Fermentation of House Crickets (Acheta domesticus): Boosting Quality and Functionality in Cricket-Based Food Ingredients
Abstract
1. Introduction
2. Why A. domesticus?
2.1. Protein Content
2.2. Fatty Acid Profile
2.3. Potential Bioactive Compounds (Vitamins, Antioxidants, and Protein Hydrolysates)
2.4. Mineral Content
2.5. Carbohydrate and Fiber Content
2.6. Techno-Functional Properties
2.7. Anti-Nutritional Factors and Allergens
3. Literature Search Strategy
3.1. Database Selection and Search Strategy
3.2. Inclusion and Exclusion Criteria
- (i)
- Publication in a peer-reviewed journal;
- (ii)
- Research related to the use of house cricket powder in combination with fermentation for the development of food products;
- (iii)
- Written and published in English;
- (iv)
- Articles published between 2018 and 2025.
3.3. Data Screening and Management
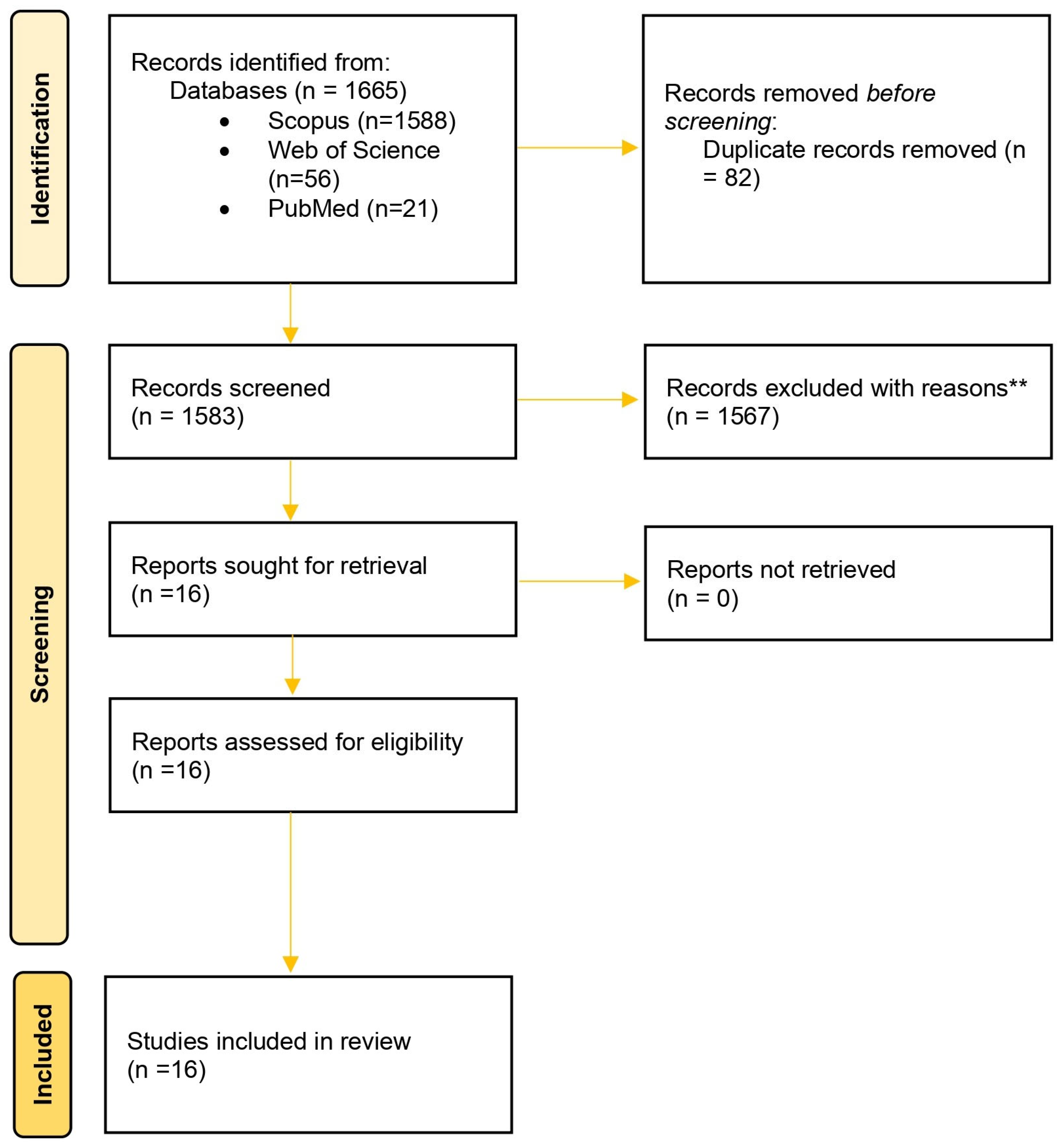
3.4. PRISMA Flow and Article Selection
3.5. Data Extraction and Synthesis
4. Processing and Fermentation Strategies for A. domesticus
5. Fermentation of A. domesticus as a Promising Biotechnological Approach for Enhancing the Functionality of Cricket-Based Ingredients (Powder or Paste)
6. Fermentation-Driven Modifications in Fermented Foods Enriched with A. domesticus
7. Consumer Acceptance Challenges for Fermented Cricket-Enriched Food Ingredients and Food Products
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BABA | β-aminobutyric acid |
| CBE | Circular Bioeconomy |
| CFU | Colony-Forming Units |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EFSA | European Food Safety Authority |
| EU | European Union |
| GABA | γ-aminobutyric acid |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| HDM | House Dust Mite |
| LAB | Lactic Acid Bacteria |
| NGS | Next-Generation Sequencing |
| PER | Protein Efficiency Ratio |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive Oxygen Species |
| TNF-α | Tumor Necrosis Factor |
| WHC | Water-Holding Capacity |
References
- Pilco-Romero, G.; Chisaguano-Tonato, A.M.; Herrera-Fontana, M.E.; Chimbo-Gándara, L.F.; Sharifi-Rad, M.; Giampieri, F.; Battino, M.; Vernaza, M.G.; Álvarez-Suárez, J.M. House Cricket (Acheta domesticus): A Review Based on Its Nutritional Composition, Quality, and Potential Uses in the Food Industry. Trends Food Sci. Technol. 2023, 142, 104226. [Google Scholar] [CrossRef]
- Vasilica, B.B.; Chiș, M.S.; Alexa, E.; Pop, C.; Păucean, A.; Man, S.; Igual, M.; Haydee, K.M.; Dalma, K.E.; Stănilă, S.; et al. The Impact of Insect Flour on Sourdough Fermentation-Fatty Acids, Amino-Acids, Minerals and Volatile Profile. Insects 2022, 13, 576. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread Enriched with Cricket Powder (Acheta domesticus): A Technological, Microbiological and Nutritional Evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Kentra, E.; Starkute, V.; Klupsaite, D.; Mockus, E.; Zokaityte, G.; Cernauskas, D.; Rocha, J.M.; Guiné, R.P.F. Characterisation of Lacto-Fermented Cricket (Acheta domesticus) Flour and Its Influence on the Quality Parameters and Acrylamide Formation in Wheat Biscuits. Fermentation 2023, 9, 153. [Google Scholar] [CrossRef]
