Cold-Pressed Walnut-Oil Adulteration with Edible Oils Detection Using Vis-NIR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV-Vis-NIR Spectroscopy Measurements
2.3. Color Measurement
2.4. Statistical Analysis
3. Results and Discussion
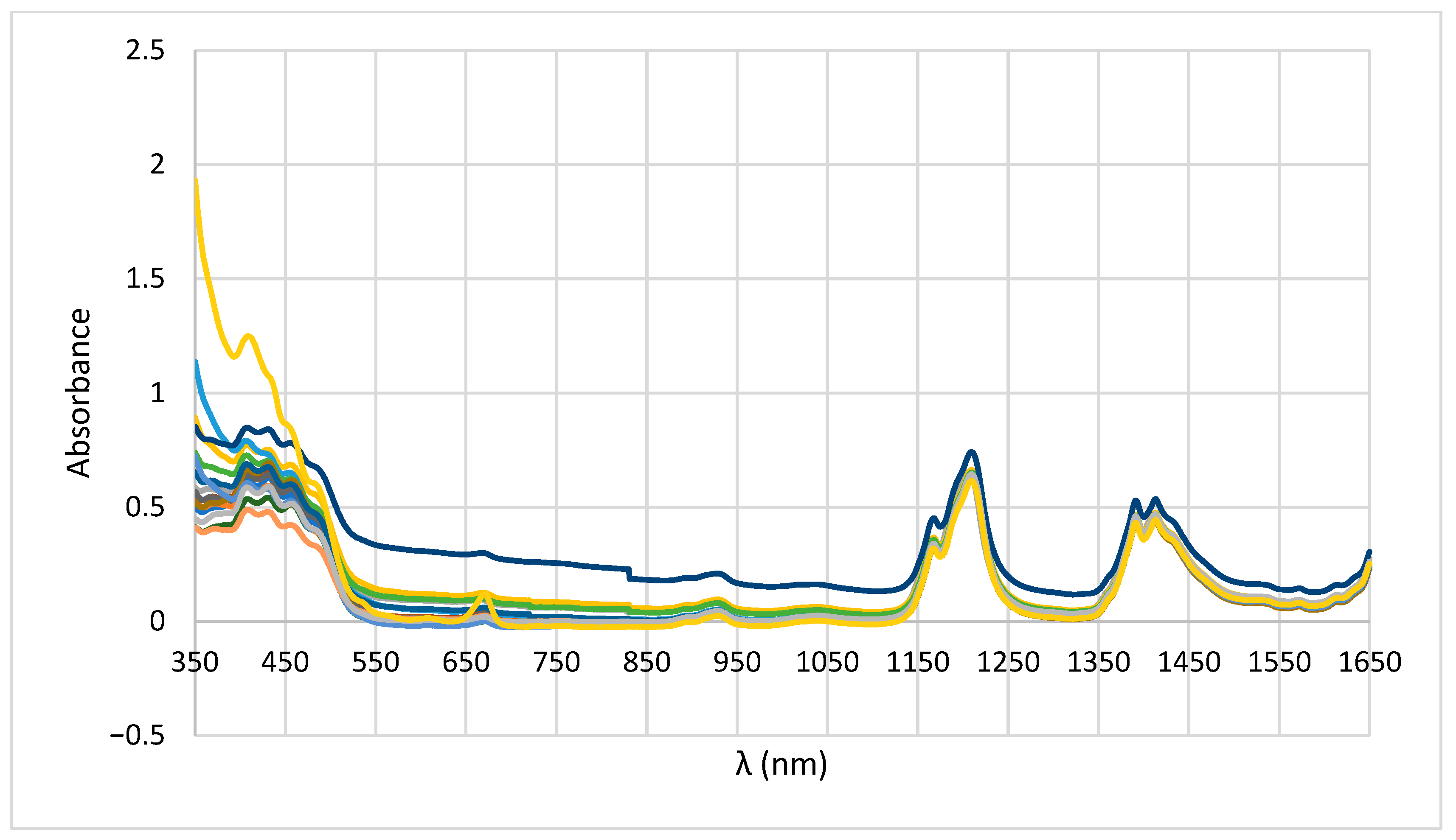
3.1. UV-Vis-NIR Spectra
3.1.1. Walnut Oil Adulterated with Sunflower Oil
3.1.2. Walnut Oil Adulterated with Rapeseed Oil
3.1.3. Walnut Oil Adulterated with Soybean Oil
3.2. Walnut Oil Color
| Parameter | Adulteration with sunflower oil (%) | F-value | ||||||
| 0 | 5 | 10 | 20 | 30 | 40 | 50 | ||
| L* | 20.84 (0.291) b | 19.16 (1.26) a | 19.19 (1.73) ab | 18.97 (0.67) a | 19.19 (0.80) a | 19.13 (0.92) a | 19.15 (0.80) a | 4.918 ns |
| a* | −0.99 (0.29) a | −1.17 (0.25) a | −1.21 (0.15) a | −1.14 (0.10) a | −1.22 (0.11) a | −1.26 (0.18) a | −1.19 (0.18) a | 1.62 ns |
| b* | 5.22 (0.52) b | 3.23 (0.14) a | 3.19 (1.07) a | 2.86 (0.68) a | 3.05 (0.68) a | 2.83 (0.86) a | 2.83 (0.77) a | 4.55 ** |
| C* | 9.8 (1.10) b | 5.59 (1.45) a | 6.00 (1.88) a | 5.49 (1.19) a | 5.86 (1.19) a | 5.49 (1.54) a | 5.48 (1.41) a | 6.53 *** |
| h* | 99.26 (2.20) a | 110.08 (5.65) b | 110.76 (4.76) b | 111.36 (4.75) b | 111.09 (4.20) b | 113.44 (3.74) b | 113.26 (3.33) b | 7.06 *** |
| ΔE* | 0 (0) a | 2.98 (1.38) b | 2.98 (1.12) b | 3.08 (1.25) b | 2.83 (1.20) b | 3.08 (1.23) b | 3.02 (1.20) b | 4.89 *** |
| WI | 7.2 (0.42) a | 9.01 (0.79) b | 9.28 (0.31) b | 9.2 (0.51) b | 9.28 (0.42) b | 9.58 (0.19) b | 9.59 (0.22) b | 18.59 *** |
| YI | 35.78 (3.19) b | 23.42 (8.8) a | 22.94 (5.51) a | 21.40 (4.47) a | 22.58 (4.28) a | 20.91 (5.24) a | 20.92 (4.81) a | 4.97 *** |
| Adulteration with rapeseed oil (%) | F-value | |||||||
| 0 | 5 | 10 | 20 | 30 | 40 | 50 | ||
| L* | 20.84 (0.29) b | 19.16 (0.56) a | 19.19 (0.74) a | 19.14 (0.73) a | 19.18 (0.72) a | 19.07 (0.67) a | 19.10 (0.71) a | 4.91 *** |
| a* | −0.99 (0.299) a | −1.14 (0.25) a | −1.09 (0.13) a | −1.01 (0.15) a | −0.96 (0.14) a | −0.96 (0.12) a | −0.97 (0.12) a | 2.05 ns |
