Evaluation of Digestion Methods in Microplastic Recovery from Mussels (Mytilus galloprovincialis) for a Standardised Microplastic Isolation Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Reference Microplastics Selection and Chemical Resistance of Plastics
2.3. Sampling and Sample Preparation
2.4. Digestion Treatment Conditions and Density Separation
2.5. Filtration, Digestion Efficiency and Recovery Rate
2.6. Quality Assurance/Quality Control Measures
2.7. Statistical Analysis
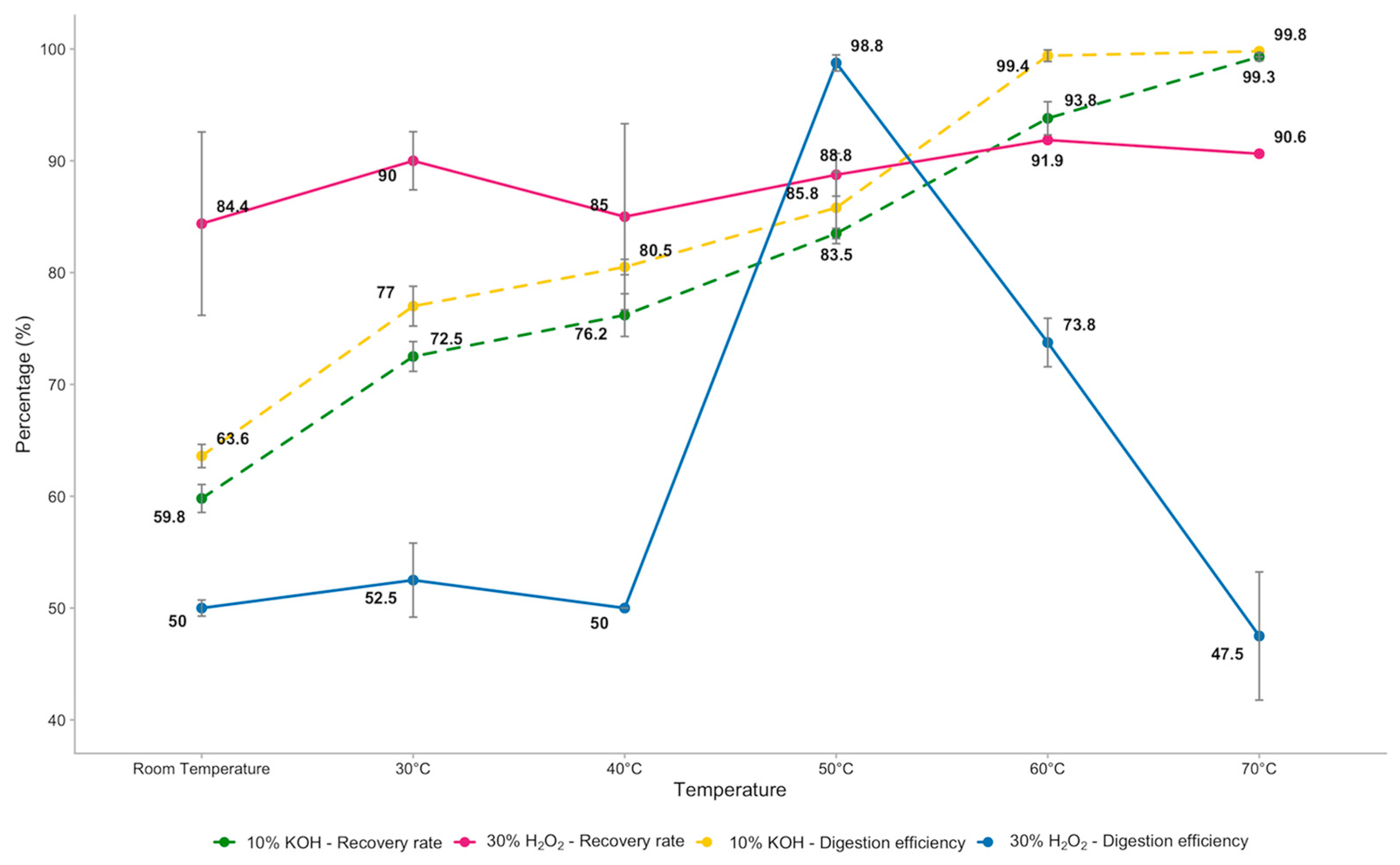
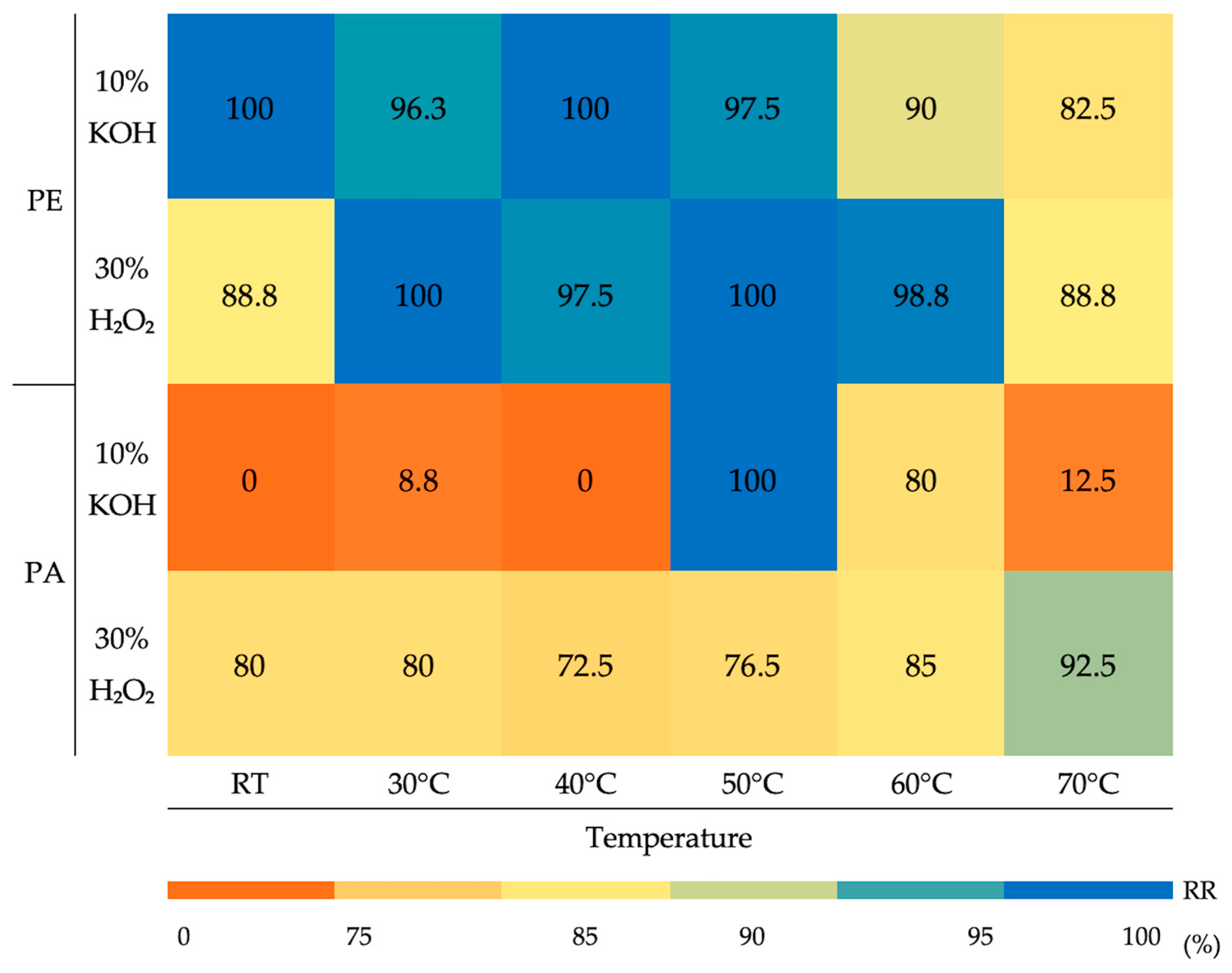
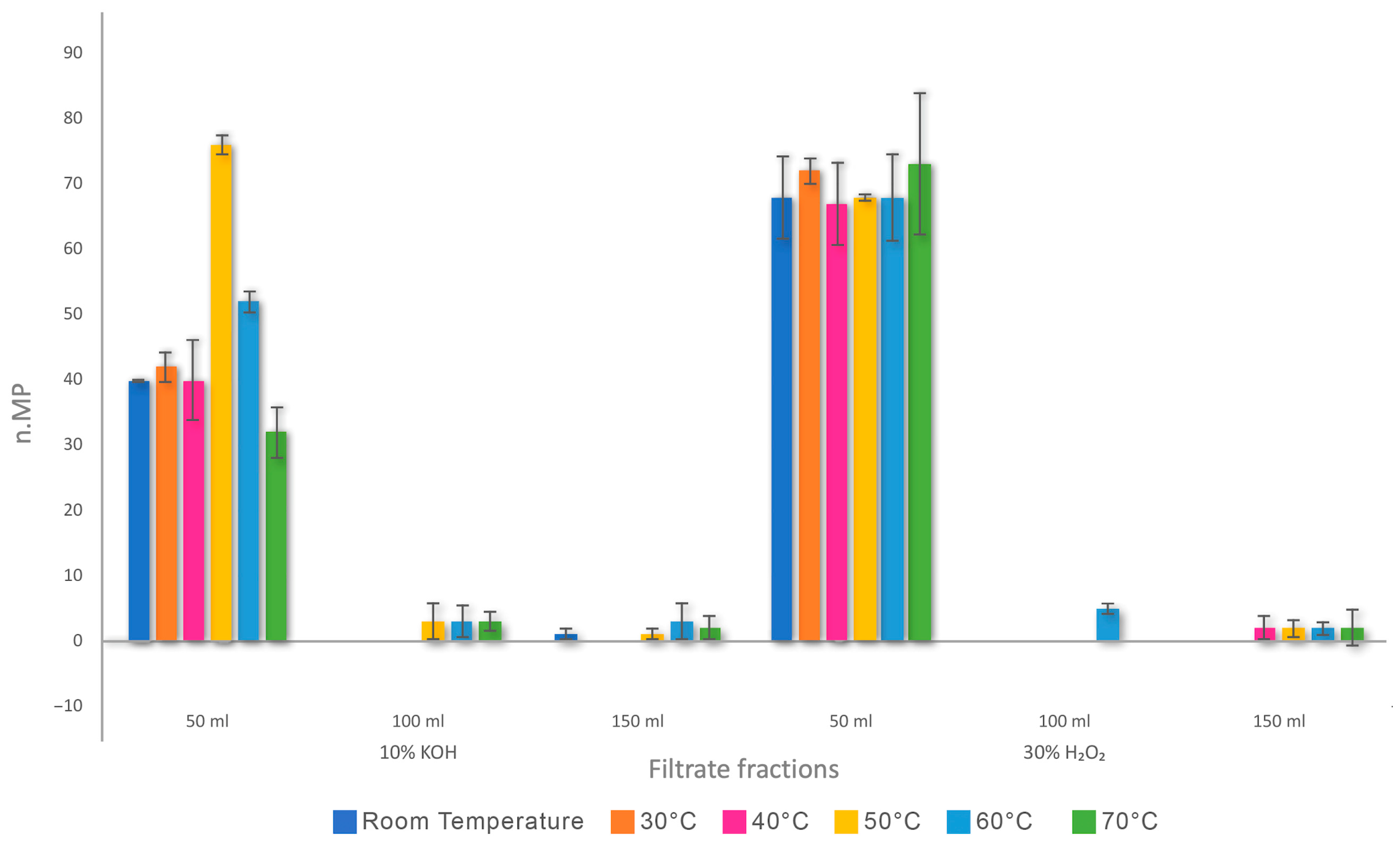
3. Results
4. Discussion
4.1. Polymers Selection
4.2. Wet Oxidative and Alkaline Treatments for Microplastic Isolation
4.3. Recovery Rates, Digestion Efficiencies and Temperature
4.4. Influence of Filtration Volume, Temperature, and Particle Size on MP Recovery
4.5. Implications for Food Safety
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Unuofin, J.O.; Igwaran, A. Microplastics in seafood: Implications for food security, safety, and human health. J. Sea Res. 2023, 194, 102410. [Google Scholar] [CrossRef]
- Shumway, S.E.; Mladinich, K.; Blaschik, N.; Holohan, B.A.; Ward, J.E. A Critical Assessment of Microplastics in Molluscan Shellfish with Recommendations for Experimental Protocols, Animal Husbandry, Publication, and Future Research. Rev. Fish. Sci. Aquac. 