An Exploratory Study of the Nutritional Composition and Caco-2 Safety Assessment of Elche Date Flour and Its Green Hydroethanolic Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents
2.3. Computer Vision System (CVS)
- Image Acquisition:
- Image Processing and Analysis:
- Segmentation:
2.4. Mineral and Heavy Metals Content
2.5. Extraction Procedure
2.6. Chemical Analysis
2.6.1. Total Carbohydrate Content
2.6.2. Total Protein Content
2.6.3. Total Phenolic Content
2.6.4. Total Antioxidant Capacity
2.6.5. Total Carotenoid Content
2.6.6. Cell Culture and Viability Assessment
- Caco-2 Cells Culture
- Cell Viability Assay
2.7. Statistical Analysis
3. Results and Discussion
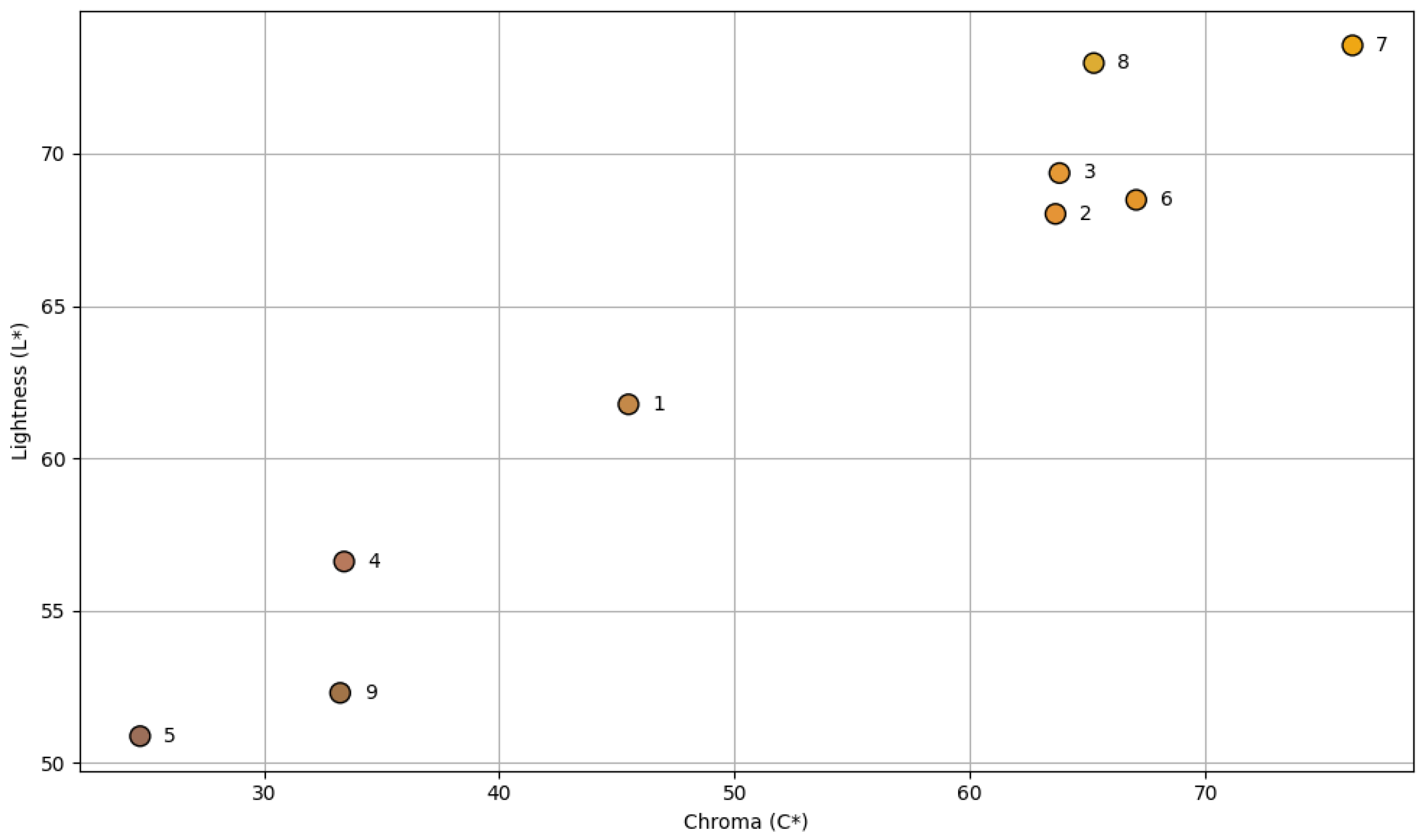
3.1. Computer Vision System Analysis

3.2. Mineral and Heavy Metal Composition
| Cultivars | As (µg/kg Dry Mass) | Cd (µg/kg Dry Mass) | Hg (µg/kg Dry Mass) | Pb (µg/kg Dry Mass) |
|---|---|---|---|---|
| D1 | 45.80 ± 1.80 a | 2.00 ± 0.40 b | <15.00 | 16.50 ± 1.00 e |
| D2 | 37.50 ± 1.20 c | <1.00 d | <15.00 | 33.90 ± 1.60 c |
| D3 | 29.40 ± 1.90 d | 1.46 ± 0.03 b | <15.00 | 43.40 ± 1.80 b |
| D4 | 29.20 ± 1.50 d | 1.29 ± 0.02 b | <15.00 | 31.90 ± 1.10 c |
| D5 | 17.70 ± 1.20 f | 1.64 ± 0.04 b | <15.00 | 17.50 ± 0.60 e |
| D6 | 19.70 ± 0.60 f | <1.00 d | <15.00 | 25.40 ± 1.10 d |
| D7 | 25.00 ± 1.00 e | <1.00 d | <15.00 | 41.00 ± 1.40 b |
| D8 | 20.40 ± 1.30 f | 15.40 ± 0.80 a | <15.00 | 113.00 ± 2.00 a |
| D9 | 41.90 ± 0.50 b | <1.00 d | <15.00 | 27.60 ± 0.90 d |
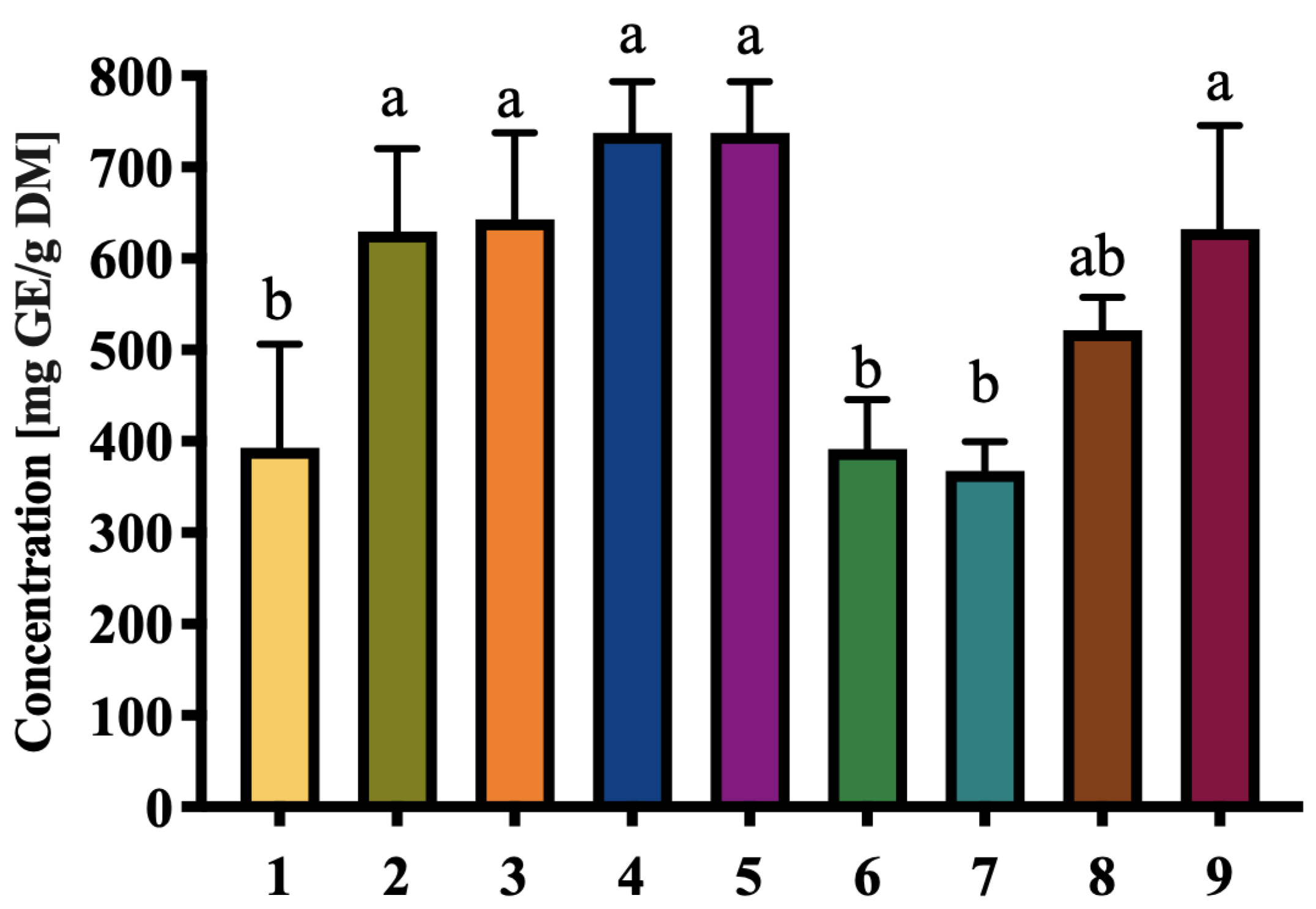
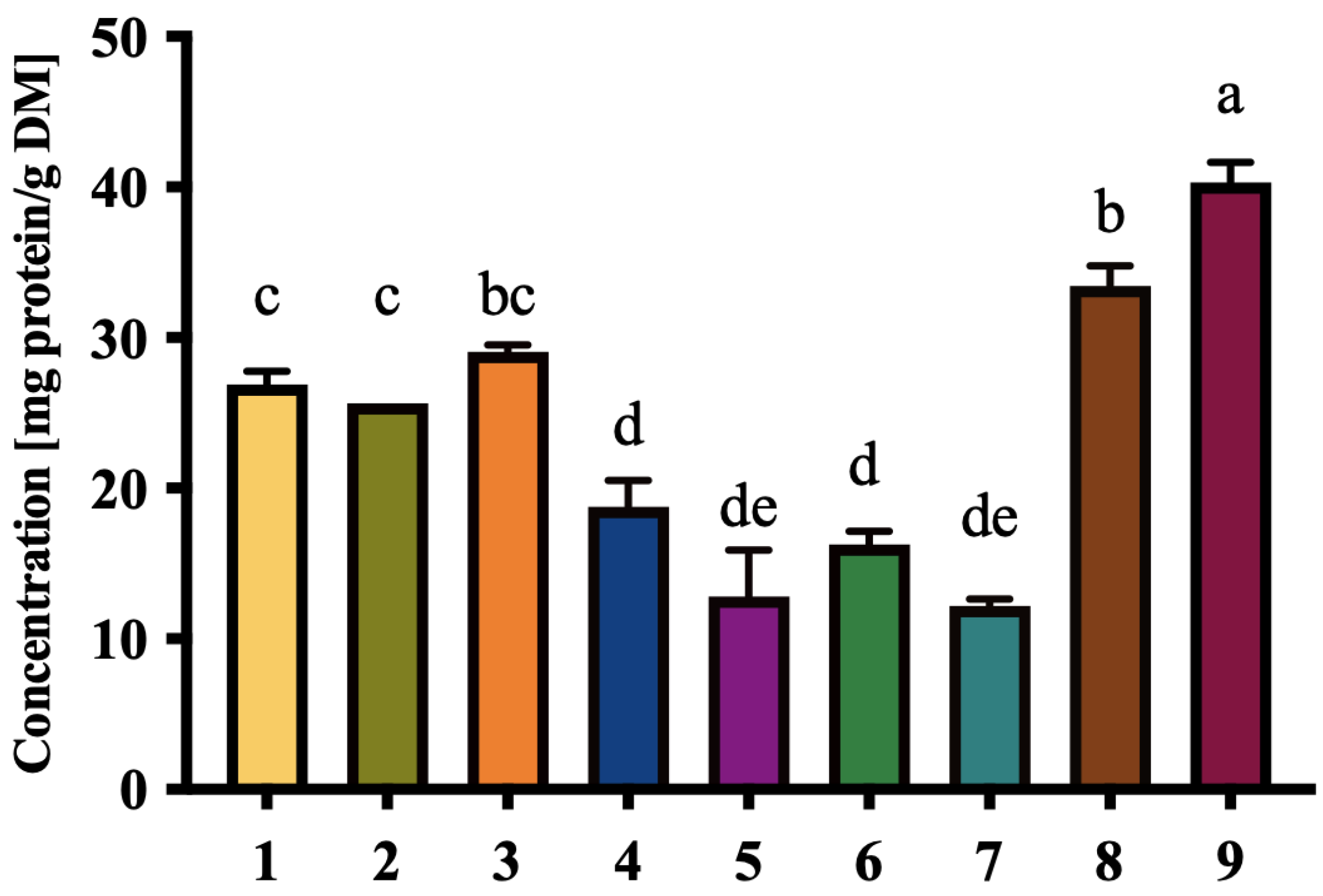
3.3. Carbohydrate and Protein Content
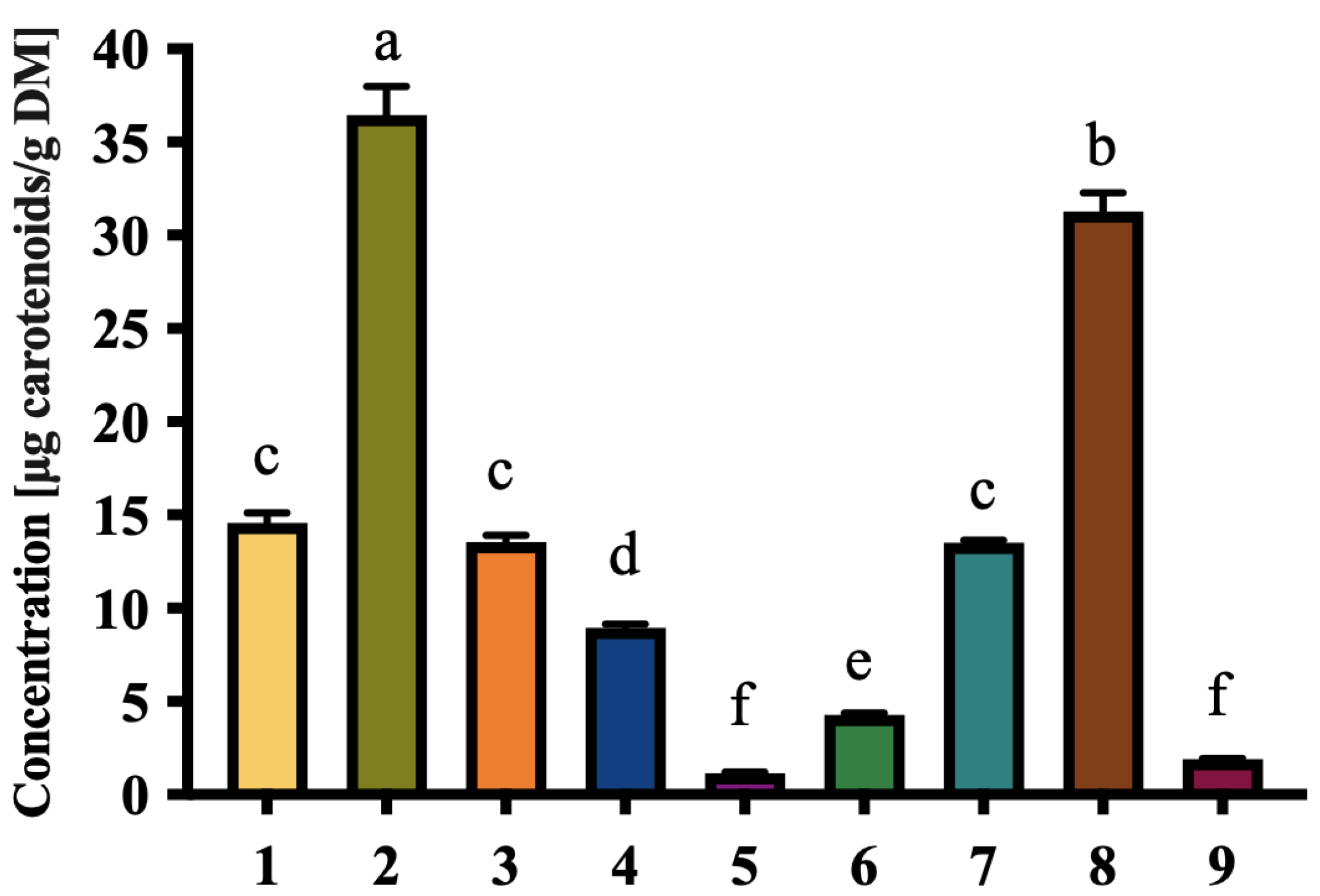
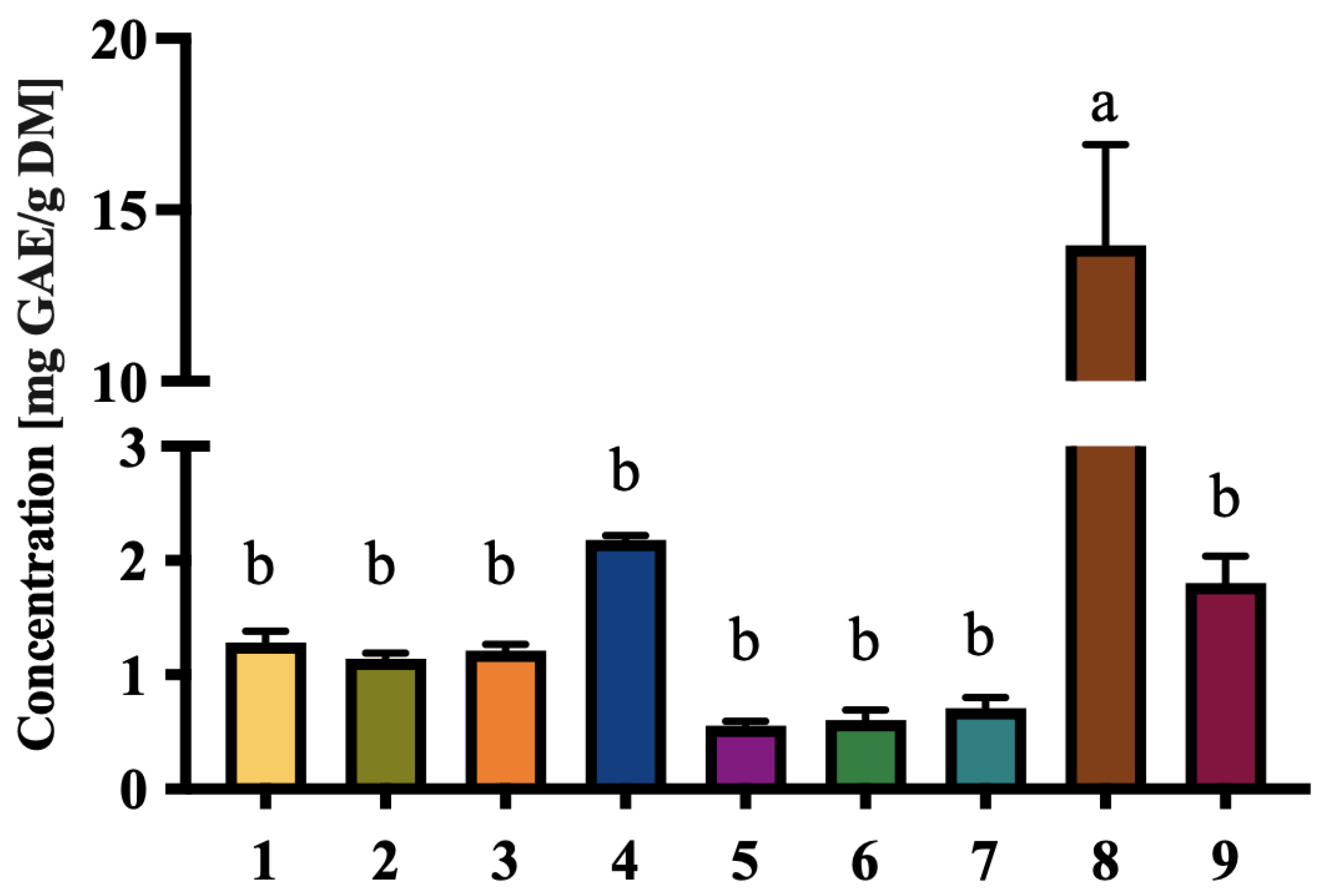
3.4. Phenolic and Carotenoid Content
3.5. Total Antioxidant Capacity
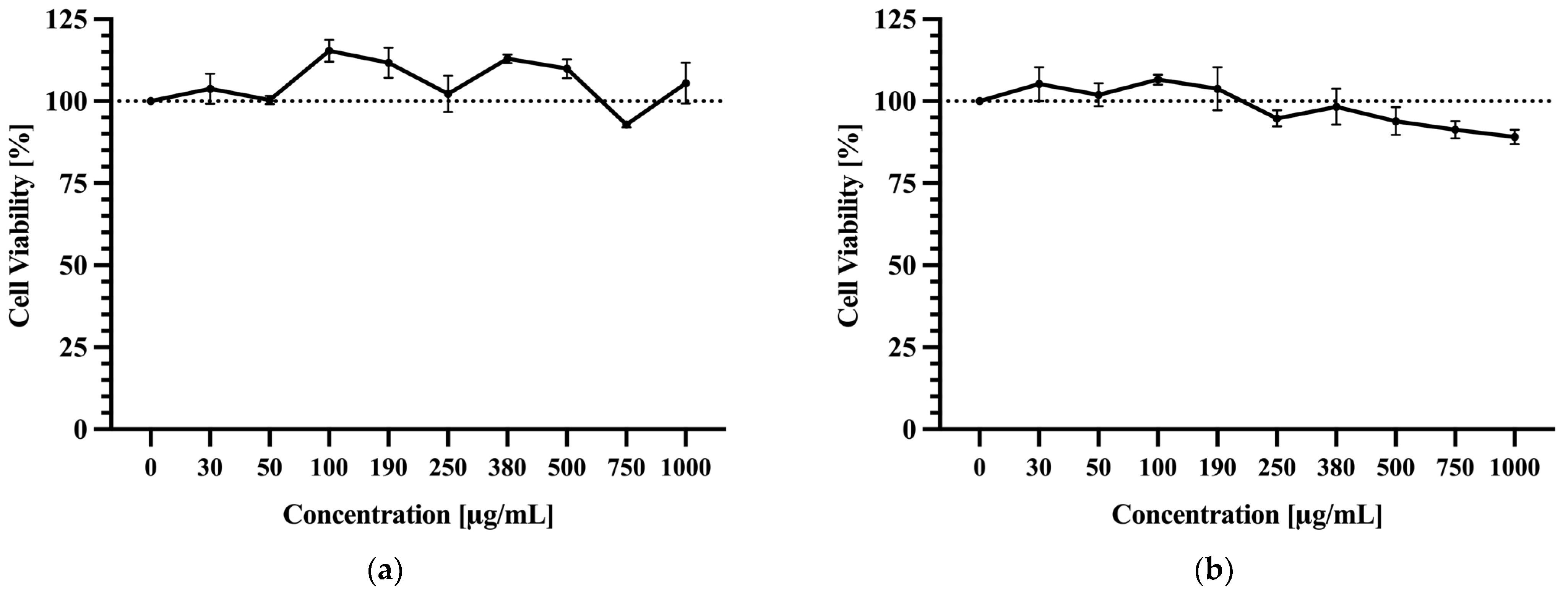
3.6. Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassani, L.; Gomez-Zavaglia, A. Sustainable Food Systems in Fruits and Vegetables Food Supply Chains. Front. Nutr. 2022, 9, 829061. [Google Scholar] [CrossRef]
