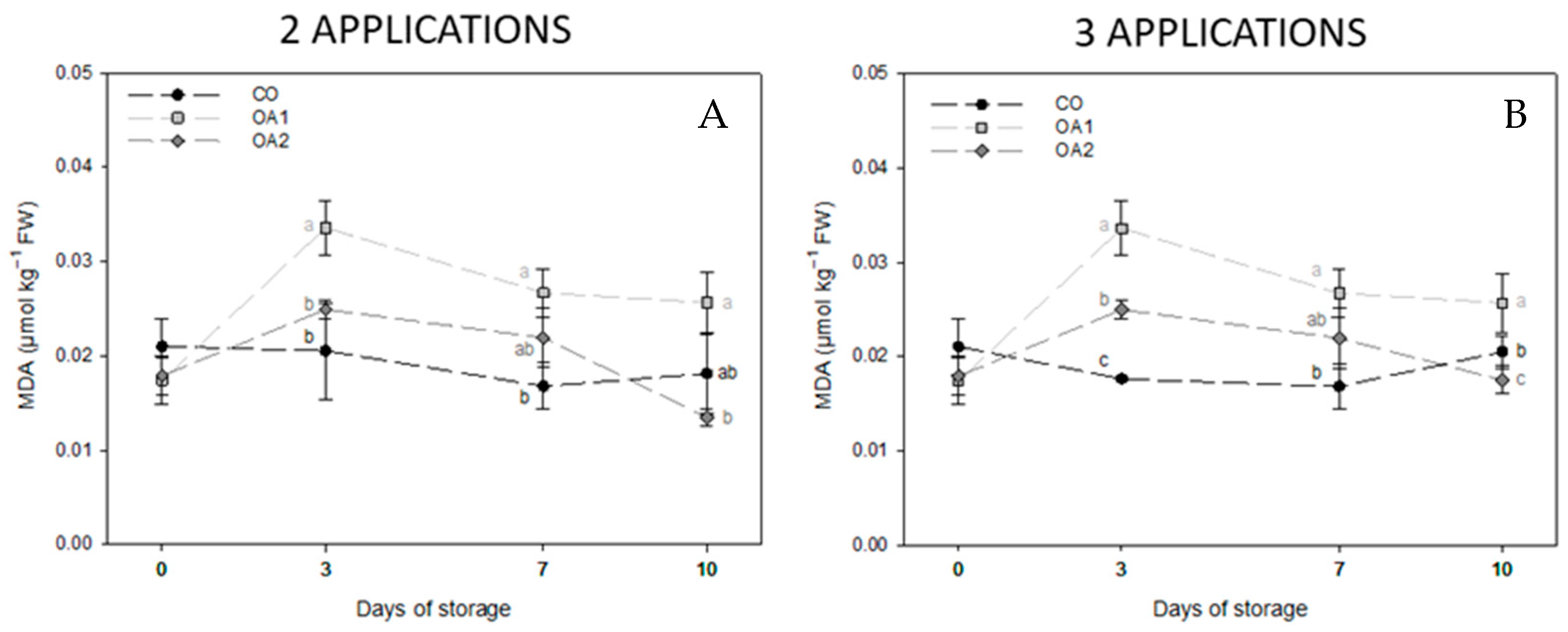
3.5. Assessment of Membrane Lipid Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | One-way analysis of variance |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| CO | Control |
| FW | Fresh weight |
| MDA | Malondialdehyde |
| PAL | Phenylalanine ammonium lyase |
| POD | Peroxidase |
| OA | Oxalic acid |
| OA1 | Oxalic acid 1 mM |
| OA2 | Oxalic acid 2 mM |
| TA | Titratable acidity |
| TAA | Total antioxidant activity |
| TPC | Total phenolic content |
| Trt | Treatment |
| TSS | Total soluble solids |
| RI | Ripening index |
References
- Aljane, F.; Neily, M.H.; Msaddak, A. Phytochemical characteristics and antioxidant activity of several fig (Ficus carica L.) ecotypes. Ital. J. Food Sci. 2020, 32, 755–768. [Google Scholar] [CrossRef]
- Koly, K.A.; Gomasta, J.; Alam, M.S.; Wahid, S.A.; Gulshan, S.S.; Kayesh, E. Morphological and physicochemical characterization of some exotic fig (Ficus carica L.) genotypes in Bangladesh. Int. J. Agron. 2024, 2024, 4735631. [Google Scholar] [CrossRef]
- Palmeira, L.; Pereira, C.; Dias, M.I.; Abreu, R.M.V.; Corrêa, R.C.G.; Pires, T.C.S.P.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Nutritional, chemical and bioactive profiles of different parts of a Portuguese common fig (Ficus carica L.) variety. Food Res. Int. 2019, 126, 108572. [Google Scholar] [CrossRef]
- Pereira, C.; López-Corrales, M.; Martín, A.; del Carmen Villalobos, M.; Córdoba, M.D.G.; Serradilla, M.J. Physicochemical and nutritional characterization of brebas for fresh consumption from nine fig varieties (Ficus carica L.) grown in Extremadura (Spain). J. Food Qual. 2017, 2017, 6302109. [Google Scholar] [CrossRef]
- Bachir-Bey, M.; Khodja, Y.K.; Meziant, L.; Benbouriche, A.; Chikhoune, A.; Louaileche, H. Impact of sun drying on the physicochemical characteristics and phytochemical contents of nine fig fruit varieties: A comparative study. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Tech. 2024, 48, 106–124. [Google Scholar] [CrossRef]
- De Bruno, A.; Mafrica, R.; Branca, V.; Piscopo, A.; Poiana, M. Evaluation of total antioxidant activity and phenolic profiles of Calabrian breba figs: A detailed study of pulp and skin from 29 Ficus carica L. accessions. Foods 2024, 13, 4035. [Google Scholar] [CrossRef]