- Vehar, A.; Potočnik, D.; Mencin, M.; Korošec, M.; Ferjančič, B.; Jagodic Hudobivnik, M.; Jamnik, P.; Ota, A.; Kouřimská, L.; Kulma, M.; et al. Evaluation of Nutritional Quality and Oxidation Stability of Fermented Edible Insects. Foods 2025, 14, 2929. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; González-Domenech, C.M.; Borrego, J.J. The Role of Fermented Vegetables as a Sustainable and Health-Promoting Nutritional Resource. Appl. Sci. 2024, 14, 10853. [Google Scholar] [CrossRef]
- Carpentier, J.; Abenaim, L.; Luttenschlager, H.; Dessauvages, K.; Liu, Y.; Samoah, P.; Francis, F.; Caparros Megido, R. Microorganism Contribution to Mass-Reared Edible Insects: Opportunities and Challenges. Insects 2024, 15, 611. [Google Scholar] [CrossRef]
- Hamam, M.; D’Amico, M.; Di Vita, G. Advances in the insect industry within a circular bioeconomy context: A research agenda. Environ. Sci. Eur. 2024, 36, 29. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, X.; Utomo, D.; Gage, E.; Xu, B. Circular bioeconomy and sustainable food systems: What are the possible mechanisms? Clean. Circ. Bioecon. 2025, 11, 100145. [Google Scholar] [CrossRef]
- Min, Y.R.; Nam, J.K.; Jang, H.W. Edible insects as sustainable food sources: Extraction techniques, nutritional profiles, and volatile characteristics. Anal. Sci. Technol. 2025, 38, 74–88. [Google Scholar] [CrossRef]
- Van Campenhout, L. Fermentation technology applied in the insect value chain: Making a win-win between microbes and insects. J. Insects Food Feed 2021, 7, 377–382. [Google Scholar] [CrossRef]
- Okaiyeto, S.A.; Yu, S.H.; Deng, L.Z.; Wang, Q.H.; Sutar, P.P.; Wang, H.; Zhao, J.H.; Mujumdar, A.S.; Ni, J.B.; Lv, W.; et al. How to enhance the acceptability of insects food—A review. Food Front. 2024, 5, 311–328. [Google Scholar] [CrossRef]
- Alejandro Ruiz, F.E.; Ortega Jácome, J.F.; Tejera, E.; Alvarez-Suarez, J.M. Edible insects as functional foods: Bioactive compounds, health benefits, safety concerns, allergenicity, and regulatory considerations. Front. Nutr. 2025, 12, 1571084. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Slimen, I.; Yerou, H.; Ben Larbi, M.; M’Hamdi, N.; Najar, T. Insects as an Alternative Protein Source for Poultry Nutrition: A Review. Front. Vet. Sci. 2023, 10, 1200031. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Altemimi, A.B.; Hashmi, A.A.; Shahzadi, S.; Mujahid, W.; Ali, A.; Bhat, Z.F.; Naz, S.; Nawaz, A.; Abdi, G.; et al. Edible Crickets as a Possible Way to Curb Protein-Energy Malnutrition: Nutritional Status, Food Applications, and Safety Concerns. Food Chem. X 2024, 23, 101533. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, W.M.; Przychodniak, D.; Gromek, W.; Majsiak, E.; Kurowski, M. Edible Insects as an Alternative Source of Nutrients: Benefits, Risks, and the Future of Entomophagy in Europe—A Narrative Review. Foods 2025, 14, 270. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Inthachat, W.; Suttisansanee, U.; Temviriyanukul, P. Road to The Red Carpet of Edible Crickets through Integration into the Human Food Chain with Biofunctions and Sustainability: A Review. Int. J. Mol. Sci. 2022, 23, 1801. [Google Scholar] [CrossRef]
- Ma, Z.; Mondor, M.; Goycoolea Valencia, F.; Hernández-Álvarez, A.J. Current State of Insect Proteins: Extraction Technologies, Bioactive Peptides and Allergenicity of Edible Insect Proteins. Food Funct. 2023, 14, 8129–8156. [Google Scholar] [CrossRef]
