CO2 and Acidification of Low-Salt Brine Promote Some Yeasts and Penalize Bacteria in Naturally Brined Black Table Olive Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Tests with Healthy Table Olives
2.1.1. Fermentation Trials with Washed Healthy Table Olives
2.1.2. Fermentation Trials with Unwashed Healthy Table Olives
2.2. Tests with Damaged Table Olives
Starter Preparation
2.3. Microbiological Analysis
2.4. Total Aerobic Bacteria Identification
2.5. Yeast Biodiversity
2.6. Physicochemical Parameters
2.7. Evaluation of Resistance of Leuconostoc Mesenteroides to Acidified Brine
2.8. Microbiological Analysis of Bactrocera Oleae Larvae
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fermentation with Healthy Olives
3.2. Fermentation with Damaged Olives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Vougiouklaki, D.; Letsiou, S.; Mavrokefalidon, I.; Tsakali, E.; Akkermans, S.; Van Impe, J.F.; Houhoula, D. Fermentation of Kalamata Natural Black Olives Using Selected Lactic Acid Bacteria as Starters. Fermentation 2024, 10, 53. [Google Scholar] [CrossRef]
- Garrido-Fernández, A.; Fernández Diaz, M.J.; Adams, M.R. Table Olives: Production and Processing, 1st ed.; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Johnson, R.L.; Mitchell, A.E. Reducing Phenolics Related to Bitterness Table Olives. J. Food Qual. 2018, 2018, 3193185. [Google Scholar] [CrossRef]
- Munir, M.T.; Mtimet, N.; Guillier, L.; Meurens, F.; Fravalo, P.; Federighi, M.; Kooh, P. Physical Treatments to Control Clostridium botulinum Hazards in Food. Foods 2023, 12, 1580. [Google Scholar] [CrossRef]
- Shoaib, M.; Ali, M.M.; Tang, M.; Weiwei, W.; Zhang, X.; He, Z.; Wu, Z.; Wang, S.; Hao, B.; Li, R. Genomic and phylogeographical analysis revealed CTX-M-55 producing Escherichia coli ST10 and ST2325 clones of One Health concern from dairy farm waste in Gansu, China. One Health 2025, 20, 101101. [Google Scholar] [CrossRef]
- Ryzhova, E.; Janine, W.; Chantelle, H.D.; Holỳ, O. Listeria monocytogenes in organic and conventional farming: Epidemiology, risks, and solutions within a One Health framework. One Health 2025, 21, 101173. [Google Scholar] [CrossRef]
- International Olive Council (IOC). Trade Standard Applying to Table Olives; IOC: Madrid, Spain, 2004. [Google Scholar]
- International Olive Council (IOC). Method for the Sensory Analysis of Table Olives; COI/OT/MO No. 1/Rev. 2 November 2011; International Olive Council: Madrid, Spain, 2011; Available online: http://www.internationaloliveoil.org/estaticos/view/70-metodos-de-evaluacion (accessed on 19 April 2023).
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization, Department of Nutrition for Health and Development: Geneva, Switzerland, 2012; Available online: https://www.who.int/publications/i/item/9789241504836 (accessed on 17 March 2023).