- Ferrara, G.; Magarelli, A.; Mazzeo, A.; Coletta, A.; Crupi, P.; Loperfido, F.; Maggi, G.; Venerito, P. Underutilized fig (Ficus carica L.) cultivars from Puglia region, southeastern Italy, for an innovative product: Dried fig disks. Processes 2023, 11, 1485. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Carbonell-Barrachina, Á.A.; Hernández, F. Phenolic compounds, antioxidant and antidiabetic activity of different cultivars of Ficus carica L. fruits. J. Funct. Foods 2016, 25, 421–432. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Guo, Y.; Jiang, Y.; Wen, L.; Yang, B. New insights of fig (Ficus carica L.) as a potential function food. Trends Food Sci. Technol. 2023, 140, 104146. [Google Scholar] [CrossRef]
- Maatallah, S.; Guizani, M.; Lahbib, K.; Montevecchi, G.; Santunione, G.; Hessini, K.; Dabbou, S. Physiological traits, fruit morphology and biochemical performance of six old fig genotypes grown in warm climates “Gafsa Oasis” in Tunisia. J. Agric. Food Res. 2024, 17, 101253. [Google Scholar] [CrossRef]
- del Carmen Villalobos, M.; Martín, A.; Serradilla, M.J.; López-Corrales, M.; Palomino-Vasco, M.; Córdoba, M.D.G. Effect of modified atmosphere packaging (MAP) on health-promoting compounds, chlorophylls and antioxidant capacity of three fig cultivars (Ficus carica L.). Eur. Food Res. Technol. 2024, 250, 2767–2780. [Google Scholar] [CrossRef]
- Pereira, C.; López-Corrales, M.; Serradilla, M.J.; del Carmen Villalobos, M.; Ruiz-Moyano, S.; Martín, A. Influence of ripening stage on bioactive compounds and antioxidant activity in nine fig (Ficus carica L.) varieties grown in Extremadura, Spain. J. Food Comp. Anal. 2017, 64, 203–212. [Google Scholar] [CrossRef]
- del Carmen Villalobos, M.; Serradilla, M.J.; Martín, A.; Hernández-León, A.; Ruíz-Moyano, S.; Córdoba, M.D.G. Characterization of microbial population of breba and main crops (Ficus carica) during cold storage: Influence of passive modified atmospheres (MAP) and antimicrobial extract application. Food Microbiol. 2017, 63, 35–46. [Google Scholar] [CrossRef]
- Sevinç Üzümcü, S.; Koyuncu, M.A.; Onursal, C.E.; Güneyli, A.; Erbas, D. Effect of pre-harvest oxalic acid treatment on shelf-life of apricot cv. ‘Roxana.’ Nevşehir Bilim. Teknol. Derg. 2020, 9, 73–80. [Google Scholar] [CrossRef]
- Boix-Fayos, C.; de Vente, J. Challenges and potential pathways towards sustainable agriculture within the European Green Deal. Agric. Syst. 2023, 207, 103634. [Google Scholar] [CrossRef]
- Karantzi, A.D.; Kafkaletou, M.; Tsaniklidis, G.; Bai, J.; Christopoulos, M.V.; Fanourakis, D.; Tsantili, E. Preharvest foliar salicylic acid sprays reduce cracking of fig fruit at harvest. Appl. Sci. 2021, 11, 11374. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Hou, D.; Guo, X.; Liu, Q.; Liu, L.; Fang, Y.; Kou, L. Quality retention and delay postharvest senescence of figs (Ficus carica L.) using 1-methylcyclopropene and modified atmosphere packaging during cold storage. Food Biosci. 2023, 53, 102748. [Google Scholar] [CrossRef]