| b* | 5.22 (0.523) b | 3.04 (0.55) a | 3.11 (0.73) a | 3.22 (0.84) a | 3.36 (0.75) a | 3.13 (0.75) a | 3.26 (0.64) a | 6.59 *** |
| C* | 9.8 (1.10) b | 5.7 9(1.005) a | 5.87 (1.35) a | 6.08 (0.52) a | 6.3 (1.38) a | 5.88 (1.39) a | 6.09 (1.17) a | 6.69 *** |
| h* | 99.26 (2.20) a | 109.59 (3.38) b | 109.11 (4.49) b | 107.01 (4.58) b | 105.34 (43.61) b | 106.43 (2.87) b | 105.71 (2.24) b | 5.98 *** |
| ΔE* | 0.52 (0.61) a | 3.19 (0.74) b | 3.13 (0.97) b | 3.06 (1.07) b | 2.93 (1.02) b | 3.17 (0.98) b | 3.07 (0.87) b | 5.94 *** |
| WI | 7.2 (0.42) a | 9.09 (0.42) b | 8.92 (0.61) b | 8.58 (0.74) b | 8.33 (0.56) b | 8.54 (0.36) b | 8.43 (0.38) b | 8.38 *** |
| YI | 35.78 (3.19) b | 232.61 (3.66) a | 23 (4.69) a | 23.87 (5.43) a | 24.85 (4.68) a | 23.31 (4.86) a | 24.27 (4.15) a | 5.79 *** |
| Adulteration with soybean oil (%) | F-value | |||||||
| 0 | 5 | 10 | 20 | 30 | 40 | 50 | ||
| L* | 20.84 (0.291) b | 19.15 (0.565) a | 18.83 (0.90) a | 18.88 (0.96) a | 19.09 (0.82) a | 19.05 (1.03) a | 18.91 (0.93) a | 3.21 ns |
| a* | −0.99 (0.29) ab | −1.09 (0.15) a | −1.06 (0.09) a | −0.95 (0.07) a | −0.89 (0.17) a | −0.86 (0.12) a | −0.77 (0.10) a | 5.39 *** |
| b* | 5.22 (0.52) b | 3.15 (0.80) a | 2.86 (0.52) ab | 2.99 (0.67) abc | 3.14 (0.90) abc | 3.06 (1.18) bc | 2.88 (0.96) c | 6.06 *** |
| C* | 9.8 (1.10) b | 5.94 (1.46) a | 5.44 (0.90) a | 5.64 (1.17) a | 5.88 (1.66) a | 5.74 (2.13) a | 5.37 (1.74) a | 5.72 *** |
| h* | 99.26 (2.20) a | 109.06 (4.87) b | 109.63 (3.09) b | 107.14 (3.69) b | 105.54 (4.82) ab | 106.42 (5.84) b | 105.34 (5.37) ab | 4.48 ** |
| ΔE* | 0.52 (0.61) a | 3.16 (1.18) b | 3.54 (0.96) b | 3.42 (1.09) b | 3.16 (1.18) b | 3.26 (1.52) b | 3.49 (1.29) b | 3.57 ** |
| WI | 7.2 (0.42) a | 8.85 (0.46) b | 8.95 (0.31) b | 8.57 (0.36) b | 8.36 (0.65) b | 8.37 (0.88) b | 8.29 (0.72) b | 5.91 *** |
| YI | 35.78 (3.19) b | 23.29 (4.99) a | 21.57 (3.12) a | 22.49 (4.06) a | 23.29 (5.75) a | 22.61 (7.51) a | 21.52 (6.10) a | 4.99 *** |
3.3. Walnut Oil Classification Using Statistical Analysis
3.3.1. Unsupervised Method—Principal Component Analysis (PCA)
