Geranium Oil Nanoemulsion Delivers More Potent and Persistent Fumigant Control of Callosobruchus maculatus in Stored Grain
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Preparation
2.1.1. Nanoemulsion Preparation
2.1.2. Particle Size Characterization
2.1.3. Insect Rearing
2.2. Experimental Setup
2.2.1. Fumigant Toxicity
2.2.2. Persistence Bioassay
2.3. Data Analysis
3. Results
3.1. Characterization of Geranium Oil Nanoemulsion
3.2. Fumigant Toxicity
3.2.1. Insect Mortality
3.2.2. The Concentration-Mortality Response
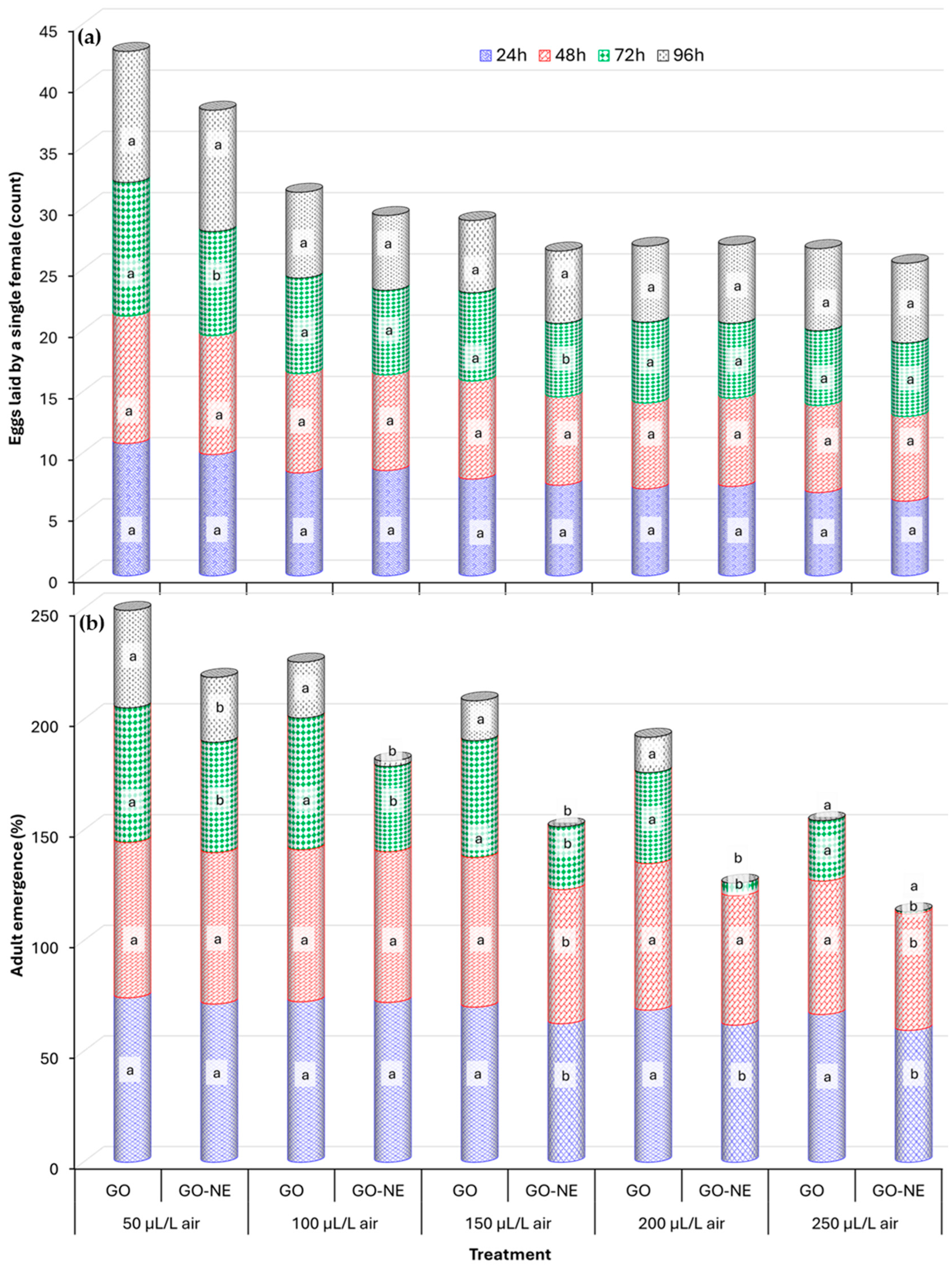
3.2.3. Progeny Development
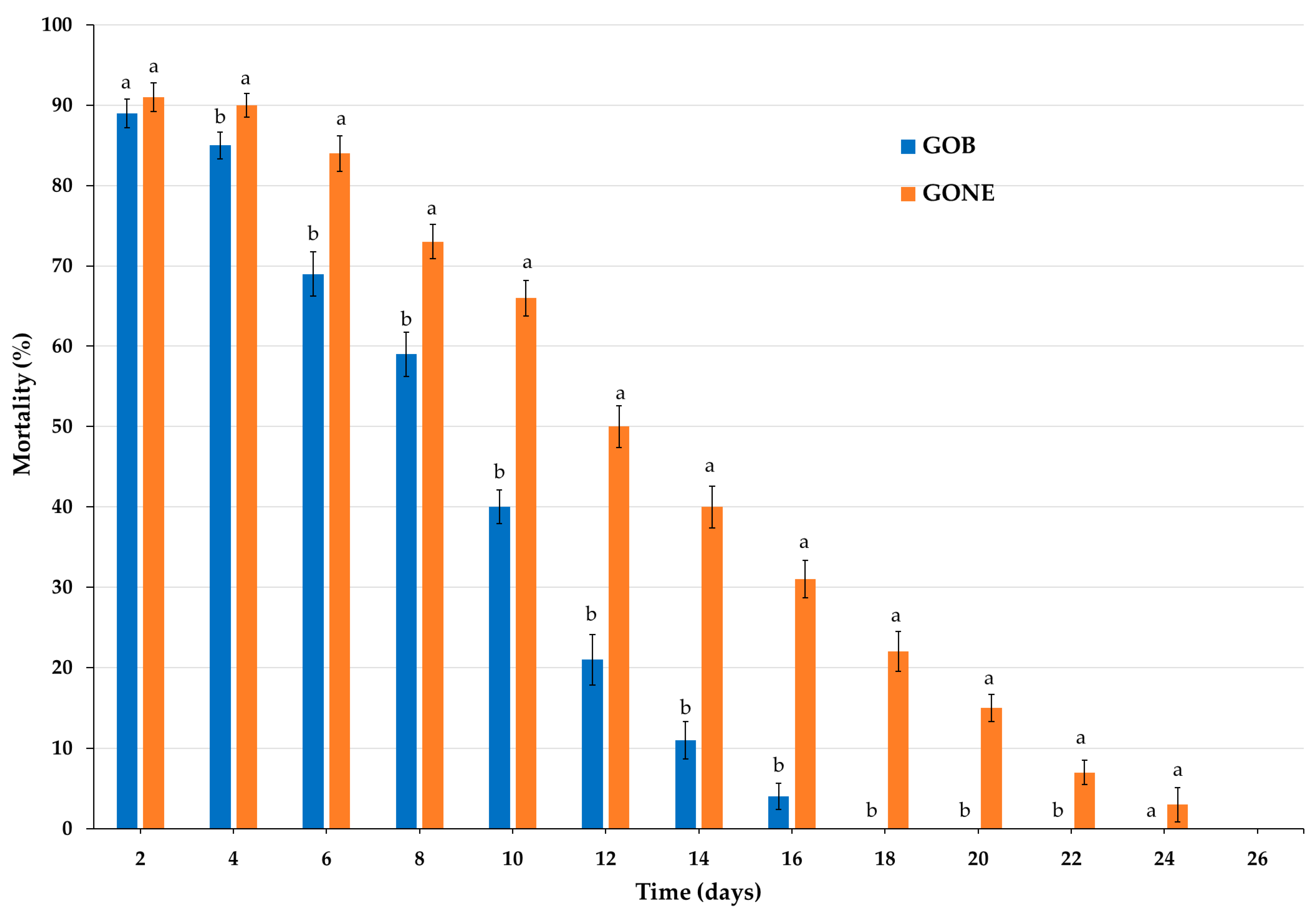
3.2.4. Persistence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nation: Rome, Italy, 2017. [Google Scholar]
- Metwally, E.; Sharshar, M.; Masoud, A.; Masry, A.; Fiad, A.; Kilian, B.; Sharma, S.; Shaw, P.D.; Raubach, S.; Rakha, M. Development of High Yielding Cowpea [Vigna unguiculata (L.) Walp.] Lines with Improved Quality Seeds through Mutation and Pedigree Selection Methods. Horticulturae 2021, 7, 271. [Google Scholar] [CrossRef]
- Baributsa, D.; Lowenberg-Deboer, J.; Murdock, L.; Moussa, B. Profitable Chemical-Free Cowpea Storage Technology for Smallholder Farmers in Africa: Opportunities and Challenges. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; Volume 425, pp. 1046–1052. [Google Scholar]
- Barbosa, D.R.e.S.; de Oliveira, J.V.; da Silva, P.H.S.; Santana, M.F.; Breda, M.O.; de França, S.M.; de Miranda, V.L. Lethal and Sublethal Effects of Chemical Constituents from Essential Oils on Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae: Bruchinae) in Cowpea Stored Grains. J. Plant Dis. Prot. 2021, 128, 1575–1586. [Google Scholar] [CrossRef]
- Gupta, H.; Deeksha; Urvashi; Reddy, S.G.E. Insecticidal and Detoxification Enzyme Inhibition Activities of Essential Oils for the Control of Pulse Beetle, Callosobruchus maculatus (F.) and Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Molecules 2023, 28, 492. [Google Scholar] [CrossRef]
- Baoua, I.B.; Amadou, L.; Margam, V.; Murdock, L.L. Comparative Evaluation of Six Storage Methods for Postharvest Preservation of Cowpea Grain. J. Stored Prod. Res. 2012, 49, 171–175. [Google Scholar] [CrossRef]
- Seni, A.; Mishra, K.M. Pulse Beetle, Callosobruchus Spp. (Coleoptera: Chrysomelidae); A Major Threat in Legume Grain Storage and Their Management. Acta Phytopathol. Entomol. Hung. 2022, 57, 49–65. [Google Scholar]