- Thorsen, M.; Skeaff, S.; Goodman-Smith, F.; Thong, B.; Bremer, P.; Mirosa, M. Upcycled Foods: A Nudge toward Nutrition. Front. Nutr. 2022, 9, 1071829. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Najda, A.; Sharma, R.; Nurzyńska-Wierdak, R.; Dhanjal, D.S.; Sharma, R.; Manickam, S.; Kabra, A.; Kuča, K.; Bhardwaj, P. Fruit and Vegetable Peel-Enriched Functional Foods: Potential Avenues and Health Perspectives. Evid.-Based Complement. Altern. Med. 2022, 2022, 8543881. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of Vegetable and Fruit By-Products as Functional Ingredient and Food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Soomro, A.H.; Marri, A.; Shaikh, N. Date Palm (Phoenix Dactylifera): A Review of Economic Potential, Industrial Valorization, Nutritional and Health Significance. In Neglected Plant Foods of South Asia; Springer International Publishing: Cham, Germany, 2023; pp. 319–350. [Google Scholar]
- Muñoz-Tebar, N.; Viuda-Martos, M.; Lorenzo, J.M.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Strategies for the Valorization of Date Fruit and Its Co-Products: A New Ingredient in the Development of Value-Added Foods. Foods 2023, 12, 1456. [Google Scholar] [CrossRef]
- Muñoz-Bas, C.; Muñoz-Tebar, N.; Candela-Salvador, L.; Pérez-Alvarez, J.A.; Lorenzo, J.M.; Viuda-Martos, M.; Fernández-López, J. Quality Characteristics of Fresh Date Palm Fruits of “Medjoul” and “Confitera” CvCv. from the Southeast of Spain (Elche Palm Grove). Foods 2023, 12, 2659. [Google Scholar] [CrossRef]
- Aljaloud, S.; Colleran, H.L.; Ibrahim, S.A. Nutritional Value of Date Fruits and Potential Use in Nutritional Bars for Athletes. Food Nutr. Sci. 2020, 11, 463–480. [Google Scholar] [CrossRef]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive Compounds from Date Fruit and Seed as Potential Nutraceutical and Functional Food Ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef]