- Lama, K.; Modi, A.; Peer, R.; Izhaki, Y.; Flaishman, M.A. On-tree ABA application synchronizes fruit ripening and maintains keeping quality of figs (Ficus carica L.). Sci. Hortic. 2019, 253, 405–411. [Google Scholar] [CrossRef]
- Souza, J.M.A.; Leonel, S.; Leonel, M.; Garcia, E.L.; Ribeiro, L.R.; Ferreira, R.B.; Martins, R.C.; de Souza Silva, M.; Monteiro, L.N.H.; Duarte, A.S. Calcium nutrition in fig orchards enhance fruit quality at harvest and storage. Horticulturae 2023, 9, 123. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; García-Viguera, C.; Castillo, S.; Serrano, M. Preharvest application of oxalic acid increased fruit size, bioactive compounds, and antioxidant capacity in sweet cherry cultivars (Prunus avium L.). J. Agric. Food Chem. 2014, 62, 3432–3437. [Google Scholar] [CrossRef]
- Eroğul, D.; Kibar, H.; Şen, F.; Gundogdu, M. Role of postharvest oxalic acid treatment on quality properties, phenolic compounds, and organic acid contents of nectarine fruits during cold storage. Horticulturae 2023, 9, 1021. [Google Scholar] [CrossRef]
- Alaboudi, N.A.H.; Hatamnia, A.A.; Mohammadi, M.; Ranjbar, M.E. Oxalic acid alleviates chilling injury and positively influences post-harvest parameters of sweet bell pepper by affecting glycine betaine accumulation, cells respiration rate and NADPH oxidase enzyme activity during cold storage. Postharvest Biol. Technol. 2024, 212, 112905. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.; Jiang, W. Changes in sugar metabolism caused by exogenous oxalic acid related to chilling tolerance of apricot fruit. Postharvest Biol. Technol. 2016, 114, 10–16. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, J.; Brecht, J.K.; Jiang, T.; Zheng, X. Pre-harvest application of oxalic acid increases quality and resistance to Penicillium Expansum in kiwifruit during postharvest storage. Food Chem. 2016, 190, 537–543. [Google Scholar] [CrossRef]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Kaur, J.; Woodward, A.; Afrifa-Yamoah, E.; Malik, A.U. Oxalic acid: A blooming organic acid for postharvest quality preservation of fresh fruit and vegetables. Postharvest Biol. Technol. 2023, 206, 112574. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Valverde, J.M.; Guillén, F.; Castillo, S.; Martínez Romero, D.