- Nachtigall, L.; Grune, T.; Weber, D. Proteins and Amino Acids from Edible Insects for the Human Diet—A Narrative Review Considering Environmental Sustainability and Regulatory Challenges. Nutrients 2025, 17, 1245. [Google Scholar] [CrossRef]
- Van Peer, M.; Berrens, S.; Coudron, C.; Noyens, I.; Verheye, G.R.; Van Miert, S. Towards Good Practices for Research on Acheta domesticus, the House Cricket. J. Insects Food Feed 2024, 10, 1235–1251. [Google Scholar] [CrossRef]
- Rossi, G.; Psarianos, M.; Ojha, S.; Schlüter, O.K. Insects as a Novel Feed Ingredient: Processing Technologies, Quality and Safety Considerations. Animal 2025, 19, 101495. [Google Scholar] [CrossRef]
- Tarahi, M.; Aghababaei, F.; McClements, D.J.; Pignitter, M.; Hadidi, M. Bioactive Peptides Derived from Insect Proteins: Preparation, Biological Activities, Potential Applications, and Safety Issues. Food Chem. 2025, 465, 142113. [Google Scholar] [CrossRef]
- Ververis, E.; Boué, G.; Poulsen, M.; Pires, S.M.; Niforou, A.; Thomsen, S.T.; Tesson, V.; Federighi, M.; Naska, A. A Systematic Review of the Nutrient Composition, Microbiological and Toxicological Profile of Acheta domesticus (House Cricket). J. Food Compos. Anal. 2022, 114, 104859. [Google Scholar] [CrossRef]
- Pan, J.; Xu, H.; Cheng, Y.; Mintah, B.K.; Dabbour, M.; Yang, F.; Chen, W.; Zhang, Z.; Dai, C.; He, R.; et al. Recent Insight on Edible Insect Protein: Extraction, Functional Properties, Allergenicity, Bioactivity, and Applications. Foods 2022, 11, 2931. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Seephua, N.; Prakitchaiwattana, C.; Liu, R.-X.; Zheng, J.-S.; Siriamornpun, S. Fortification of cricket and silkworm pupae powders to improve nutritional quality and digestibility of rice noodles. Food Chem. X 2025, 26, 102279. [Google Scholar] [CrossRef] [PubMed]
- Cruz, V.A.; Vicentini-Polette, C.M.; Magalhaes, D.R.; de Oliveira, A.L. Extraction, Characterization, and Use of Edible Insect Oil—A Review. Food Chem. 2025, 463, 141199. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.; Martins, L.L.; Mourato, M.P.; Lourenço, H.; Ramos, A.C.; Roseiro, C.; Pereira, N.; Costa, G.J.; Lucas, R.; Alvarenga, N.; et al. Nutritional and Microbial Quality of Edible Insect Powder from Plant-Based Industrial By-Product and Fish Biowaste Diets. Foods 2025, 14, 1242. [Google Scholar] [CrossRef] [PubMed]
- Gantner, M.; Sadowska, A.; Piotrowska, A.; Kulik, K.; Sionek, B.; Kostyra, E. Wheat Bread Enriched with House Cricket Powder (Acheta domesticus L.) as an Alternative Protein Source. Molecules 2024, 29, 711. [Google Scholar] [CrossRef]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Wangtueai, S.; Chaiyana, W. Optimization of Hydrolysis Conditions, Isolation, and Identification of Biologically Active Peptides Derived from Acheta domesticus for Antioxidant and Collagenase Inhibition. Antioxidants 2024, 13, 367. [Google Scholar] [CrossRef]
- Aiello, D.; Barbera, M.; Bongiorno, D.; Cammarata, M.; Censi, V.; Indelicato, S.; Mazzotti, F.; Napoli, A.; Piazzese, D.; Saiano, F. Edible Insects an Alternative Nutritional Source of Bioactive Compounds: A Review. Molecules 2023, 28, 699. [Google Scholar] [CrossRef]
- Brai, A.; Pasqualini, C.; Poggialini, F.; Vagaggini, C.; Dreassi, E. Insects as Source of Nutraceuticals with Antioxidant, Antihypertensive, and Antidiabetic Properties: Focus on the Species Approved in Europe up to 2024. Foods 2025, 14, 1383. [Google Scholar] [CrossRef] [PubMed]