2023, 1–133. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Courtene-Jones, W.; Quinn, B.; Murphy, F.; Gary, S.F.; Narayanaswamy, B.E. Optimisation of enzymatic digestion and validation of specimen preservation methods for the analysis of ingested microplastics. Anal. Methods 2017, 9, 1437–1445. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, P.M.; Serra-Gonçalves, C.; Lia Ferreira, J.; Catry, T.; Granadeiro, J.P. Plastic and other microfibers in sediments, macroinvertebrates and shorebirds from three intertidal wetlands of southern Europe and west Africa. Environ. Pollut. 2017, 231, 123–133. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, A.; Strafella, P.; Øysæd, K.B.; Fabi, G. First occurrence and composition assessment of microplastics in native mussels collected from coastal and offshore areas of the northern and central Adriatic Sea. Environ. Sci. Pollut. Res. 2019, 26, 24407–24416. [Google Scholar] [CrossRef]
- Jang, M.; Shim, W.J.; Cho, Y.; Han, G.M.; Song, Y.K.; Hong, S.H. A close relationship between microplastic contamination and coastal area use pattern. Water Res. 2020, 171, 115400. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, C.; Prosser, R.S. Investigation of Microplastics in Freshwater Mussels (Lasmigona costata) from the Grand River Watershed in Ontario, Canada. Water Air Soil Pollut. 2020, 231, 405. [Google Scholar] [CrossRef]