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. Eur. Union 2011, 304, 18–63. [Google Scholar]
- Chrysant, S.G. Effects of high salt intake on blood pressure and cardiovascular disease. The role of COX inhibitors. Clin. Cardiol. 2016, 39, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Callego, J.; Arroyo-López, F.N.; Durán-Quintana, M.C.; Garrido-Fernández, A. Fermentation profiles of Manzanilla- Aloreña cracked green table olives in different choride salt mixtures. Food Microbiol. 2010, 27, 403–412. [Google Scholar] [CrossRef]
- Bautista-Callego, J.; Arroyo-López, F.N.; Romero-Gil, V.; Rodríguez-Gómez, F.; García-García, P.; Garrido-Fernández, A. Chloride salt mixture affect Gordal cv. Green Spanish-style table olive fermentation. Food Microb. 2011, 28, 1316–1325. [Google Scholar] [CrossRef]
- Zinno, P.; Guantario, B.; Perozzi, G.; Pastore, G.; Devirgiliis, C. Impact of NaCl reduction on lactic acid bacteria during fermentation of Nocellara del Belice table olives. Food Microb. 2017, 63, 239–247. [Google Scholar] [CrossRef]
- López-López, A.; Moreno-Baquero, J.M.; Garrido-Fernández, A. The desalting process for table olives and its effect on their physicochemical characteristics and nutrient mineral content. Foods 2023, 12, 2307. [Google Scholar] [CrossRef]
- Haas, G.J.; Prescott, H.E.; Dudley, E.; Dik, R.; Hintlian, C.; Keane, L. Inactivation of microorganisms by carbon dioxide under pressure. J. Food Saf. 1989, 9, 253–265. [Google Scholar] [CrossRef]
- Zullo, B.A.; Ciafardini, G. Use of Slightly Pressurized Carbon Dioxide to Enhance the Antimicrobial Properties of Brines in Naturally Processed Black Table Olives. Microorganisms 2022, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Ciafardini, G.; Zullo, B.A. A New Natural Processing System Based on Slight Carbon Dioxide Pressure for Producing Black Table Olives with Low Salt Content. Foods 2023, 12, 3950. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Use of air-protected headspace to prevent yeast film formation on the brine of Leccino and Taggiasca black table olives processed in industrial-scale plastic barrels. Foods 2020, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Uceda, M.; Frias, L. Harvest dates. Evolution of the fruit oil content, oil composition and oil quality. In Proceedings of the II Seminario Oleicola Internacional, International Olive Oil Council, Cordoba, Spain, 6–17 October 1975; pp. 125–130. [Google Scholar]