- Shahdadi, F.; Mirzaei, H.O.; Daraei Garmakhany, A. Study of Phenolic Compound and Antioxidant Activity of Date Fruit as a Function of Ripening Stages and Drying Process. J. Food Sci. Technol. 2015, 52, 1814–1819. [Google Scholar] [CrossRef]
- Zhang, C.R.; Aldosari, S.A.; Vidyasagar, P.S.P.V.; Shukla, P.; Nair, M.G. Health-Benefits of Date Fruits Produced in Saudi Arabia Based on in Vitro Antioxidant, Anti-Inflammatory and Human Tumor Cell Proliferation Inhibitory Assays. J. Saudi Soc. Agric. Sci. 2017, 16, 287–293. [Google Scholar] [CrossRef]
- Sebastià, A.; Dawidowicz, K.; Pallarés, N.; Ferrer, E.; Castagnini, J.M.; Martí-Quijal, F.J.; Salgado-Ramos, M. Chemical Characterization and Safety Assessment of Black Truffle (Tuber Melanosporum) Leftovers Extracts Obtained through Non-Conventional Extraction Techniques. Food Chem. 2025, 495, 146367. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E. Colorimetric Quantification of Carbohydrates. Curr. Protoc. Food Anal. Chem. 2001, 1, E1.1.1–E1.1.8. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio Molitor, Alphitobius Diaperinus, and Hermetia Illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Saint-Denis, T.; Goupy, J. Optimization of a Nitrogen Analyser Based on the Dumas Method. Anal. Chim. Acta 2004, 515, 191–198. [Google Scholar] [CrossRef]
- Al Khawli, F.; Martí-Quijal, F.J.; Pallarés, N.; Barba, F.J.; Ferrer, E. Ultrasound Extraction Mediated Recovery of Nutrients and Antioxidant Bioactive Compounds from Phaeodactylum Tricornutum Microalgae. Appl. Sci. 2021, 11, 1701. [Google Scholar] [CrossRef]
- Liu, Y.; Berrada, H.; Wang, M.; Zhou, J.; Kousoulaki, K.; Barba, F.J.; Castagnini, J.M. Is Pulsed Electric Field (PEF) a Useful Tool for the Valorization of Solid and Liquid Sea Bass Side Streams?: Evaluation of Nutrients and Contaminants. Food Bioprocess Technol. 2024, 18, 1873–1892. