Effects of Crushing, Vacuum Nano-Collision, and Steam Explosion on the Flavor and Physical Properties of Solid Spices
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Reagents
2.2. Major Instruments and Equipment
2.3. Materials and Methods
2.3.1. Vacuum Nano-Collision Method
2.3.2. Steam Explosion Treatment
2.3.3. Natural Solid Spice Treatment Method
2.3.4. Moisture Content Analysis of Vacuum Nano-Collision Treated Spices
2.3.5. Solubility Analysis of Spices
2.3.6. Determination of Spice Particle Size
2.3.7. GC-MS Analysis
Sample Preparation
GC-MS Chromatographic Conditions
Mass Spectrometry Conditions
Analysis of Volatile Flavor Compounds
2.3.8. HPLC-MS Analysis
Preparation of Sample Solution
Preparation of Standard Solution
Chromatographic Conditions
Mass Spectrometry Conditions
2.4. Electronic Tongue Analysis of Braised Chicken with Spices
Sample Preparation of Chicken Meat
2.5. Data Analysis
3. Results and Discussion
3.1. Impact of Processing Methods on Spice Physical Properties
3.1.1. Analysis of Moisture Content in Spices
3.1.2. Analysis of Spice Solubility
3.1.3. Analysis of Spice Particle Size
3.2. Analysis of Volatile Compounds in Spices
3.3. Effects of Processing Methods on Key Volatile Compounds of Spices
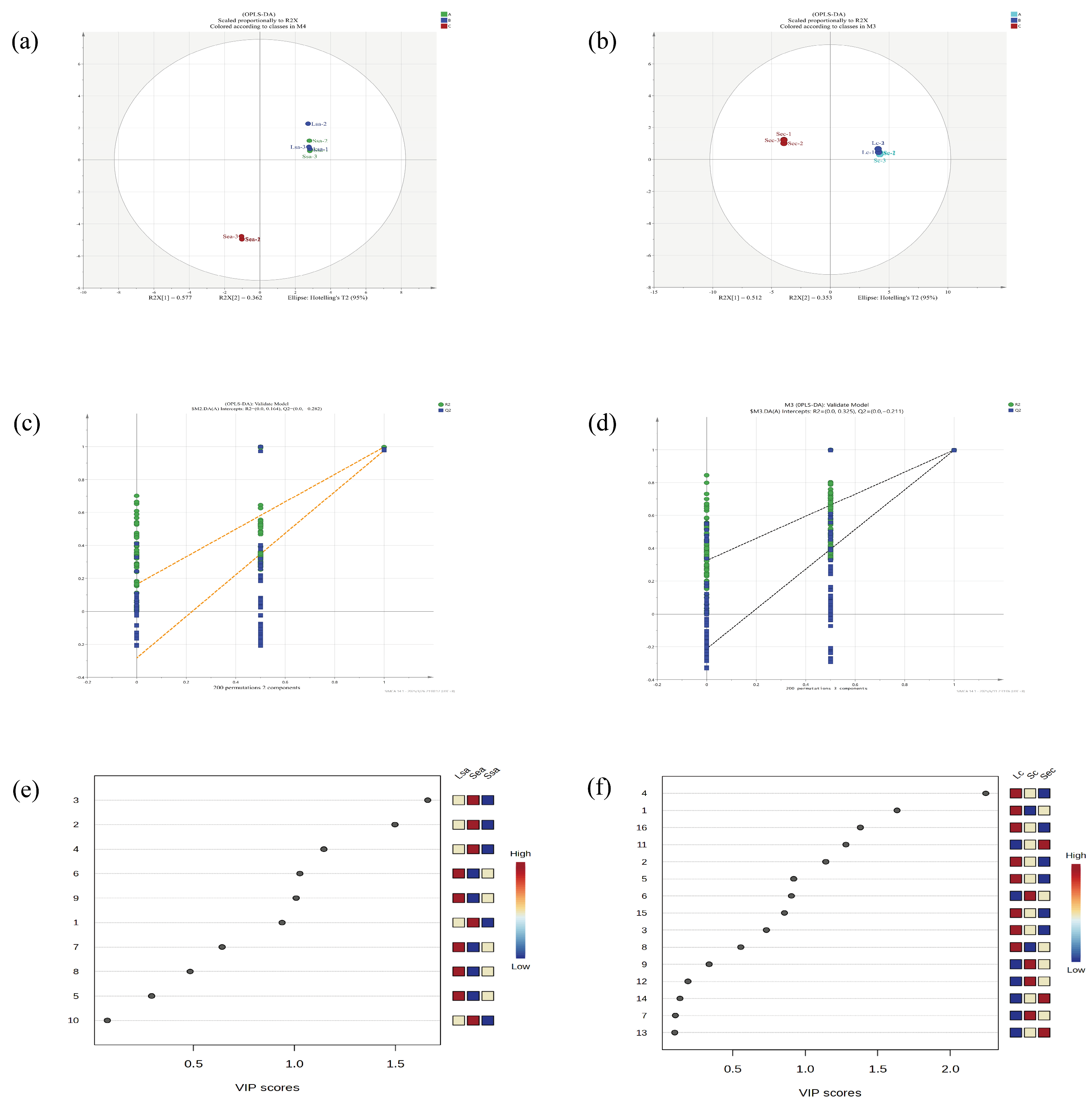
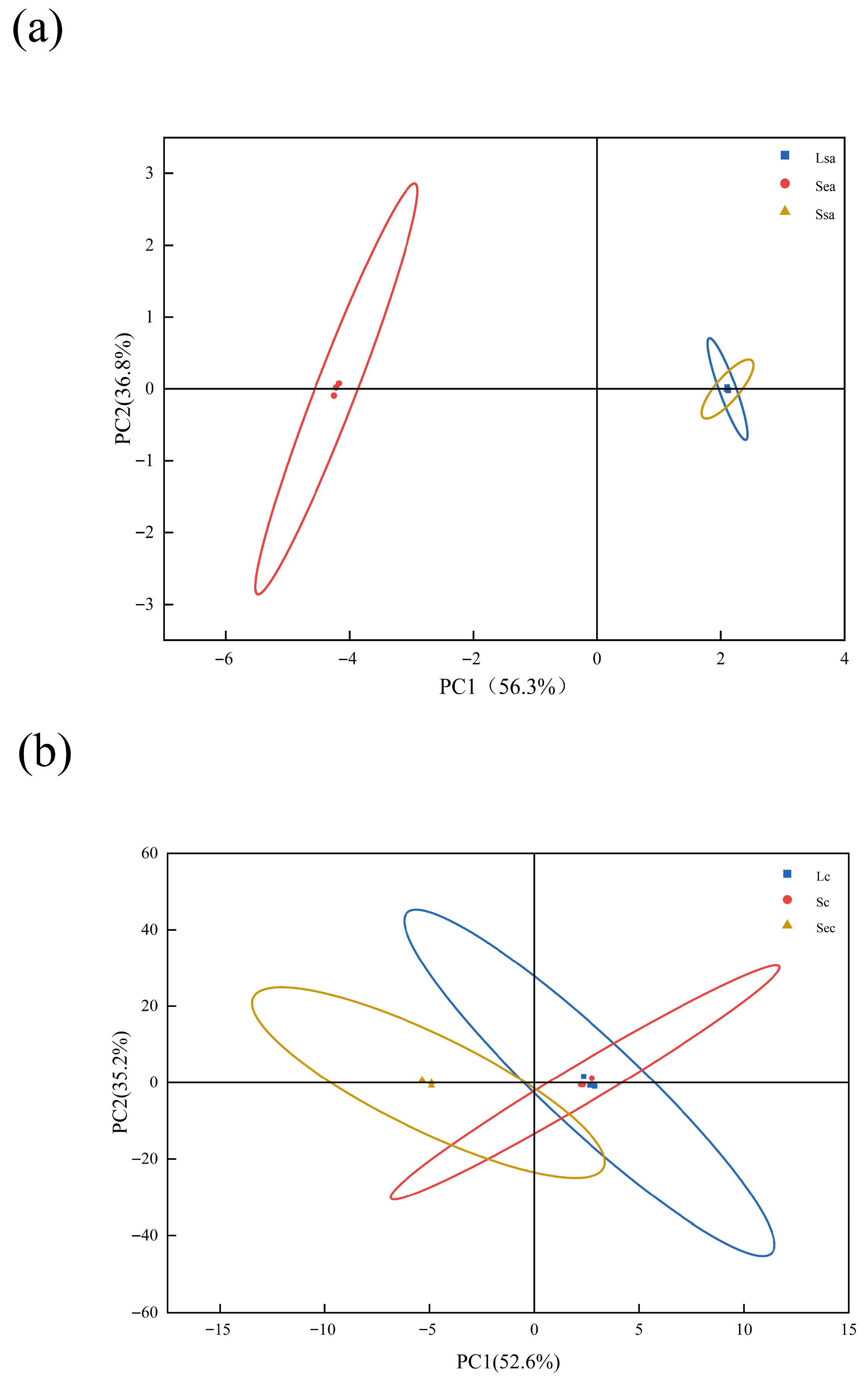
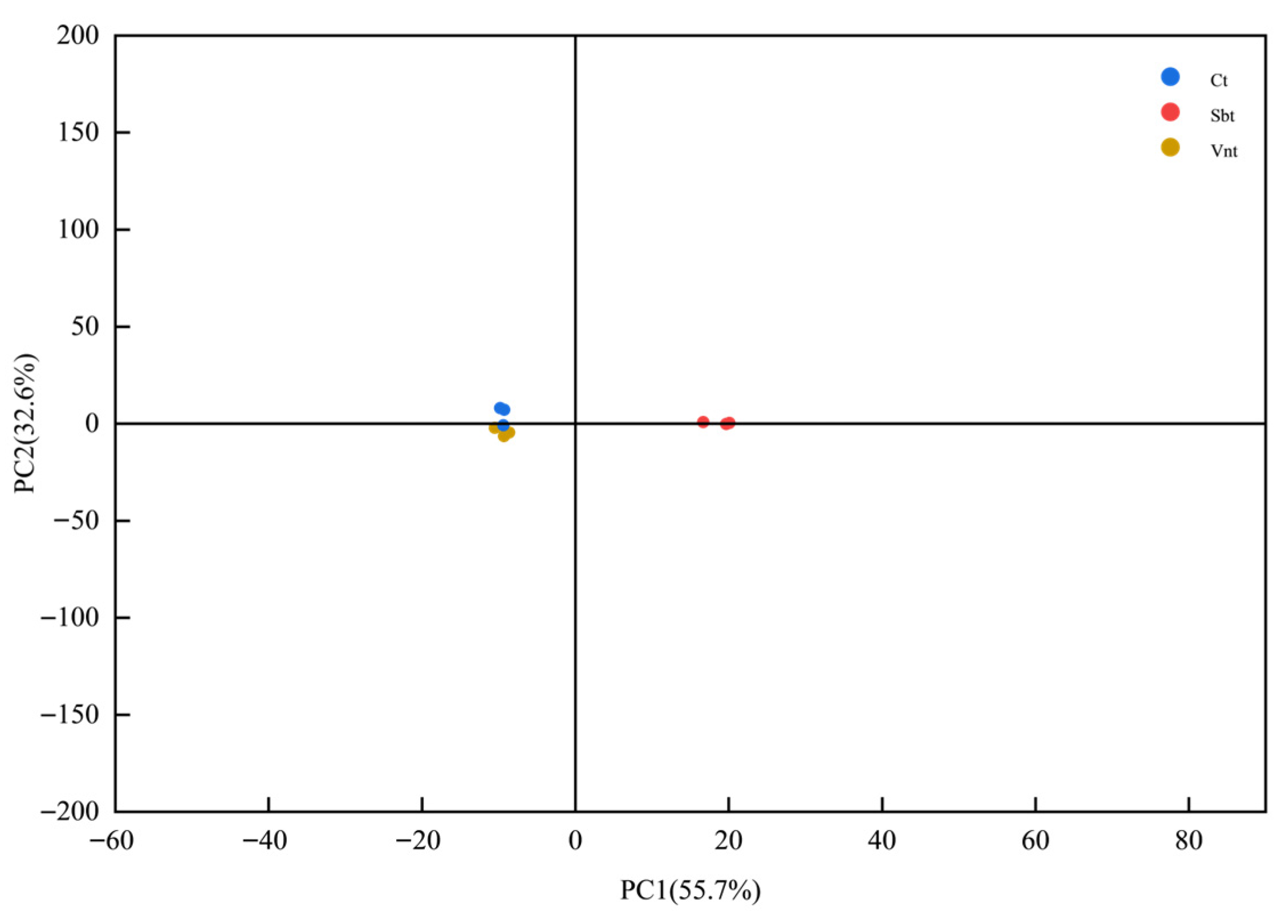
3.4. PCA-Based Analysis of Major Compound Differences in Spices
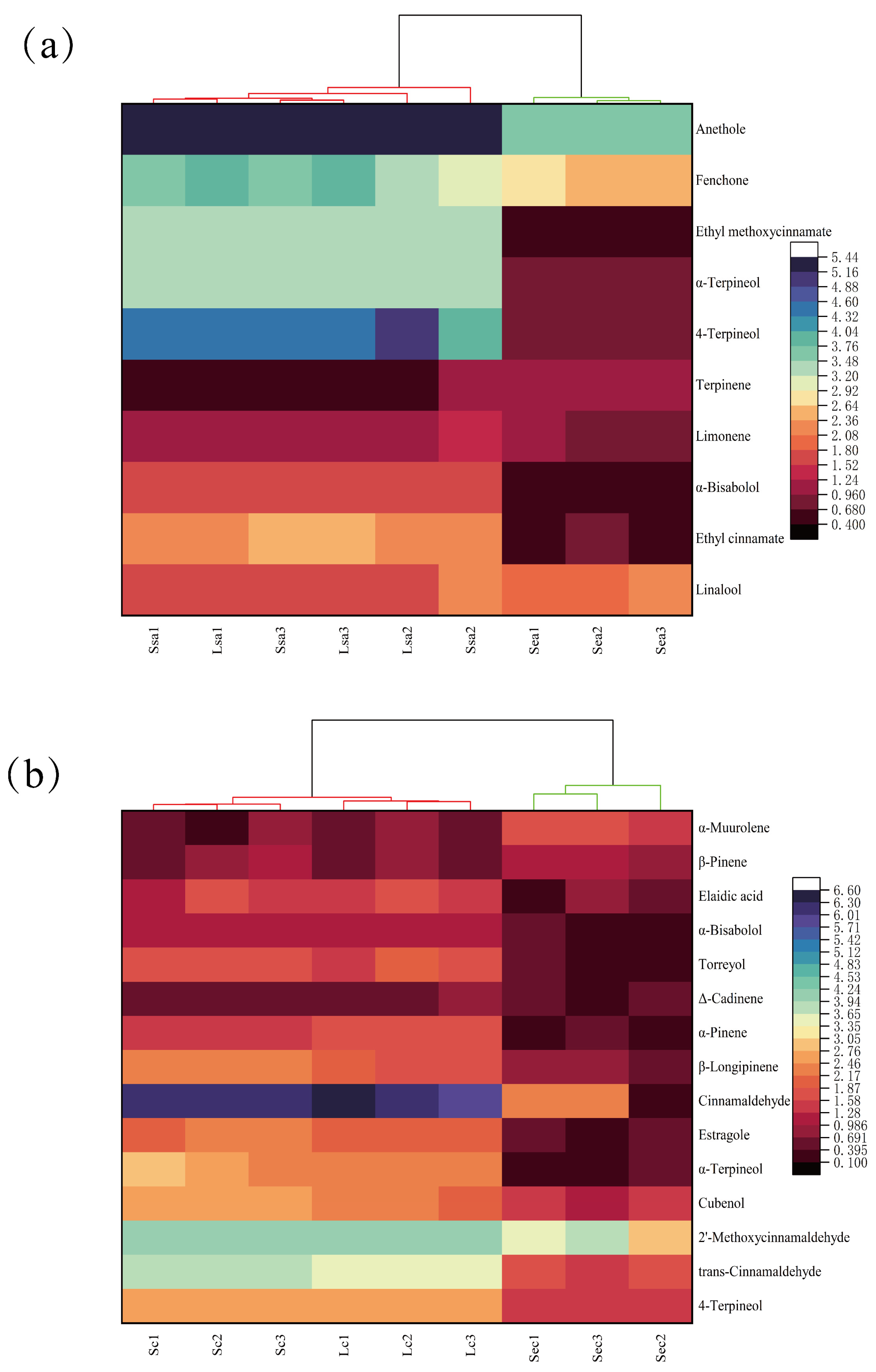
3.5. Heatmap Clustering of Key Flavor Compounds Across Processing Methods
3.6. Analysis of Major Bioactive Component Contents in Spices
3.7. E-Tongue Assessment of Spice-Braised Chicken Thighs Under Three Treatment Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Flavor Compound Name | Flavor Description | Threshold | OAV | ||
|---|---|---|---|---|---|
| Sc | Lc | Sec | |||
| Eugenol | Spicy, sweet, woody aroma | 2 | 4.8072 ± 0.0006 b | 4.3780 ± 0.0007 b | 19.2155 ± 0.0011 a |
| 4-Methoxycinnamaldehyde | Woody, citrus aroma | 0.11 | 29.0618 ± 0.0017 a | 25.1679 ± 0.0014 ab | 13.2037 ± 0.0011 b |
| trans-Cinnamaldehyde | Spicy, medicinal, woody bitterness | 2 | 36.4698 ± 0.0025 a | 34.4034 ± 0.0023 a | 16.9234 ± 0.0008 b |
| 2′-Methoxycinnamaldehyde | Sweet-spicy, meaty, woody | 7 | 41.6396 ± 0.0029 a | 40.5837 ± 0.0028 a | 35.9715 ± 0.0024 b |
| Bicyclogermacrene | Woody, spicy, bitter-medicinal | 3 | 25.8372 ± 0.0015 a | 22.0907 ± 0.0014 ab | 14.6342 ± 0.0009 b |
| α-Pinene | Woody, spicy, bitter-medicinal | 2.75 | 27.8412 ± 0.0017 a | 23.9473 ± 0.018 ab | / |