- Anaduaka, E.G.; Uchendu, N.O.; Asomadu, R.O.; Ezugwu, A.L.; Okeke, E.S.; Chidike Ezeorba, T.P. Widespread Use of Toxic Agrochemicals and Pesticides for Agricultural Products Storage in Africa and Developing Countries: Possible Panacea for Ecotoxicology and Health Implications. Heliyon 2023, 9, e15173. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Zhang, H.; Lempérière, G. A Review of Control Methods and Resistance Mechanisms in Stored-Product Insects. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef]
- Gbaye, O.A.; Oyeniyi, E.A.; Ojo, O.B. Resistance of Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) Populations in Nigeria to Dichlorvos. Jordan J. Biol. Sci. 2016, 9, 41–46. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Akbar, R.; Khan, I.A.; Alajmi, R.A.; Ali, A.; Faheem, B.; Usman, A.; Ahmed, A.M.; El-Shazly, M.; Farid, A.; Giesy, J.P.; et al. Evaluation of Insecticidal Potentials of Five Plant Extracts against the Stored Grain Pest, Callosobruchus maculatus (Coleoptera: Bruchidae). Insects 2022, 13, 1047. [Google Scholar] [CrossRef]
- Bandi, S.M.; Mishra, P.; Venkatesha, K.T.; Aidbhavi, R.; Singh, B. Insecticidal, Residual and Sub-Lethal Effects of Some Plant Essential Oils on Callosobruchus analis (F.) Infesting Stored Legumes. Int. J. Trop. Insect Sci. 2023, 43, 383–395. [Google Scholar] [CrossRef]
- Elbehery, H.H.; Ibrahim, S.S. Potential Fumigant Toxicity of Essential Oils against Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) and Its Egg Parasitoid Trichogramma evanescens (Hymenoptera: Trichogrammatidae). Sci. Rep. 2024, 14, 6253. [Google Scholar] [CrossRef]
- Đukić, N.; Marković, T.; Mikić, S.; Čutović, N. Repellent Activity of Basil, Clary Sage and Celery Essential Oils on Tribolium castaneum (Herbst). J. Stored Prod. Res. 2023, 103, 102150. [Google Scholar] [CrossRef]
- Abouelatta, A.M.; Keratum, A.Y.; Ahmed, S.I.; El-Zun, H.M. Repellent, Contact and Fumigant Activities of Geranium (Pelargonium graveolens L.’Hér) Essential Oils against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.). Int. J. Trop. Insect Sci. 2020, 40, 1021–1030. [Google Scholar] [CrossRef]
- Fan, G.W.; Wang, P.; Liu, Y.S.; Sang, Y.L.; Liu, N.; Hao, Y.J. Insecticidal Activity of Two Pelargonium Essential Oils and Head Transcriptome Analysis of Stored-Product Pest Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) in Response to Citronellyl Formate Fumigation. Pestic. Biochem. Physiol. 2025, 208, 106278. [Google Scholar] [CrossRef]
