Genetic Algorithm-Driven Optimization of Mixed-Strain Fermentation for Improving the Physicochemical, Antioxidant, and Sensory Properties of Wampee (Clausena lansium (Lour.) Skeels) Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Growth Conditions
2.3. Sample Preparation
2.4. Experimental Design for the Mixed Fermentation of WJ
2.5. Optimization of Mixed Fermentation Models
2.6. Microbiological and Physicochemical Analyses of FWJ
2.6.1. Measurement of Viable Cell Counts of LAB
2.6.2. Analyses of pH and Organic Acid Composition
2.6.3. Determination of Color Indices
2.7. Analyses of Total Polyphenols Content (TPC) and Total Flavonoid Content (TFC) in FWJ
2.8. Antioxidant Activity Analysis of FWJ
2.9. E-Nose Analysis of FWJ
2.10. E-Tongue Analysis of FWJ
2.11. Sensory Evaluation of FWJ
2.12. HS-SPME-GC–MS Analysis of FWJ
2.13. HS-GC-IMS Analysis of FWJ
2.14. Statistical Analyses
3. Results
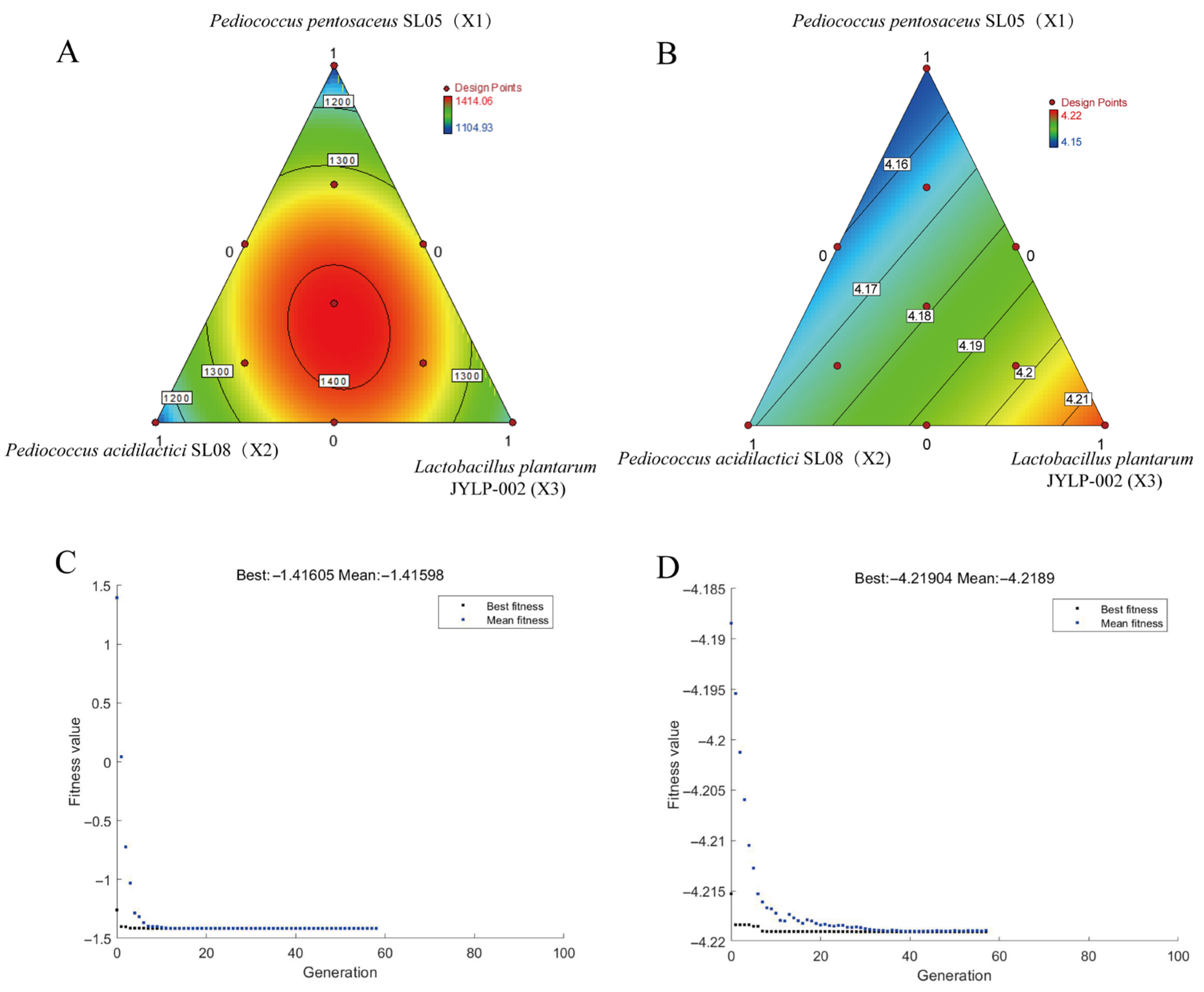
3.1. Optimization of Inoculation Ratio in Mixed LAB Fermentation
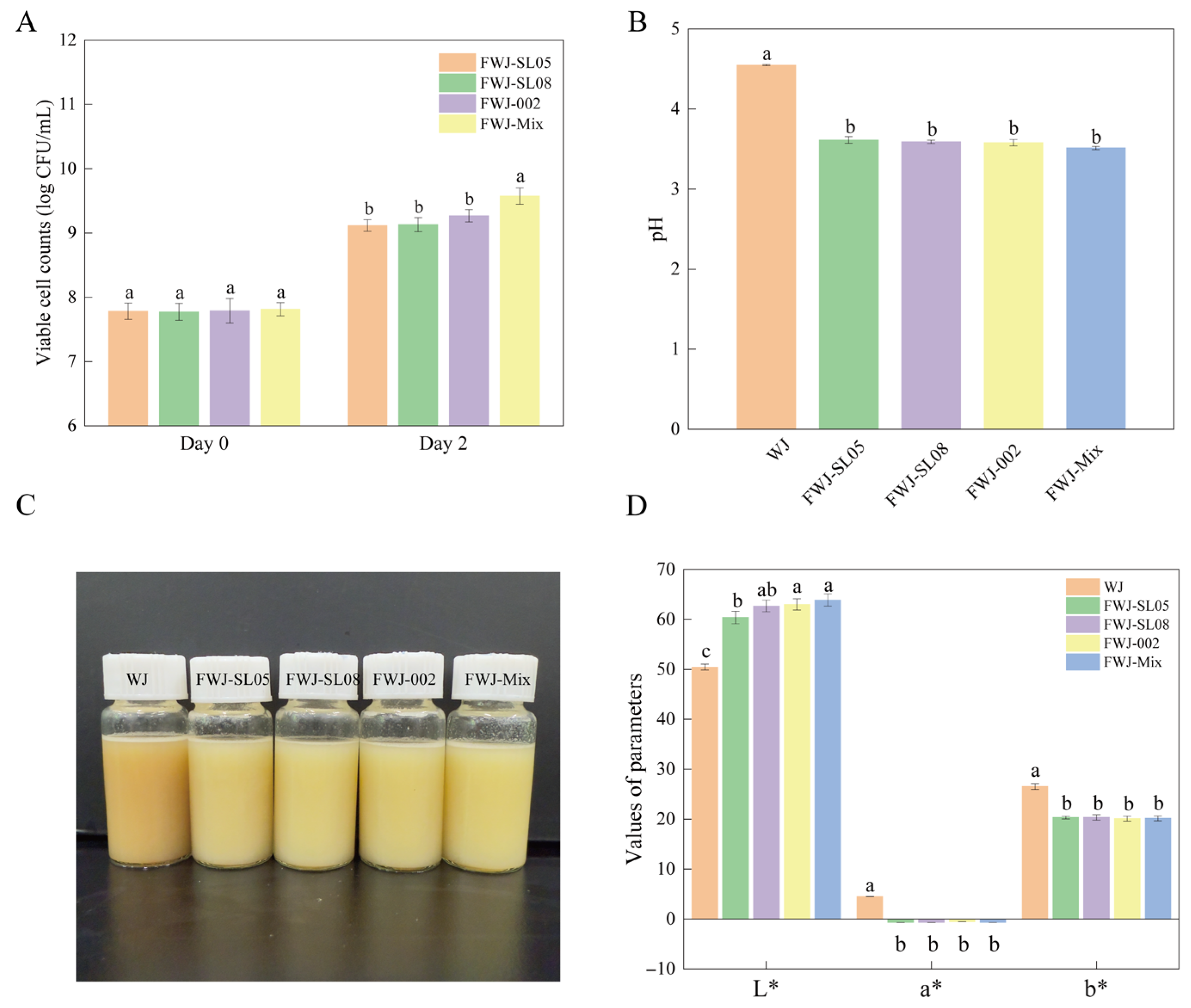
3.2. Physicochemical Characterization of FWJ Prepared via Single and Mixed LAB Fermentations
3.2.1. Comparison of Viable Cell Counts and pH
3.2.2. Comparison of Organic Acid Composition
3.2.3. Comparison of Color Indices
3.3. Comparison Analyses of the TPC, TFC, and Antioxidant Capacity of FWJ Prepared via Single and Mixed LAB Fermentations
3.4. Sensory Evaluation of FWJ Prepared via Single and Mixed LAB Fermentations
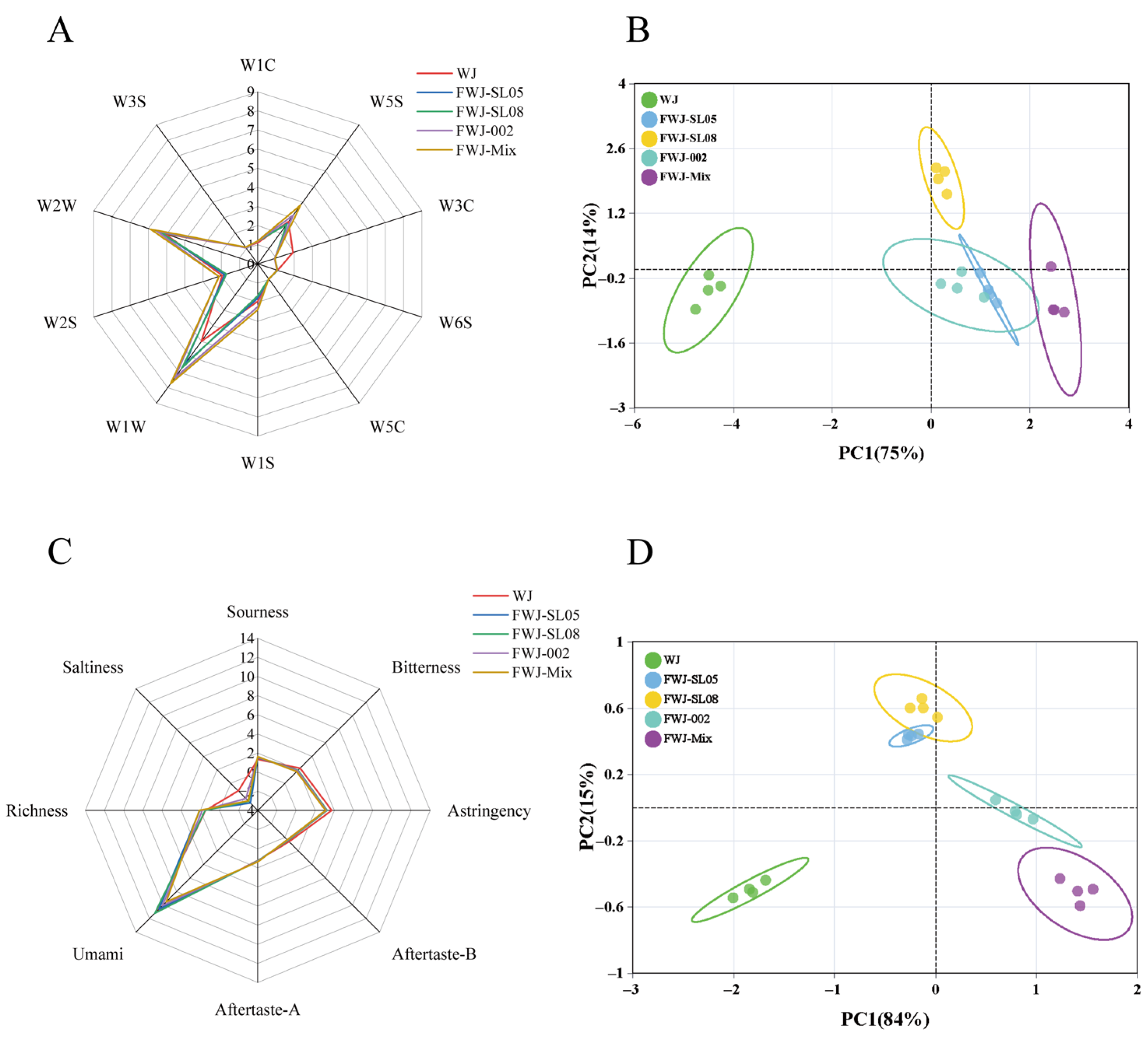
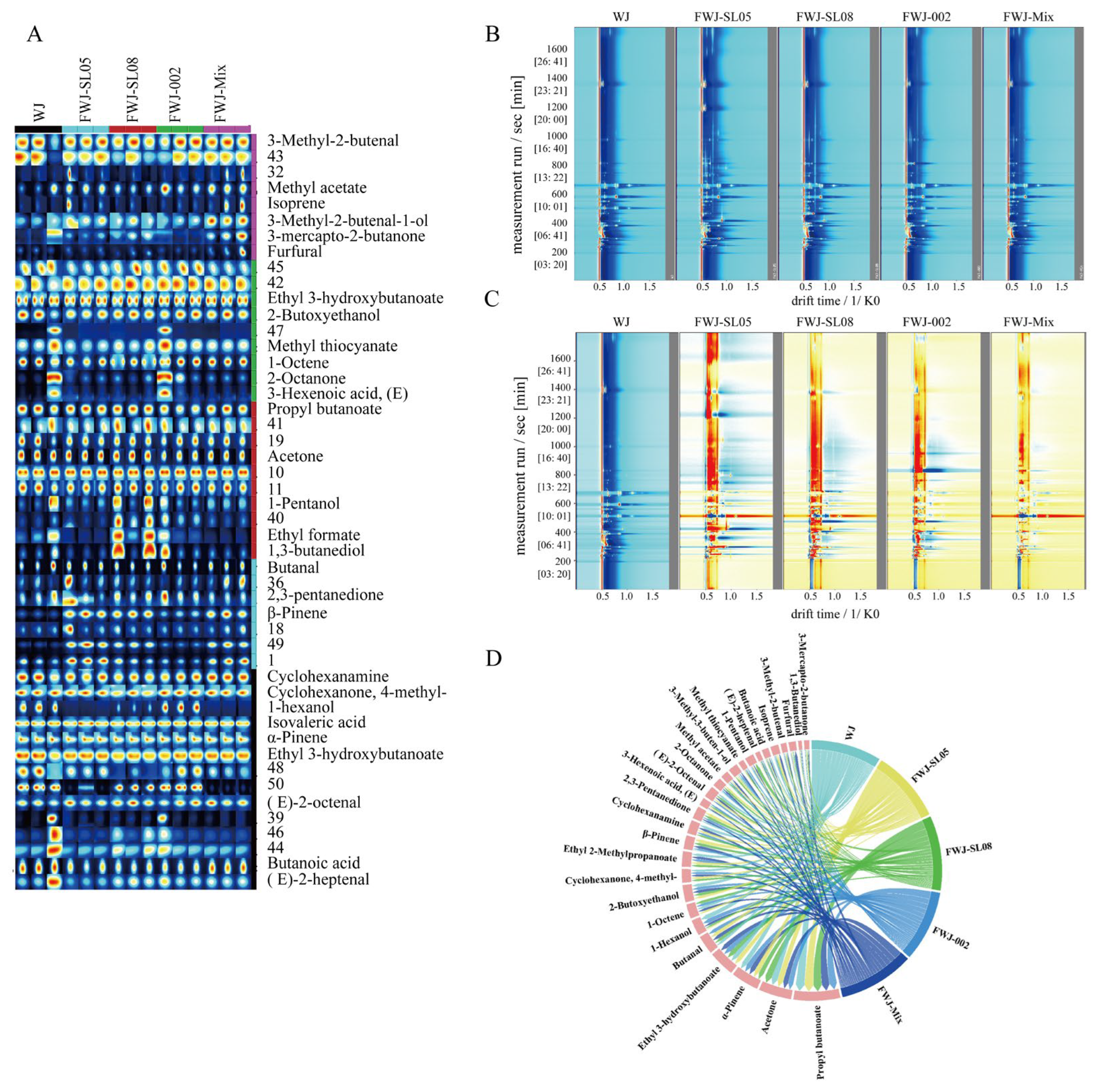
3.5. Flavor Profile Characterization of FWJ Prepared via Single and Mixed LAB Fermentations
3.6. Taste Profile Characterization of FWJ Prepared via Single and Mixed LAB Fermentations
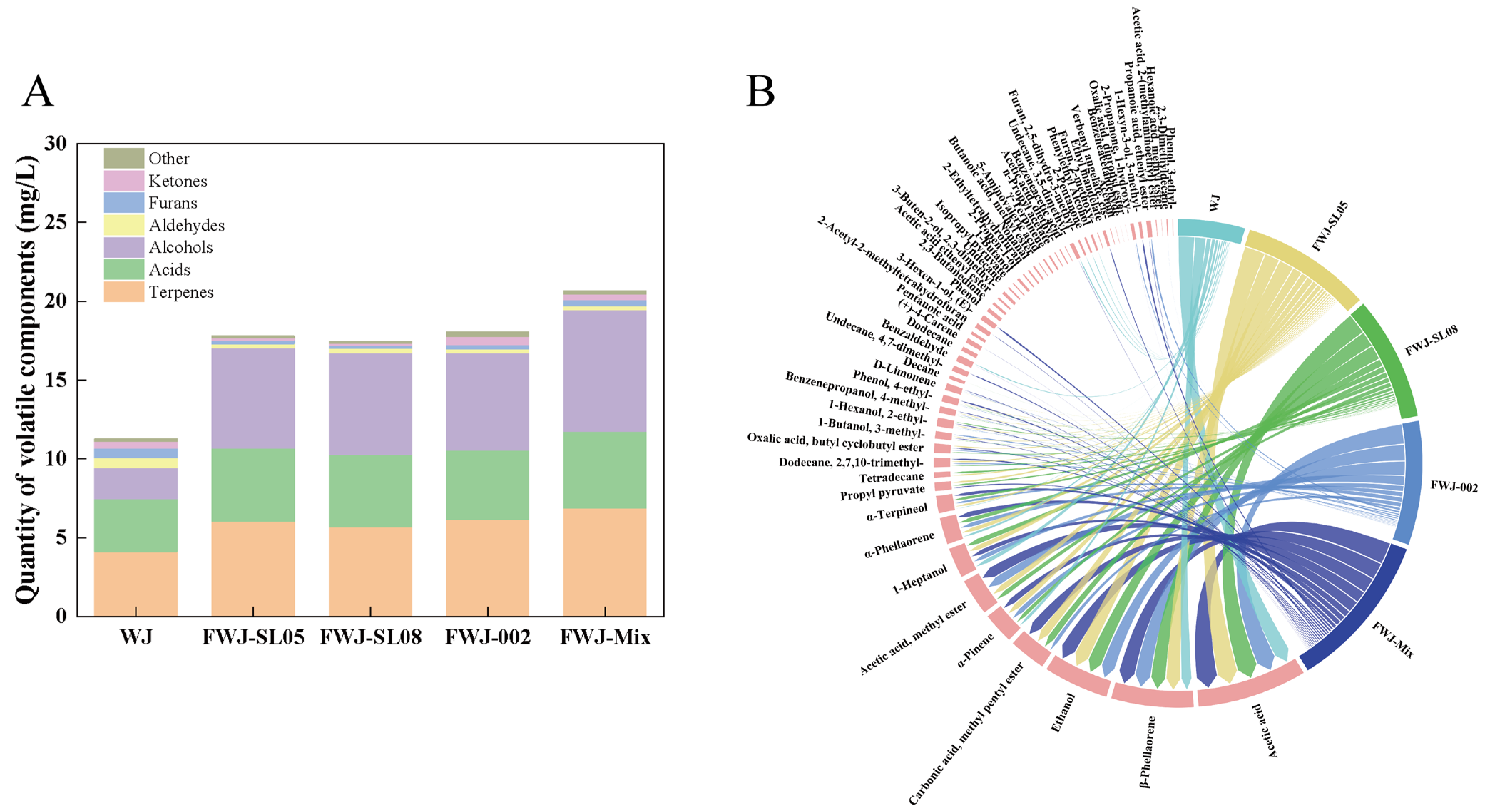
3.7. Analysis of Volatile Components in FWJ Prepared via Single and Mixed LAB Fermentations
3.7.1. Qualitative and Quantitative Analysis of VOCs via HS-SPME-GC-MS
3.7.2. Qualitative and Quantitative Analysis of VOCs via HS-GC-IMS
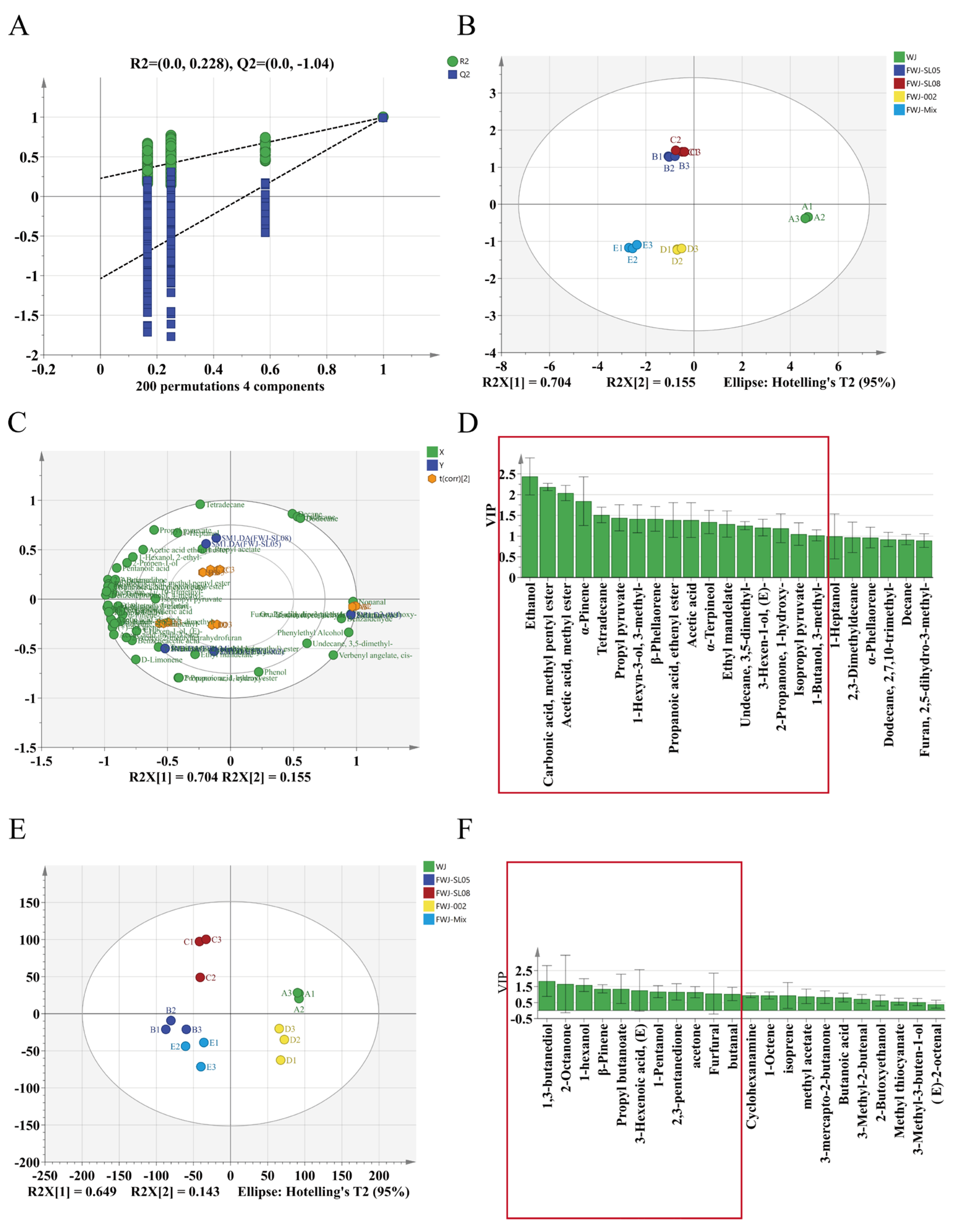
3.8. OPLS-DA Analysis of VOCs in FWJ Prepared via Single and Mixed LAB Fermentations
3.9. Identification of Key Aroma Contributors in FWJ Prepared via Single and Mixed LAB Fermentations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Xie, H.; Wei, X. Jasmonoid Glucosides, Sesquiterpenes and Coumarins from the Fruit of Clausena lansium. LWT-Food Sci. Technol. 2014, 59, 65–69. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Guo, J.-M.; Liu, Y.-Y.; Hu, S.; Yan, G.; Qiang, L.; Fu, Y.-H. Carbazole Alkaloids with Potential Neuroprotective Activities from the Fruits of Clausena lansium. J. Agric. Food Chem. 2019, 67, 5764–5771. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Yan, G.; Xie, Y.-T.; Lin, T.-C.; Zhang, W.; Li, J.; Wu, Y.-J.; Zhou, J.-Y.; Fu, Y.-H. Bioactive Prenylated Coumarins as Potential Anti-Inflammatory and Anti-HIV Agents from Clausena lenis. Bioorg. Chem. 2020, 97, 103699. [Google Scholar] [CrossRef]