- von Friesen, L.W.; Granberg, M.E.; Hassellöv, M.; Gabrielsen, G.W.; Magnusson, K. An efficient and gentle enzymatic digestion protocol for the extraction of microplastics from bivalve tissue. Mar. Pollut. Bull. 2019, 142, 129–134. [Google Scholar] [CrossRef]
- Vandermeersch, G.; Van Cauwenberghe, L.; Janssen, C.R.; Marques, A.; Granby, K.; Fait, G.; Kotterman, M.J.J.; Diogène, J.; Bekaert, K.; Robbens, J.; et al. A critical view on microplastic quantification in aquatic organisms. Environ. Res. 2015, 143, 46–55. [Google Scholar] [CrossRef]
- Santana, M.F.M.; Ascer, L.G.; Custódio, M.R.; Moreira, F.T.; Turra, A. Microplastic contamination in natural mussel beds from a Brazilian urbanized coastal region: Rapid evaluation through bioassessment. Mar. Pollut. Bull. 2016, 106, 183–189. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.L. A Comparison of Microplastics in Farmed and Wild Shellfish near Vancouver Island and Potential Implications for Contaminant Transfer to Humans. Master’s Thesis, Royal Roads University, Victoria, BC, Canada, 2018. [Google Scholar]
- Naidu, S.A. Preliminary study and first evidence of presence of microplastics and colorants in green mussel, Perna viridis (Linnaeus, 1758), from southeast coast of India. Mar. Pollut. Bull. 2019, 140, 416–422. [Google Scholar] [CrossRef]