- M’hamdi, Z.; Davì, F.; Elhourri, M.; Amechrouq, A.; Mondello, F.; Cacciola, F.; Laganà Vinci, R.; Mondello, L.; Miceli, N.; Taviano, M.F. Phytochemical Investigations, Antioxidant and Insecticidal Properties of Essential Oil and Extracts from the Aerial Parts of Pelargonium graveolens from Morocco. Molecules 2024, 29, 4036. [Google Scholar] [CrossRef]
- Gaire, S.; Lewis, C.D.; Booth, W.; Scharf, M.E.; Zheng, W.; Ginzel, M.D.; Gondhalekar, A.D. Bed Bugs, Cimex lectularius L., Exhibiting Metabolic and Target Site Deltamethrin Resistance Are Susceptible to Plant Essential Oils. Pestic. Biochem. Physiol. 2020, 169, 104667. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Gad, H.A.; Ramadan, G.R.M.; El-Bakry, A.M.; El-Sabrout, A.M. Monoterpenes: Chemistry, Insecticidal Activity against Stored Product Insects and Modes of Action—A Review. Int. J. Pest Manag. 2024, 70, 267–289. [Google Scholar] [CrossRef]
- Jaradat, N.; Hawash, M.; Qadi, M.; Abualhasan, M.; Odetallah, A.; Qasim, G.; Awayssa, R.; Akkawi, A.; Abdullah, I.; Al-Maharik, N. Chemical Markers and Pharmacological Characters of Pelargonium graveolens Essential Oil from Palestine. Molecules 2022, 27, 5721. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Kameli, A.; Ferhat, M.A.; Saidi, F.; Mekarnia, M. Rose Geranium Essential Oil as a Source of New and Safe Anti-Inflammatory Drugs. Libyan J. Med. 2013, 8, 22520. [Google Scholar] [CrossRef]
- Mori-mestanza, D.; Valqui-rojas, I.; Caetano, A.C.; Culqui-arce, C.; Cruz-lacerna, R.; Cayo-colca, I.S.; Castro-alayo, E.M.; Balcázar-zumaeta, C.R. Physicochemical Properties of Nanoencapsulated Essential Oils: Optimizing D-Limonene Preservation. Polymers 2025, 17, 348. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.A.; Fahmy, H.M.; Arafa, F.N.; Abd Allah, M.Y.; Tawfik, Y.M.; El Halwany, K.K.; El-Ashmanty, B.A.; Al-anany, F.S.; Mohamed, M.A.; Bassily, M.E. Nanotechnology in Pest Management: Advantages, Applications, and Challenges. Int. J. Trop. Insect Sci. 2023, 43, 1387–1399. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.X. Nanoemulsions for Drug Delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Sahu, U.; Malik, T.; Ibrahim, S.S.; Vendan, S.E.; Karthik, P. Pest Management with Green Nanoemulsions. Bio-Based Nanoemulsions Agri-Food Appl. 2022, 177–195. