Establishing a Detection Method Based on Multiplex PCR for Identification of Sheep Meat, Goat Meat and Common Adulterant Meats
Abstract
1. Introduction
2. Materials and Methods
2.1. Meat Sample Sources
2.2. DNA Extraction
2.3. Establishment of Multiplex PCR Detection Method
2.3.1. Selection of Specific Primers
2.3.2. Optimization Testing of Multiplex PCR Amplification Annealing Temperature
2.3.3. Agarose Gel Electrophoresis
2.4. Specificity Experiment
2.5. Reproducibility Experiment
2.6. Sensitivity Experiment
2.7. Analog Simulation Experiment
2.7.1. Simulation of Duck Meat Adulteration in Sheep and Goat Meat
2.7.2. Heat-Treated Meat Product Adulteration Experiment
3. Results
3.1. Optimization Ressult of Multiplex PCR Annealing Temperature
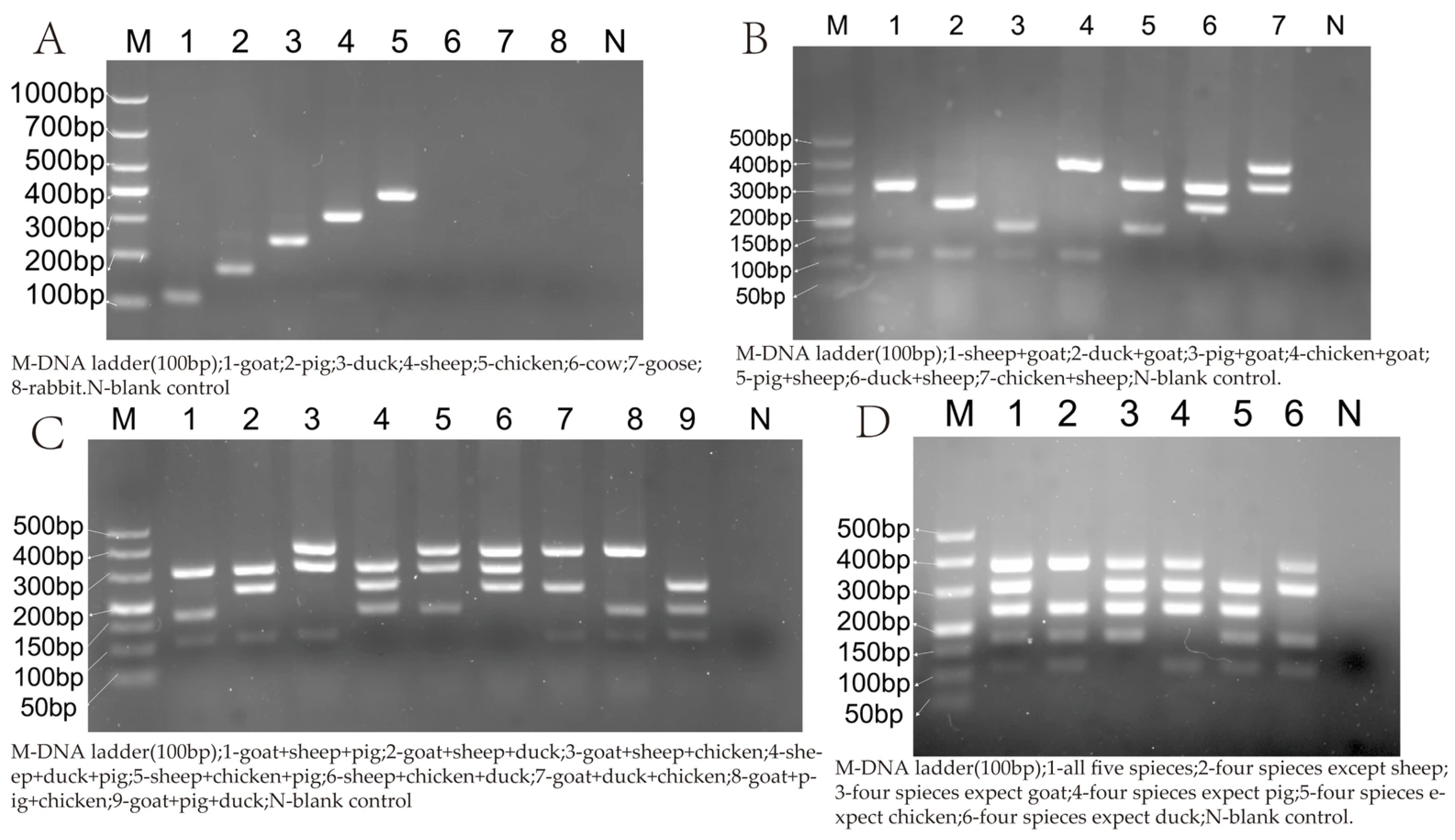