- He, X.; Zhang, L.; Chen, J.; Sui, J.; Yi, G.; Wu, J.; Ma, Y. Correlation between Chemical Composition and Antifungal Activity of Clausena lansium Essential Oil against Candida spp. Molecules 2019, 24, 1394. Molecules 2019, 24, 1394. [Google Scholar] [CrossRef]
- Mo, X.; Cai, D.; Yang, H.; Chen, Q.; Xu, C.; Wang, J.; Tong, Z.; Xu, B. Changes in Fruit Quality Parameters and Volatile Compounds in Four Wampee Varieties at Different Ripening Stages. Food Chem. X 2025, 27, 102377. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Mao, X.; Liu, Z.; Wang, R.; Guo, L.; Fang, L.; Zhou, J. Improving the Flavor Components, Metabolic Characteristics, and Antioxidant Activity of American Ginseng by Lactic Acid Bacteria and Yeast Fermentation. LWT 2025, 231, 118363. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, X.; Liu, Q.; Yang, J.; Li, J.; Xiao, M.; Xiong, T.; Xie, M. Lactic Acid Bacteria Fermentation Improves Sensory Properties, Bioactivity, and Metabolic Profiles of Carrot Pulp. Food Biosci. 2025, 66, 106303. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; He, X.; Guo, M.; Chen, J.; Luo, L.; Xiang, J. Characterizations of Lactic Acid Bacteria Derived from Pickles and the Effects of Fermentation on Phenolic Compounds in Peony Flowers. Food Chem. X 2025, 27, 102430. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Li, Y.; Xie, Y.; Cui, M.; Hu, Y.; Yin, R.; Ma, X.; Niu, J.; Cheng, W.; et al. Enhancing Physicochemical Properties, Organic Acids, Antioxidant Capacity, Amino Acids and Volatile Compounds for ‘Summer Black’ Grape Juice by Lactic Acid Bacteria Fermentation. LWT 2024, 209, 116791. [Google Scholar] [CrossRef]
- Xing, Z.; Fu, X.; Huang, H.; Xu, Y.; Wei, L.; Shan, C.; Du, Y. Recent Advances in Lactobacillus plantarum Fermentation in Modifying Fruit-based Products: Flavor Property, Bioactivity, and Practical Production Applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70160. [Google Scholar] [CrossRef]
- Liu, N.; Qin, L.; Lu, X.; Zhao, Y.; Miao, S. Physicochemical Components and Flavor Properties of Acid Rice Soup (Rice-Acid) Fermented with Lactobacillus paracasei and/or Kluyveromyces marxianus. Food Biosci. 2021, 43, 101278. [Google Scholar] [CrossRef]
- Lv, M.; Liu, X.; Liu, R.; Aihaiti, A.; Hong, J.; Zheng, L.; Xing, J.; Cui, Y.; Wang, L. Untargeted Metabolomics Reveals Flavor and Metabolic Changes in Mixed Lactobacillus-Fermented Black Mulberry Juice. Food Chem. X 2025, 27, 102367. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, W.; Wang, L.; Chen, Y.; Cai, Z.; Wu, D.; Chen, N.; Jiang, Q.; Zheng, Z.; Zhu, J.; et al. Co-Fermentation of Lactobacillus plantarum and Lactobacillus casei Improves In Vitro Antioxidant Capacity and Quality of Apple Juice. Fermentation 2025, 11, 161. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Y.; Wang, W.; Ye, H.; Zhang, Q. Enhancing the Quality of Low-Alcohol Navel Orange Wine through Simultaneous Co-Fermentation Using Saccharomyces cerevisiae SC-125, Angel Yeast SY, and Lactiplantibacillus plantarum BC114. Molecules 2024, 29, 1781. [Google Scholar] [CrossRef]