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Singh, I.R.; Pulikkal, A.K. Preparation, Stability and Biological Activity of Essential Oil-Based Nano Emulsions: A Comprehensive Review. OpenNano 2022, 8, 100066. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physicochemical Properties and Antimicrobial Efficacy of Carvacrol Nanoemulsions Formed by Spontaneous Emulsification. J. Agric. Food Chem. 2013, 61, 8906–8913. [Google Scholar] [CrossRef]
- Yang, Y.; Marshall-Breton, C.; Leser, M.E.; Sher, A.A.; McClements, D.J. Fabrication of Ultrafine Edible Emulsions: Comparison of High-Energy and Low-Energy Homogenization Methods. Food Hydrocoll. 2012, 29, 398–406. [Google Scholar] [CrossRef]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Hormesis-Inducing Essential Oil Nanodelivery System Protects Plants against Broad Host-Range Necrotrophs. ACS Nano 2021, 15, 8338–8349. [Google Scholar] [CrossRef] [PubMed]
- Yuliani, S.; Wahyuningsih, K.; Hernani; Herawati, H.; Hoerudin; Rahmini; Noveriza, R. Spontaneous Emulsification of Citronella Oil: Effect of Processing Conditions and Production Scale. IOP Conf. Ser. Earth Environ. Sci. 2023, 1172, 012053. [Google Scholar] [CrossRef]
- Giunti, G.; Campolo, O.; Laudani, F.; Zappalà, L.; Palmeri, V. Bioactivity of Essential Oil-Based Nano-Biopesticides toward Rhyzopertha dominica (Coleoptera: Bostrichidae). Ind. Crops Prod. 2021, 162, 113257. [Google Scholar] [CrossRef]
- Hagag, H.; El-Sawah, M.H.A.; Raddy, H.M.; Gad, H.A. Chemical Composition, Insecticidal Activities of Origanum Majorana L. Essential Oil Nanoemulsion against Callosobruchus maculatus and Callosobruchus chinensis. Egypt. J. Chem. 2024, 67, 371–381. [Google Scholar] [CrossRef]
- Aisyah, Y.; Haryani, S.; Safriani, N.; El Husna, N. Optimization of Emulsification Process Parameters of Cinnamon Oil Nanoemulsion. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 2092–2098. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis. J. Pharm. Sci. 1971, 60, 1432. [Google Scholar] [CrossRef]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-Energy Formation of Edible Nanoemulsions: Factors Influencing Droplet Size Produced by Emulsion Phase Inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and Stability of Nano-Emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of Vitamin E-Enriched Nanoemulsions by Spontaneous Emulsification: Effect of Propylene Glycol and Ethanol on Formation, Stability, and Properties. Food Res. Int. 2013, 54, 812–820. [Google Scholar] [CrossRef]