- Vehar, A.; Potocnik, D.; Strojnik, L.; Zuliani, T.; Heath, D.; Mencin, M.; Vrhovsek, U.; Skvorová, P.; Kourimská, L.; Kulma, M.; et al. Nutritional Composition of Farmed Insects: Impact of Species, Developmental Stage, and Sex. J. Insects Food Feed 2025, 11, 2763–2785. [Google Scholar] [CrossRef]
- Izadi, H.; Asadi, H.; Bemani, M. Chitin: A Comparison between Its Main Sources. Front. Mater. 2025, 12, 1537067. [Google Scholar] [CrossRef]
- Nino, M.C.; Reddivari, L.; Ferruzzi, M.G.; Liceaga, A.M. Targeted Phenolic Characterization and Antioxidant Bioactivity of Extracts from Edible Acheta domesticus. Foods 2021, 10, 2295. [Google Scholar] [CrossRef]
- Umebara, I.; Akutsu, K.; Kubo, M.; Iijima, A.; Sakurai, R.; Masutomi, H.; Ishihara, K. Analysis of Fatty Acid Composition and Volatile Profile of Powder from Edible Crickets (Acheta domesticus) Reared on Apple By-Products. Foods 2024, 13, 1668. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, C.; Smetana, S.; Zhao, M.; Zhang, H.; Zhang, F.; Du, Y. Minerals in Edible Insects: A Review of Content and Potential for Sustainable Sourcing. Food Sci. Hum. Wellness 2024, 13, 65–74. [Google Scholar] [CrossRef]
- Mabelebele, M.; Kolobe, S.D.; Malematja, E.; Sebola, N.A.; Manyelo, T.G. A Comprehensive Review of the Importance of Selected Trace Elements Present in Edible Insects. Biol. Trace Elem. Res. 2023, 201, 3520–3527. [Google Scholar] [CrossRef]
- Malematja, E.; Sebola, N.A.; Manyelo, T.G.; Kolobe, S.D.; Mabelebele, M. Copping out of Novel Feeds: HOW Climate Change Pledgers and Food Summits Overlooked Insect Protein. Heliyon 2023, 9, e22773. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.A.B.; Hummel, M.; Utami, D.A.; Gunawan, L.; Veenenbos, M.; Zeder, C.; Cercamondi, C.I.; Zimmermann, M.B.; Van Loon, J.J.A.; et al. Absorption of Iron from Edible House Crickets: A Randomized Crossover Stable-Isotope Study in Humans. Am. J. Clin. Nutr. 2022, 116, 1146–1156. [Google Scholar] [CrossRef]
- Cunha, N.; Andrade, V.; Macedo, A.; Ruivo, P.; Lima, G. Methods of Protein Extraction from House Crickets (Acheta domesticus) for Food Purposes. Foods 2025, 14, 1164. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The Use of Edible Insect Proteins in Food: Challenges and Issues Related to Their Functional Properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- López-Gámez, G.; del Pino-García, R.; López-Bascón, M.A.; Verardo, V. From Feed to Functionality: Unravelling the Nutritional Composition and Techno-Functional Properties of Insect-Based Ingredients. Food Res. Int. 2024, 178, 113985. [Google Scholar] [CrossRef]
- Mannozzi, C.; Foligni, R.; Mozzon, M.; Aquilanti, L.; Cesaro, C.; Isidoro, N.; Osimani, A. Nonthermal Technologies Affecting Techno-Functional Properties of Edible Insect-Derived Proteins, Lipids, and Chitin: A Literature Review. Innov. Food Sci. Emerg. Technol. 2023, 88, 103453. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Nogueira Silva, N.F.; Jessen, F.; Mohammadifar, M.A.; Stephani, R.; Fernandes de Carvalho, A.; Perrone, Í.T.; Casanova, F. Edible Insect as an Alternative Protein Source: A Review on the Chemistry and Functionalities of Proteins under Different Processing Methods. Heliyon 2023, 9, e14831. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Ebrahimi, M.; Assadpour, E.; Jafari, S.M. Application of Edible Insects in Bread Enrichment; Emerging Techno-Functional Opportunities and Potential Challenges. Future Foods 2025, 11, 100638. [Google Scholar] [CrossRef]