| Myristicin | Piney, camphor, minty | 0.075 | 20.8179 ± 0.0018 a | 21.2672 ± 0.0019 a | / |
| Cinnamic alcohol | Piney, camphor, minty | 2.39 | 638.674 ± 0.0035 a | 624.872 ± 0.0032 a | 220.365 ± 0.0012 b |
| β-Caryophyllene | Minty aroma | 0.4 | 23.7860 ± 0.0018 a | 19.3389 ± 0.0009 ab | / |
| α-Phellandrene | Cinnamic, spicy, woody | 0.41 | 15.7506 ± 0.0011 a | 16.8464 ± 0.0012 a | 10.3582 ± 0.0008 b |
| α-Cubebene | Bitter aroma | 3.6 | 24.4993 ± 0.0015 a | 25.3015 ± 0.0019 a | / |
| trans-β-Ocimene | Woody, spicy, floral | 3 | 16.1548 ± 0.0015 a | 13.8214 ± 0.0014 b | / |
| α-Bisabolol | Woody, spicy, medicinal | 3 | 10.1464 ± 0.0009 a | 11.6848 ± 0.0010 a | / |
| trans-Isoeugenol | Spicy, sweet, acidic-floral | 0.039 | 12.7422 ± 0.0011 ab | 14.8718 ± 0.0012 a | / |
| α-Curcumene | Fruity, sweet, floral aroma | 9.01 | / | / | 16.5327 ± 0.0014 a |
Appendix B
| Flavor Compound Name | Flavor Description | Threshold | OAV | ||
|---|---|---|---|---|---|
| Ssa | Lsa | Sea | |||
| Linalool | Floral, woody aroma | 2.38 | 12.1504 ± 0.0016 a | 13.7321 ± 0.0015 a | 7.5640 ± 0.0008 b |
| Methyl chavicol | Sweet, herbal, bitter | 1 | 498.8312 ± 0.0031 ab | 512.738 ± 0.0036 a | 331.272 ± 0.0025 b |
| Myrtenol | Piney, spicy | 0.2 | 37.6230 ± 0.0027 a | 35.7100 ± 0.0026 ab | / |
| α-Terpineol | Woody pine aroma | 2.75 | 12.3387 ± 0.0013 ab | 14.0569 ± 0.0014 a | / |
| Cinnamyl acetate | Cinnamic, floral, spicy aroma | 3.6 | 22.0761 ± 0.0027 a | / | / |
| 4-Terpineol | Minty, woody, herbal | 1.1 | 32.2828 ± 0.0023 ab | 34.5754 ± 0.0026 a | 30.1526 ± 0.0019 ab |
| α-Bisabolol | Woody, spicy, herbal-medicinal aroma | 3 | 15.7750 ± 0.0016 ab | 17.2610 ± 0.0013 a | / |
| Terpinolene | Piney, woody aroma | 1 | 5.6103 ± 0.004 b | 6.3587 ± 0.007 b | 12.0069 ± 0.0012 a |
| Citral | Citrus, herbal aroma | 5 | 9.0810 ± 0.0008 ab | 11.7271 ± 0.0012 a | / |
| Methyl cinnamate | Spicy, sweet, herbal | 5 | 41.7452 ± 0.0032 a | 39.1275 ± 0.0023 a | / |
References
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [PubMed]
- Djiazet, S.; Kenfack, L.B.M.; Ngangoum, E.S.; Nzali, H.G.; Tchiegang, C. Indigenous spices consumed in the food habits of the populations living in some countries of Sub-Saharan Africa: Utilisation value, nutritional and health potentials for the development of functional foods and drugs: A review. Food Res. Int. 2022, 157, 111280. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ren, X.; Li, Y.; Mao, L. The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. Lwt-Food Sci. Technol. 2019, 101, 25–31. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control 2018, 92, 399–411. [Google Scholar] [CrossRef]