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest application of oxalic acid improved pomegranate fruit yield, quality, and bioactive compounds at harvest in a concentration’ dependent manner. Agronomy 2020, 10, 1522. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Adaos, G.; Cedeño-García, G.; Ospino-Olivella, S.C.; Vergara-Retamales, R.; Lopéz, M.D.; Olivares, R.; Hirzel, J.; Olivares-Soto, H.; Betancur, M. Preharvest applications of oxalic acid and salicylic acid increase fruit firmness and polyphenolic content in blueberry (Vaccinium corymbosum L.). Horticulturae 2023, 9, 639. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, S.; Razzaq, K.; Rajwana, I.A.; Akhtar, G.; Naz, A.; Amin, M.; Khalid, M.S.; Khalid, S. Pre-harvest oxalic acid application improves fruit size at harvest, physico-chemical and sensory attributes of ‘Red Flesh’ apricot during fruit ripening. J. Hortic. Sci. Technol. 2021, 4, 48–55. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Oxalic acid preharvest treatment increases antioxidant systems and improves plum quality at harvest and during postharvest storage. J. Sci. Food Agric. 2019, 99, 235–243. [Google Scholar] [CrossRef]
- Cui, Y.; Zhai, Y.; Flaishman, M.; Li, J.; Chen, S.; Zheng, C.; Ma, H. Ethephon induces coordinated ripening acceleration and divergent coloration responses in fig (Ficus carica L.) flowers and receptacles. Plant Mol. Biol. 2021, 105, 347–364. [Google Scholar] [CrossRef]
- Freiman, Z.E.; Doron-Faigenboim, A.; Dasmohapatra, R.; Yablovitz, Z.; Flaishman, M.A. High-throughput sequencing analysis of common fig (Ficus carica L.) transcriptome during fruit ripening. Tree Genet. Genomes 2014, 10, 923–935. [Google Scholar] [CrossRef]
- Freiman, Z.E.; Rosianskey, Y.; Dasmohapatra, R.; Kamara, I.; Flaishman, M.A. The ambiguous ripening nature of the fig (Ficus carica L.) fruit: A gene-expression study of potential ripening regulators and ethylene-related genes. J. Exp. Bot. 2015, 66, 3309–3324. [Google Scholar] [CrossRef]
- Pereira, C.; Martín, A.; López-Corrales, M.; de Guía Córdoba, M.; Galván, A.I.; Serradilla, M.J. Evaluation of the physicochemical and sensory characteristics of different fig cultivars for the fresh fruit market. Foods 2020, 9, 619. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Lozano, M.; Bernalte, M.J.; Ayuso, M.C.; López-Corrales, M.; González-Gómez, D. Physicochemical and bioactive properties evolution during ripening of “Ambrunés” sweet cherry cultivar. LWT 2011, 44, 199–205. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Martínez-Romero, D.; Guillén, F.; Zapata, P.J.; Serrano, M.; Valero, D. Melatonin pre-harvest treatments leads to maintenance of sweet cherry quality during storage by increasing antioxidant systems. Front. Plant Sci. 2022, 13, 863467. [Google Scholar] [CrossRef]
