Preparation, Structural Characterization of Octenyl Succinic Anhydride-Modified Bamboo Shoot-Derived Cellulose Nano-Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bamboo Shoot CNC
2.3. OSA Modification of Bamboo Shoot CNC
2.4. Characterization of Modified and Unmodified Cellulose Particles
2.4.1. Degree of Substitution (DS) Determination
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4.3. X-Ray Diffraction (XRD) Analysis
2.4.4. Scanning Electron Microscopy (SEM) Observation
2.4.5. Zeta Potential Analysis
2.4.6. Contact Angle Measurement
2.4.7. Thermogravimetric Analysis (TGA)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Degree of Substitution (DS)
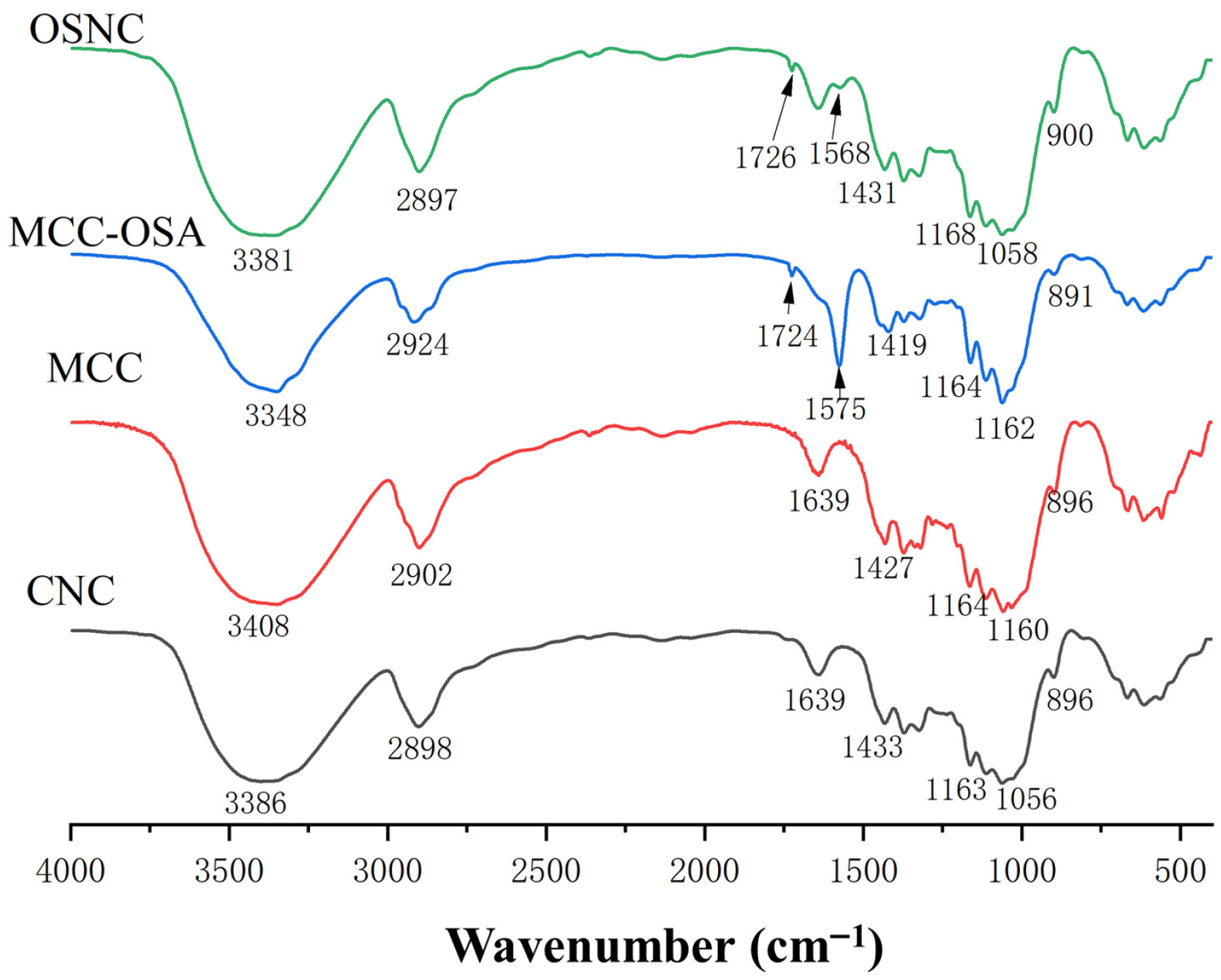
3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
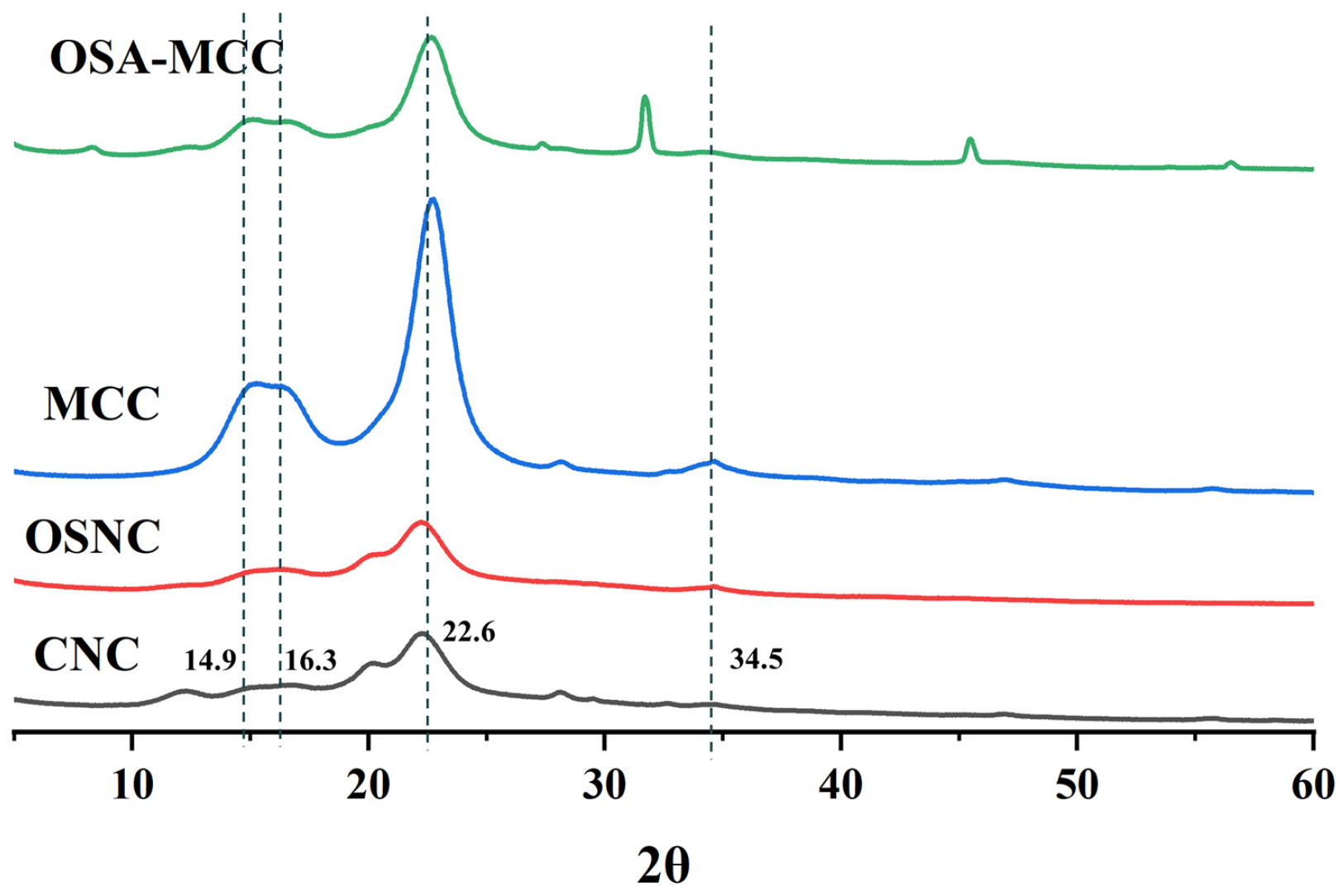
3.3. X-Ray Diffraction (XRD) Analysis
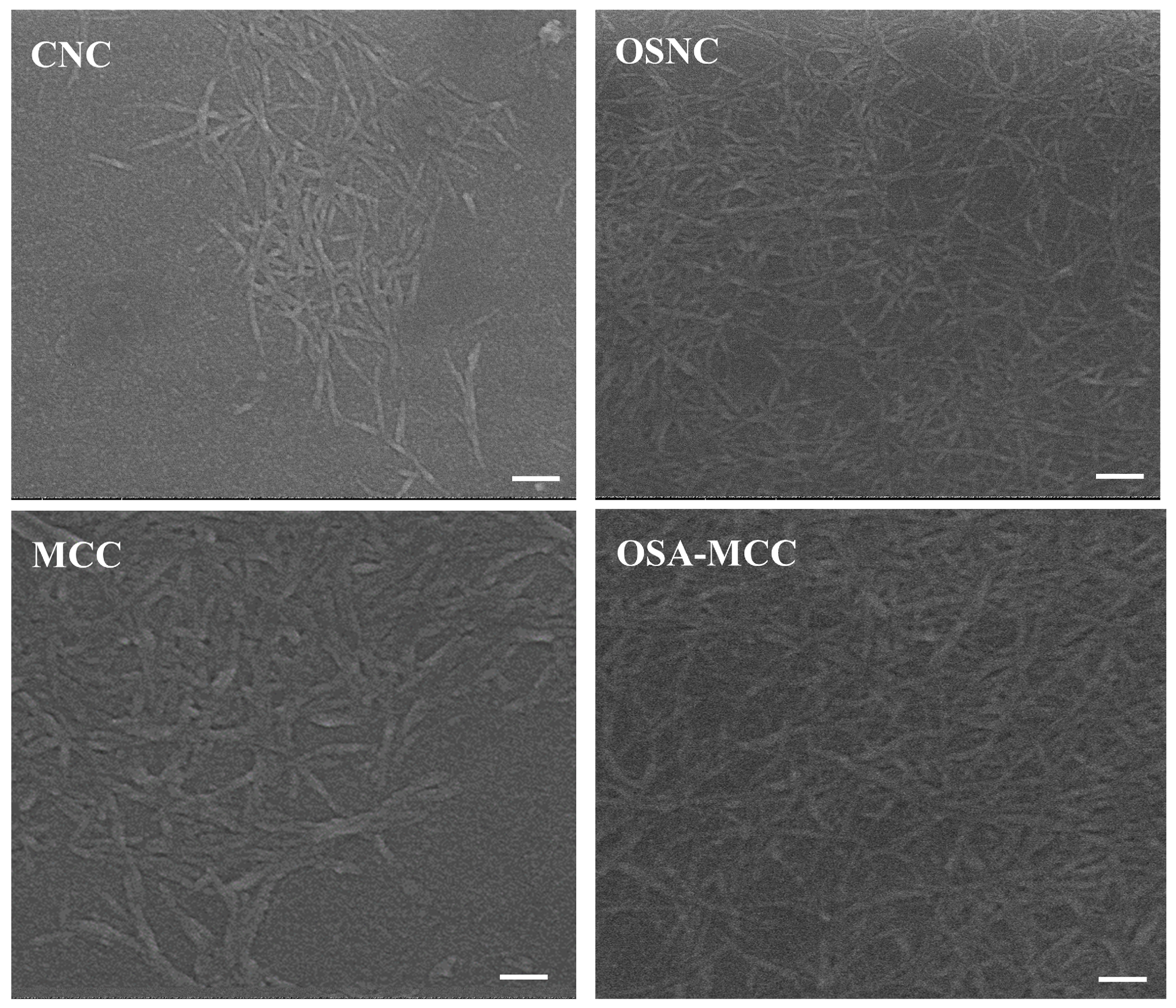
3.4. Scanning Electron Microscopy (SEM) Analysis
3.5. Zeta Potential Analysis
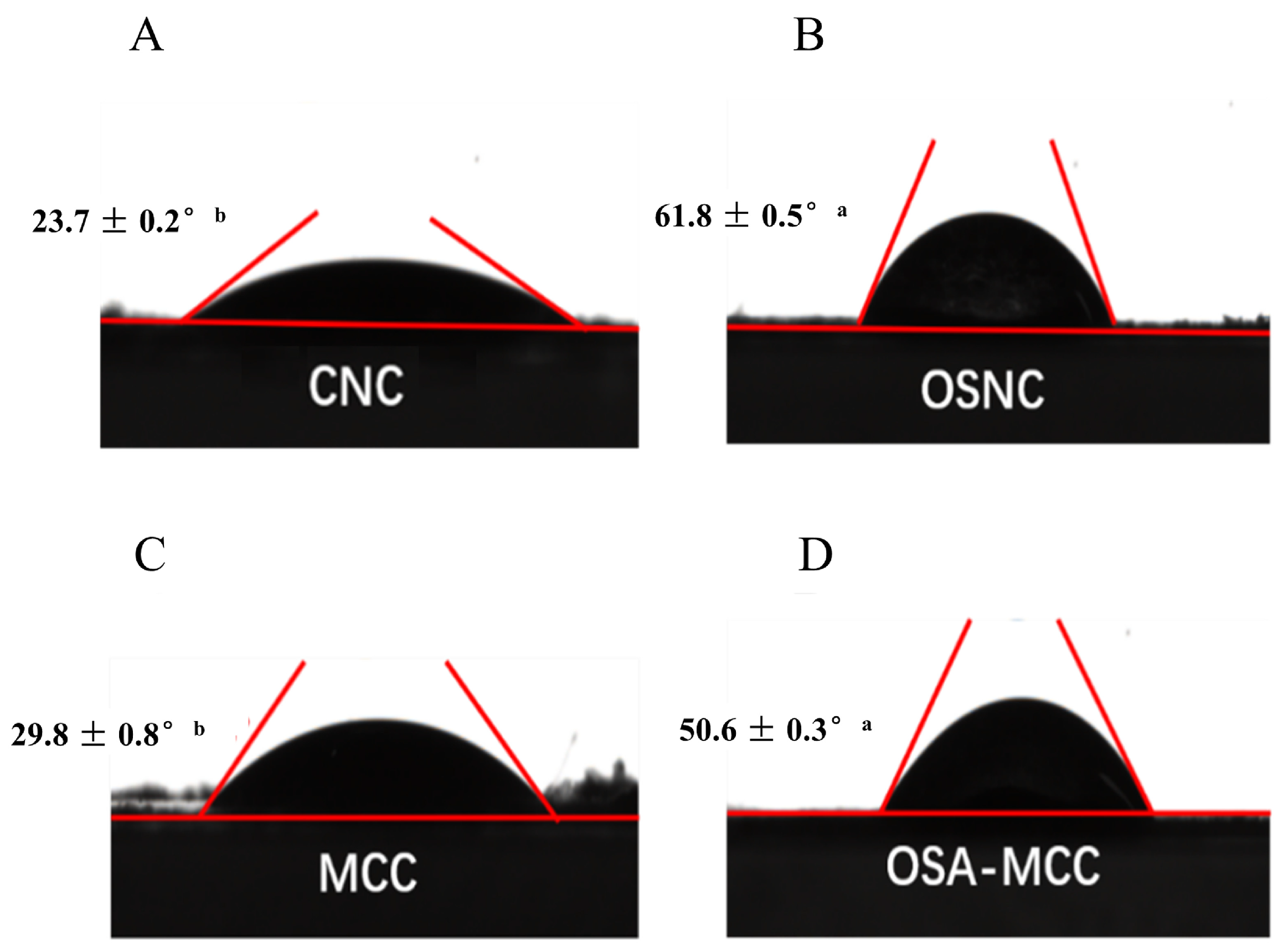
3.6. Contact Angle Analysis
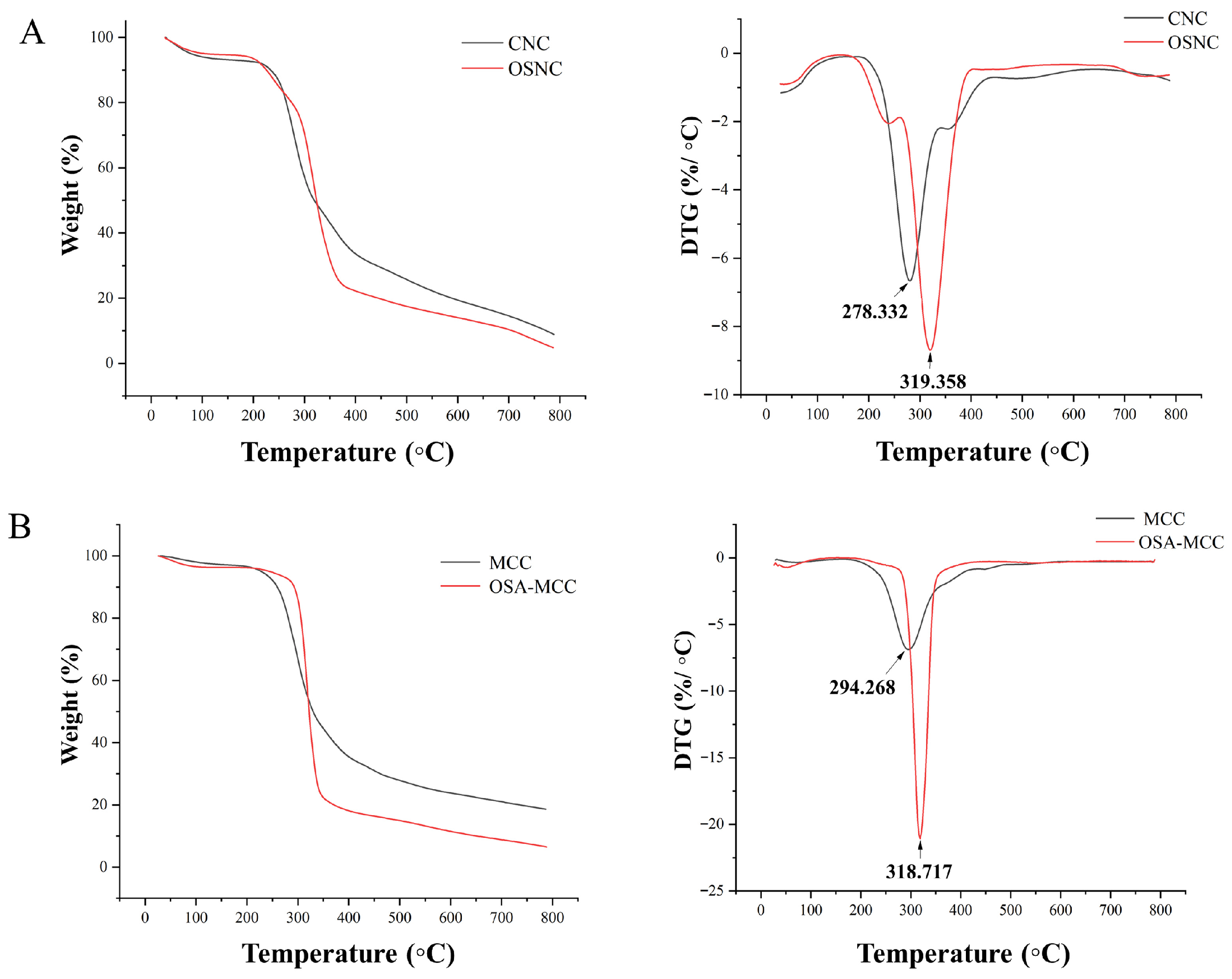
3.7. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNC | Cellulose Nanocrystals |
| OSA | Octenyl Succinic Anhydride |
| OSNC | OSA-Modified Bamboo Shoot CNC |
| MCC | Microcrystalline Cellulose |
| DS | Degree of Substitution |
| AGU | Anhydroglucose Unit |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XRD | X-Ray Diffraction |