- Kelmann, R.G.; Kuminek, G.; Teixeira, H.F.; Koester, L.S. Carbamazepine Parenteral Nanoemulsions Prepared by Spontaneous Emulsification Process. Int. J. Pharm. 2007, 342, 231–239. [Google Scholar] [CrossRef]
- Marzuki, N.H.C.; Wahab, R.A.; Hamid, M.A. An Overview of Nanoemulsion: Concepts of Development and Cosmeceutical Applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Sharma, U.C.; Hariprasad, P.; Satya, S. Efficacy Evaluation of Eucalyptus Globulus Essential Oil-Based Nanoemulsion- a Green Insecticide against Callosobruchus maculatus. Int. J. Trop. Insect Sci. 2024, 44, 2361–2370. [Google Scholar] [CrossRef]
- Maurya, A.; Yadav, A.; Soni, M.; Paul, K.K.; Banjare, U.; Jha, M.K.; Dwivedy, A.K.; Dubey, N.K. Nanoencapsulated Essential Oils for Post-Harvest Preservation of Stored Cereals: A Review. Foods 2024, 13, 4013. [Google Scholar] [CrossRef] [PubMed]
- Draz, K.A.; Tabikha, R.M.; Eldosouky, M.I.; Darwish, A.A.; Abdelnasser, M. Biotoxicity of Essential Oils and Their Nano-Emulsions against the Coleopteran Stored Product Insect Pests Sitophilus oryzae L. and Tribolium castaneum Herbst. Int. J. Pest Manag. 2022, 70, 729–743. [Google Scholar] [CrossRef]
- Nenaah, G.E.; Ibrahim, S.I.A.; Al-Assiuty, B.A. Chemical Composition, Insecticidal Activity and Persistence of Three Asteraceae Essential Oils and Their Nanoemulsions against Callosobruchus maculatus (F.). J. Stored Prod. Res. 2015, 61, 9–16. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- de Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of Nanotechnology for the Encapsulation of Botanical Insecticides for Sustainable Agriculture: Prospects and Promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Sahu, U.; Karthik, P.; Vendan, S.E. Eugenol Nanoemulsion as Bio-Fumigant: Enhanced Insecticidal Activity against the Rice Weevil, Sitophilus oryzae Adults. J. Food Sci. Technol. 2023, 60, 1435–1445. [Google Scholar] [CrossRef]
- Kedia, A.; Dubey, N.K. Nanoencapsulation of Essential Oils: A Possible Way for an Eco-Friendly Strategy to Control Postharvest Spoilage of Food Commodities From Pests; Elsevier Inc.: New York, NY, USA, 2018; Volume 1, ISBN 9780128116463. [Google Scholar]