- Huang, Y.; Pu, D.; Hao, Z.; Liang, L.; Zhao, J.; Tang, Y.; Zhang, Y. Characterization of Taste Compounds and Sensory Evaluation of Soup Cooked with Sheep Tail Fat and Prickly Ash. Foods 2022, 11, 896. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Sun, L.; Chen, J.; Li, M.; Liu, Y.; Zhao, G. Effect of Star Anise (Illicium verum) on the Volatile Compounds of Stewed Chicken. J. Food Process Eng. 2014, 37, 131–145. [Google Scholar] [CrossRef]
- Qin, Y.-X.; Cal, D.-D.; Zhang, D.-N.; Liu, Y.; Lai, K.-Q. Characteristics of volatile flavor components in stewed meat and meat broths prepared with repeatedly used broths containing star anise. J. Food Meas. Charact. 2020, 14, 557–572. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Saldanha, T.; Soares, R.A.M.; Torres, E.A.F.S. Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chem. 2012, 135, 1383–1390. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Xing, J.; Yang, C.; Zhang, L. Characterization of key flavor compounds in cinnamon bark oil extracts using principal component analysis. Food Res. Int. 2025, 200, 115446. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Zhang, M.; Chen, J.; He, Z.; Qin, F.; Xu, Z.; Cao, D.; Chen, J. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018, 239, 111–118. [Google Scholar] [CrossRef]
- Zhong, Q.; Xing, Z.; Teng, F.; Wu, T.; Pan, S.; Xu, X. Evaluation of the aroma and taste contributions of star anise (I. Verum hook. f.) in braised duck leg via flavor omics combined with multivariate statistics. Food Res. Int. 2024, 184, 114209. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, N.; Rosiak, A.; Kaluzna-Czaplinska, J. The Potential Role of Cinnamon in Human Health. Forests 2021, 12, 648. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Ma, Y.Y.; Zhang, J.Y.; Li, M.Y.; Yan, L.G.; Zhao, G.M.; Liu, Y.X.; Zhang, Y.Y. The inhibitory effects of spice essential oils and rapidly prediction on the growth of Clostridium perfringens in cooked chicken breast. Food Control 2020, 113, 106978. [Google Scholar] [CrossRef]
- Mohammadi-Moghaddam, T.; Razavi, S.M.A.; Taghizadeh, M.; Pradhan, B.; Sazgarnia, A.; Shaker-Ardekani, A. Hyperspectral imaging as an effective tool for prediction the moisture content and textural characteristics of roasted pistachio kernels. J. Food Meas. Charact. 2018, 12, 1493–1502. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhu, F. Physicochemical properties of quinoa starch. Carbohydr. Polym. 2016, 137, 328–338. [Google Scholar] [CrossRef]