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Cravotto, G.; Moreno, A.; Barba, F.J. Sequential Extraction of Almond Hull Biomass with Pulsed Electric Fields (PEF) and Supercritical CO2 for the Recovery of Lipids, Carbohydrates and Antioxidants. Food Bioprod. Process. 2023, 139, 216–226. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Barba, F.J.; Ruiz, M.J. Enhancement of the Antioxidant Effect of Natural Products on the Proliferation of Caco-2 Cells Produced by Fish Protein Hydrolysates and Collagen. Int. J. Mol. Sci. 2023, 24, 6871. [Google Scholar] [CrossRef]
- Jaouhari, Y.; Ferreira-Santos, P.; Disca, V.; Oliveira, H.; Martoccia, M.; Travaglia, F.; Gullón, B.; Mateus, N.; Coïsson, J.D.; Bordiga, M. Carbohydrases Treatment on Blueberry Pomace: Influence on Chemical Composition and Bioactive Potential. LWT 2024, 206, 116573. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.J. Role of Quercetin on Caco-2 Cells against Cytotoxic Effects of Alternariol and Alternariol Monomethyl Ether. Food Chem. Toxicol. 2016, 89, 60–66. [Google Scholar] [CrossRef]
- Hazzouri, K.M.; Gros-Balthazard, M.; Flowers, J.M.; Copetti, D.; Lemansour, A.; Lebrun, M.; Masmoudi, K.; Ferrand, S.; Dhar, M.I.; Fresquez, Z.A.; et al. Genome-Wide Association Mapping of Date Palm Fruit Traits. Nat. Commun. 2019, 10, 116573. [Google Scholar] [CrossRef]
- Salomón-Torres, R.; Ortiz-Uribe, N.; Valdez-Salas, B.; Rosas-González, N.; García-González, C.; Chávez, D.; Córdova-Guerrero, I.; Díaz-Rubio, L.; del Haro-Vázquez, M.P.; Mijangos-Montiel, J.L.; et al. Nutritional Assessment, Phytochemical Composition and Antioxidant Analysis of the Pulp and Seed of Medjool Date Grown in Mexico. PeerJ 2019, 7, e6821. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; MacGregor, G.A. Beneficial Effects of Potassium on Human Health. Physiol. Plant. 2008, 133, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Pethő, Á.G.; Fülöp, T.; Orosz, P.; Tapolyai, M. Magnesium Is a Vital Ion in the Body—It Is Time to Consider Its Supplementation on a Routine Basis. Clin. Pract. 2024, 14, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P. Calcium and Phosphate: A Duet of Ions Playing for Bone Health. J. Am. Coll. Nutr. 2011, 30, 438S–448S. [Google Scholar] [CrossRef]