| SEM | Scanning Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| DTG | derivative thermogravimetric |
References
- Pan, X.; Ji, H.; Gong, X.X.; Yang, W.T.; Jin, Z.; Zheng, Y.; Ding, S.; Xia, H.; Shen, Z.; Shao, J.F. Screening and evaluation of bamboo shoots: Comparing the content of trace elements from 100 species. Food Chem. X 2024, 21, 101071. [Google Scholar] [CrossRef]
- Ma, T.; Mo, W.; Lv, B.; Wang, W.; He, H.; Jian, C.; Liu, X.; Li, S.; Guo, Y. A review of the nutritional composition, storage challenges, processing technology and widespread use of bamboo shoots. Foods 2024, 13, 3539. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Q.; Liu, W.; Wu, Y.; Yang, J.; Chen, P.; Zhuang, W.; Zheng, Y. Characterization and safety assessment of bamboo shoot shell cellulose nanofiber: Prepared by acidolysis combined with dynamic high-pressure microfluidization. Carbohydr. Polym. 2024, 335, 122082. [Google Scholar] [CrossRef]
- Silva, F.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose bio-based composites for food packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wang, Q.; Zheng, X.; Chang, Y.; Cao, H.; Zheng, Y. Investigation of the structural, thermal, and physicochemical properties of nanocelluloses extracted from bamboo shoot processing byproducts. Front. Chem. 2022, 10, 922437. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Xu, Y.; He, Y.T.; Wen, J.L.; Yuan, T.Q. Lignocellulosic biomass-derived functional nanocellulose for food-related applications: A review. Int. J. Biol. Macromol. 