3.3.2. Supervised Methods—Discriminant Analysis (DA) vs. Partial Least Squares Linear Discriminant Analysis (PLS-LDA)
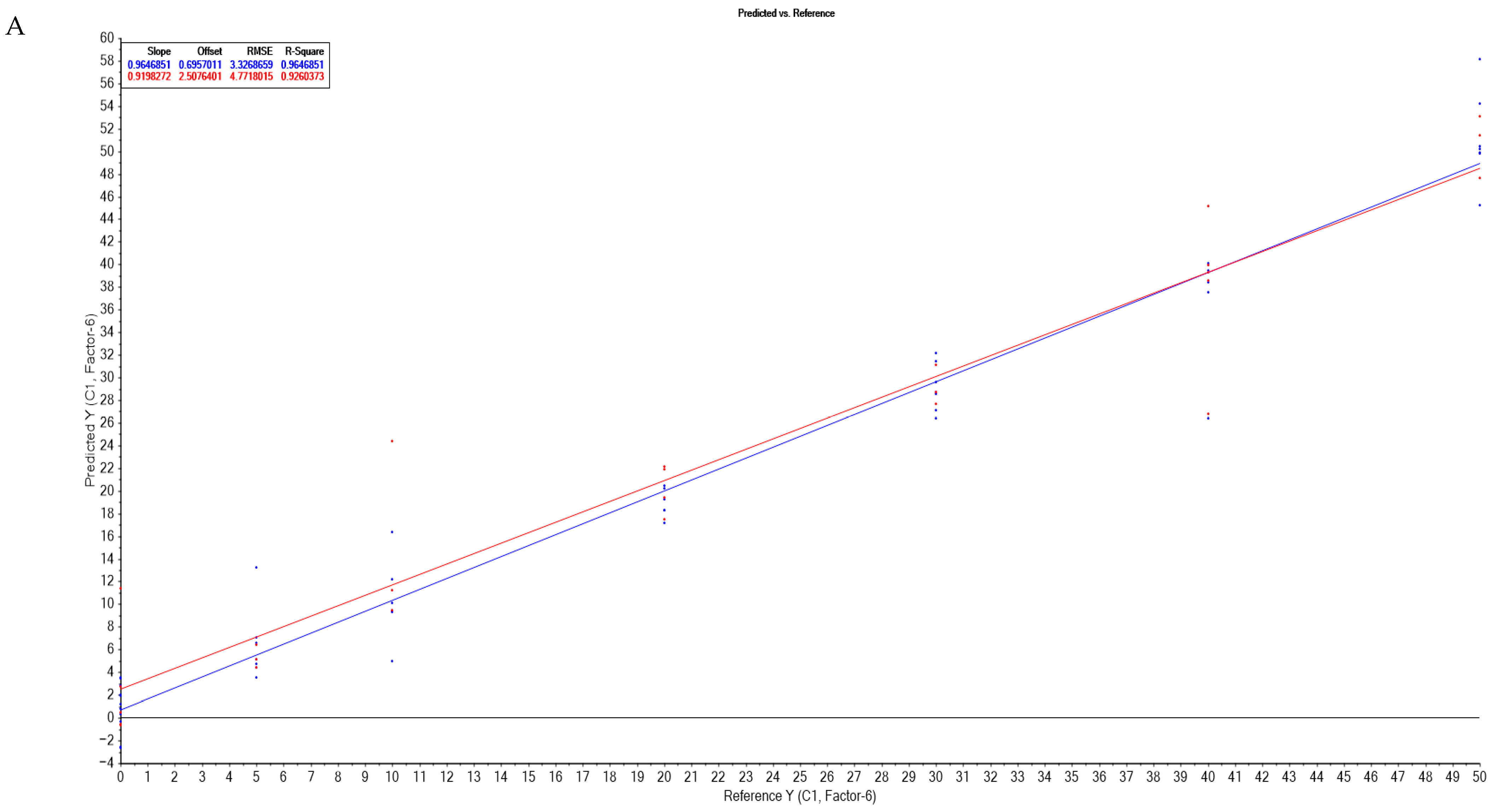
3.3.3. Partial Least Squares Regression Correlation of UV-Vis-NIR Spectra with the Degree of Adulteration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Özdikicierler, O.; Akcan, T.; Ergönül, P.G.; Karacan, E. Rapid Detection of Virgin Olive Oil Adulteration via Chemometric Analysis of Raw Differential Scanning Calorimetry Data and Pretreated Fourier Transform Infrared Spectroscopy. Eur. Food Res. Technol. 2025, 251, 2617–2631. [Google Scholar] [CrossRef]
- Pan, M.; Sun, S.; Zhou, Q.; Chen, J. A Simple and Portable Screening Method for Adulterated Olive Oils Using the Hand-Held FTIR Spectrometer and Chemometrics Tools. J. Food Sci. 2018, 83, 1605–1612. [Google Scholar] [CrossRef]
- Tan, C.H.; Kong, I.; Irfan, U.; Solihin, M.I.; Pui, L.P. Edible Oils Adulteration: A Review on Regulatory Compliance and Its Detection Technologies. J. Oleo Sci. 2021, 70, ess21109. [Google Scholar] [CrossRef]
- Ali, H.; Saleem, M.; Anser, M.R.; Khan, S.; Ullah, R.; Bilal, M. Validation of Fluorescence Spectroscopy to Detect Adulteration of Edible Oil in Extra Virgin Olive Oil (EVOO) by Applying Chemometrics. Appl. Spectrosc. 2018, 72, 1371–1379. [Google Scholar] [CrossRef]
- McGrath, T.F.; Haughey, S.A.; Patterson, J.; Fauhl-Hassek, C.; Donarski, J.; Alewijn, M.; van Ruth, S.; Elliott, C.T. What Are the Scientific Challenges in Moving from Targeted to Non-Targeted Methods for Food Fraud Testing and How Can They Be Addressed?—Spectroscopy Case Study. Trends Food Sci. Technol. 2018, 76, 38–55. [Google Scholar] [CrossRef]
- Vicario, G.; Francini, A.; Cifelli, M.; Domenici, V.; Sebastiani, L. Near UV-Vis and NMR Spectroscopic Methods for Rapid Screening of Antioxidant Molecules in Extra-Virgin Olive Oil. Antioxidants 2020, 9, 1245. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, J.A.; Yousfi, K.; Carmen Martínez, M.; García, J.M. Rapid Determination of Olive Oil Chlorophylls and Carotenoids by Using Visible Spectroscopy. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1677–1689. [Google Scholar] [CrossRef]