- Matikainen, N.; Pekkarinen, T.; Ryhänen, E.M.; Schalin-Jäntti, C. Physiology of Calcium Homeostasis: An Overview. Endocrinol. Metab. Clin. N. Am. 2021, 50, 575–590. [Google Scholar] [CrossRef]
- Serna, J.; Bergwitz, C. Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients 2020, 12, 3001. [Google Scholar] [CrossRef]
- Beard, J.L. Iron-Deficiency Anemia: Reexamining the Nature and Magnitude of the Public Health Problem Iron Biology in Immune Function, Muscle Metabolism and Neuronal Functioning. J. Nutr. 2001, 131, 568S–580S. [Google Scholar] [CrossRef]
- Asenova, S.; Koleva-Kolarova, R. Koleva-Kolarova. Copp. Hum. Org. 2011, 9, 88–98. [Google Scholar]
- Kiouri, D.P.; Tsoupra, E.; Peana, M.; Perlepes, S.P.; Stefanidou, M.E.; Chasapis, C.T. Multifunctional Role of Zinc in Human Health: An Update. EXCLI J. 2023, 22, 809–827. [Google Scholar] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Bouhlali, E.D.T.; Ramchoun, M.; Alem, C.; Ghafoor, K.; Ennassir, J.; Zegzouti, Y.F. Functional Composition and Antioxidant Activities of Eight Moroccan Date Fruit Varieties (Phoenix dactylifera L.). J. Saudi Soc. Agric. Sci. 2017, 16, 257–264. [Google Scholar] [CrossRef]
- Kiran; Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Jaouhari, Y.; Disca, V.; Ferreira-Santos, P.; Alvaredo-López-Vizcaíno, A.; Travaglia, F.; Bordiga, M.; Locatelli, M. Valorization of Date Fruit (Phoenix dactylifera L.) as a Potential Functional Food and Ingredient: Characterization of Fiber, Oligosaccharides, and Antioxidant Polyphenols. Molecules 2024, 29, 4606. [Google Scholar] [CrossRef]
- Mirghani, H.O. Dates Fruits Effects on Blood Glucose among Patients with Diabetes Mellitus: A Review and Meta-Analysis. Pak. J. Med. Sci. 2021, 37, 1230–1236. [Google Scholar] [CrossRef]