- Zullo, B.A.; Ciafardini, G. Technological Improvement of Brined Black Table Olives Processed Using Two-Phase and Single-Phase Methods Under Slight CO2 Pressure and Low Salt Content. Foods 2024, 13, 3799. [Google Scholar] [CrossRef]
- Ghoddusi, H.B.; Sherburn, R.E.; Adoaba, O.O. Growth limiting pH, water activity, and temperature for neuroxigenic strains of Clostridium butyricum. ISRN Microbiol. 2013, 2013, 731430. [Google Scholar] [CrossRef]
- Harrigan, W.F.; McCance, M.E. Culture media composition. In Laboratory Methods in Microbiology; Editorial Academic: Leon, Spain, 1979; p. 46. [Google Scholar]
- Sarwat, F.; Qader, S.A.; Aman, A.; Ahmed, N. Production and characterization of a unique Dextran from an Indigenous Leuconostoc mesenteroides CMG713. Int. J. Biol. Sci. 2008, 4, 379–386. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. B-glucosidase activity in Leuconostoc mesenteroides associated with fermentation of “Coratina” cultivar olives. Ital. J. Food Sci. 2001, 13, 41–52. [Google Scholar]
- Tornai-Lehoczki, J.; Péter, G.; Dlauchy, D. CHROMagar medium as a practical tool for the differentiation and presuntive identification of yeast species isolated from salad. Int. J. Food Microbiol. 2003, 86, 189–200. [Google Scholar] [CrossRef]
- Valero, A.; Olague, E.; Medina-Pradas, E.; Garrido-Fernández, A.; Romero-Gil, V.; Cantalejo, M.J.; García-Gimeno, R.M.; Pérez-Rodríguez, F.; Posada-Itquierdo, G.D.; Arroyo-López, F.N. Influence of Acid Adaptation on the Probability of Germination of Clostridium sporogenes Spores Against pH, NaCl and Time. Foods 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.L.C.; Hocking, A.D.; Stewart, C.M.; Buckle, K.E.; Graham, H.F. Baroprotective effect of increased solute concentration on yeasts and moulds during high pressure processing. Inn.Food Sci. Emer. Technol. 2007, 8, 535–542. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.; Miao, B.; Xie, C.; Tang, Y.Q. Saccharomyces cerevisiae employs complex regulation strategies to tolerate low pH stress during ethanol production. Microb. Cell. Fact. 2022, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-López, F.N.; Romero-Gil, V.; Bautista-Gallego, J.; Rodriguez-Gómez, F.; Jiménez- Díaz, R.; García-García, P.; Garrido-Fernández, A. Yeasts in table olive processing desirable or spoilage microorganisms. Int. J. Food Microbiol. 2012, 160, 42–49. [Google Scholar] [CrossRef]
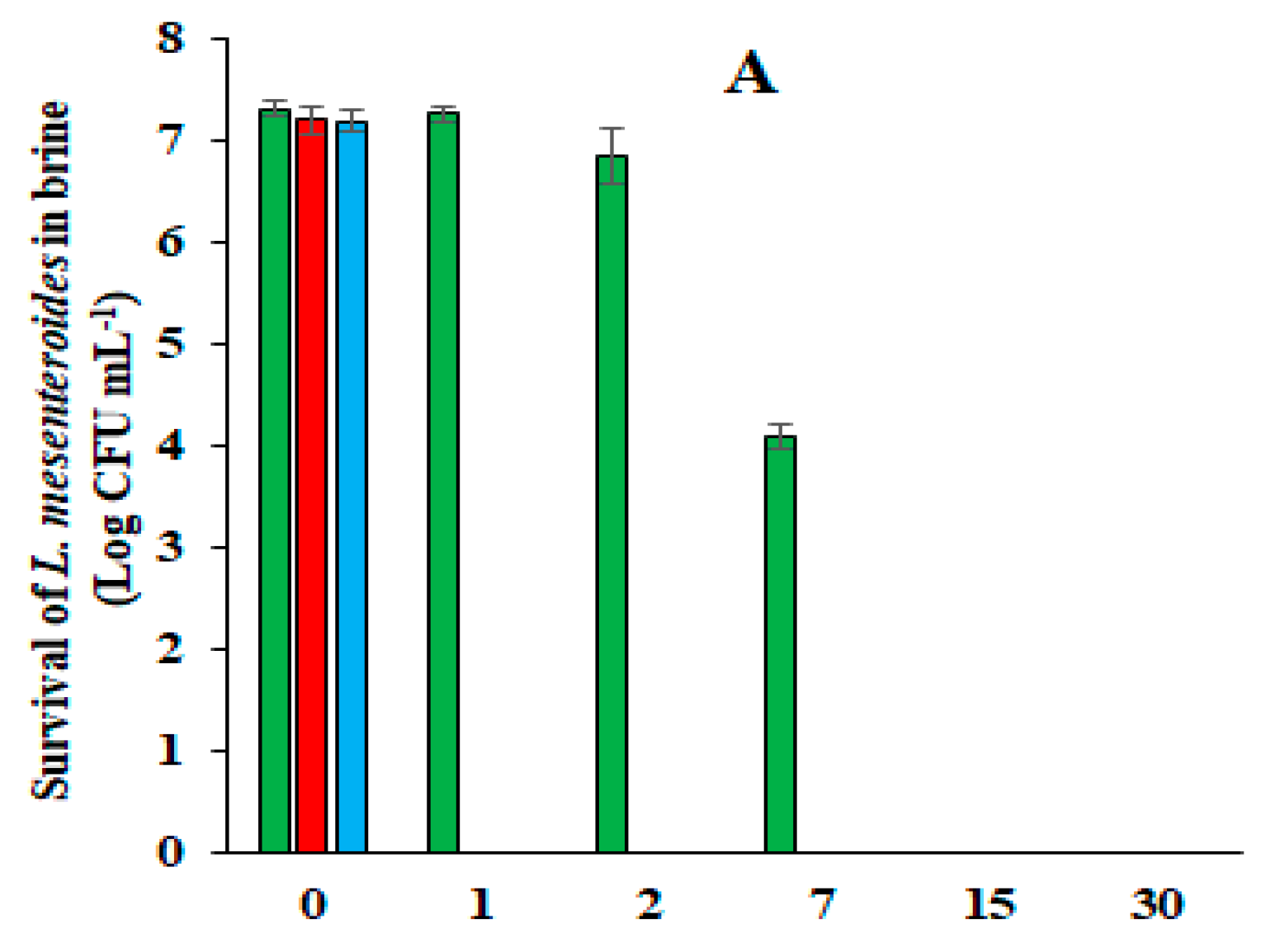
 ), acidified with HCl (
), acidified with HCl ( ), or not acidified (
), or not acidified ( ).