- Bi, X.; Dai, Y.; Zhou, Z.; Xing, Y.; Che, Z. Combining natamycin and 1-methylcyclopropene with modified atmosphere packaging to evaluate plum (Prunus Salicina cv. ‘Cuihongli’) quality. Postharvest Biol. Technol. 2022, 183, 111749. [Google Scholar] [CrossRef]
- del Carmen Villalobos, M.; Serradilla, M.J.; Martín, A.; López Corrales, M.; Pereira, C.; Córdoba, M.D.G. preservation of different fig cultivars (Ficus carica L.) under modified atmosphere packaging during cold storage. J. Sci. Food Agric. 2016, 96, 2103–2115. [Google Scholar] [CrossRef]
- Subramaniyan, V.; Sellamuthu, P.S.; Sadiku, E.R.; Jarugala, J. Effect of flax seed mucilage and guar gum coating enriched with postbiotics on postharvest storage of fig fruits (Ficus carica L.). S Afr. J. Bot. 2024, 166, 636–647. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Castillo, S.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D.; Zapata, P.J. preharvest treatment with oxalic acid improves postharvest storage of lemon fruit by stimulation of the antioxidant system and phenolic content. Antioxidants 2021, 10, 963. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; García-Pastor, M.E.; Zapata, P.J.; Guillén, F.; Serrano, M.; Valero, D.; Gironés-Vilaplana, A. Preharvest application of oxalic acid improves quality and phytochemical content of artichoke (Cynara Scolymus L.) at harvest and during storage. Food Chem. 2017, 230, 343–349. [Google Scholar] [CrossRef]
- Pereira, C.; Serradilla, M.J.; Martín, A.; del Carmen Villalobos, M.; Pérez-Gragera, F.; López-Corrales, M. Agronomic behaviour and quality of six fig cultivars for fresh consumption. Sci. Hortic. 2015, 185, 121–128. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Legua, P.; Martínez-Nicolás, J.J.; Melgarejo, P. Breba fruits characterization from four varieties (Ficus carica L.) with important commercial interest in Spain. Foods 2021, 10, 3138. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Gull, S.; Nafees, M.; Amin, M.; Hussain, Z.; Sattar Khan, A.; Ullah Malik, A. Pre-harvest foliar application of oxalic acid improves strawberry plant growth and fruit quality. J. Hortic. Sci. Technol. 2018, 1, 35–41. [Google Scholar] [CrossRef]