3.2. Specificity of Multiplex PCR
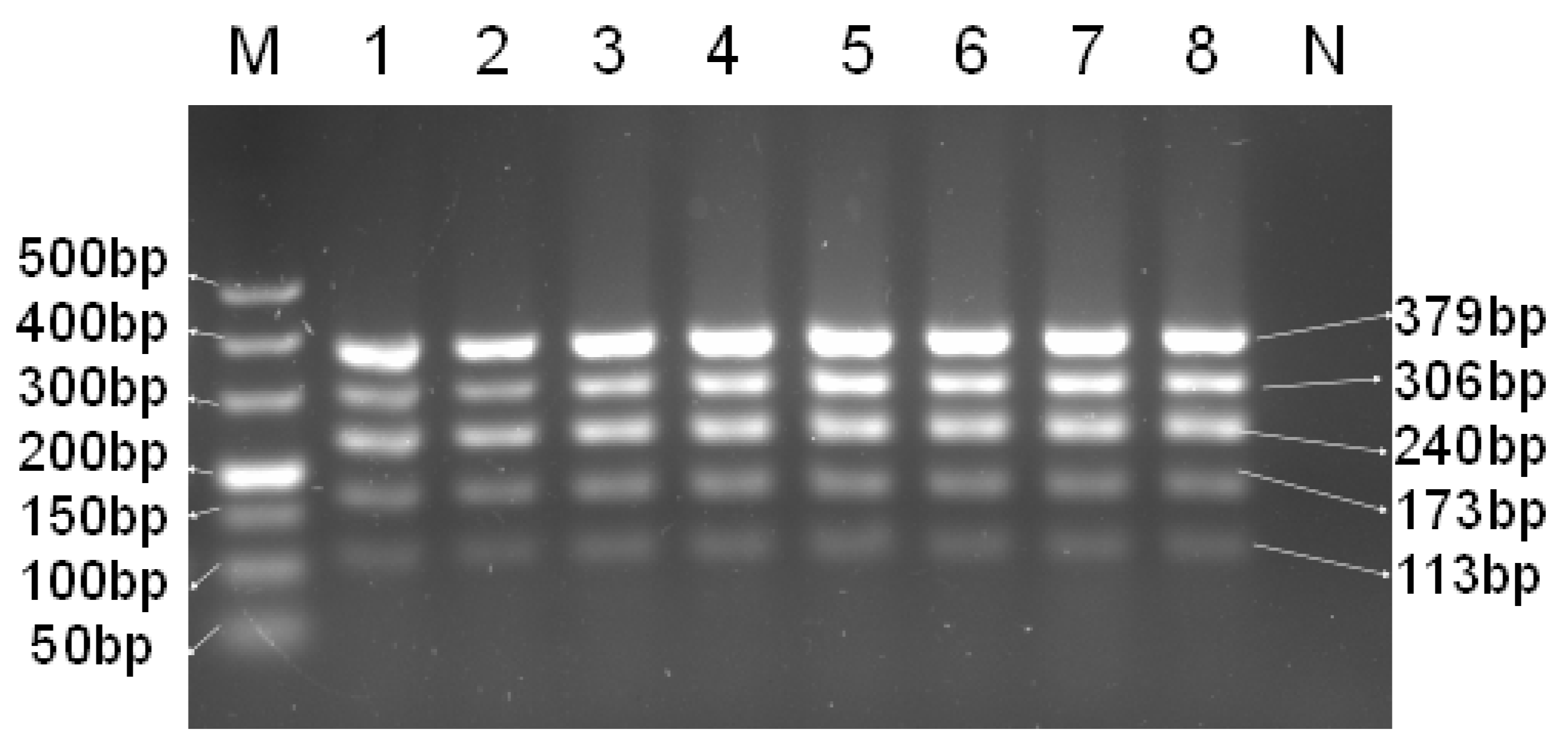
3.3. Reproducibility of Multiplex PCR
3.4. Sensitivity of Multiplex PCR
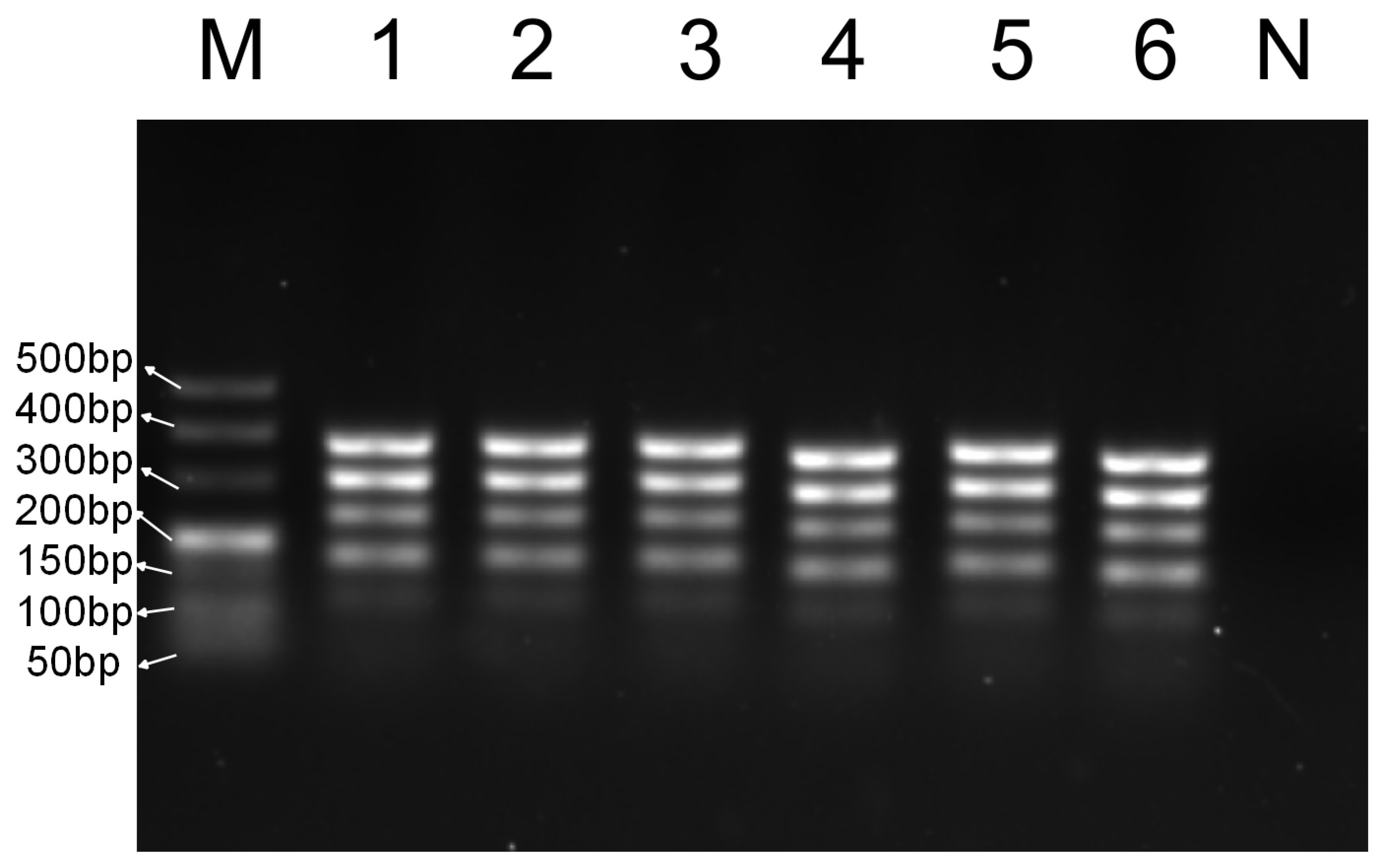
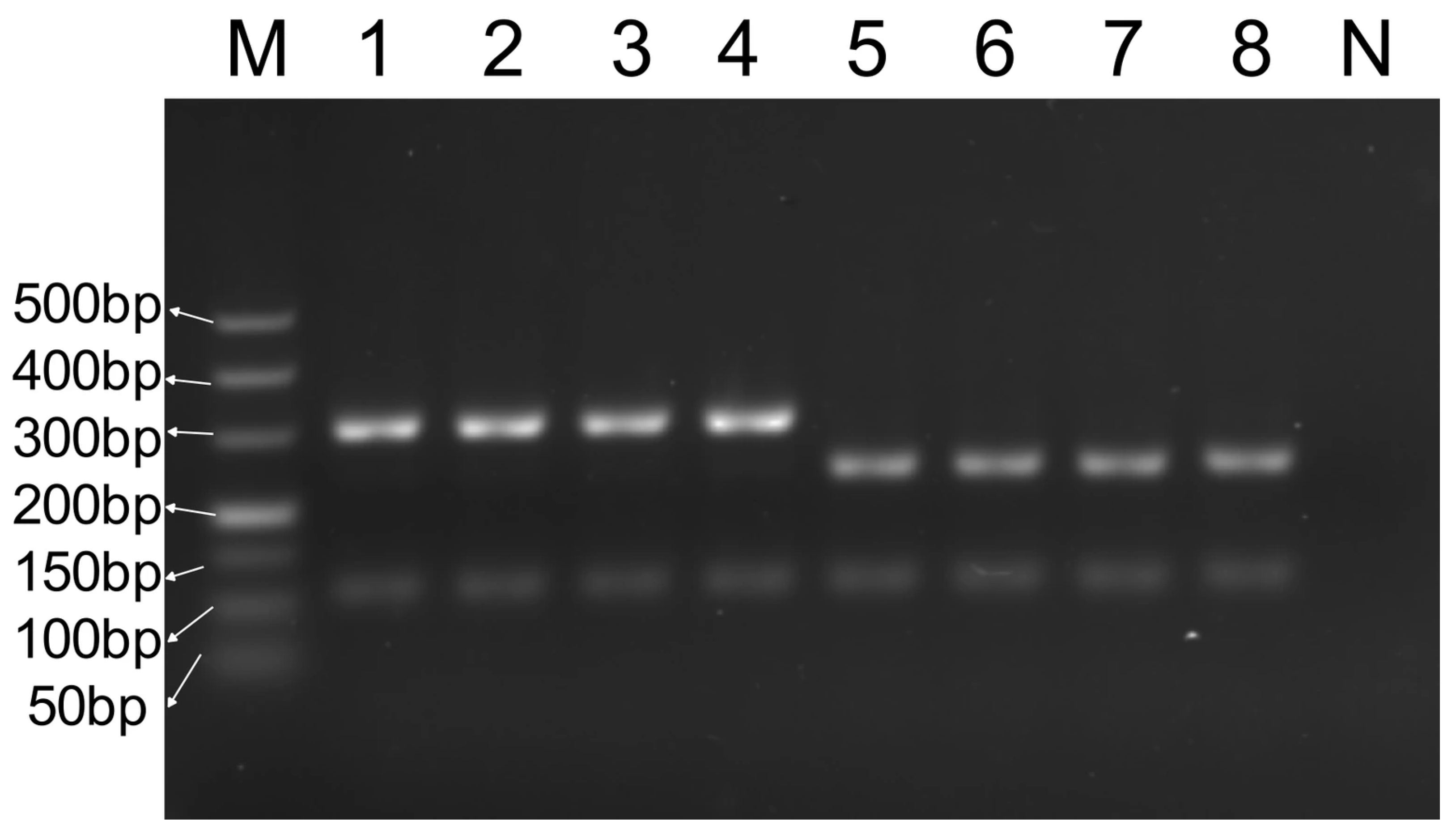
3.5. Market Adulteration Simulation Experiment
3.5.1. Simulation of Duck Meat Adulteration of Sheep and Goat Meat, and Sheep Meat Adulteration of Goat Meat
3.5.2. Analysis of Heat-Treated Meat Product Adulteration Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Meat Market Review—Overview of Global Market Developments in 2024; Food and Agriculture Organization of the United Nations: Rome, Italy, 2025; Available online: https://openknowledge.fao.org/handle/20.500.14283/cd5077en (accessed on 1 October 2025).