).
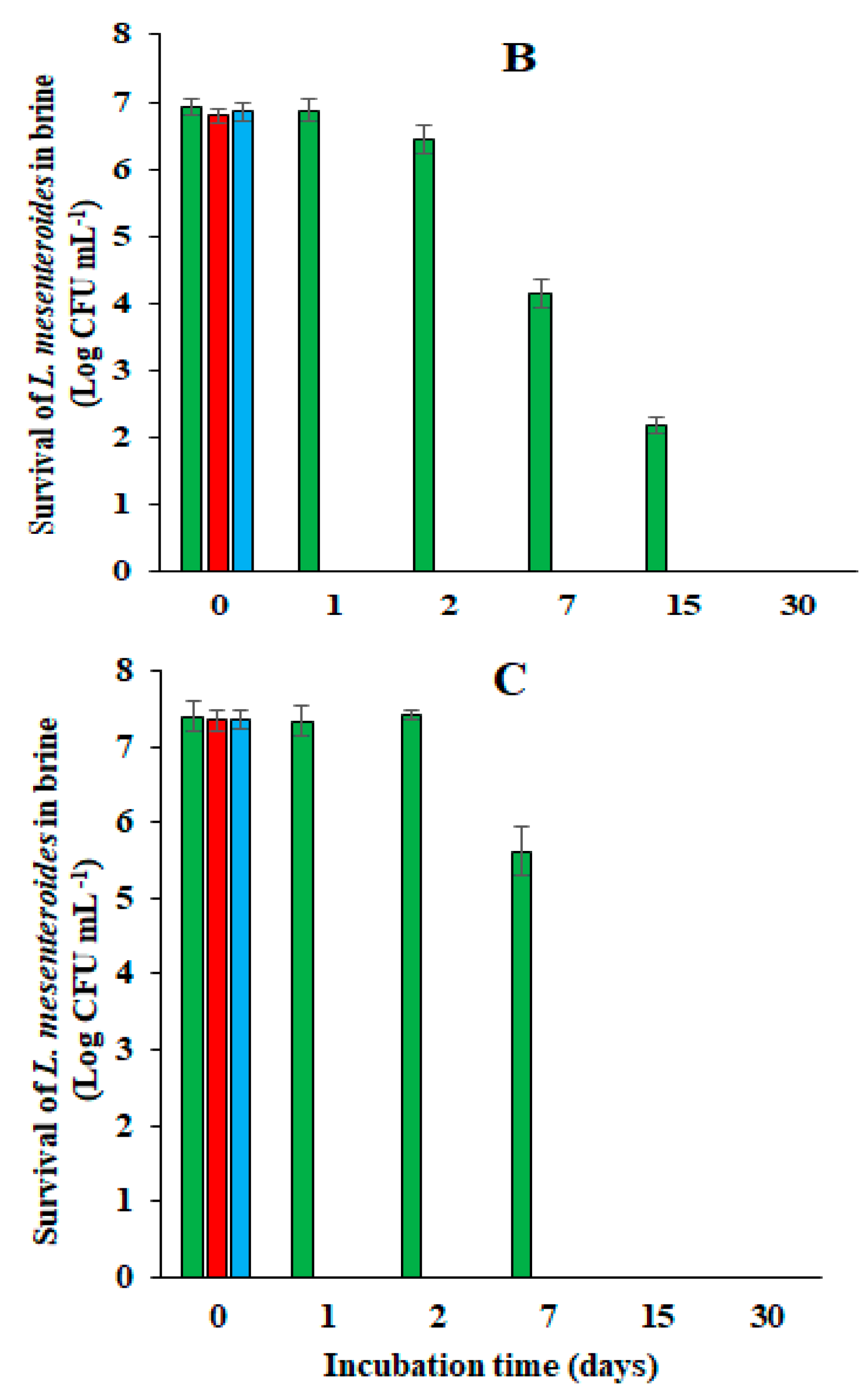
 ), acidified with HCl (
), acidified with HCl ( ), or not acidified (
), or not acidified ( ).