- Moyano, M.J.; Meléndez-Martínez, A.J.; Alba, J.; Heredia, F.J. A Comprehensive Study on the Colour of Virgin Olive Oils and Its Relationship with Their Chlorophylls and Carotenoids Indexes (II): CIELUV and CIELAB Uniform Colour Spaces. Food Res. Int. 2008, 41, 513–521. [Google Scholar] [CrossRef]
- Vucane, S.; Cinkmanis, I.; Šabovics, M. Colorimetric Measurements of Vegetable Oils by Smartphone-Based Image Analysis. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2022, 76, 110–115. [Google Scholar] [CrossRef]
- Escolar, D.; Haro, M.R.; Saucedo, A.; Ayuso, J.; Jiménez, A.; Alvarez, J.A. Color Determination in Olive Oils. J. Am. Oil Chem. Soc. 1994, 71, 1333–1337. [Google Scholar] [CrossRef]
- Minguez-Mosquera, I.M.; Rejano-Navarro, L.; Gandul-Rojas, B.; SanchezGomez, A.H.; Garrido-Fernandez, J. Color-Pigment Correlation in Virgin Olive Oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Christy, A.A.; Kasemsumran, S.; Du, Y.; Ozaki, Y. The Detection and Quantification of Adulteration in Olive Oil by Near-Infrared Spectroscopy and Chemometrics. Anal. Sci. 2004, 20, 935–940. [Google Scholar] [CrossRef]
- Ge, F.; Chen, C.; Liu, D.; Zhao, S. Rapid Quantitative Determination of Walnut Oil Adulteration with Sunflower Oil Using Fluorescence Spectroscopy. Food Anal. Methods 2014, 7, 146–150. [Google Scholar] [CrossRef]
- Dais, P.; Hatzakis, E. Quality Assessment and Authentication of Virgin Olive Oil by NMR Spectroscopy: A Critical Review. Anal. Chim. Acta 2013, 765, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Nanou, E.; Bekogianni, M.; Stamatoukos, T.; Couris, S. Detection of Adulteration of Extra Virgin Olive Oil via Laser-Induced Breakdown Spectroscopy and Ultraviolet-Visible-Near-Infrared Absorption Spectroscopy: A Comparative Study. Foods 2025, 14, 321. [Google Scholar] [CrossRef]