- FAO. Agricultural Production Statistics 2010–2023; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024; Available online: https://openknowledge.fao.org/items/ab36b259-d641-4ded-8832-32f579685be7 (accessed on 1 October 2025).
- FAO. Food Outlook—Biannual Report on Global Food Markets; Food and Agriculture Organization of the United Nations: Rome, Italy, 2025; Available online: https://openknowledge.fao.org/items/9c0817c6-e826-4588-a4f6-0fa1e6c64b6e (accessed on 1 October 2025).
- FAO. FAO Meat Price Index. Markets and Trade; Food and Agriculture Organization of the United Nations: Rome, Italy, 2025; Available online: https://www.fao.org/markets-and-trade/commodities-overview/basic-foods/fao-meat-price-index/en (accessed on 1 October 2025).
- OECD-FAO. OECD-FAO Agricultural Outlook 2025–2034; OECD Publishing: Paris, France; FAO: Rome, Italy, 2025; Available online: https://www.oecd.org/en/publications/oecd-fao-agricultural-outlook-2025-2034_601276cd-en.html (accessed on 1 October 2025).
- Xu, J.; Zhao, W.; Zhu, M.; Wen, Y.; Xie, T.; He, X.; Zhang, Y.; Cao, S.; Niu, L.; Zhang, H.; et al. Molecular identification of adulteration in mutton based on mitochondrial 16S rRNA gene. Mitochondrial DNA Part A 2016, 27, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, L.; Ding, W.; Zhang, D.Q.; Wang, J.Y.; Reed, K.; Zhang, B.C. Adulterant identification in mutton by electronic nose and gas chromatography-mass spectrometer. Food Control 2019, 98, 431–438. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024; Available online: https://www.fao.org/faostat/en/#data/EA (accessed on 1 October 2025).