- Scheffe, H. Experiments with mixtures. J. R. Stat. Soc. Ser. B. Methodol. 1958, 20, 344–360. [Google Scholar] [CrossRef]
- Liao, W.; Shen, J.; Manickam, S.; Li, S.; Tao, Y.; Li, D.; Liu, D.; Han, Y. Investigation of Blueberry Juice Fermentation by Mixed Probiotic Strains: Regression Modeling, Machine Learning Optimization and Comparison with Fermentation by Single Strain in the Phenolic and Volatile Profiles. Food Chem. 2023, 405, 134982. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Z.; Sun, Z.; Xu, J.; Meng, X.; Wang, H.; Wang, R.; Chen, F.; Hu, X.; Zhang, J. The Fermentation of Carambola Juice with Lactic Acid Bacteria Improves Its Flavor, Bioactive Properties, and Metabolic Composition. Food Biosci. 2025, 66, 106307. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Li, S. Enhancement in the Physicochemical Properties, Antioxidant Activity, Volatile Compounds, and Non-Volatile Compounds of Watermelon Juices through Lactobacillus plantarum JHT78 Fermentation. Food Chem. 2023, 420, 136146. [Google Scholar] [CrossRef]
- Borahan, T.; Girgin, A.; Atsever, N.; Zaman, B.T.; Chormey, D.S.; Bakırdere, S. Development of a Double-Monitoring Method for the Determination of Total Antioxidant Capacity as Ascorbic Acid Equivalent Using CUPRAC Assay with RP-HPLC and Digital Image-Based Colorimetric Detection. Eur. Food Res. Technol. 2022, 248, 707–713. [Google Scholar] [CrossRef]
- Liao, Y.; Cheng, Y.; Zheng, J.; Li, Z.; Wang, F.; Li, L. ·Lactic Acid Bacteria Fermentation Advances in Fruit-Vegetables: Enhancing Quality and Bioactivity. Food Bioprocess Technol. 2025, 18, 10004–10024. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of Blueberry Juices Using Autochthonous Lactic Acid Bacteria Isolated from Fruit Environment: Fermentation Characteristics and Evolution of Phenolic Profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef]
- Gao, B.; Wang, J.; Wang, Y.; Xu, Z.; Li, B.; Meng, X.; Sun, X.; Zhu, J. Influence of Fermentation by Lactic Acid Bacteria and in Vitro Digestion on the Biotransformations of Blueberry Juice Phenolics. Food Control 2022, 133, 108603. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, M.; Yang, X.; Xu, J.; Yu, Z. Effect of Cutting and Storage Temperature on Sucrose and Organic Acids Metabolism in Postharvest Melon Fruit. Postharvest Biol. Technol. 2020, 161, 111081. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, R.; Wang, W.; Sun, T.; Han, X.; Ai, M.; Huang, S. Study on Nutritional Characteristics, Antioxidant Activity, and Volatile Compounds in Non-Saccharomyces cerevisiae–Lactiplantibacillus plantarum Co-Fermented Prune Juice. Foods 2025, 14, 1966. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Sun, S.; Chen, H.Y.; Ji, C.; Zhou, Z. Metabolomic and Bioactivity Analysis of Plum Pulp Fermented with Lactiplantibacillus, Lacticaseibacillus, and Bifidobacterium Strains. Food Chem. X 2025, 30, 102969. [Google Scholar] [CrossRef] [PubMed]