- Moura, E.d.S.; Faroni, L.R.D.A.; Zanuncio, J.C.; Heleno, F.F.; Prates, L.H.F. Insecticidal Activity of Vanillosmopsis arborea Essential Oil and of Its Major Constituent α-Bisabolol against Callosobruchus maculatus (Coleoptera: Chrysomelidae). Sci. Rep. 2019, 9, 3723. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella anisum Essential Oil Nanoemulsions against Tribolium castaneum—Insecticidal Activity and Mode of Action. Environ. Sci. Pollut. Res. 2018, 25, 18802–18812. [Google Scholar] [CrossRef]
- Ibrahim, S.S. Polyethylene Glycol Nanocapsules Containing Syzygium aromaticum Essential Oil for the Management of Lesser Grain Borer, Rhyzopertha dominica. Food Biophys. 2022, 17, 523–534. [Google Scholar] [CrossRef]
- Louni, M.; Shakarami, J.; Negahban, M. Insecticidal Efficacy of Nanoemulsion Containing Mentha longifolia Essential Oil against Ephestia kuehniella (Lepidoptera: Pyralidae). J. Crop Prot. 2018, 7, 171–182. [Google Scholar]
- Ziaee, M.; Moharramipour, S.; Mohsenifar, A. MA-Chitosan Nanogel Loaded with Cuminum cyminum Essential Oil for Efficient Management of Two Stored Product Beetle Pests. J. Pest Sci. 2014, 87, 691–699. [Google Scholar] [CrossRef]
| Formulation Characterization | Formulation 2 (F2) Storage | |||||
|---|---|---|---|---|---|---|
| Formulation | Particle Size (d.nm) | PDI | Storage Time | Particle Size (d.nm) | PDI | |
| F1 † | 126.03 ± 0.98 a * | 0.16 ± 0.004 a | Initial | 91.85 ± 1.02 a | 0.16 ± 0.02 a | |
| F2 | 91.85 ± 0.02 c | 0.16 ± 0.02 a | 9 °C for 30 days | 92.17 ± 0.87 a | 0.06 ± 0.03 b | |
| F3 | 113.66 ± 0.63 b | 0.06 ± 0.002 b | 25 °C for 30 days | 94.35 ± 0.91 a | 0.10 ± 0.008 ab | |
| Average Mortality (%) | ||||
|---|---|---|---|---|
| Treatment | 24 h | 48 h | 72 h | 96 h |
| GOB-50 µL/L air | 18.00 ± 2.00 aC * | 41.00 ± 1.79 aB | 73.00 ± 4.22 aA | 78.00 ± 3.59 bA |
| GONE-50 µL/L air | 20.00 ± 1.49 aC | 42.00 ± 2.49 aB | 83.00 ± 2.13 aA | 89.00 ± 1.79 aA |
| GOB-100 µL/L air | 46.00 ± 2.21 aC | 65.00 ± 2.23 aB | 80.00 ± 2.98 aA | 84.00 ± 2.21 bA |
| GONE-100 µL/L air | 45.00 ± 1.67 aD | 69.00 ± 2.76 aC | 88.00 ± 2.90 aB | 97.00 ± 1.52 aA |
| GOB-150 µL/L air | 56.00 ± 2.21 aB | 70.00 ± 2.58 aB | 86.00 ± 4.52 bA | 91.00 ± 2.33 bA |
| GONE-150 µL/L air | 57.00 ± 2.60 aC | 75.00 ± 1.66 aB | 98.00 ± 1.33 aA | 100.00 ± 0.00 aA |