- Long, Z.; Zhao, M.; Liu, N.; Liu, D.; Sun-Waterhouse, D.; Zhao, Q. Physicochemical properties of peanut oil-based diacylglycerol and their derived oil-in-water emulsions stabilized by sodium caseinate. Food Chem. 2015, 184, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, Y.; Wang, Z.; Akhtar, K.H.; Saleh, A.S.M.; Li, W.; Zhang, D. Insights into flavor formation of braised chicken: Based on E-nose, GC–MS GC-IMS, and UPLC-Q-Exactive-MS/MS. Food Chem. 2024, 448, 138972. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Duan, J.; Wang, B.; Ma, T.; Jiang, Y.; Zhang, B. Discrimination and characterization of the volatile organic compounds in red and black raspberry wines fermented with different commercial Saccharomyces cerevisiae: An integrated analysis using E-nose, GC-MS, GC-IMS, and multivariate statistical models. Food Chem. 2025, 478, 143678. [Google Scholar] [CrossRef]
- Jia, Z.; Shi, C.; Wang, Y.; Yang, X.; Zhang, J.; Ji, Z. Nondestructive determination of salmon fillet freshness during storage at different temperatures by electronic nose system combined with radial basis function neural networks. Int. J. Food Sci. Technol. 2020, 55, 2080–2091. [Google Scholar] [CrossRef]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef]
- van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter: Zeist, The Netherlands, 2003. [Google Scholar]
- Buttery, R.G. Flavor Chemistry and Odor Thresholds. In Flavor Chemistry: Thirty Years of Progress; Teranishi, R., Wick, E.L., Hornstein, I., Eds.; Springer: Boston, MA, USA, 1999; pp. 353–365. [Google Scholar]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. Lwt-Food Sci. Technol. 2021, 140, 110764. [Google Scholar] [CrossRef]
- Han, D.; Zhang, C.-H.; Fauconnier, M.-L. Effect of Seasoning Addition on Volatile Composition and Sensory Properties of Stewed Pork. Foods 2021, 10, 83. [Google Scholar] [CrossRef]
- Prado, R.; Hartung, A.C.M.; Gastl, M.; Becker, T. Identification of potential odorant markers to monitor the aroma formation in kilned specialty malts. Food Chem. 2022, 392, 133251. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Mei, X.; Wang, Z.; Chen, X.; Zhang, R.; Chen, Q.; Kan, J. Comprehensive identification of non-volatile bitter-tasting compounds in Zanthoxylum bungeanum Maxim. by untargeted metabolomics combined with sensory-guided fractionation technique. Food Chem. 2021, 347, 129085. [Google Scholar] [CrossRef]