- Alvi, T.; Khan, M.K.I.; Maan, A.A.; Razzaq, Z.U. Date Fruit as a Promising Source of Functional Carbohydrates and Bioactive Compounds: A Review on Its Nutraceutical Potential. J. Food Biochem. 2022, 46, e14325. [Google Scholar] [CrossRef]
- Shanmugam, G. Polyphenols: Potent Protectors against Chronic Diseases. Nat. Prod. Res. 2024, 1–3. [Google Scholar] [CrossRef]
- Assirey, E.A. The Chemical Composition, Total Phenolic and Antioxidant Content of Four Date Palm Saudi Cultivars. J. Taibah Univ. Sci. 2021, 15, 282–287. [Google Scholar] [CrossRef]
- Ouamnina, A.; Alahyane, A.; Elateri, I.; Boutasknit, A.; Abderrazik, M. Relationship between Phenolic Compounds and Antioxidant Activity of Some Moroccan Date Palm Fruit Varieties (Phoenix dactylifera L.): A Two-Year Study. Plants 2024, 13, 1119. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O.; Francis, F.J. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Takagi, T.; Hong, H.; Dillon, N.; Crisp, P.; Cozzolino, D.; O’Hare, T. The Association Between the Flesh Colour and Carotenoid Profile of 25 Cultivars of Mangoes. Molecules 2025, 30, 1661. [Google Scholar] [CrossRef]
- Abdeen, E.S.M.M. Enhancement of Functional Properties of Dairy Products by Date Fruits. Egypt. J. Food 2018, 46, 197–206. [Google Scholar]
- AlFaris, N.A.; AlTamim, J.Z.; AlMousa, L.A.; Albarid, N.A.; AlGhamidi, F.A. Nutritional Values, Nutraceutical Properties, and Health Benefits of Arabian Date Palme Fruit. Emir. J. Food Agric. 2023, 35, 488–510. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Dini, S.; Esmaeili, Y.; Roshanak, S.; Redha, A.A.; Wani, S.A. Uses of Carotenoid-Rich Ingredients to Design Functional Foods: A Review. J. Food Bioact. 2023, 21, 3–20. [Google Scholar] [CrossRef]
- Milella, R.A.; De Rosso, M.; Gasparro, M.; Gigante, I.; Debiase, G.; Forleo, L.R.; Marsico, A.D.; Perniola, R.; Tutino, V.; Notarnicola, M.; et al. Correlation between Antioxidant and Anticancer Activity and Phenolic Profile of New Apulian Table Grape Genotypes (V. vinifera L.). Front. Plant Sci. 2023, 13, 1064023. [Google Scholar] [CrossRef]