- FAO. Food Balance Sheets 2010–2022: Global, Regional and Country Trends; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024; Available online: https://www.fao.org/statistics/highlights-archive/highlights-detail/food-balance-sheets-2010-2022-global-regional-and-country-trends/en (accessed on 1 October 2025).
- Rosario, I.L.D.; Vieira, C.P.; Salgado, M.J.G.; Monteiro, N.B.; Alzate, K.G.; de Araújo, G.C.; Delgado, K.F.; Conte, C.A., Jr.; da Costa, M.P. Assessment of plain yoghurt quality parameters affected by milk adulteration: Implications for culture kinetics, physicochemical properties, and sensory perception. Int. J. Dairy Technol. 2024, 77, 827–842. [Google Scholar] [CrossRef]
- Rady, A.; Adedeji, A. Assessing different processed meats for adulterants using visible-near infrared spectroscopy. Meat Sci. 2018, 136, 59–67. [Google Scholar] [CrossRef]
- Rady, A.M.; Adedeji, A.; Watson, N.J. Feasibility of utilizing color imaging and machine learning for adulteration detection in minced meat. J. Agric. Food Res. 2021, 6, 100251. [Google Scholar] [CrossRef]
- Quan, P.L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef]
- Hossain, M.A.M.; Abidin, S.; Bujang, A.; Taib, M.N.; Sagadevan, S.; Johan, M.R.; Nizar, N.N.A. TaqMan multiplex qPCR for detecting animal species in meat and meat products: Development, recent advances and future prospects. Food Control 2023, 150, 109761. [Google Scholar] [CrossRef]
- Roslan, N.D.; Vadamalai, G.; Idris, A.S.; Ling, K.L.; Sundram, S. Comparison of Real-Time PCR, Conventional PCR and RT-LAMP for the Detection Of Coconut Cadang-cadang Viroid Variant in Oil Palm. J. Oil Palm Res. 2022, 35, 121–132. [Google Scholar] [CrossRef]
- Liu, H.B.; Cao, T.T.; Wang, J.; Yuan, Y.; Li, H.J.; He, K.; Chen, H.S.; Wang, L. Accurate and simultaneous detection of pork and horse meat adulteration by double tailed recombinase polymerase amplification integrated with SERS based two-color lateral flow nucleic acid hybridization strip. J. Food Compos. Anal. 2024, 134, 106562. [Google Scholar] [CrossRef]
- Huang, J.H.; Dai, J.S.; Liu, M.; Huang, S.X.; Wu, Y.W.; Rong, D.L.; Li, Y.Y.; Zhao, M.; Li, Y.; Zhang, J.M. Mining of novel target genes through Pan-genome analysis for Conventional PCR and Real-Time PCR detection of Staphylococcus argenteus in food. Food Control 2024, 164, 110550. [Google Scholar] [CrossRef]
- De Barba, M.; Miquel, C.; Boyer, F.; Mercier, C.; Rioux, D.; Coissac, E.; Taberlet, P. DNA metabarcoding multiplexing and validation of data accuracy for diet assessment: Application to omnivorous diet. Mol. Ecol. Resour. 2014, 14, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.J.; Du, B.Y.; Ma, Q.H.; Ma, Y.H.; Yu, W.Y.; Li, T.; Liu, Y.; Yuan, G.X. Multiplex-PCR method application to identify duck blood and its adulterated varieties. Food Chem. 2024, 444, 138673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Semi-nested multiplex PCR enhanced method sensitivity of species detection in further-processed meats. Food Control 2013, 31, 326–330. [Google Scholar] [CrossRef]
- Pu, K.Y.; Qiu, J.M.; Li, J.Y.; Huang, W.; Lai, X.P.; Liu, C.; Lin, Y.; Ng, K.M. MALDI-TOF MS Protein Profiling Combined with Multivariate Analysis for Identification and Quantitation of Beef Adulteration. Food Anal. Methods 2023, 16, 132–142. [Google Scholar] [CrossRef]
- Jiang, Z.-W.; Xia, R.-C.; Tao, R.-Y.; Li, C.-T. Establishment and Validation of a Multiplex PCR Detection System for the Identification of Six Common Edible Meat Components. Fa Yi Xue Za Zhi 2024, 40, 254–260. [Google Scholar]
- Ali, M.E.; Hashim, U.; Kashif, M.; Mustafa, S.; Man, Y.B.C.; Abd Hamid, S.B. Development of swine-specific DNA markers for biosensor-based halal authentication. Genet. Mol. Res. 2012, 11, 1762–1772. [Google Scholar] [CrossRef]
- Galal-Khallaf, A. Multiplex PCR and 12S rRNA gene sequencing for detection of meat adulteration: A case study in the Egyptian markets. Gene 2021, 764, 145062. [Google Scholar] [CrossRef]
- Vallone, P.M.; Butler, J.M. AutoDimer: A screening tool for primer-dimer and hairpin structures. BioTechniques 2004, 37, 226–231. [Google Scholar] [CrossRef]
- Karabasanavar, N.S.; Singh, S.P.; Umapathi, V.; Kumar, D.; Patil, G.; Shebannavar, S.N. A highly specific PCR assay for identification of raw and heat treated mutton (Ovis aries). Small Rumin. Res. 2011, 100, 153–158. [Google Scholar] [CrossRef]
- Laib, S.; Rankl, M.; Ruckstuhl, T.; Seeger, S. Sizing of single fluorescently stained DNA fragments by scanning microscopy. Nucleic Acids Res. 2003, 31, e138. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Economically Motivated Adulteration (Food Fraud). Available online: https://www.fda.gov/food/compliance-enforcement-food/economically-motivated-adulteration-food-fraud (accessed on 20 January 2025).