- Vieira, J.; Rodrigues Rui de Sousa, D. Qualitative characteristics of seven cultivars producing breba figs. Rev. Ciênc. Agrár. 2023, 4, 332–337. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Valentão, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus carica L.: Metabolic and biological screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar] [CrossRef]
- Pande, G.; Akoh, C.C. Organic acids, antioxidant capacity, phenolic content and lipid characterisation of Georgia-grown underutilized fruit crops. Food Chem. 2010, 120, 1067–1075. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Serna-Escolano, V.; Guillén, F.; Valero, D.; Serrano, M.; García-Martínez, S.; Terry, L.A.; Alamar, M.C.; Zapata, P.J. Oxalic acid preharvest treatment improves colour and quality of seedless table grape ‘Magenta’ upregulating on-vine abscisic acid metabolism, relative VvNCED1 gene expression, and the antioxidant system in berries. Front. Plant Sci. 2021, 12, 740240. [Google Scholar] [CrossRef]
- Li, P.; Yin, F.; Song, L.; Zheng, X. Alleviation of chilling injury in tomato fruit by exogenous application of oxalic acid. Food Chem. 2016, 202, 125–132. [Google Scholar] [CrossRef]
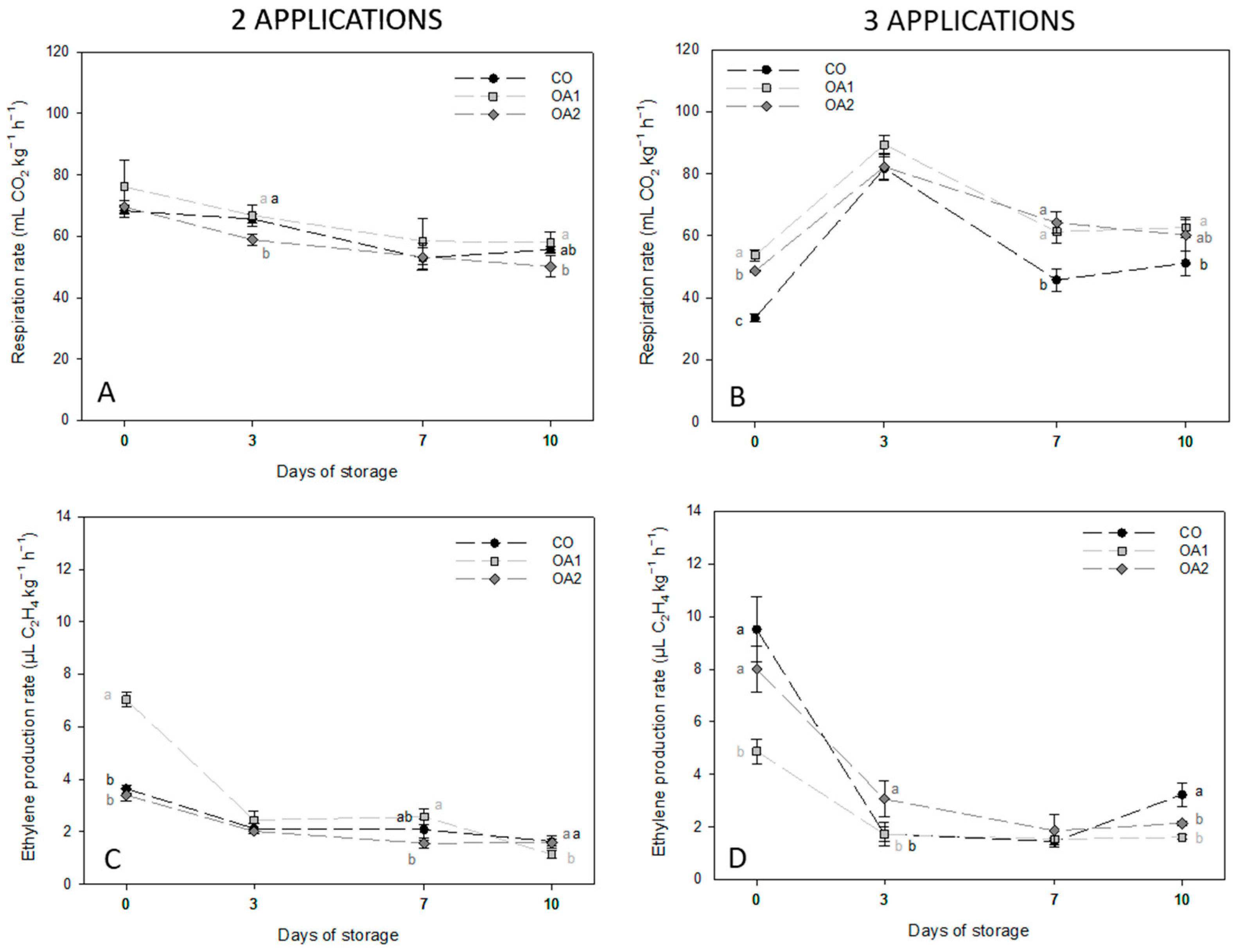
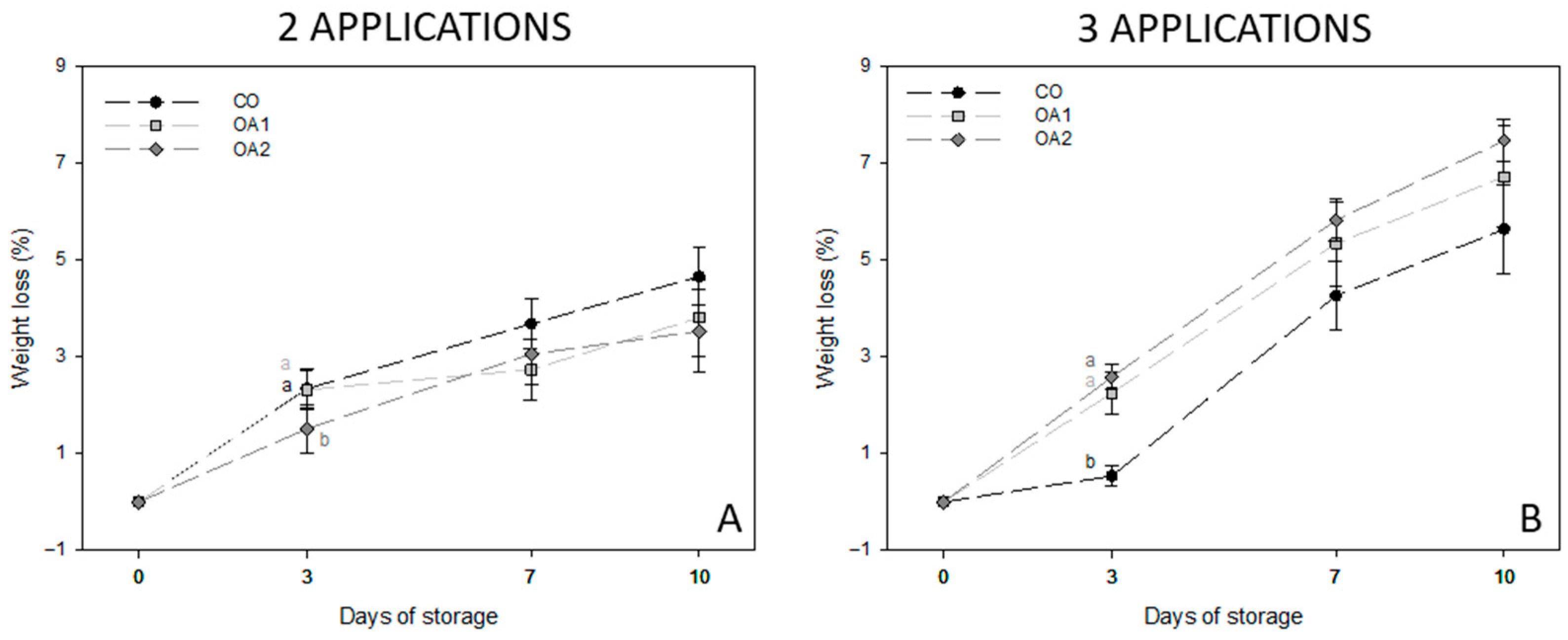
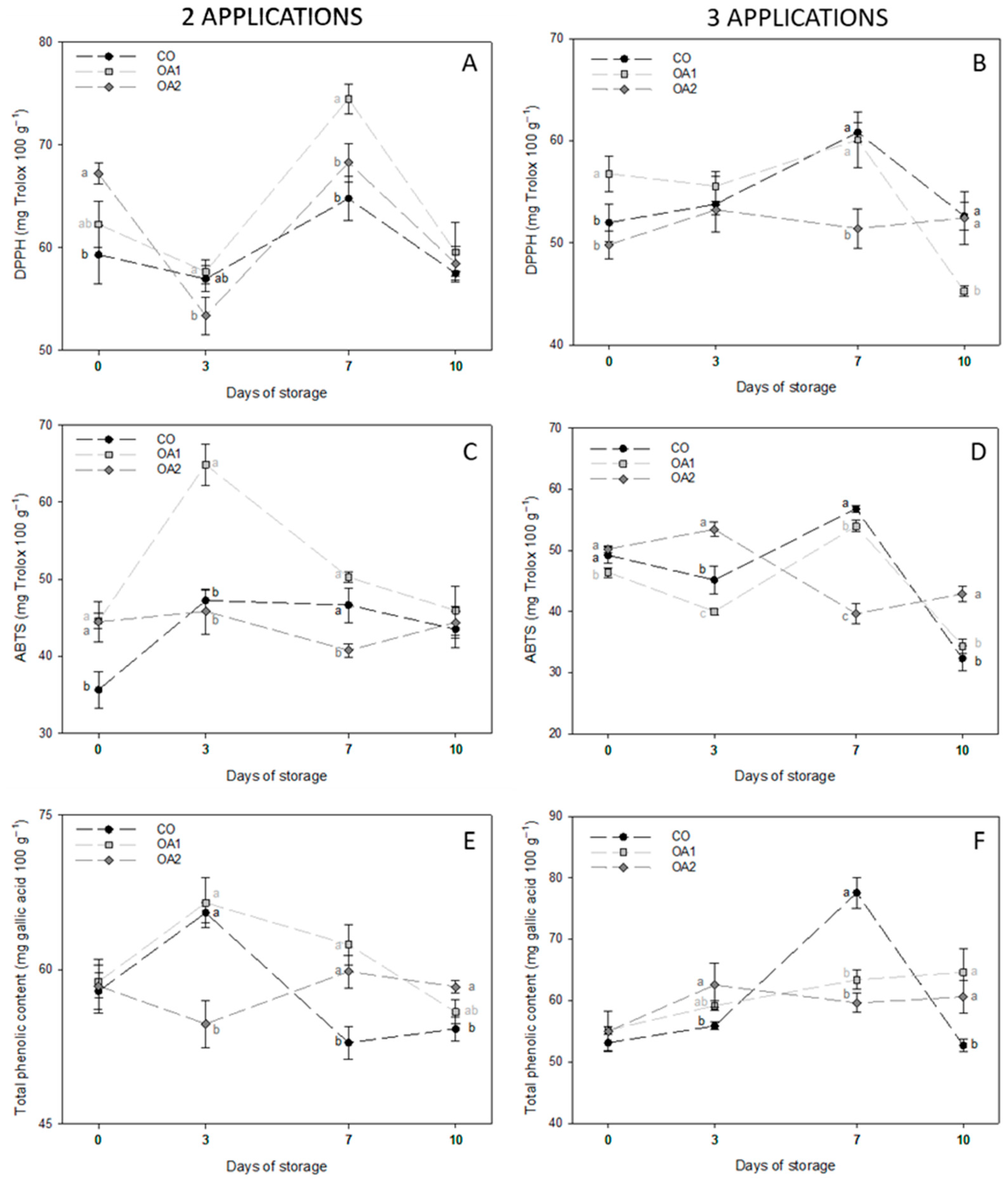
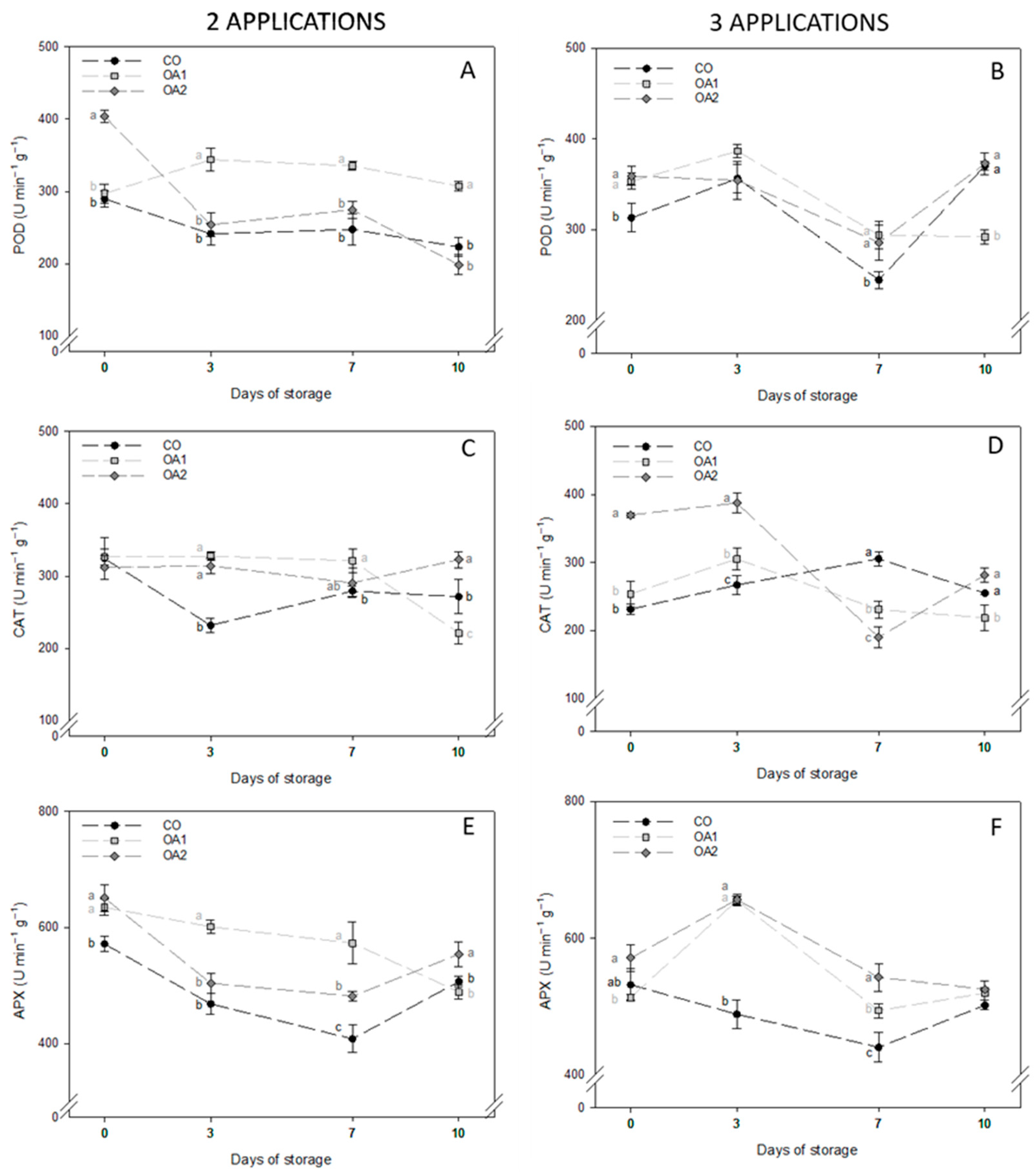
| Day | Trt | Firmness (N mm−1) | TSS (°Brix) | TA (% Citric Acid) | RI (TSS TA−1) | |
|---|---|---|---|---|---|---|
| 2 APPLICATIONS | 0 | CO | 0.63 ± 0.19 | 20.7 ± 2.9 | 0.071 b ± 0.003 | 279.1 a ± 13.2 |
| OA1 | 0.63 ± 0.09 | 19.9 ± 1.4 | 0.083 a ± 0.006 | 224.5 b ± 8.5 | ||
| OA2 | 0.62 ± 0.16 | 20.2 ± 1.0 | 0.072 b ± 0.002 | 279.3 a ± 11.6 | ||
| 3 | CO | 0.62 ± 0.16 | 23.5 a ± 2.2 | 0.079 b ± 0.001 | 288.4 a ± 13.3 | |
| OA1 | 0.70 ± 0.14 | 21.5 ab ± 0.7 | 0.100 a ± 0.005 | 206.2 b ± 2.1 | ||
| OA2 | 0.63 ± 0.06 | 18.7 b ± 1.0 | 0.087 b ± 0.005 | 202.6 b ± 4.5 | ||
| 7 | CO | 0.68 ± 0.11 | 18.9 ± 0.5 | 0.055 ± 0.002 | 342.0 a ± 16.7 | |
| OA1 | 0.74 ± 0.06 | 19.9 ± 0.5 | 0.068 ± 0.007 | 289.2 b ± 16.6 | ||
| OA2 | 0.64 ± 0.10 | 19.0 ± 0.6 | 0.065 ± 0.008 | 280.4 b ± 21.1 | ||
| 10 | CO | 0.49 ± 0.03 | 19.5 ± 1.1 | 0.051 b ± 0.003 | 368.6 a ± 10.3 | |
| OA1 | 0.52 ± 0.02 | 20.1 ± 0.6 | 0.055 b ± 0.001 | 367.7 a ± 9.1 | ||
| OA2 | 0.53 ± 0.02 | 20.7 ± 0.5 | 0.061 a ± 0.002 | 341.3 b ± 4.2 | ||
| 3 APPLICATIONS | 0 | CO | 0.84 ± 0.02 | 21.0 ± 0.1 | 0.061 b ± 0.002 | 342.7 a ± 11.3 |
| OA1 | 0.83 ± 0.10 | 18.3 ± 1.4 | 0.066 ab ± 0.001 | 275.8 b ± 19.2 | ||
| OA2 | 0.74 ± 0.14 | 19.9 ± 1.8 | 0.070 a ± 0.003 | 272.3 b ± 10.2 | ||