).
| Days | Total Yeasts (Log CFU mL−1 or g−1) | Preeminent Yeast Species (%) | Other Microorganisms (Log CFU mL−1 or g−1) | |||
|---|---|---|---|---|---|---|
| Brine | Flesh | Brine | Flesh | Brine | Flesh | |
| 7 | 2.81 ± 0.13 a | n.d. | S.c. (100) | n.d. | <1 1 | <1 |
| 60 | 4.85 ± 0.24 b | n.d. | S.c. (100) | n.d. | <1 | <1 |
| 120 | 5.59 ± 0.15 b | n.d. | S.c. (98) C.b. (2) | n.d. | <1 | <1 |
| 180 | 5.99 ± 0.26 b | n.d. | S.c. (94) C.b. (6) | n.d. | <1 | <1 |
| 240 | 4.65 ± 0.12 b | 4.48 ± 0.18 | S.c. (89) C.b. (11) | S.c. (100) | <1 | <1 |
| Days | pH | NaCl (%, w v−1 or w−1) | Free CO2 (g kg−1) | Total Polar Phenols (mg CAE mL−1 or g−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Brine | Flesh | Brine | Flesh | Brine | Flesh | Brine | Flesh | |
| 7 | 3.44 ± 0.06 | n.d. | 3.50 ± 0.10 a | n.d. | 1.40 ± 0.20 a | n.d. | 1.20 ± 0.10 a | 5.00 ± 0.10 d |
| 60 | 4.02 ± 0.06 | n.d. | 3.75 ± 0.10 a | n.d. | 1.70 ± 0.19 a | n.d. | 1.43 ± 0.21 a | 4.52 ± 0.09 c |
| 120 | 4.04 ± 0.04 | n.d. | 4.05 ± 0.04 ab | n.d. | 1.89 ± 0.02 ab | n.d. | 2.03 ± 0.00 b | 3.61 ± 0.08 b |
| 180 | 4.24 ± 0.02 | n.d. | 4.95 ± 0.02 b | n.d. | 1.96 ± 0.06 ab | n.d. | 2.61 ± 0.12 c | 2.61 ± 0.00 a |
| 240 | 4.32 ± 0.09 | 4.50 ± 0.08 | 4.40 ± 0.10 b | 3.73 ± 0.15 | 2.64 ± 0.01 b | n.d. | 2.79 ± 0.07 c | 2.79 ± 0.02 a |
| Days | Total Yeasts (Log CFU mL−1) | Preeminent Yeast Species (%) | Total Molds (Log CFU mL−1) | Others 1 |
|---|---|---|---|---|
| 1 | 2.68 ± 0.08 a | W.a. (89) Other (11) | 2.51 ± 0.20 | <1 2 |
| 2 | 2.19 ± 0.15 a | K.a. (64) W.a. (36) | 3.33 ± 0.38 | <1 |
| 3 | 2.31 ± 0.11 a | K.a. (50) W.a. (40) C.b. (10) | 2.63 ± 0.24 | <1 |
| 4 | 2.44 ± 0.37 a | C.b. (50) Other (50) | <1 | <1 |
| 5 | 2.15 ± 0.17 a | C.b. (70) Other (30) | <1 | <1 |
| 6 | 2.10 ± 0.15 a | C.b. (75) Other (25) | <1 | <1 |
| 7 | 1.90 ± 0.20 a | n.d. | <1 | <1 |
| 8 | 1.85 ± 0.21 a | n.d. | <1 | <1 |
| 9 | 1.90 ± 0.19 a | n.d. | <1 | <1 |
| 10 | 1.89 ± 0.13 a | n.d. | <1 | <1 |
| 11 | 3.52 ± 0.92 b | C.b. (98) S.c. (2) | <1 | <1 |
| 12 | 3.50 ± 0.19 b | S.c. (70) C.b. (30) | <1 | <1 |
| 13 | 3.48 ± 0.10 b | S.c. (75) C.b. (25) | <1 | <1 |
| 14 | 3.40 ± 0.11 b | S.c. (77) C.b. (23) | <1 | <1 |