- Parada, R.B.; Marguet, E.; Campos, C.; Vallejo, M. Improved Antioxidant Capacity of Three Brassica Vegetables by Two-Step Controlled Fermentation Using Isolated Autochthone Strains of the Genus Leuconostoc spp. and Lactiplantibacillus spp. Food Chem. Mol. Sci. 2023, 6, 100163. [Google Scholar] [CrossRef]
- Quan, Q.; Liu, W.; Guo, J.; Ye, M.; Zhang, J. Effect of Six Lactic Acid Bacteria Strains on Physicochemical Characteristics, Antioxidant Activities and Sensory Properties of Fermented Orange Juices. Foods 2022, 11, 1920. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-B.; Lei, Y.-T.; Li, Q.-Z.; Li, Y.-C.; Deng, Y.; Liu, D.-Y. Effect of Lactobacillus plantarum and Lactobacillus acidophilus Fermentation on Antioxidant Activity and Metabolomic Profiles of Loquat Juice. LWT 2022, 171, 114104. [Google Scholar] [CrossRef]
- Yaqoob, S.; Gouda, M.M.; Mubeen, B.; Imtiaz, A.; Murtaza, M.S.; Awan, K.A.; Rehman, A.; Alsulami, T.; Khalifa, I.; Ma, Y. Synergistic Enhancement in the Mulberry Juice’s Antioxidant Activity by Using Lactic Acid Bacteria Co-Culture Fermentation. Sci. Rep. 2025, 15, 19453. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Li, C.; Zhang, X.; Wang, G.; Shen, Y.; Wang, Y.; Liu, X.; Sun, L. Addition of Cyperus Esculentus (Tiger Nut) Milk Improved the Flavor and Gelation Properties of Set Yogurt: The Main Contribution of Volatile Constituents, Starch and Proteins. Food Hydrocoll. 2024, 155, 110212. [Google Scholar] [CrossRef]
- Varghese, C.; Srivastav, P.P. Formulation and Sensory Characterization of Low-Cost, High-Energy, Nutritious Cookies for Undernourished Adolescents: An Approach Using Linear Programming and Fuzzy Logic. Innov. Food Sci. Emerg. Technol. 2022, 75, 102904. [Google Scholar] [CrossRef]
- Hu, A.; Wu, S. Formulation of Gracilaria Lemaneiformis Jelly Using Fuzzy Mathematics Evaluation. Food Chem. X 2025, 28, 102612. [Google Scholar] [CrossRef]
- Cai, H.; Liu, Y.; Jin, W.; Li, F.; Chen, X.; Yang, G.; Shen, W. Construction of Sensory Evaluation System of Purple Sweet Potato Rice Steamed Sponge Cake Based on Fuzzy Mathematics. Foods 2024, 13, 3527. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Zhang, Y.-H.; Chen, G.-S.; Yin, J.-F.; Chen, J.-X.; Wang, F.; Xu, Y.-Q. Effects of Phenolic Acids and Quercetin-3-O-Rutinoside on the Bitterness and Astringency of Green Tea Infusion. npj Sci. Food 2022, 6, 8. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Tan, W.; Ren, J.; Hou, M.; Wang, Z.; Guo, C.; Gao, Z. Lactic Acid Bacteria Fermentation of Prebiotic-Supplemented Apple Juice: Viable Counts and Flavor Evolution Revealed by HS-SPME-GC–MS Coupled with Electronic Sensory and Chemometrics. Food Chem. 2025, 486, 144634. [Google Scholar] [CrossRef]
- Schanzmann, H.; Gaar, S.; Keip, S.; Telgheder, U.; Sielemann, S. Comparison of the Quantification Performance of Thermal Desorption GC-IMS and GC-MS in VOC Analysis. Anal. Bioanal. Chem. 2025, 417, 4179–4198. [Google Scholar] [CrossRef]