| 3 | CO | 0.78 ± 0.07 | 18.9 ± 0.9 | 0.057 b ± 0.002 | 330.9 ± 28.1 | |
| OA1 | 0.70 ± 0.11 | 21.4 ± 1.5 | 0.060 b ± 0.003 | 341.9 ± 10.9 | ||
| OA2 | 0.72 ± 0.04 | 20.8 ± 0.2 | 0.069 a ± 0.002 | 300.1 ± 8.7 | ||
| 7 | CO | 0.62 ± 0.05 | 19.4 ± 1.0 | 0.059 ab ± 0.003 | 342.8 b ± 10.9 | |
| OA1 | 0.55 ± 0.06 | 21.3 ± 1.1 | 0.056 b ± 0.005 | 408.2 a ± 16.2 | ||
| OA2 | 0.63 ± 0.02 | 20.3 ± 0.8 | 0.066 a ± 0.004 | 295.3 c ± 9.8 | ||
| 10 | CO | 0.76 ± 0.12 | 19.4 ± 0.6 | 0.053 ab ± 0.001 | 375.3 b ± 8.5 | |
| OA1 | 0.77 ± 0.05 | 21.3 ± 2.2 | 0.048 b ± 0.003 | 417.8 a ± 23.8 | ||
| OA2 | 1.03 ± 0.25 | 18.4 ± 1.6 | 0.059 a ± 0.003 | 337.9 c ± 37.0 |
| Day | Trt | Sugars (g kg−1 FW) | Organic Acids (g kg−1 FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Sucrose | Oxalic Acid | Citric Acid | Malic Acid | Succinic Acid | |||
| 2 APPLICATIONS | 0 | CO | 77.7 ± 1.7 | 75.4 ± 1.5 | 0.38 ab ± 0.66 | 0.11 a ± 0.01 | 0.87 b ± 0.04 | 7.3 ab ± 0.1 | 4.5 ± 0.2 |
| OA1 | 80.4 ± 4.1 | 77.3 ± 8.9 | 1.5 a ± 0.4 | 0.11 a ± 0.03 | 1.14 a ± 0.05 | 7.7 a ± 0.2 | 4.5 ± 0.3 | ||
| OA2 | 75.3 ± 9.4 | 73.3 ± 4.5 | 0.00 b ± 0.00 | 0.09 b ± 0.07 | 0.80 b ± 0.04 | 7.1 b ± 0.3 | 4.4 ± 0.1 | ||
| 3 | CO | 96.0 a ± 3.4 | 93.8 a ± 3.6 | 0.00 ± 0.00 | 0.157 b ± 0.003 | 1.32 ab ± 0.07 | 12.1 a ± 0.6 | 6.6 a ± 0.3 | |
| OA1 | 89.5 ab ± 2.6 | 85.9 ab ± 3.8 | 0.06 ± 0.09 | 0.179 a ± 0.007 | 1.48 a ± 0.03 | 10.4 b ± 0.4 | 5.5 b ± 0.3 | ||
| OA2 | 79.4 b ± 6.3 | 77.2 b ± 5.9 | 0.00 ± 0.00 | 0.153 b ± 0.003 | 1.19 b ± 0.09 | 9.5 b ± 0.2 | 4.7 c ± 0.2 | ||
| 7 | CO | 86.1 a ± 3.4 | 83.9 a ± 3.4 | 0.21 ± 0.37 | 0.105 a ± 0.004 | 0.74 b ± 0.02 | 6.87 ± 0.09 | 3.3 ± 0.2 | |
| OA1 | 69.0 b ± 4.7 | 66.5 b ± 4.0 | 0.18 ± 0.32 | 0.10 a ± 0.01 | 0.91 a ± 0.02 | 6.9 ± 0.2 | 3.5 ± 0.4 | ||
| OA2 | 68.7 b ± 6.2 | 67.1 b ± 6.0 | 0.00 ± 0.00 | 0.064 b ± 0.005 | 0.80 ab ± 0.09 | 6.6 ± 0.4 | 3.5 ± 0.1 | ||
| 10 | CO | 85.4 a ± 3.9 | 81.3 a ± 8.5 | 0.00 ± 0.00 | 0.095 a ± 0.007 | 0.87 a ± 0.05 | 7.1 a ± 0.2 | 3.5 ± 0.2 | |
| OA1 | 69.0 b ± 2.3 | 68.3 b ± 2.4 | 0.00 ± 0.00 | 0.085 ab ± 0.005 | 0.68 b ± 0.04 | 6.5 a ± 0.2 | 3.2 ± 0.3 | ||
| OA2 | 66.8 b ± 1.6 | 64.7 b ± 1.6 | 0.28 ± 0.49 | 0.077 b ± 0.005 | 0.68 b ± 0.02 | 5.4 b ± 0.3 | 2.9 ± 0.3 | ||
| 3 APPLICATIONS | 0 | CO | 91.1 a ± 5.5 | 88.3 a ± 5.1 | 0.78 ± 1.4 | 0.044 ± 0.004 | 0.49 a ± 0.02 | 5.1 a ± 0.3 | 3.5 a ± 0.3 |
| OA1 | 42.8 b ± 5.1 | 41.2 b ± 4.7 | 0.00 ± 0.00 | 0.047 ± 0.008 | 0.33 b ± 0.02 | 3.2 b ± 0.3 | 2.4 b ± 0.3 | ||