2024, 277, 134536. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, H.; Li, Y. Characterization of pickering emulsions stabilized by osa-modified sweet potato residue cellulose: Effect of degree of substitute and concentration. Food Hydrocoll. 2020, 108, 105915. [Google Scholar] [CrossRef]
- Peng, S.; Luo, Q.; Zhou, G.; Xu, X. Recent advances on cellulose nanocrystals and their derivatives. Polymers 2021, 13, 3247. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, E.; Bertelli, D.; Bilia, A.R.; Vanti, G.; Maretti, E.; Leo, E. Combination of nanodelivery systems and constituents derived from novel foods: A comprehensive review. Pharmaceutics 2023, 15, 2614. [Google Scholar] [CrossRef]
- Liu, S.; Sun, S.; Chen, W.; Jia, R.; Zheng, B.; Guo, Z. Structural, physicochemical properties, and digestibility of lotus seed starch-conjugated linoleic acid complexes. Int. J. Biol. Macromol. 2022, 214, 601–609. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Zheng, J.; Xu, Y.-T.; Yin, S.-W.; Liu, F.; Tang, C.-H. Surface modification improves fabrication of pickering high internal phase emulsions stabilized by cellulose nanocrystals. Food Hydrocoll. 2018, 75, 125–130. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, Y.; Zhang, B.; Fang, Y.; Lin, Q.; Ding, Y. Application of pickering emulsions stabilized by corn, potato and pea starch nanoparticles: Effect of environmental conditions and approach for curcumin release. Int. J. Biol. Macromol. 