- Market Data Forecast. Food Authenticity Testing Market Size, Share & Trends Analysis Report 2024–2032. Available online: https://www.marketdataforecast.com/market-reports/food-authenticity-testing-market (accessed on 20 January 2025).
- Zhang, W.; Liu, Y.; Wang, X.; Zhang, H.; Wang, Y.; Li, X.; Dong, X. Meat food fraud risk in Chinese markets 2012–2021. npj Sci. Food 2023, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Saleem, M.S.; Imran, M.; Khan, W.A.; Ashraf, K.; Zahoor, M.Y.; Rashid, I.; Rehman, H.U.; Nadeem, A.; Ali, S.; et al. Single tube multiplex PCR assay for the identification of banned meat species. Food Addit. Contam. Part B-Surveill. 2020, 13, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.D.; Fröder, H.; Righi, E. Gluten Detection by Real-Time PCR: An Alternative for Tracking Processed Foods. Braz. Arch. Biol. Technol. 2025, 68, e25230869. [Google Scholar] [CrossRef]
- Zhu, T.Y.; Zhou, X.F.; Zhang, W.; Wu, Y.; Yang, J.J.; Xu, L.Y.; Chen, M.; Dong, W.R.; Xu, H.S. Multiplex and real-time PCR for qualitative and quantitative donkey meat adulteration. J. Food Meas. Charact. 2021, 15, 1161–1168. [Google Scholar] [CrossRef]
- Liu, W.W.; Wang, X.N.; Tao, J.; Xi, B.S.; Xue, M.; Sun, W.P. A Multiplex PCR Assay Mediated by Universal Primers for the Detection of Adulterated Meat in Mutton. J. Food Prot. 2019, 82, 325–330. [Google Scholar] [CrossRef]
- Bai, Z.X.; Gu, J.F.; Zhu, R.G.; Yao, X.D.; Kang, L.C.; Ge, J.B. Discrimination of Minced Mutton Adulteration Based on Sized-Adaptive Online NIRS Information and 2D Conventional Neural Network. Foods 2022, 11, 2977. [Google Scholar] [CrossRef]
- Yao, Y.L.; Zhou, Y.D.; Wang, W.Y. Study on Detection Method for Pig, Cow and Sheep Derived Componentsby Multiple Fluorescence PCR in Meat Skewer Products. J. Anhui Agric. Sci. 2024, 52, 184–187. (In Chinese) [Google Scholar]
- Fan, B.B.; Zhu, R.G.; He, D.Y.; Wang, S.C.; Cui, X.M.; Yao, X.D. Evaluation of Mutton Adulteration under the Effect of Mutton Flavour Essence Using Hyperspectral Imaging Combined with Machine Learning and Sparrow Search Algorithm. Foods 2022, 11, 2278. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.J.; Bai, Y.; Zhao, Y.; Guo, J.; Chen, A.L.; Yang, S.M.; Zhao, S.S.; Tan, L.Q. Research progress on mutton origin tracing and authenticity. Food Chem. 2022, 373, 131387. [Google Scholar] [CrossRef]