- Ji, G.; Liu, G.; Li, B.; Tan, H.; Zheng, R.; Sun, X.; He, F. Influence on the Aroma Substances and Functional Ingredients of Apple Juice by Lactic Acid Bacteria Fermentation. Food Biosci. 2023, 51, 102337. [Google Scholar] [CrossRef]
- Chen, Y.; Ouyang, X.; Laaksonen, O.; Liu, X.; Shao, Y.; Zhao, H.; Zhang, B.; Zhu, B. Effect of Lactobacillus acidophilus, Oenococcus oeni, and Lactobacillus brevis on Composition of Bog Bilberry Juice. Foods 2019, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, Y.; Aihaiti, A.; Wang, L.; Wang, Y.; Xing, J.; Zhu, M.; Hong, J. The Metabolic Pathways of Yeast and Acetic Acid Bacteria during Fruit Vinegar Fermentation and Their Influence on Flavor Development. Microorganisms 2025, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chan, L.-J.; Li, X.; Liu, S.-Q. Effects of Different Inoculation Strategies of Saccharomyces cerevisiae and Williopsis saturnus on Chemical Components of Mango Wine. LWT 2018, 87, 85–92. [Google Scholar] [CrossRef]
- Yuan, H.-W.; Zhang, C.; Chen, S.-Y.; Zhao, Y.; Tie, Y.; Yin, L.-G.; Chen, J.; Wu, Q.-D.; Wang, Y.-T.; Xu, Z.; et al. Effect of Different Moulds on Oenological Properties and Flavor Characteristics in Rice Wine. LWT 2023, 173, 114201. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; Garcia, H.S.; Reyes-Díaz, R.; Estrada-Montoya, M.C.; Torres-Llanez, M.J.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B. Cooperation between Lactococcus lactis NRRL B-50571 and NRRL B-50572 for Aroma Formation in Fermented Milk. Foods 2019, 8, 645. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic Acid Fermentation Drives the Optimal Volatile Flavor-Aroma Profile of Pomegranate Juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS Discriminant Analysis: Combining the Strengths of PLS-DA and SIMCA Classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Xu, H.; Pan, S.; Wang, J.; Ye, T.; Yan, M.; Liang, X.; Qian, G.; Yan, T.; Xin, G. Comparative Characterization of Volatile Organic Compounds and Aroma Profiles in 10 Actinidia arguta Cultivars by Gas Chromatography-Mass Spectrometry (GC–MS), Sensory Analysis, and Odor Activity Value (OAV) Combined with Chemometrics. J. Food Compos. Anal. 2024, 133, 106450. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, Y.; Liu, Y.; Shi, Y.; Lin, X.; Wang, L.; Wen, P.; Hu, X.; Li, J. Comprehensive Evaluation of Aroma and Taste Properties of Different Parts from the Wampee Fruit. Food Chem. X 2023, 19, 100835. [Google Scholar] [CrossRef]
| Index | WJ | FWJ-SL05 | FWJ-SL08 | FWJ-002 | FWJ-Mix |
|---|---|---|---|---|---|
| Lactic acid (mg/mL) | ND | 4.16 ± 0.17 a | 4.10 ± 0.11 a | 4.21 ± 0.13 a | 4.22 ± 0.13 a |
| Acetic acid (mg/mL) | 0.49 ± 0.05 b | 0.83 ± 0.06 a | 0.85 ± 0.06 a | 0.81 ± 0.02 a | 0.85 ± 0.08 a |
| Succinic acid (mg/mL) | 0.64 ± 0.02 c | 1.32 ± 0.06 b | 1.31 ± 0.07 b | 1.41 ± 0.04 ab | 1.46 ± 0.01 a |
| Pyruvic acid (mg/mL) | 0.007 ± 0.001 a | 0.010 ± 0.001 a | 0.010 ± 0.001 a | 0.011 ± 0.001 a | 0.010 ± 0.001 a |
| Tartaric acid (mg/mL) | 0.19 ± 0.01 b | 0.35 ± 0.07 a | 0.38 ± 0.06 a | 0.49 ± 0.07 a | 0.52 ± 0.09 a |