2023, 238, 124115. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, M.; Qin, C.; Guo, D. Effect of octenyl succinic anhydride (Osa) substitution degree on the thermal stability and oral delivery efficiency of β-carotene in emulsions stabilized by high-amylose starch nanocrystals. Food Chem. 2025, 475, 143288. [Google Scholar] [CrossRef]
- Le, H.D.; Loveday, S.M.; Singh, H. Pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Responsiveness to ph and ionic strength. Food Hydrocoll. 2020, 99, 105344. [Google Scholar] [CrossRef]
- Dewi, A.M.P.; Santoso, U.; Pranoto, Y.; Marseno, D.W. Dual modification of sago starch via heat moisture treatment and octenyl succinylation to improve starch hydrophobicity. Polymers 2022, 14, 1086. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.; Ramzan, R.; Abdullah; Abbas, H.M.K.; Sun, W.; Zhang, G. Integrating the modified amphiphilic eleocharis tuberosa starch to stabilize curcuminoid-enriched pickering emulsions for enhanced bioavailability, thermal stability, and retention of the hydrophobic bioactive compound. Carbohydr. Polym. 2025, 352, 123199. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gao, J.; Qi, L.; Gao, Q.; Fan, L. Preparation and properties of starch-cellulose composite aerogel. Polymers 2023, 15, 4294. [Google Scholar] [CrossRef]