- Zhan, F.; Sun, L.; Zhao, G.; Li, M.; Zhu, C. Multiple Technologies Combined to Analyze the Changes of Odor and Taste in Daokou Braised Chicken during Processing. Foods 2022, 11, 963. [Google Scholar] [CrossRef]
- Zhao, X.; Du, F.; Zhu, Q.; Qiu, D.; Yin, W.; Ao, Q. Effect of superfine pulverization on properties of Astragalus membranaceus powder. Powder Technol. 2010, 203, 620–625. [Google Scholar] [CrossRef]
- Matheis, K.; Granvogl, M. Characterization of Key Odorants Causing a Fusty/Musty Off-Flavor in Native Cold-Pressed Rapeseed Oil by Means of the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 8168–8178. [Google Scholar] [CrossRef]
- Chen, F.; Shen, L.; Shi, X.; Deng, Y.; Qiao, Y.; Wu, W.; Xiong, G.; Wang, L.; Li, X.; Ding, A.; et al. Characterization of flavor perception and characteristic aroma of traditional dry-cured fish by flavor omics combined with multivariate statistics. Lwt-Food Sci. Technol. 2023, 173, 114240. [Google Scholar] [CrossRef]
- Chen, Y.P.; Li, W.; Yu, Y.; Wang, M.; Blank, I.; Zhang, Y.; Liu, Y. Elucidation of the Impact of Steaming on the Key Odorants of Jinhua Dry-Cured Ham Using the Sensomics Approach. J. Agric. Food Chem. 2023, 71, 4932–4942. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Liu, H.; Zhang, G.; Tu, Y.; Zhao, Y. Changes in physicochemical properties and lipid oxidation lead to the formation of mud on salted egg yolks during storage. Food Chem. 2023, 409, 135341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fu, J.-J.; Mao, J.-L.; Dong, X.-P.; Chen, Y.-W. Lipidomics reveals the relationship between lipid oxidation and flavor formation of basic amnio acids participated Low-Sodium cured large yellow croaker. Food Chem. 2023, 429, 136888. [Google Scholar] [CrossRef] [PubMed]
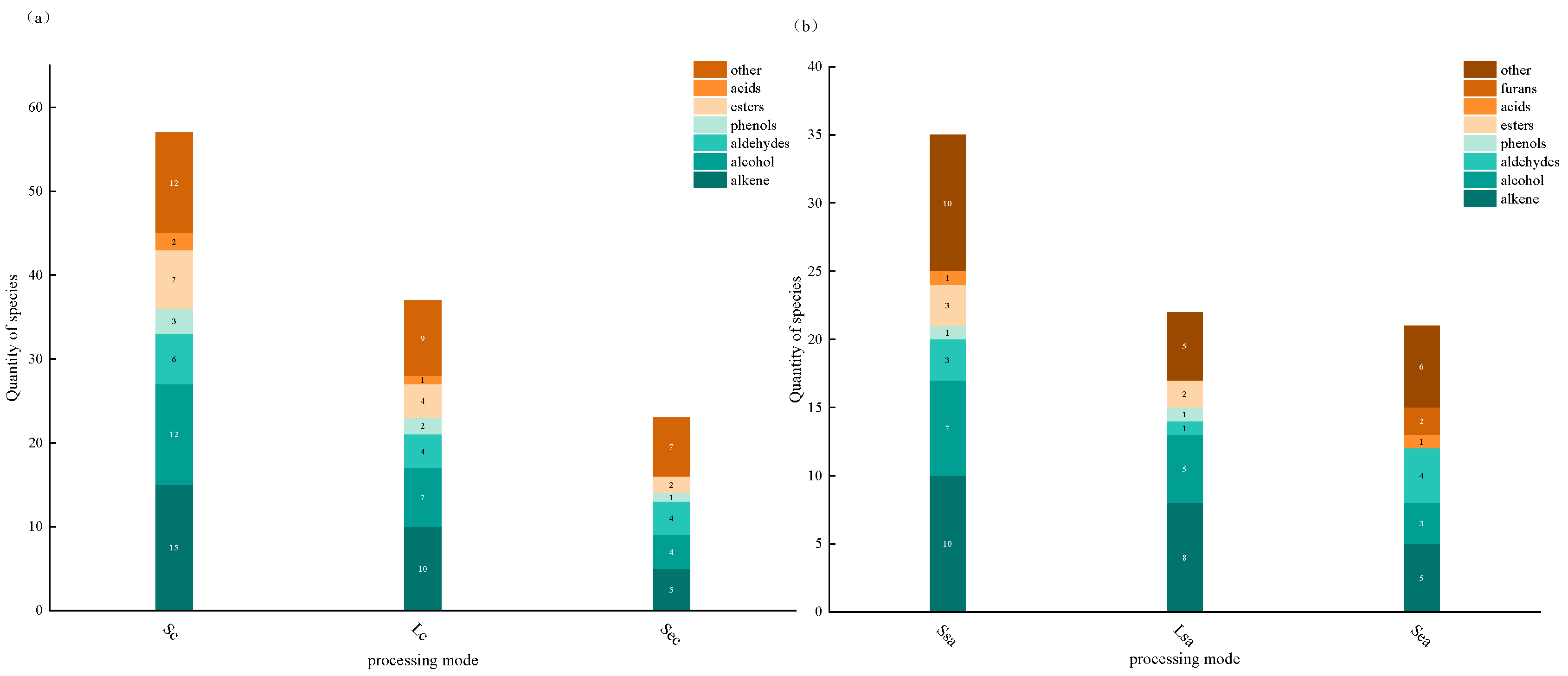
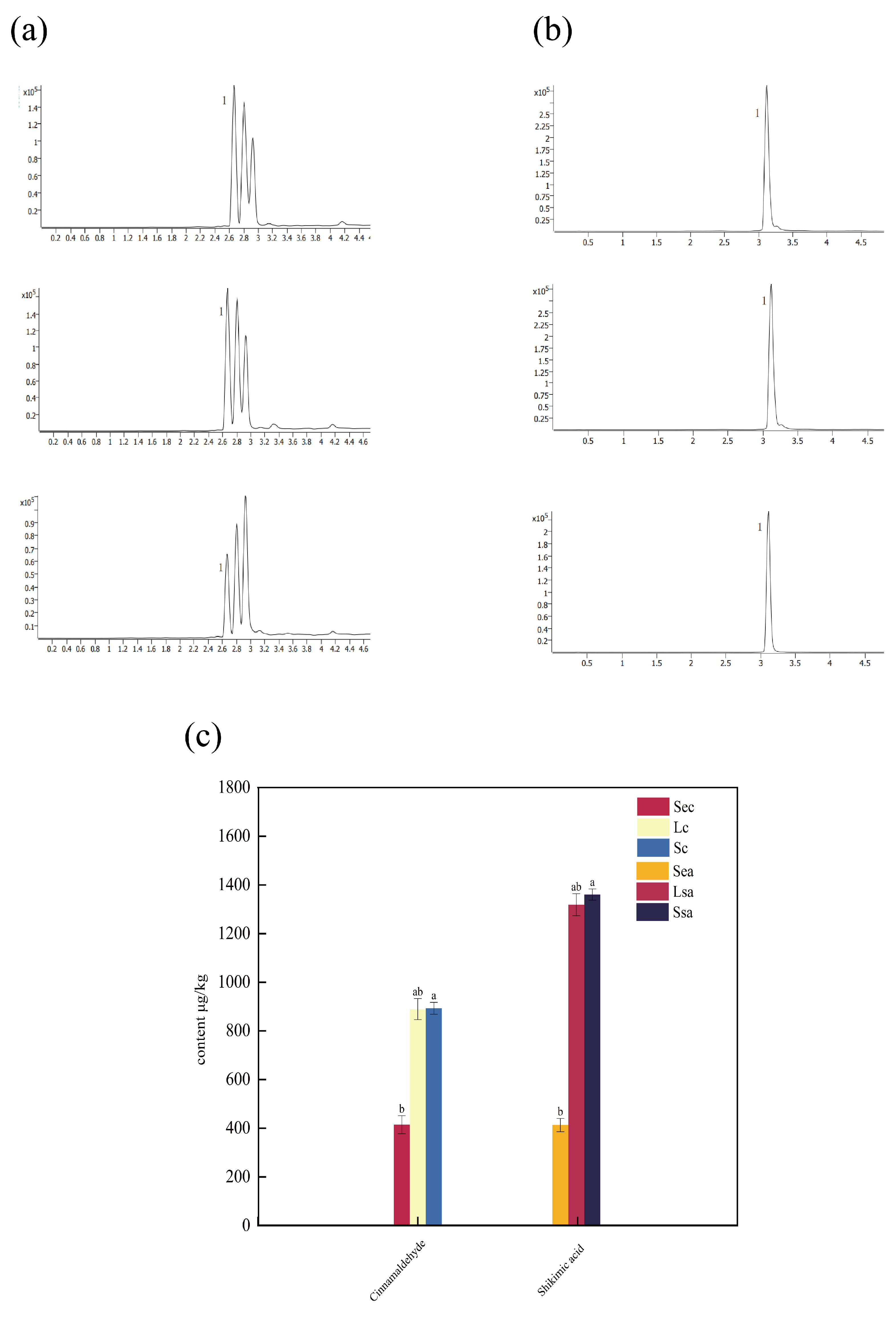
| Various Indicators | The Name of the Spice | |||||
|---|---|---|---|---|---|---|
| Lsa | Ssa | Sea | Lc | Sc | Sec | |
| MC% | 78 ± 2.37 a | 68.63 ± 3.11 b | 75 ± 3.51 a | 66.57 ± 2.66 b | ||
| WSI/% | 64.96 ± 1.12 c | 93.41 ± 2.83 a | 78.36 ± 1.28 b | 93.5 ± 0.71 a | 71.94 ± 2.09 bc | 75.37 ± 2.29 b |
| GS/nm | 13.65 ± 0.22 c | 879.33 ± 3.37 b | 10.40 ± 0.65 c | 935.19 ± 2.56 a | ||
| Flavor Ingredient Name | Concentration (μg/kg) | ||
|---|---|---|---|
| Sc | Lc | Sec | |
| Limonene | 40.02 ± 0.0009 ab | 50.665 ± 0.0009 ab | 3.4465 ± 0.0014 a |
| Eucalyptus oil alcohol | 1.6537 ± 0.0008 b | 1.6537 ± 0.008 b | 8.5000 ± 0.0016 a |
| linalool | 1.5921 ± 0.0006 a | / | / |
| L-camphor | 3.8227 ± 0.0015 a | / | / |