- Lin, X.; Wang, F.; Lu, Y.; Wang, J.; Chen, J.; Yu, Y.; Tao, X.; Xiao, Y.; Peng, Y. A Review on Edible Insects in China: Nutritional Supply, Environmental Benefits, and Potential Applications. Curr. Res. Food Sci. 2023, 7, 100596. [Google Scholar] [CrossRef]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-Reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef]
- de Matos, F.M.; Rasera, G.B.; Soares De Castro, R.J. Insects as a Sustainable Source of Emerging Proteins and Their Processing to Obtain Bioactive Compounds: An Updated Review. Sustain. Food Technol. 2024, 2, 19–31. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Vanhecke, T.E. Zotero. J. Med. Libr. Assoc. 2008, 96, 275–276. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Rossi, S.; Parrotta, L.; Del Duca, S.; Rosa, M.D.; Patrignani, F.; Schluter, O.; Lanciotti, R. Effect of Yarrowia Lipolytica RO25 Cricket-Based Hydrolysates on Sourdough Quality Parameters. LWT 2021, 148, 111760. [Google Scholar] [CrossRef]
- Patrignani, F.; Parrotta, L.; Del Duca, S.; Vannini, L.; Camprini, L.; Dalla Rosa, M.; Schlüter, O.; Lanciotti, R. Potential of Yarrowia Lipolytica and Debaryomyces Hansenii Strains to Produce High Quality Food Ingredients Based on Cricket Powder. LWT 2020, 119, 108866. [Google Scholar] [CrossRef]
- Rossi, S.; Parrotta, L.; Gottardi, D.; Glicerina, V.T.; Del Duca, S.; Rosa, M.D.; Patrignani, F.; Schlüter, O.; Lanciotti, R. Unravelling the Potential of Cricket-Based sHydrolysed Sourdough on the Quality of an Innovative Bakery Product. J. Insects Food Feed 2022, 8, 921–936. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Starkute, V.; Zokaityte, G.; Kaminskaite, A.; Mockus, E.; Klupsaite, D.; Cernauskas, D.; Rocha, J.M.; Özogul, F.; et al. Crickets (Acheta domesticus) as Wheat Bread Ingredient: Influence on Bread Quality and Safety Characteristics. Foods 2023, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Gumienna, M.; Rybicka, I.; Górna, B.; Sarbak, P.; Dziedzic, K.; Kmiecik, D. Nutritional Value and Biological Activity of Gluten-Free Bread Enriched with Cricket Powder. Molecules 2021, 26, 1184. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten Free Sourdough Bread Enriched with Cricket Flour for Protein Fortification: Antioxidant Improvement and Volatilome Characterization. Food Chem. 2020, 333, 127410. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Pini, N.; Granchi, L. Technological Feature Assessment of Lactic Acid Bacteria Isolated from Cricket Powder’s Spontaneous Fermentation as Potential Starters for Cricket-Wheat Bread Production. Foods 2020, 9, 1322. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Bonaccorsi, G.; Lorini, C.; Cini, E. Assessment of the Rheological Properties and Bread Characteristics Obtained by Innovative Protein Sources (Cicer arietinum, Acheta domesticus, Tenebrio molitor): Novel Food or Potential Improvers for Wheat Flour? LWT 2020, 118, 108867. [Google Scholar] [CrossRef]
- Belleggia, L.; Foligni, R.; Ferrocino, I.; Biolcati, F.; Mozzon, M.; Aquilanti, L.; Osimani, A.; Harasym, J. Morphotextural, Microbiological, and Volatile Characterization of Flatbread Containing Cricket (Acheta domesticus) Powder and Buckwheat (Fagopyrum esculentum) Flour. Eur. Food Res. Technol. 2023, 249, 2777–2795. [Google Scholar] [CrossRef]