- Du, J.H.; Gan, M.L.; Xie, Z.W.; Zhou, C.P.; Li, M.L.; Wang, M.; Dai, H.D.; Huang, Z.Y.; Chen, L.; Zhao, Y.; et al. Current progress on meat food authenticity detection methods. Food Control 2023, 152, 109842. [Google Scholar] [CrossRef]
- Hossain, M.A.M.; Uddin, S.M.K.; Sultana, S.; Wahab, Y.A.; Sagadevan, S.; Johan, M.R.; Ali, M.E. Authentication of Halal and Kosher meat and meat products: Analytical approaches, current progresses and future prospects. Crit. Rev. Food Sci. Nutr. 2022, 62, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, X.M.; Ma, W.J.; Li, H.M.; Sun, Y.X.; Qiu, J.H.; Guo, H.J. Establishment and Application of Multiplex PCR Scheme for Simultaneously Distinguishing Muscle Tissues of Mink, Fox, and Raccoon Dog from Conventional Livestock and Poultry. Food Anal. Methods 2024, 17, 226–235. [Google Scholar] [CrossRef]
- Mori, C.; Matsumura, S. Development and validation of simultaneous identification of 26 mammalian and poultry species by a multiplex assay. Int. J. Leg. Med. 2022, 136, 1–12. [Google Scholar] [CrossRef]
- Meyer, R.; Candrian, U.; Lüthy, J. Detection of pork in heated meat products by the polymerase chain reaction. Aoac Int. 1994, 77, 617–622. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ye, J.R.; Li, X.M.; Liu, Y.; Dai, R.T. Detection of Meat Component and Meat Species in Food and Feedstuff Using PCR Technique. Storage Process 2006, 4, 33–36. [Google Scholar]
- Cutrufelli, M.E.; Mageau, R.P.; Schwab, B.; Johnston, R.W. Detection of beef and poultry by serological field screening tests (ORBIT and PROFIT): Collaborative study. J. Assoc. Off. Anal. Chem. 1987, 70, 230–233. [Google Scholar] [CrossRef]
- Yu, N.; Xing, R.R.; Wang, P.; Deng, T.T.; Zhang, J.K.; Zhao, G.M.; Chen, Y. A novel duplex droplet digital PCR assay for simultaneous authentication and quantification of Panax notoginseng and its adulterants. Food Control 2022, 132, 108493. [Google Scholar] [CrossRef]
- Nhari, R.; Soh, J.H.; Mokhtar, N.F.K.; Mohammad, N.A.; Hashim, A.M. Halal authentication using lateral flow devices for detection of pork adulteration in meat products: A review. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2023, 40, 971–980. [Google Scholar] [CrossRef]
- Li, J.C.; Li, J.P.; Xu, S.G.; Xiong, S.Y.; Yang, J.N.; Chen, X.; Wang, S.W.; Qiao, X.L.; Zhou, T. A rapid and reliable multiplex PCR assay for simultaneous detection of fourteen animal species in two tubes. Food Chem. 2019, 295, 395–402. [Google Scholar] [CrossRef]