- Abu-Thabit, N.; Hakeem, A.S.; Mezghani, K.; Ratemi, E.; Elzagheid, M.; Umar, Y.; Primartomo, A.; Al Batty, S.; Azad, A.K.; Al Anazi, S.; et al. Preparation of pH-indicative and flame-retardant nanocomposite films for smart packaging applications. Sensors 2020, 20, 5462. [Google Scholar] [CrossRef]
- Eshag Osman, M.F.; Mohamed, A.A.; Alamri, M.S.; Ahmed, I.A.M.; Hussain, S.; Ibraheem, M.I.; Qasem, A.A. Quality characteristics of beef patties prepared with octenyl-succinylated (Osan) starch. Foods 2021, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Chen, Y.; Zhu, M.; Zhao, Y.; Zhang, K.; Xu, X. Spatial-temporal heterogeneity in large three-dimensional nanofibrillar cellulose hydrogel for human pluripotent stem cell culture. Gels 2023, 9, 324. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, H.; Wu, Y. Improving the decorative performance of uv-curable coatings with iridescent cellulose nanocrystal film. RSC Adv. 2023, 13, 22569–22578. [Google Scholar] [CrossRef] [PubMed]
- No, J.; Mun, S.; Shin, M. Properties and digestibility of octenyl succinic anhydride-modified japonica-type waxy and non-waxy rice starches. Molecules 2019, 24, 765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, M.; Tao, L.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Octenyl succinic anhydride modification of bovine bone and fish skin gelatins and their application for fish oil-loaded emulsions. Food Hydrocoll. 2020, 108, 106041. [Google Scholar] [CrossRef]