| 2-Ziol | 4.2784 ± 0.0013 a | 2.6359 ± 0.0009 b | 4.8319 ± 0.0013 a |
| Cinnamyl | 9.6144 ± 0.0008 b | 8.7559 ± 0.0012 b | 38.4309 ± 0.042 a |
| Synthetic dextrotron | 2.6359 ± 0.0006 a | / | / |
| 4-terpene alcohols | 3.1968 ± 0.0015 a | 2.768 ± 0.0003 ab | 1.4524 ± 0.0002 b |
| α-terpineol | 76.5634 ± 0.0046 a | 65.8552 ± 0.0037 b | / |
| Myristicin | 1.5613 ± 0.0003 a | 1.5950 ± 0.0002 a | / |
| Trans-cinnamaldehyde | 72.9396 ± 0.0064 a | 68.8068 ± 0.0056 ab | 33.8468 ± 0.0021 b |
| 3-Phenylpropanol | 11.4955 ± 0.0012 a | / | 10.9805 ± 0.0008 a |
| 3-Methoxybenzaldehyde | 0.8736 ± 0.0001 a | / | / |
| Cinnamic alcohol | 1526.4309 ± 0.1037 a | 1493.4441 ± 0.1139 ab | 526.6724 ± 0.0763 b |
| β-Caryophyllene | 9.5144 ± 0.0018 a | 7.7356 ± 0.0013 b | / |
| α-Phellandrene | 6.4577 ± 0.0013 a | 6.9070 ± 0.0011 ab | 4.2469 ± 0.0008 b |
| Eugenol | 9.6144 ± 0.0011 b | 8.7560 ± 0.0015 b | 38.431 ± 0.0024 a |
| (+)-Cyclic Alfalpene | 8.0260 ± 0.0017 b | / | 14.1002 ± 0.0011 a |
| γ-mulene | 9.2908 ± 0.0019 a | / | / |
| Longifoliene | 5.5152 ± 0.0013 b | 8.3227 ± 0.0019 a | 7.8302 ± 0.0016 ab |
| β-Caryophyllene | 6.3813 ± 0.0015 b | 8.3882 ± 0.0017 a | / |
| (±)-β-cobaene | 5.0058 ± 0.0012 a | / | / |
| Γ-juniperene | 7.5718 ± 0.0016 a | / | / |
| α-Rhythmene | 7.5276 ± 0.0018 a | 1.9092 ± 0.0006 b | / |
| Ethyl cinnamate | 13.1002 ± 0.0023 a | / | / |
| Guiaoxin is a form of xylol | 14.3322 ± 0.0027 a | / | / |
| Alpha-cobaene | 7.2007 ± 0.0015 a | / | / |
| α-Eylene | 10.4180 ± 0.0011 a | 4.8114 ± 0,0013 b | / |
| Valencian oranges | 0.8736 ± 0.0001 a | / | / |
| α-Caryophyllene | 16.3934 ± 0.0029 a | / | / |
| Delta-juniperene | 88.1975 ± 0.0067 ab | 91.0855 ± 0.0087 a | / |
| Dehydroleucene | 8.2811 ± 0.0017 b | 42.6684 ± 0.0038 a | / |
| 4′-Methoxycinnamaldehyde | 3.1968 ± 0.0006 a | 2.7685 ± 0.0002 ab | 1.4524 ± 0.0001 b |
| Glycene | 6.9697 ± 0.0013 a | / | / |
| Melaleuca alcohol | 7.6575 ± 0.0015 a | / | / |
| Eggplant enol | 77.5115 ± 0.0065 a | 66.2722 ± 0.0034 b | 43.9026 ± 0.0054 ab |
| T-juniper alcohol | 19.2349 ± 0.0026 a | 16.1339 ± 0.0122 a | / |
| trans-β-Ocimene | 48.4644 ± 0.0033 a | 41.4642 ± 0.0032 ab | / |
| α-Bisabolol | 30.4392 ± 0.0028 b | 35.0544 ± 0.0021 a | / |
| Citron mellow | 27.1747 ± 0.0069 a | 28.9666 ± 0.0031 b | / |
| Bisabolol | 9.6760 ± 0.0012 a | 9.8129 ± 0.0008 a | / |
| eucalyptole enol | 8.1477 ± 0.0014 a | / | / |
| Ethyl p-methoxycinnamate | 2.7395 ± 0.0012 a | 3.6324 ± 0.0013 a | / |
| Dehydrolignolide | 3.4878 ± 0.0015 a | 2.7873 ± 0.0009 a | / |
| trans-Isoeugenol | 0.4969 ± 0.0001 a | 0.5800 ± 0.0002 a | / |