| 15 | 3.42 ± 0.20 b | S.c. (80) C.b. (20) | <1 | <1 |
| 16 | 3.43 ± 0.19 b | S.c. (98) C.b. (2) | <1 | <1 |
| 17 | 3.37 ± 0.15 b | S.c. (100) | <1 | <1 |
| 18 | 4.17 ± 0.26 c | S.c. (100) | <1 | <1 |
| 19 | 4.49 ± 0.23 c | S.c. (100) | <1 | <1 |
| 20 | 4.10 ± 0.10 c | S.c. (100) | <1 | <1 |
| 21 | 4.30 ± 0.20 c | S.c. (100) | <1 | <1 |
| 22 | 4.17 ± 0.18 c | S.c. (100) | <1 | <1 |
| 23 | 4.44 ± 0.22 c | S.c. (100) | <1 | <1 |
| 24 | 4.27 ± 0.93 c | S.c. (98) C.b. (2) | <1 | <1 |
| 25 | 4.48 ± 0.15 c | S.c. (100) | <1 | <1 |
| Days | pH | Free CO2 (g kg−1) | Total Polar Phenols (mg CAE mL−1) |
|---|---|---|---|
| 1 | 2.25 ± 0.09 a | 1.22 ± 0.09 a | 0.09 ± 0.00 a |
| 2 | 2.32 ± 0.04 a | 1.26 ± 0.07 a | 0.17 ± 0.02 a |
| 3 | 2.42 ± 0.03 a | 1.29 ± 0.05 a | 0.33 ± 0.05 a |
| 4 | 2.56 ± 0.08 ab | 1.39 ± 0.09 a | 0.37 ± 0.03 a |
| 5 | 2.65 ± 0.06 ab | 1.48 ± 0.10 a | 0.49 ± 0.02 a |
| 6 | 3.00 ± 0.06 b | 1.48 ± 0.11 a | 0.57 ± 0.06 a |
| 7 | 3.06 ± 0.07 b | 1.49 ±0.06 a | 0.59 ± 0.07 ab |
| 8 | 3.06 ± 0.06 b | 1.49 ± 0.25 a | 0.69 ± 0.05 ab |
| 9 | 3.07 ± 0.01 b | 1.54 ± 0.12 a | 0.98 ± 0.08 b |
| 10 | 3.17 ± 0.05 b | 1.59 ± 0.06 a | 1.08 ± 0.05 b |
| 11 | 3.31 ± 0.01 bc | 1.62 ± 0.07 ab | 1.15 ± 0.06 b |
| 12 | 3.37 ± 0.02 bc | 1.70 ± 0.03 ab | 1.38 ± 0.01 b |
| 13 | 3.37 ± 0.03 bc | 1.72 ± 0.06 ab | 1.50 ± 0.07 b |
| 14 | 3.40 ± 0.02 bc | 1.76 ± 0.08 ab | 1.66 ± 0.08 b |
| 15 | 3.45 ± 0.04 bc | 1.80 ± 0.04 ab | 1.72 ± 0.03 bc |
| 16 | 3.50 ± 0.13 c | 1.82 ± 0.03 ab | 1.88 ± 0.05 bc |
| 17 | 3.74 ± 0.09 c | 1.84 ± 0.11 b | 1.88 ± 0.01 bc |
| 18 | 3.78 ± 0.00 c | 1.85 ± 0.09 b | 1.89 ± 0.00 bc |
| 19 | 3.80 ± 0.03 c | 1.88 ± 0.06 b | 1.91 ± 0.02 c |
| 20 | 3.84 ± 0.01 c | 1.88 ± 0.05 b | 1.92 ± 0.05 c |
| 21 | 3.86 ± 0.07 cd | 1.89 ± 0.09 b | 1.94 ± 0.01 c |
| 22 | 3.88 ± 0.06 cd | 1.90 ± 0.13 b | 1.94 ± 0.07 c |
| 23 | 3.89 ± 0.04 cd | 1.85 ± 0.05 b | 1.96 ± 0.06 c |
| 24 | 3.91 ± 0.05 d | 1.80 ± 0.11 ab | 1.98 ± 0.02 c |
| 25 | 3.94 ± 0.04 d | 1.74 ± 0.03 ab | 1.99 ± 0.03 c |
| Total Yeasts (Log CFU mL−1) | Preeminent Yeast Species (%) | Total Molds (Log CFU mL−1) | TAB (Log CFU mL−1) | TANB (Log CFU mL−1) | Enterobacteria (Log CFU mL−1) |