| Malic acid (mg/mL) | 0.66 ± 0.04 a | 0.05 ± 0.01 b | 0.05 ± 0.01 b | 0.04 ± 0.01 b | 0.08 ± 0.02 b |
| Citric acid (mg/mL) | 1.59 ± 0.15 a | 0.31 ± 0.02 b | 0.37 ± 0.03 b | 0.33 ± 0.07 b | 0.39 ± 0.09 b |
| Total organic acid (mg/mL) | 3.87 ± 0.19 c | 7.37 ± 0.11 b | 7.52 ± 0.17 b | 7.62 ± 0.21 ab | 7.91 ± 0.15 a |
| TPC (mg GAE/L) | 847.23 ± 14.90 e | 1121.93 ± 6.67 d | 1182.12 ± 11.02 c | 1211.7 ± 5.18 b | 1421.85 ± 36.68 a |
| TFC (mg RE/L) | 93.25 ± 0.30 d | 143.26 ± 1.77 c | 140.87 ± 1.10 c | 151.95 ± 0.82 b | 163.25 ± 0.57 a |
| ABTS (μmol TE/mL) | 1.13 ± 0.05 b | 1.45 ± 0.04 a | 1.43 ± 0.04 a | 1.46 ± 0.07 a | 1.54 ± 0.08 a |
| CURAP (μmol TE/mL) | 3.39 ± 0.09 c | 3.62 ± 0.07 b | 3.65 ± 0.06 b | 3.66 ± 0.07 b | 3.97 ± 0.11 a |
| Sensory evaluation score | 81.88 | 88.5 | 89 | 90.125 | 91 |
| Category | RI1 | RI2 | VOCs | Formula | Content (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|---|
| WJ | FWJ-SL05 | FWJ-SL08 | FWJ-002 | FWJ-Mix | |||||
| Aldehydes | 887 | 887 | Nonanal | C9H18O | 0.19 ± 0.01 a | 0.06 ± 0.00 b | 0.03 ± 0.00 b | 0.04 ± 0.00 b | 0.01 ± 0.00 b |
| 900 | 900 | Benzeneacetaldehyde | C8H8O | 0.02 ± 0.00 a | ND | ND | ND | ND | |
| 940 | 943 | Benzaldehyde | C7H6O | 0.42 ± 0.02 a | 0.20 ± 0.01 b | 0.21 ± 0.01 b | 0.19 ± 0.01 b | 0.24 ± 0.01 b | |
| Terpenes | 877 | 877 | D-Limonene | C10H16 | 0.20 ± 0.01 a | 0.25 ± 0.01 a | 0.22 ± 0.01 a | 0.29 ± 0.01 b | 0.34 ± 0.01 b |
| 858 | 858 | γ-Terpinene | C10H16 | 0.03 ± 0.00 b | 0.04 ± 0.00 b | 0.04 ± 0.00 b | 0.04 ± 0.00 b | 0.19 ± 0.01 a | |
| 908 | 908 | β-Phellandrene | C10H16 | 2.32 ± 0.13 b | 3.16 ± 0.13 a | 3.18 ± 0.13 a | 3.37 ± 0.13 a | 3.45 ± 0.14 a | |
| 893 | 893 | α-Pinene | C10H16 | 0.83 ± 0.05 d | 1.39 ± 0.06 a | 1.01 ± 0.04 c | 1.23 ± 0.05 b | 1.45 ± 0.06 a | |
| 905 | 905 | α-Phellandrene | C10H16 | 0.68 ± 0.04 c | 1.03 ± 0.04 b | 1.06 ± 0.04 b | 1.06 ± 0.04 b | 1.22 ± 0.05 a | |
| 912 | 912 | (+)-4-Carene | C10H16 | 0.10 ± 0.01 c | 0.18 ± 0.01 b | 0.17 ± 0.01 b | 0.19 ± 0.01 b | 0.25 ± 0.01 a | |
| Ketones | 843 | 843 | 2-Pentanone | C5H10O | 0.36 ± 0.02 a | ND | ND | ND | ND |
| 889 | 889 | 2,3-Butanedione | C4H6O2 | ND | 0.13 ± 0.01 a | 0.12 ± 0.00 a | 0.11 ± 0.00 a | 0.13 ± 0.01 a | |
| 916 | 916 | 2-Propanone, 1-hydroxy- | C3H6O2 | ND | ND | ND | 0.41 ± 0.02 a | 0.23 ± 0.01 b | |
| 963 | 963 | Acetoin | C3H6O | 0.06 ± 0.00 a | ND | ND | ND | ND | |
| Alcohols | 883 | 883 | Phenylethyl Alcohol | C8H10O | 0.3 ± 0.02 a | ND | ND | 0.1 ± 0.00 b | ND |
| 923 | 923 | Ethanol | C2H6O | ND | 3.11 ± 0.12 a | 3.02 ± 0.12 a | 2.94 ± 0.12 a | 3.18 ± 0.13 a | |
| 863 | 863 | 3-Hexen-1-ol, (E)- | C6H12O | ND | 0.15 ± 0.01 b | 0.17 ± 0.01 b | 0.12 ± 0.00 b | 0.56 ± 0.02 a | |
| 984 | 984 | 2-Propen-1-ol | C3H6O | ND | 0.08 ± 0.00 a | 0.07 ± 0.00 a | 0.02 ± 0.00 b | 0.09 ± 0.00 a | |
| 917 | 917 | Benzenepropanol, 4-methyl- | C10H14O | ND | 0.32 ± 0.01 a | 0.27 ± 0.01 a | 0.29 ± 0.01 a | 0.36 ± 0.01 a | |