| Methyl trans-9-octadecaenoate | 0.4358 ± 0.0001 a | / | / |
| Phenethyl acetate | 2.7675 ± 0.0013 a | / | / |
| Perillalactone | 2.8908 ± 0.0012 a | / | / |
| Oleptyl | 1.8210 ± 0.0009 a | / | / |
| Trumpet tea alcohol | 5.1833 ± 0.0016 b | 8.6547 ± 0.0019 a | / |
| Green flowers and melaleuca alcohol | 4.4191 ± 0.0013 a | / | / |
| (-)-isolongol | 4.1899 ± 0.0013 a | / | / |
| geraniol | 1.7216 ± 0.0008 a | / | / |
| 1-Indenone | / | 1.4085 ± 0.0007 a | / |
| n-propyl cinnamate | / | 1.4495 ± 0.0005 a | / |
| (+)-Alfalpene | / | 2.9660 ± 0.0009 a | / |
| α-cypressene | / | 2.8388 ± 0.0011 a | / |
| coumarin | / | 41.5196 ± 0.0039 b | 68.2397 ± 0.0056 a |
| Bicyclic geraniene | 77.5116 ± 0.0023 a | 66.2721 ± 0.0019 ab | 43.9026 ± 0.0016 b |
| Alpha-di-dehydrocalamus alem | / | 16.3934 ± 0.0024 a | / |
| mannitol | / | 10.2691 ± 0.00019 a | / |
| cedar alcohol | / | 9.6841 ± 0.0018 a | / |
| Alpha-calamus alcohol | / | 2.7395 ± 0.0011 a | / |
| α pinene | 76.5633 ± 0.0025 a | 65.8551 ± 0.0021 ab | / |
| Isopropyltoluene | / | / | 1.2516 ± 0.0007 a |
| anethole | / | / | 13.5331 ± 0.0008 a |
| Aninene | / | / | 1.3167 ± 0.0007 a |
| α-Curcumene | / | / | 148.9596 ± 0.0159 a |
| Methyl linoleate | / | / | 17.0585 ± 0.0021 a |
| 2′-Methoxycinnamaldehyde | 291.4772 ± 0.0123 a | 284.0859 ± 0.0118 ab | 251.8005 ± 0.0112 b |
| Flavor Ingredient Name | Concentration (μg/kg) | ||
|---|---|---|---|
| Ssa | Lsa | Sea | |
| Cressellin | 1.8329 ± 0.0006 b | 2.1545 ± 0.0009 b | 12.0154 ± 0.0024 a |
| pinene | 19.6048 ± 0.0012 ab | 23.2812 ± 0.0031 a | 14.4500 ± 0.0026 b |
| Cypress | 14.2616 ± 0.0023 a | / | / |
| α-thujagone | 12.1143 ± 0.0021 a | / | / |
| 4-Isopropyltoluene | 9.6621 ± 0.0015 b | 28.1913 ± 0.0038 a | / |
| Citral | 45.4050 ± 0.0056 b | 58.6355 ± 0.0126 a | / |
| Eucalyptus oil alcohol | 38.8992 ± 0.0045 b | 100.1467 ± 0.0089 a | / |
| γ-terpinene | 4.7048 ± 0.0011 c | 68.7413 ± 0.0076 a | 11.0732 ± 0.0021 b |
| linalool | 28.9180 ± 0.0014 ab | 32.6823 ± 0.0041 a | 18.0024 ± 0.0028 b |
| 2-Ziol | 3.6147 ± 0.0012 a | / | / |
| 4-terpineol | 35.5111 ± 0.0016 a | 38.0329 ± 0.0044 a | 33.1679 ± 0.0042 b |
| α-terpineol | 33.9314 ± 0.0043 ab | 38.6565 ± 0.0048 a | / |
| 4-Allyl anisole | 7.9823 ± 0.0089 a | 7.2044 ± 0.0226 a | 8.3013 ± 0.0654 a |
| 3-Methoxybenzaldehyde | 117.1289 ± 0.0108 a | / | / |
| Methyl chavicol | 498.8312 ± 0.031 ab | 512.738 ± 0.036 a | 331.272 ± 0.0025 b |
| α-Lymphatic solene | 58.3498 ± 0.0067 b | 77.8220 ± 0.0061 a | / |