| OA2 | 50.7 b ± 2.4 | 48.7 b ± 2.6 | 0.29 ± 0.50 | 0.040 ± 0.007 | 0.46 a ± 0.02 | 4.8 a ± 0.2 | 2.5 b ± 0.4 | ||
| 3 | CO | 80.2 c ± 5.9 | 77.3 b ± 6.0 | 0.57 b ± 0.49 | 0.069 c ± 0.003 | 0.66 b ± 0.03 | 7.88 b ± 0.06 | 4.2 ± 0.1 | |
| OA1 | 98.8 b ± 1.1 | 96.4 a ± 1.1 | 0.00 b ± 0.00 | 0.13 a ± 0.01 | 0.858 a ± 0.002 | 9.1 a ± 0.1 | 4.07 ± 0.04 | ||
| OA2 | 110.4 a ± 2.6 | 105.3 a ± 2.4 | 4.2 a ± 0.9 | 0.090 b ± 0.004 | 0.67 b ± 0.02 | 7.7 b ± 0.4 | 4.24 ± 0.05 | ||
| 7 | CO | 66.7 b ± 3.5 | 64.1 b ± 3.9 | 0.10 ± 0.17 | 0.050 c ± 0.001 | 0.53 b ± 0.04 | 6.5 b ± 0.2 | 2.9 b ± 0.2 | |
| OA1 | 81.3 a ± 7.6 | 78.7 a ± 7.3 | 0.26 ± 0.45 | 0.106 a ± 0.007 | 0.635 a ± 0.002 | 7.9 a ± 0.1 | 3.7 b ± 0.3 | ||
| OA2 | 66.3 b ± 1.8 | 64.0 b ± 2.0 | 0.27 ± 0.23 | 0.069 b ± 0.004 | 0.56 b ± 0.03 | 6.6 b ± 0.3 | 3.4 ab ± 0.2 | ||
| 10 | CO | 64.3 b ± 3.3 | 62.0 b ± 2.8 | 0.64 ± 0.11 | 0.064 a ± 0.003 | 0.56 a ± 0.03 | 6.6 a ± 0.2 | 3.6 a ± 0.2 | |
| OA1 | 69.5 ab ± 3.1 | 66.6 ab ± 3.2 | 0.95 ± 1.26 | 0.063 a ± 0.003 | 0.52 a ± 0.02 | 7.0 a ± 0.2 | 3.5 a ± 0.3 | ||
| OA2 | 73.5 a ± 3.5 | 70.8 a ± 2.7 | 0.00 ± 0.00 | 0.041 b ± 0.003 | 0.44 b ± 0.04 | 5.5 b ± 0.3 | 2.84 b ± 0.09 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraga-Lozano, C.; Fernández-León, A.M.; López-Corrales, M.; Rodríguez, A.; Serradilla, M.J.; Palomino-Vasco, M. Preharvest Application of Oxalic Acid to ‘Calabacita’ Fresh Figs: Effects on Physicochemical and Antioxidant Profile During Cold Storage. Foods 2025, 14, 4061. https://doi.org/10.3390/foods14234061
Moraga-Lozano C, Fernández-León AM, López-Corrales M, Rodríguez A, Serradilla MJ, Palomino-Vasco M. Preharvest Application of Oxalic Acid to ‘Calabacita’ Fresh Figs: Effects on Physicochemical and Antioxidant Profile During Cold Storage. Foods. 2025; 14(23):4061. https://doi.org/10.3390/foods14234061
Chicago/Turabian StyleMoraga-Lozano, Carlos, Ana María Fernández-León, Margarita López-Corrales, Alicia Rodríguez, Manuel J. Serradilla, and Mónica Palomino-Vasco. 2025. "Preharvest Application of Oxalic Acid to ‘Calabacita’ Fresh Figs: Effects on Physicochemical and Antioxidant Profile During Cold Storage" Foods 14, no. 23: 4061. https://doi.org/10.3390/foods14234061
APA StyleMoraga-Lozano, C., Fernández-León, A. M., López-Corrales, M., Rodríguez, A., Serradilla, M. J., & Palomino-Vasco, M. (2025). Preharvest Application of Oxalic Acid to ‘Calabacita’ Fresh Figs: Effects on Physicochemical and Antioxidant Profile During Cold Storage. Foods, 14(23), 4061. https://doi.org/10.3390/foods14234061