- Karwacka, K.; Łobacz, A.; Ziajka, J.; Lis, A.; Małkowska-Kowalczyk, M.; Baranowska, M. Use of House Cricket (Acheta domesticus) Powder in Yoghurt Products. Foods 2024, 13, 2426. [Google Scholar] [CrossRef]
- Dridi, C.; Millette, M.; Uscanga, B.R.A.; Salmieri, S.; Allahdad, Z.; Lacroix, M. Evaluation of the Nutritional Quality and In Vivo Digestibility of Probiotic Beverages Enriched with Cricket Proteins. Food Bioprocess Technol. 2023, 16, 1992–2000. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Whanmek, K.; Santivarangkna, C. Physicochemical, Microbiological and Nutritional Quality of Fermented Cricket (Acheta domesticus) Paste. LWT 2023, 189, 115444. [Google Scholar] [CrossRef]
- Dhakal, M.; Kemsawasd, V.; Whanmek, K.; Chathiran, W.; Intawong, S.; Srichamnong, W.; Suttisansanee, U.; Kittibunchakul, S. Physicochemical Characteristics, Volatile Components and Bioactivities of Fermented Seasoning Sauce Produced from Cricket (Acheta domesticus) Meal. Future Foods 2025, 11, 100505. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhang, L.; Zhou, S.; You, L.; Song, J. Application of Edible Insects to Food Products: A Review on the Functionality, Bioactivity and Digestibility of Insect Proteins under High-Pressure/Ultrasound Processing. Food Chem. 2025, 468, 142469. [Google Scholar] [CrossRef] [PubMed]
- Roncolini, A.; Milanović, V.; Cardinali, F.; Osimani, A.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; et al. Protein Fortification with Mealworm (Tenebrio Molitor L.) Powder: Effect on Textural, Microbiological, Nutritional and Sensory Features of Bread. PLoS ONE 2019, 14, e0211747. [Google Scholar] [CrossRef]

| Type of Fermented Ingredient | Microorganism Used | Obtained Enhancement | Reference |
|---|---|---|---|
| Acheta domesticus powder | Lactiplantibacillus. plantarum and Lacticaseibacillus casei (48 h at 30 °C) | Nutritional: - Enhancing fatty acid profile by increasing linoleic, oleic, palmitic, and linolenic acids, with the highest value of 26.28% increase after 48 h for oleic acid. - Decrease in biogenic amines by a decrease in cadaverine and putrescine. Sensory: - Increase in volatile compounds such as acetoin and 3-methylbutanoic acid and decrease in hexanal. - Decrease in lightness and yellowness. Functional: n/a | Bartkiene et al. [4] |
| A. domesticus powder | L. plantarum (48 h at 37 °C) | Nutritional: - Organic acid profile improvement by reduction in citric acid and an increase in succinic acid - Increase in amino acid content particularly Ala (1.76-fold), Gly (3.67-fold), Leu (1.99-fold), and Met (2.89-fold). - Increase in fatty acids particularly PUFAs (polyunsaturated fatty acids) - Reduction in anti-nutritional factors like tannins and phytates. Sensory: - Production of aroma compounds such as aldehydes, ketones, and alcohols. Functional: n/a | Vasilica et al. [2] |
| Cricket powder-based hydrolysate | Yarrowia lipolytica (72 h at 25 °C) | Nutritional: - Rise in fatty acid content especially arachidonic and linoleic acids. - High release of volatile precursors such as C18:1, C18:2 and C20:4 Sensory: - Identification of around 60 volatile compounds mostly aldehydes and ketones. Functional: n/a | Rossi et al. [52] |