| 914 | 914 | 1-Hexanol, 2-ethyl- | C8H18O | 0.24 ± 0.01 b | 0.35 ± 0.01 a | 0.39 ± 0.02 a | 0.28 ± 0.01 b | 0.38 ± 0.02 a | |
| 924 | 924 | 1-Heptanol | C7H16O | 1.06 ± 0.06 b | 1.17 ± 0.05 a | 1.26 ± 0.05 a | 1.09 ± 0.04 b | 1.13 ± 0.05 b | |
| 903 | 903 | 1-Butanol, 3-methyl- | C5H12O | ND | 0.36 ± 0.01 b | 0.27 ± 0.01 c | 0.43 ± 0.02 a | 0.34 ± 0.01 b | |
| 834 | 855 | 1-Butanol | C4H10O | ND | 0.09 ± 0.00 b | 0.11 ± 0.00 a | 0.14 ± 0.01 a | 0.15 ± 0.01 a | |
| 904 | 904 | 3-Buten-2-ol, 2,3-dimethyl- | C6H12O | 0.07 ± 0.00 b | 0.11 ± 0.00 a | 0.12 ± 0.00 a | 0.14 ± 0.01 a | 0.13 ± 0.01 a | |
| 863 | 863 | 1-Hexyn-3-ol, 3-methyl- | C7H12O | ND | ND | ND | ND | 0.56 ± 0.02 a | |
| 854 | 854 | α-Terpineol | C10H18O | 0.31 ± 0.02 c | 0.63 ± 0.03 b | 0.79 ± 0.03 a | 0.66 ± 0.03 b | 0.84 ± 0.03 a | |
| Esters | 906 | 906 | Verbenyl angelate, cis- | C15H22O2 | 0.07 ± 0 a | ND | ND | 0.03 ± 0.00 b | 0.02 ± 0.00 b |
| 902 | 902 | Propyl pyruvate | C6H10O3 | ND | 0.63 ± 0.03 a | 0.55 ± 0.02 b | ND | 0.44 ± 0.02 c | |
| 822 | 822 | Carbonic acid, methyl pentyl ester | C7H14O3 | ND | 1.97 ± 0.08 b | 1.59 ± 0.06 c | 0.98 ± 0.04 d | 2.43 ± 0.10 a | |
| 850 | 852 | Propanoic acid, ethenyl ester | C5H8O2 | ND | ND | ND | 0.56 ± 0.02 a | 0.31 ± 0.01 b | |
| 999 | 999 | Acetic acid, methyl ester | C3H6O2 | ND | 1.39 ± 0.06 c | 1.16 ± 0.05 d | 1.98 ± 0.08 b | 2.37 ± 0.09 a | |
| 806 | 806 | Ethyl mandelate | C10H12O3 | 0.11 ± 0.01 b | ND | ND | ND | 0.43 ± 0.02 a | |
| 864 | 864 | Acetic acid, 2-(methylaminoethyl) ester | C5H11NO2 | ND | ND | ND | ND | 0.13 ± 0.01 a | |
| 949 | 949 | Acetic acid ethenyl ester | C4H6O2 | ND | 0.13 ± 0.01 a | 0.11 ± 0.00 a | ND | 0.13 ± 0.01 a | |
| 898 | 898 | Oxalic acid, butyl cyclobutyl ester | C10H16O4 | 0.09 ± 0.01 b | 0.42 ± 0.02 a | 0.38 ± 0.02 a | 0.39 ± 0.02 a | 0.42 ± 0.02 a | |
| 995 | 995 | Isopropyl pyruvate | C6H10O3 | ND | 0.10 ± 0.00 a | ND | ND | 0.11 ± 0.00 a | |
| 916 | 916 | Butanoic acid, methyl ester | C5H10O2 | ND | 0.05 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.05 ± 0.00 a | |
| 986 | 986 | n-Propyl acetate | C5H10O2 | 0.03 ± 0.00 a | 0.04 ± 0.00 a | 0.05 ± 0.00 a | ND | 0.05 ± 0.00 a | |
| 824 | 824 | Hexanoic acid, methyl ester | C7H14O2 | ND | ND | ND | ND | 0.05 ± 0.00 a | |
| 878 | 878 | Oxalic acid, dipropyl ester | C8H14O4 | 0.02 ± 0.00 a | ND | ND | ND | ND | |
| Acids | 955 | 955 | Acetic acid | C2H4O2 | 3.38 ± 0.19 b | 4.34 ± 0.17 a | 4.33 ± 0.17 a | 4.09 ± 0.16 a | 4.47 ± 0.18 a |
| 917 | 917 | Acetic acid, diethyl- | C6H12O2 | ND | 0.04 ± 0.00 c | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.08 ± 0.00 a | |
| 883 | 883 | Benzeneacetic acid | C8H8O2 | ND | 0.01 ± 0.00 c | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.06 ± 0.00 a | |
| 827 | 827 | Pentanoic acid | C5H10O2 | ND | 0.18 ± 0.01 a | 0.12 ± 0.00 b | 0.11 ± 0.00 b | 0.14 ± 0.01 b | |
| 806 | 806 | 5-Aminovaleric acid | C5H11NO2 | ND | 0.05 ± 0.00 a | 0.08 ± 0.00 a | 0.09 ± 0.00 a | 0.08 ± 0.00 a | |