- Sumarago, E.C.; Dela Cerna, M.F.M.; Leyson, A.K.B.; Tan, N.P.B.; Magsico, K.F. Production and characterization of nanocellulose from maguey (Agave cantala) fiber. Polymers 2024, 16, 1312. [Google Scholar] [CrossRef] [PubMed]
- Ridella, F.; Carpintero, M.; Marcet, I.; Matos, M.; Gutiérrez, G.; Rendueles, M.; Díaz, M. Esterification of dextran by octenyl succinic anhydride (Osa): Physicochemical characterization and functional properties assessment. Carbohydr. Polym. 2024, 340, 122300. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Mariano, M.; Rabelo, S.C.; Gouveia, R.F.; Lona, L.M.F. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro- to a nano-scale view. Appl. Surf. Sci. 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Harini, K.; Chandra Mohan, C. Isolation and characterization of micro and nanocrystalline cellulose fibers from the walnut shell, corncob and sugarcane bagasse. Int. J. Biol. Macromol. 2020, 163, 1375–1383. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Yu, Y.; Tang, M.; Li, Y.; Hu, W.; Liu, T.; Zhang, Y. Food-grade gelatin nanoparticles: Preparation, characterization, and preliminary application for stabilizing pickering emulsions. Foods 2019, 8, 479. [Google Scholar] [CrossRef]