| Cricket powder-based hydrolysate | Y. lipolytica and Debaryomyces hansenii (72 h at 25 °C) | Nutritional: - Significant increase in protein content, with the highest value of 26.35% after 48 h for D. hansenii SPL612. - Reduction in chitin content. - Increase in antimicrobial substances and health-promoting molecules. - Detection of bioactive compounds like γ-aminobutyricacid (GABA) and β-aminobutyricacid (BABA). - Increased fatty acid profile, especially unsaturated fatty acids. - High matrix digestibility due to the release of amino acids. Sensory: - Identification of over 80 aroma compounds such as alcohols, ketones, and pyrazines. Functional: n/a | Patrignani et al. [53] |
| Cricket powder-based hydrolysate | Y. lipolytica | Nutritional: - Reduction in chitin content. - High protein content. - High concentration of polyunsaturated free fatty acids. - Reduction in biogenic amine levels compared to the non-hydrolyzed breads. Sensory: - Possessing a diverse volatile compound profile by identification of more than 120 molecules. - Enhanced texture and flavor. Functional: - Improvement of rheology. | Rossi et al. [54] |
| Product Type (% Inclusion) | Form of Cricket Used | Microorganism Used | Obtained Enhancement | Reference |
|---|---|---|---|---|
| Cricket-enriched bread (10, 20, 30%) | Acheta domesticus powder | Lactiplantibacillus plantarum | Nutritional: - Fatty acids profile enhancement by increasing in saturated and monosaturated fatty acids, decreasing in polyunsaturated fatty acids. - Reduction in biogenic amines (13.1%). - Disappearance of cadaverine and putrescine after 48 h. Sensory: - Increase in volatile compounds like acetoin, 2,3-butanediol, and pyrazines. - Decrease in lightness, redness, and yellowness. Functional: n/a | Bartkiene et al. [55] |
| Cricket-powder enriched sourdough bread (10, 30%) | A. domesticus powder | Lactobacillus sanfranciscensis PB276, L. sanfranciscensis PB223, Lactobacillus plantarum PB11, L. plantarum PB24, Lactobacillus fermentum PB162 | Nutritional: - Increase in protein content. - High amount of essential amino acids. - Improved fatty acid profile. Sensory: n/a Functional: n/a | Osimani et al. [3] |
| Cricket-enriched gluten-free bread (2, 6, 10%) | A. domesticus powder | n/a | Nutritional: -Increase in protein content. - Higher mineral content. - Increase in antioxidant activities and polyphenols. - Decreasing markers activities such as β-glucuronidase. - No inhibitory effect on the growth of microflora. Sensory: n/a Functional: n/a | Kowalczewski et al. [56] |
| Cricket-fortified gluten-free sourdough bread (n/a %) | A. domesticus powder | L. plantarum 98a, L. sanfranciscensis Bb12 and Saccharomyces cerevisiae LBS | Nutritional: -Increased antioxidant activity by protein hydrolysates. - Decrease in lipid oxidation by antioxidants. - Unique volatile compounds profile. Sensory: n/a Functional: n/a | Nissen et al. [57] |
| Cricket-enriched bread (20%) | Cricket powder | L. plantarum CR L1, Latilactobacillus curvatus CR L13, Lactococcus spp., Enterococcus spp., and Weissella spp. | Nutritional: - Higher protein and fat content in bread. - Identification of L. plantarum and L. cuvatus as suitable microbial starters. Sensory: n/a Functional: n/a | Galli et al. [58] |
| Cricket-enriched bread (5, 10, 15%) | A. domesticus powder | n/a | Nutritional: n/a Sensory: n/a Functional: - Increase in stability. - Reduction in softening. | Cappelli et al. [59] |