- Bagdonaitė, L.; Leder, E.H.; Lifjeld, J.T.; Johnsen, A.; Mauvisseau, Q. Assessing reliability and accuracy of qPCR, dPCR and ddPCR for estimating mitochondrial DNA copy number in songbird blood and sperm cells. PeerJ 2025, 13, e19278. [Google Scholar] [CrossRef]
- He, Y.X.; Dong, L.M.; Yan, W.; Xing, Z.J.; Xia, W.; Li, C.C.; Long, L.K.; Li, F.W. A Multiplex PCR System for the Detection and Quantification of Four Genetically Modified Soybean Events. Food Anal. Methods 2025, 18, 621–633. [Google Scholar]
- Xia, L.P.; Gui, Y.H.; Yin, R.; Li, N.; Yue, M.; Mu, Y. Concanavalin A-assisted multiplex digital PCR assay for rapid capture and accurate quantification detection of foodborne pathogens. Talanta 2024, 277, 126351. [Google Scholar] [CrossRef]


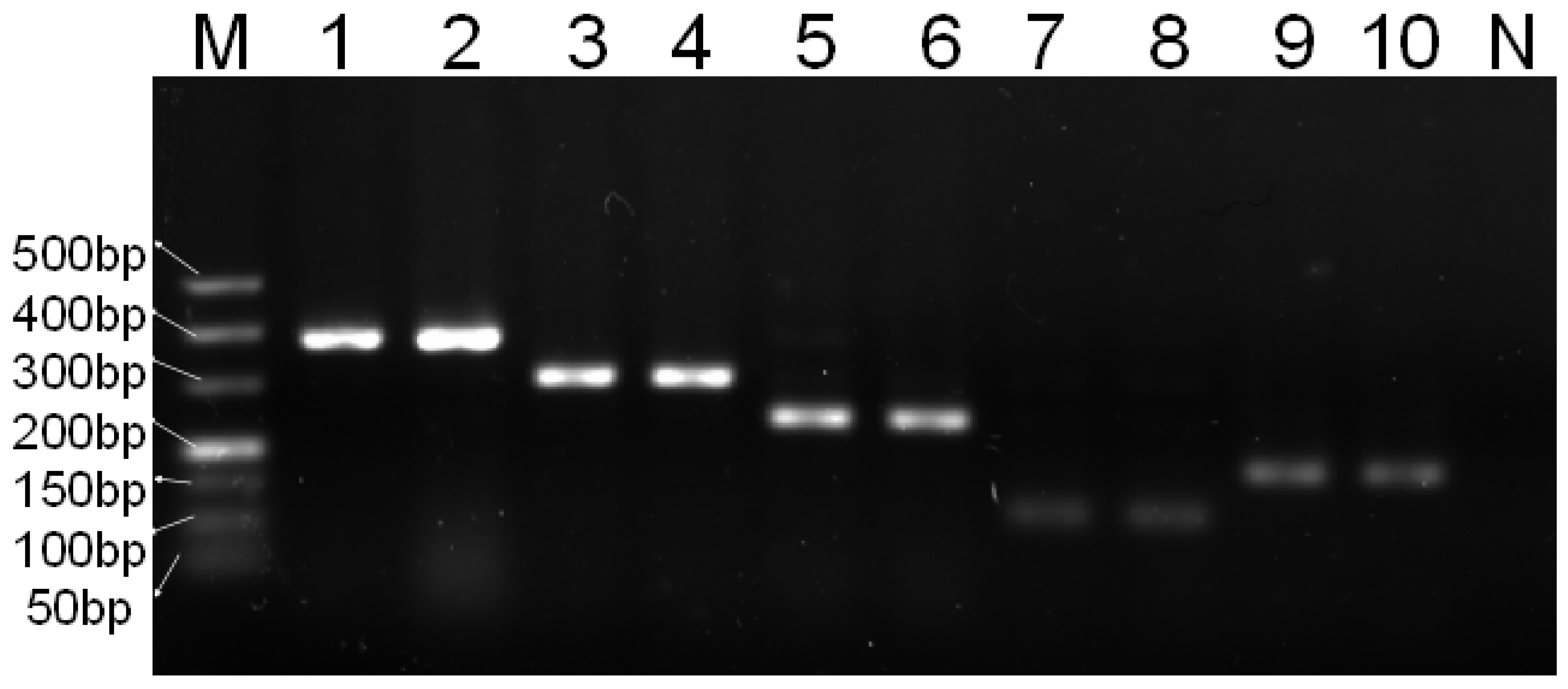
| Species | Target Gene | Primer Sequence (5′→3′) | Product Length |
|---|---|---|---|
| Chicken | 16S rRNA | F: TGCGTCAAAGCTCCCTCATT R: TTCGCACGGTTAGGATACCG | 379 bp |
| Sheep | COX-2 | F: TGCTCTTCCATCCTTGCGAAT R: CGACCTGGAATTGCGTCTGT | 306 bp |
| Pig | 16S rRNA | F: TCGCACACGCTTACATCAGT R: TTGGTAAACAGGCGGGGTTT | 173 bp |
| Goat | ND6 | F: CTCATCCTCGTCACCGCAAA R: GTGTTTGCGTCTGTTCGTCC | 113 bp |
| Duck | ATP6 | F: AAAACGGCCACAAATGAGCC R: GGATTAGTGCGGGGATCAGG | 240 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Quan, K.; Yang, H.; Song, Y.; Zhang, X.; Wang, B.; Lv, X.; Sun, W. Establishing a Detection Method Based on Multiplex PCR for Identification of Sheep Meat, Goat Meat and Common Adulterant Meats. Foods 2025, 14, 3875. https://doi.org/10.3390/foods14223875
Yang Y, Quan K, Yang H, Song Y, Zhang X, Wang B, Lv X, Sun W. Establishing a Detection Method Based on Multiplex PCR for Identification of Sheep Meat, Goat Meat and Common Adulterant Meats. Foods. 2025; 14(22):3875. https://doi.org/10.3390/foods14223875
Chicago/Turabian StyleYang, Yanbing, Kai Quan, Huiguo Yang, Yuxuan Song, Xiyun Zhang, Bo Wang, Xiaoyang Lv, and Wei Sun. 2025. "Establishing a Detection Method Based on Multiplex PCR for Identification of Sheep Meat, Goat Meat and Common Adulterant Meats" Foods 14, no. 22: 3875. https://doi.org/10.3390/foods14223875
APA StyleYang, Y., Quan, K., Yang, H., Song, Y., Zhang, X., Wang, B., Lv, X., & Sun, W. (2025). Establishing a Detection Method Based on Multiplex PCR for Identification of Sheep Meat, Goat Meat and Common Adulterant Meats. Foods, 14(22), 3875. https://doi.org/10.3390/foods14223875