- Teo, S.H.; Ching, Y.C.; Fahmi, M.Z.; Lee, H.V. Surface functionalization of sugarcane-bagasse-derived cellulose nanocrystal for pickering emulsion gel: Microstructural properties and stability efficiency. Gels 2023, 9, 734. [Google Scholar] [CrossRef]

| Sample | Cellulose:OSA | DS |
|---|---|---|
| OSNC | 1:0.175 | 0.019 ± 0.02 c |
| 1:0.200 | 0.026 ± 0.01 b | |
| 1:0.225 | 0.029 ± 0.01 a | |
| 1:0.250 | 0.030 ± 0.02 a | |
| OSA-MCC | 1:0.225 | 0.024 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Chen, W.; Zhang, Z.; Wang, Q.; Li, Y.; Zheng, Y. Preparation, Structural Characterization of Octenyl Succinic Anhydride-Modified Bamboo Shoot-Derived Cellulose Nano-Crystals. Foods 2025, 14, 3876. https://doi.org/10.3390/foods14223876
Huang M, Chen W, Zhang Z, Wang Q, Li Y, Zheng Y. Preparation, Structural Characterization of Octenyl Succinic Anhydride-Modified Bamboo Shoot-Derived Cellulose Nano-Crystals. Foods. 2025; 14(22):3876. https://doi.org/10.3390/foods14223876
Chicago/Turabian StyleHuang, Maokun, Wen Chen, Zichen Zhang, Qi Wang, Yunlong Li, and Yafeng Zheng. 2025. "Preparation, Structural Characterization of Octenyl Succinic Anhydride-Modified Bamboo Shoot-Derived Cellulose Nano-Crystals" Foods 14, no. 22: 3876. https://doi.org/10.3390/foods14223876
APA StyleHuang, M., Chen, W., Zhang, Z., Wang, Q., Li, Y., & Zheng, Y. (2025). Preparation, Structural Characterization of Octenyl Succinic Anhydride-Modified Bamboo Shoot-Derived Cellulose Nano-Crystals. Foods, 14(22), 3876. https://doi.org/10.3390/foods14223876