| Furans | 887 | 887 | 2-Acetyl-2-methyltetrahydrofuran | C7H12O2 | 0.09 ± 0.01 c | 0.16 ± 0.01 b | 0.17 ± 0.01 b | 0.18 ± 0.01 b | 0.31 ± 0.01 a |
| 933 | 935 | Furan, 2-methoxy- | C5H6O2 | 0.13 ± 0.01 a | ND | ND | ND | ND | |
| 846 | 846 | Furan, 2,5-dihydro-3-methyl- | C5H8O | 0.39 ± 0.02 a | ND | ND | ND | ND | |
| 840 | 840 | 2-Ethyltetrahydrofuran | C6H12O | ND | 0.08 ± 0.00 a | 0.06 ± 0.00 a | 0.08 ± 0.00 a | 0.09 ± 0.00 a | |
| Hydrocarbon | 929 | 929 | Decane | C10H22 | 0.21 ± 0.01 a | 0.22 ± 0.01 a | 0.23 ± 0.01 a | ND | ND |
| 856 | 856 | Undecane | C11H24 | 0.1 ± 0.01 a | 0.1 ± 0.00 a | 0.1 ± 0.00 a | ND | ND | |
| 846 | 846 | Undecane, 4,7-dimethyl- | C13H28 | ND | 0.21 ± 0.01 b | 0.2 ± 0.01 b | 0.21 ± 0.01 b | 0.43 ± 0.04 a | |
| 868 | 868 | Undecane, 3,5-dimethyl- | C13H28 | 0.46 ± 0.03 a | ND | ND | ND | 0.29 ± 0.01 b | |
| 826 | 826 | Dodecane | C12H26 | 0.20 ± 0.01 a | 0.20 ± 0.01 a | 0.20 ± 0.01 a | 0.12 ± 0.00 b | 0.11 ± 0.00 b | |
| 867 | 867 | Dodecane, 2,7,10-trimethyl- | C15H32 | ND | 0.45 ± 0.02 b | 0.41 ± 0.02 b | 0.43 ± 0.02 b | 0.49 ± 0.02 a | |
| 918 | 918 | 2,3-Dimethyldecane | C12H26 | ND | ND | ND | 0.23 ± 0.01 a | ND | |
| 805 | 805 | Tetradecane | C14H30 | ND | 0.47 ± 0.02 b | 0.57 ± 0.02 a | ND | ND | |
| Other | 922 | 922 | Phenol, 4-ethyl- | C8H10O | ND | 0.29 ± 0.01 b | 0.28 ± 0.01 b | 0.27 ± 0.01 b | 0.49 ± 0.02 a |
| 883 | 883 | Phenol, 3-ethyl- | C8H10O | ND | ND | ND | 0.13 ± 0.01 a | ND | |
| 907 | 907 | Phenol | C6H6O | 0.17 ± 0.01 a | 0.13 ± 0.01 b | 0.11 ± 0.00 b | 0.14 ± 0.01 a | 0.18 ± 0.01 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Zhang, L.; Liu, R.; Chen, H.; Zhao, Z.; Li, X.; Cai, K.; Zhang, W.; Hu, X.; Lin, X. Genetic Algorithm-Driven Optimization of Mixed-Strain Fermentation for Improving the Physicochemical, Antioxidant, and Sensory Properties of Wampee (Clausena lansium (Lour.) Skeels) Juice. Foods 2025, 14, 4001. https://doi.org/10.3390/foods14234001
Zhong X, Zhang L, Liu R, Chen H, Zhao Z, Li X, Cai K, Zhang W, Hu X, Lin X. Genetic Algorithm-Driven Optimization of Mixed-Strain Fermentation for Improving the Physicochemical, Antioxidant, and Sensory Properties of Wampee (Clausena lansium (Lour.) Skeels) Juice. Foods. 2025; 14(23):4001. https://doi.org/10.3390/foods14234001
Chicago/Turabian StyleZhong, Xianquan, Lin Zhang, Rong Liu, Hua Chen, Zhiheng Zhao, Xiaonuo Li, Kun Cai, Weimin Zhang, Xiaoping Hu, and Xue Lin. 2025. "Genetic Algorithm-Driven Optimization of Mixed-Strain Fermentation for Improving the Physicochemical, Antioxidant, and Sensory Properties of Wampee (Clausena lansium (Lour.) Skeels) Juice" Foods 14, no. 23: 4001. https://doi.org/10.3390/foods14234001
APA StyleZhong, X., Zhang, L., Liu, R., Chen, H., Zhao, Z., Li, X., Cai, K., Zhang, W., Hu, X., & Lin, X. (2025). Genetic Algorithm-Driven Optimization of Mixed-Strain Fermentation for Improving the Physicochemical, Antioxidant, and Sensory Properties of Wampee (Clausena lansium (Lour.) Skeels) Juice. Foods, 14(23), 4001. https://doi.org/10.3390/foods14234001