- Zhang, W.; Li, N.; Feng, Y.; Su, S.; Li, T.; Liang, B. A Unique Quantitative Method of Acid Value of Edible Oils and Studying the Impact of Heating on Edible Oils by UV-Vis Spectrometry. Food Chem. 2015, 185, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Weng, S.; Wang, L.; Xu, T. Reflectance Spectroscopy with Multivariate Methods for Non-Destructive Discrimination of Edible Oil Adulteration. Biosensors 2021, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Pan, F.; Wang, L.; Weng, S. Rapid Detection of Fatty Acids in Edible Oils Using Vis-Nir Reflectance Spectroscopy with Multivariate Methods. Biosensors 2021, 11, 261. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Effects of Processing Methods on the Chemical Composition and Antioxidant Capacity of Walnut (Juglans regia L.) Oil. LWT 2021, 135, 109958. [Google Scholar] [CrossRef]
- Leahu, A.; Oroian, M.; Ropciuc, S. The Quality and Stability of Walnut Oil Under the Influence of Storage Conditions; University of Agricultural Sciences and Veterinary Medicine Iasi: Iasi, Romania, 2016; pp. 230–236. [Google Scholar]
- Zaukuu, J.L.Z.; Adam, M.N.; Nkansah, A.A.; Mensah, E.T. Detection and Quantification of Groundnut Oil Adulteration with Machine Learning Using a Comparative Approach with NIRS and UV–VIS. Sci. Rep. 2024, 14, 20931. [Google Scholar] [CrossRef] [PubMed]
- Malavi, D.; Nikkhah, A.; Raes, K.; Van Haute, S. Hyperspectral Imaging and Chemometrics for Authentication of Extra Virgin Olive Oil: A Comparative Approach with FTIR, UV-VIS, Raman, and GC-MS. Foods 2023, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, I.; Bakır, M.R.; Kalkan, O.; Kara, H. Multivariate Modeling for Quantifying Adulteration of Sunflower Oil with Low Level of Safflower Oil Using ATR-FTIR, UV-Visible, and Fluorescence Spectroscopies: A Comparative Approach. Food Anal. Methods 2021, 14, 361–371. [Google Scholar] [CrossRef]





| Pre-Treatment | Model | Step | Samples (%) | Total | |||
|---|---|---|---|---|---|---|---|
| Authentic | Sunflower | Rapeseed | Soybean | ||||
| Baseline spectra 350–1650 nm | PLS-DA | Calibration | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Validation | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | ||