|---|---|---|---|---|---|
| 6.31 ± 0.16 | C.b. (80) Others (20) | <1 1 | <1 | <1 | <1 |
| Days | Total Yeasts (Log CFU mL−1) | Preeminent Yeast Species (%) | Total Molds (Log CFU mL−1) | TAB (Log CFU mL−1) | Other Bacteria 1 |
|---|---|---|---|---|---|
| 3 | 5.10 ± 0.16 a | C.b. (76) S.c. (22) W.a. (2) | <1 2 | 4.66 ± 0.50 a | <1 |
| 4 | 5.66 ± 0.12 ab | S.c. (58) C.b. (42) | <1 | 4.70 ± 0.23 a | <1 |
| 5 | 6.07 ± 0.08 b | S.c. (84) C.b. (16) | <1 | 4.74 ± 0.64 a | <1 |
| 6 | 6.21 ± 0.17 b | S.c. (98) C.b. (2) | <1 | 4.88 ± 0.55 ab | <1 |
| 9 | 6.44 ± 0.21 b | S.c. (86) C.b. (14) | <1 | 5.63 ± 0.53 b | <1 |
| 10 | 6.29 ± 0.19 b | S.c. (90) C.b. (10) | <1 | 5.33 ± 0.83 b | <1 |
| 11 | 6.19 ± 0.13 b | S.c. (90) C.b. (10) | <1 | 5.72 ± 0.18 b | <1 |
| 12 | 6.15 ± 0.27 b | S.c. (98) C.b. (2) | <1 | 5.08 ± 0.47 b | <1 |
| 16 | 6.26 ± 0.26 b | S.c. (100) | <1 | 5.60 ± 0.33 b | <1 |
| 18 | 6.37 ± 0.07 b | S.c. (100) | <1 | 5.74 ± 0.17 b | <1 |
| 20 | 6.48 ± 0.11 b | S.c. (98) C.b. (2) | <1 | 4.40 ± 0.12 a | <1 |
| 23 | 6.55 ± 0.11 b | S.c. (98) C.b. (2) | <1 | 4.09 ± 0.12 a | <1 |
| 50 | 6.31 ± 0.18 b | S.c. (100) | <1 | 4.77 ± 0.36 a | <1 |
| 57 | 6.24 ± 0.09 b | S.c. (100) | <1 | 4.22 ± 0.91 a | <1 |
| 64 | 6.33 ± 0.06 b | S.c. (92) C.b. (8) | <1 | <1 | <1 |
| 71 | 5.98 ± 0.27 ab | S.c. (100) | <1 | <1 | <1 |
| 85 | 5.75 ± 0.26 ab | S.c. (100) | <1 | <1 | <1 |
| 180 | 5.48 ± 0.20 a | S.c. (100) | <1 | <1 | <1 |
| Tests | Observations |
|---|---|
| Shape | Coccus |
| Gram’s stain | + |
| Growth at 45 °C | − |
| Growth at 15 °C | + |
| Growth with 4.0% (w v−1) NaCl | + |
| Growth with 6.5% (w v−1) NaCl | − |
| Catalase test | − |
| Gas from glucose | + |
| NH3 production | − |
| NO3 reduction | − |
| Dextran production | + |
| Litmus milk coagulation | + |
| Fermentation of: | |
| maltose | + |
| fructose | + |
| trehalose | + |
| raffinose | +/− |
| lactose | +/− |
| arabinose | + |
| Days | pH | Free CO2 (g kg−1) | Total Polar Phenols (mg CAE mL−1) |
|---|---|---|---|
| 1 | 3.71 ± 0.05 a | 1.62 ± 0.14 a | 0.11 ± 0.02 a |
| 2 | 3.75 ± 0.01 a | 1.67 ± 0.16 a | 0.20 ± 0.08 a |
| 3 | 3.79 ± 0.04 a | 1.71 ± 0.12 a | 0.36 ± 0.07 a |