| β-Caryophyllene | 49.8234 ± 0.0056 b | 108.4960 ± 0.0082 a | 56.4314 ± 0.0062 b |
| α-cis-bo-limonene | 23.0367 ± 0.0038 c | 81.0613 ± 0.0067 a | 48.8155 ± 0.0051 b |
| α-Rhythmene | 15.9122 ± 0.0026 a | / | / |
| Cinnamyl acetate | 79.4740 ± 0.0069 a | / | / |
| α-Curcumene | 33.5702 ± 0.0045 a | / | / |
| β-Serene | 81.7368 ± 0.0078 a | / | / |
| γ juniperene | 71.3309 ± 0.0065 a | / | / |
| Delta-juniperene | 89.7035 ± 0.0071 a | 27.6374 ± 0.0031 b | / |
| Elemiol | 13.4937 ± 0.0023 a | / | / |
| Neroli tertiary alcohol | 63.2022 ± 0.0059 a | 17.1422 ± 0.0029 b | / |
| Dianthus plain | 51.5094 ± 0.0042 a | / | / |
| T-ylang ylang alcohol | 48.4736 ± 0.0057 a | / | / |
| Cyclodiene | 59.9278 ± 0.0048 a | / | / |
| α-Bisabolol | 47.325 ± 0.0071 a | 51.783 ± 0.0038 a | / |
| Methyl cinnamate | 208.7260 ± 0.0172 a | 195.6375 ± 0.0098 ab | / |
| palmitic acid | 54.2902 ± 0.0062 a | / | / |
| 3-Cruthene | / | / | 13.4096 ± 0.0026 a |
| terpinlene | 5.6103 ± 0.0006 b | 6.3587 ± 0.0009 b | 12.0069 ± 0.0029 a |
| furfural | / | / | 21.35831 ± 0.0035 a |
| 5-Methylfurfural | / | / | 26.40385 ± 0.0031 a |
| cyclohexene | / | / | 73.23692 ± 0.0082 a |
| m-chlorobenzoic acid | / | / | 11.17162 ± 0.0008 a |
| 3-Methoxybenzaldehyde | / | / | 139.4462 ± 0.0088 a |
| Myrtenol | 7.5246 ± 0.0037 a | 7.1420 ± 0.0025 a | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Zhang, D.; Liu, Y.; Zhu, Y.; Li, M.; Zhao, L.; Dong, F.; Zhao, G.; Hong, N.; Liu, S.; et al. Effects of Crushing, Vacuum Nano-Collision, and Steam Explosion on the Flavor and Physical Properties of Solid Spices. Foods 2025, 14, 4010. https://doi.org/10.3390/foods14234010
Chen K, Zhang D, Liu Y, Zhu Y, Li M, Zhao L, Dong F, Zhao G, Hong N, Liu S, et al. Effects of Crushing, Vacuum Nano-Collision, and Steam Explosion on the Flavor and Physical Properties of Solid Spices. Foods. 2025; 14(23):4010. https://doi.org/10.3390/foods14234010
Chicago/Turabian StyleChen, Kunyang, Dezi Zhang, Yanxia Liu, Yaodi Zhu, Miaoyun Li, Lijun Zhao, Fukang Dong, Gaiming Zhao, Niancheng Hong, Shijie Liu, and et al. 2025. "Effects of Crushing, Vacuum Nano-Collision, and Steam Explosion on the Flavor and Physical Properties of Solid Spices" Foods 14, no. 23: 4010. https://doi.org/10.3390/foods14234010
APA StyleChen, K., Zhang, D., Liu, Y., Zhu, Y., Li, M., Zhao, L., Dong, F., Zhao, G., Hong, N., Liu, S., & Du, S. (2025). Effects of Crushing, Vacuum Nano-Collision, and Steam Explosion on the Flavor and Physical Properties of Solid Spices. Foods, 14(23), 4010. https://doi.org/10.3390/foods14234010