- Al-Qarni, S.S.M.; Bazzi, M.D. Date Fruit Ripening with Degradation of Chlorophylls, Carotenes, and Other Pigments. Int. J. Fruit Sci. 2020, 20, S827–S839. [Google Scholar] [CrossRef]
- Yildiz, A.Y.; Öztekin, S.; Anaya, K. Effects of Plant-Derived Antioxidants to the Oxidative Stability of Edible Oils under Thermal and Storage Conditions: Benefits, Challenges and Sustainable Solutions. Food Chem. 2025, 479, 143752. [Google Scholar] [CrossRef]
- Barrera-Chamorro, L.; Fernandez-Prior, A.; Gonzalez-de la Rosa, T.; Rivero-Pino, F.; Claro-Cala, C.M.; Montserrat-de la Paz, S. Evaluation of Immunomodulatory Properties of Phenolic Extracts from Olive Mill By-Products Using Caco-2 Cells and Molecular Docking Analysis. J. Agric. Food Res. 2024, 18, 101399. [Google Scholar] [CrossRef]

| Cultivars | Mg (mg/g Dry Mass) | P (mg/g Dry Mass) | K (mg/g Dry Mass) | Ca (mg/g Dry Mass) |
|---|---|---|---|---|
| D1 | 1.026 ± 0.008 b | 0.605 ± 0.007 b | 10.820 ± 0.160 c | 1.940 ± 0.040 a |
| D2 | 0.698 ± 0.011 e | 0.490 ± 0.007 d | 11.530 ± 0.180 c | 1.030 ± 0.020 d |
| D3 | 0.972 ± 0.009 c | 0.594 ± 0.005 b | 13.250 ± 0.110 b | 1.320 ± 0.030 c |
| D4 | 0.374 ± 0.006 i | 0.500 ± 0.010 d | 22.100 ± 0.300 a | 0.453 ± 0.013 f |
| D5 | 0.608 ± 0.007 f | 0.505 ± 0.004 d | 9.190 ± 0.070 e | 0.710 ± 0.020 e |
| D6 | 0.565 ± 0.008 g | 0.549 ± 0.008 c | 8.800 ± 0.200 e | 0.451 ± 0.016 f |
| D7 | 0.476 ± 0.005 h | 0.488 ± 0.017 d | 13.400 ± 0.200 b | 0.524 ± 0.012 f |
| D8 | 1.232 ± 0.012 a | 0.776 ± 0.007 a | 13.000 ± 0.400 b | 1.560 ± 0.070 b |
| D9 | 0.810 ± 0.030 d | 0.480 ± 0.005 d | 10.000± 0.400 d | 0.750 ± 0.030 e |
| Cultivars | Fe (mg/kg Dry Mass) | Zn (mg/kg Dry Mass) | Cu (mg/kg Dry Mass) | Se (µg/kg Dry Mass) |
|---|---|---|---|---|
| D1 | 9.54 ± 0.11 d | 8.00 ± 0.40 b | 2.97 ± 0.03 e | 48.00 ± 3.00 b |
| D2 | 9.03 ± 0.05 de | 8.20 ± 0.30 b | 4.86 ± 0.04 b | 44.00 ± 3.00 b |