| 4 | 3.83 ± 0.10 a | 1.79 ± 0.03 a | 0.40 ± 0.01 a |
| 5 | 3.92 ± 0.04 ab | 1.88 ± 0.21 b | 0.52 ± 0.05 ab |
| 6 | 3.82 ± 0.03 a | 1.92 ± 0.07 b | 0.60 ± 0.03 ab |
| 9 | 3.92 ± 0.00 ab | 2.02 ± 0.03 b | 1.08 ± 0.06 b |
| 10 | 3.89 ± 0.04 ab | 1.99 ± 0.13 b | 1.12 ± 0.09 b |
| 11 | 3.82 ± 0.01 a | 1.97 ± 0.08 b | 1.20 ± 0.05 b |
| 12 | 3.88 ± 0.04 ab | 1.95 ± 0.07 b | 1.42 ± 0.04 b |
| 16 | 4.03 ± 0.12 b | 1.96 ± 0.13 b | 1.91 ± 0.04 bc |
| 18 | 4.08 ± 0.10 b | 1.84 ± 0.11 b | 1.93 ± 0.06 bc |
| 20 | 4.10 ± 0.09 b | 1.72 ± 0.09 a | 1.95 ± 0.01 bc |
| 23 | 4.16 ± 0.06 b | 1.68 ± 0.23 a | 1.98 ± 0.05 bc |
| 50 | 4.44 ± 0.01 b | 1.72 ± 0.48 a | 2.25 ± 0.10 c |
| 57 | 4.44 ± 0.02 b | 1.79 ± 0.23 a | 2.32 ± 0.09 c |
| 64 | 4.46 ± 0.03 b | 1.91 ± 0.15 b | 2.39 ± 0.11 c |
| 71 | 4.47 ± 0.05 b | 1.86 ± 0.08 bc | 2.46 ± 0.01 cd |
| 85 | 4.47 ± 0.08 b | 1.84 ± 0.16 bc | 3.02 ± 0.11 d |
| 180 | 4.47 ± 0.12 b | 1.80 ± 0.13 a | 3.57 ± 0.08 d |
| Total Yeasts (Log CFU g−1) | Total Molds (Log CFU g−1) | Enterobacteria (Log CFU g−1) | TAB (Log CFU g−1) | TANB (Log CFU g−1) |
|---|---|---|---|---|
| <1 1 | <1 | 6.53 ± 0.16 | 8.02 ± 0.26 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zullo, B.A.; Ciafardini, G. CO2 and Acidification of Low-Salt Brine Promote Some Yeasts and Penalize Bacteria in Naturally Brined Black Table Olive Fermentation. Foods 2025, 14, 4062. https://doi.org/10.3390/foods14234062
Zullo BA, Ciafardini G. CO2 and Acidification of Low-Salt Brine Promote Some Yeasts and Penalize Bacteria in Naturally Brined Black Table Olive Fermentation. Foods. 2025; 14(23):4062. https://doi.org/10.3390/foods14234062
Chicago/Turabian StyleZullo, Biagi Angelo, and Gino Ciafardini. 2025. "CO2 and Acidification of Low-Salt Brine Promote Some Yeasts and Penalize Bacteria in Naturally Brined Black Table Olive Fermentation" Foods 14, no. 23: 4062. https://doi.org/10.3390/foods14234062
APA StyleZullo, B. A., & Ciafardini, G. (2025). CO2 and Acidification of Low-Salt Brine Promote Some Yeasts and Penalize Bacteria in Naturally Brined Black Table Olive Fermentation. Foods, 14(23), 4062. https://doi.org/10.3390/foods14234062