| GOB-200 µL/L air | 64.00 ± 2.21 aB | 74.00 ± 3.05 bB | 93.00 ± 1.52 bA | 96.00 ± 2.21 aA |
| GONE-200 µL/L air | 63.00 ± 2.13 aC | 82.00 ± 1.33 aB | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA |
| GOB-250 µL/L air | 68.00 ± 2.91 aB | 76.00 ± 7.91 aB | 97.00 ± 1.52 aA | 99.00 ± 1.00 aA |
| GONE-250 µL/L air | 70.00 ± 1.49 aC | 88.00 ± 1.33 aB | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA |
| Oil Type | Exposure Time (Hours) | LC a | LC Value (LCL b–UCL c) | Slope (SE) | X2 (df) d |
|---|---|---|---|---|---|
| GOB | 24 | 50 | 130.45 (113.14–149.81) | 1.94 ± 0.24 | 1.95 (3) |
| 90 | 596.35 (431.88–1005.68) | ||||
| 48 | 50 | 65.88 (46.29–81.98) | 1.51 ± 0.23 | 1.37 (3) | |
| 90 | 462.28 (326.05–868.01) | ||||
| 72 | 50 | 22.77 (8.76–36.11) | 1.51 ± 0.28 | 3.93 (3) | |
| 90 | 159.77 (127.84–225.36) | ||||
| 96 | 50 | 20.26 (7.69–32.40) | 1.69 ± 0.31 | 4.37 (3) | |
| 90 | 115.13 (92.91–148.13) | ||||
| GONE | 24 | 50 | 127.65 (110.27–146.85) | 1.90 ± 0.24 | 0.85 (3) |
| 90 | 600.39 (432.29–1025.52 | ||||
| 48 | 50 | 61.44 (46.04–74.49) | 1.87 ± 0.24 | 0.92 (3) | |
| 90 | 296.35 (235.03–426.66) | ||||
| 72 | 50 | 22.64 (0.00–47.57) | 2.39 ± 0.42 | 8.61 (3) | |
| 90 | 77.84 (4.55–264.67) | ||||
| 96 | 50 | 19.86 (6.40–30.35) | 2.97 ± 0.72 | 1.43 (3) | |
| 90 | 53.56 (38.55–65.76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.S.; Gondhalekar, A.D.; Ristroph, K.; Baributsa, D. Geranium Oil Nanoemulsion Delivers More Potent and Persistent Fumigant Control of Callosobruchus maculatus in Stored Grain. Foods 2025, 14, 3514. https://doi.org/10.3390/foods14203514
Ibrahim SS, Gondhalekar AD, Ristroph K, Baributsa D. Geranium Oil Nanoemulsion Delivers More Potent and Persistent Fumigant Control of Callosobruchus maculatus in Stored Grain. Foods. 2025; 14(20):3514. https://doi.org/10.3390/foods14203514
Chicago/Turabian StyleIbrahim, Samar Sayed, Ameya D. Gondhalekar, Kurt Ristroph, and Dieudonne Baributsa. 2025. "Geranium Oil Nanoemulsion Delivers More Potent and Persistent Fumigant Control of Callosobruchus maculatus in Stored Grain" Foods 14, no. 20: 3514. https://doi.org/10.3390/foods14203514
APA StyleIbrahim, S. S., Gondhalekar, A. D., Ristroph, K., & Baributsa, D. (2025). Geranium Oil Nanoemulsion Delivers More Potent and Persistent Fumigant Control of Callosobruchus maculatus in Stored Grain. Foods, 14(20), 3514. https://doi.org/10.3390/foods14203514