| D3 | 11.41 ± 0.17 b | 8.40 ± 0.20 b | 2.58 ± 0.04 f | 43.10 ± 1.80 b |
| D4 | 8.83 ± 0.09 e | 2.83 ± 0.08 f | 3.62 ± 0.04 c | 30.00 ± 2.00 cd |
| D5 | 6.29 ± 0.13 g | 4.54 ± 0.10 e | 3.41 ± 0.02 d | 26.30 ± 1.20 d |
| D6 | 9.35 ± 0.04 d | 5.93 ± 0.09 d | 3.39 ± 0.02 d | 40.00 ± 3.00 bc |
| D7 | 7.11 ± 0.07 f | 6.70 ± 0.20 c | 2.23 ± 0.03 g | 22.90 ± 1.40 d |
| D8 | 15.32 ± 0.17 a | 9.20 ± 0.30 a | 5.22 ± 0.05 a | 68.00 ± 4.00 a |
| D9 | 9.94 ± 0.18 c | 6.40 ± 0.20 cd | 3.39 ± 0.06 d | 33.90 ± 1.60 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawidowicz, K.; Martinez-Terol, S.; Sayas-Barberá, E.; Pérez-Álvarez, J.Á.; Marti-Quijal, F.J.; Roig, P.; Castagnini, J.M. An Exploratory Study of the Nutritional Composition and Caco-2 Safety Assessment of Elche Date Flour and Its Green Hydroethanolic Extracts. Foods 2025, 14, 3908. https://doi.org/10.3390/foods14223908
Dawidowicz K, Martinez-Terol S, Sayas-Barberá E, Pérez-Álvarez JÁ, Marti-Quijal FJ, Roig P, Castagnini JM. An Exploratory Study of the Nutritional Composition and Caco-2 Safety Assessment of Elche Date Flour and Its Green Hydroethanolic Extracts. Foods. 2025; 14(22):3908. https://doi.org/10.3390/foods14223908
Chicago/Turabian StyleDawidowicz, Katarzyna, Sergio Martinez-Terol, Estrella Sayas-Barberá, José Ángel Pérez-Álvarez, Francisco J. Marti-Quijal, Patricia Roig, and Juan Manuel Castagnini. 2025. "An Exploratory Study of the Nutritional Composition and Caco-2 Safety Assessment of Elche Date Flour and Its Green Hydroethanolic Extracts" Foods 14, no. 22: 3908. https://doi.org/10.3390/foods14223908
APA StyleDawidowicz, K., Martinez-Terol, S., Sayas-Barberá, E., Pérez-Álvarez, J. Á., Marti-Quijal, F. J., Roig, P., & Castagnini, J. M. (2025). An Exploratory Study of the Nutritional Composition and Caco-2 Safety Assessment of Elche Date Flour and Its Green Hydroethanolic Extracts. Foods, 14(22), 3908. https://doi.org/10.3390/foods14223908