| Detrend spectra 500–1650 nm | DA | Calibration | 91.67 | 77.50 | 72.22 | 71.43 | 78.20 |
| Validation | 75.00 | 90.00 | 70.83 | 61.11 | 74.24 | ||
| SNV spectra 350–1650 nm | DA | Calibration | 100.00 | 94.29 | 95.00 | 97.73 | 96.75 |
| Validation | 80.00 | 80.00 | 95.00 | 87.50 | 86.36 | ||
| MSC spectra 500–1650 nm | PLS-DA | Calibration | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Validation | 100.00 | 94.74 | 100.00 | 100.00 | 98.48 | ||
| Adulterant | Pre-Treatment | No. Factor | Calibration | Cross-Validation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Offset | RMSE | R2 | Slope | Offset | RMSE | R2 | |||
| All the samples 500–1650 nm | Detrend | 17 | 0.915 | 2.0927 | 4.965 | 0.915 | 0.810 | 6.106 | 6.691 | 0.828 |
| Rapeseed oils 350–1650 nm | 1st derivate | 7 | 0.955 | 1.006 | 3.945 | 0.955 | 0.990 | 2.947 | 5.672 | 0.876 |
| Soybean oils 500–1650 nm | 2nd derivate | 6 | 0.964 | 0.695 | 3.332 | 0.964 | 0.919 | 2.507 | 4.771 | 0.926 |
| Sunflower oils 350–1650 nm | No pre-treatment | 18 | 0.989 | 0.195 | 1.774 | 0.989 | 0.943 | 0.463 | 5.547 | 0.906 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fediuc, G.; Spinei, M.; Oroian, M. Cold-Pressed Walnut-Oil Adulteration with Edible Oils Detection Using Vis-NIR Spectroscopy. Foods 2025, 14, 3877. https://doi.org/10.3390/foods14223877
Fediuc G, Spinei M, Oroian M. Cold-Pressed Walnut-Oil Adulteration with Edible Oils Detection Using Vis-NIR Spectroscopy. Foods. 2025; 14(22):3877. https://doi.org/10.3390/foods14223877
Chicago/Turabian StyleFediuc, Georgiana, Mariana Spinei, and Mircea Oroian. 2025. "Cold-Pressed Walnut-Oil Adulteration with Edible Oils Detection Using Vis-NIR Spectroscopy" Foods 14, no. 22: 3877. https://doi.org/10.3390/foods14223877
APA StyleFediuc, G., Spinei, M., & Oroian, M. (2025). Cold-Pressed Walnut-Oil Adulteration with Edible Oils Detection Using Vis-NIR Spectroscopy. Foods, 14(22), 3877. https://doi.org/10.3390/foods14223877