| Cricket-enriched flatbread (20%) | A. domesticus powder | Lactic acid bacteria and yeasts naturally contained in the sourdough used as leavening agent or baker’s yeast | Nutritional: - Identification of numerous aromatic compounds (more than 540) such as alcohols, aldehydes, esters, ketones, acids, pyrazines, furans, and sulfur compounds. Sensory: - Softer texture of flatbreads due to the fiber content. - Darker color of breads which could be advantageous in consumer perception. Functional: - Low water-activity of flatbreads, preventing growth of pathogenic microorganisms. | Belleggia et al. [60] |
| Cricket-enriched yoghurt (1.5, 3, 5%) | A. domesticus powder | Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus | Nutritional: - Increase in protein (7.83%) and fat (5.18%) content of the yoghurt. Sensory: - Negatively affecting texture, appearance, and losing hardness and consistency. Functional: n/a | Karwacka et al. [61] |
| Beverage (n/a %) | Cricket powder | Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 | Nutritional: - Improved protein efficiency. - Complete amino acid profile. - Improvement of the growth parameters, food intake and protein efficiency ratio. Sensory: - Higher digestibility (94%) according to the presence of cricket protein hydrolysates. Functional: n/a | Dridi et al. [62] |
| Cricket paste (n/a %) | A. domesticus powder | Microorganisms naturally contained in Kapi used as starter | Nutritional: - Increase in total essential amino acids (11.18%) compared to the whole cricket powder. - Flavor development due to protein hydrolysis. Sensory: - Soft and pasty texture. - Unique flavor and texture properties due to the protein hydrolysis. - Increase in lightness after salting. Functional: - Decrease in moisture content. - Lower water activity. | Kittibunchakul et al. [63] |
| Seasoning sauce (n/a %) | A. domesticus | Staphylococcus piscifermentans TISTR 824 and Halobacillus sp. TISTR 1860 | Nutritional: - Physiochemical properties such as degree of hydrolysis, pH, absorbance at 420 nm and water activity. - Unique flavor profile - Increase in antioxidant potential. - Enhanced bioactivity due to the release of bioactive peptides. - High anti-diabetic effects compared to Thai fish sauce. Sensory: n/a Functional: n/a | Dhakal et al. [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghayeghi, S.M.H.; Osimani, A.; Aquilanti, L. Fermentation of House Crickets (Acheta domesticus): Boosting Quality and Functionality in Cricket-Based Food Ingredients. Foods 2025, 14, 4003. https://doi.org/10.3390/foods14234003
Haghayeghi SMH, Osimani A, Aquilanti L. Fermentation of House Crickets (Acheta domesticus): Boosting Quality and Functionality in Cricket-Based Food Ingredients. Foods. 2025; 14(23):4003. https://doi.org/10.3390/foods14234003
Chicago/Turabian StyleHaghayeghi, Seyed Mohammad Hasan, Andrea Osimani, and Lucia Aquilanti. 2025. "Fermentation of House Crickets (Acheta domesticus): Boosting Quality and Functionality in Cricket-Based Food Ingredients" Foods 14, no. 23: 4003. https://doi.org/10.3390/foods14234003
APA StyleHaghayeghi, S. M. H., Osimani, A., & Aquilanti, L. (2025). Fermentation of House Crickets (Acheta domesticus): Boosting Quality and Functionality in Cricket-Based Food Ingredients. Foods, 14(23), 4003. https://doi.org/10.3390/foods14234003