- Reguera, P.; Vinas, L.; Gago, J. Microplastics in wild mussels (Mytilus spp.) from the north coast of Spain. Sci. Mar. 2019, 83, 337–347. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Lahbib, Y.; El Menif, N.T. Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar. Pollut. Bull. 2019, 142, 243–252. [Google Scholar] [CrossRef]
- Teng, J.; Wang, Q.; Ran, W.; Wu, D.; Liu, Y.; Sun, S.; Liu, H.; Cao, R.; Zhao, J. Microplastic in cultured oysters from different coastal areas of China. Sci. Total Environ. 2019, 653, 1282–1292. [Google Scholar] [CrossRef]
- Ribeiro, F.; Okoffo, E.D.; O’Brien, J.W.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.F.; Galloway, T.; et al. Quantitative analysis of selected plastics in high-commercial-value Australian seafood by pyrolysis gas chromatography mass spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.P.; Chiu, C.C.; Huang, H.W. Assessment of microplastics in oysters in coastal areas of Taiwan. Environ. Pollut. 2021, 286, 117437. [Google Scholar] [CrossRef]
- Dambrosio, A.; Cometa, S.; Capuozzo, F.; Ceci, E.; Derosa, M.; Quaglia, N.C. Occurrence and characterization of microplastics in commercial mussels (Mytilus galloprovincialis) from Apulia Region (Italy). Foods 2023, 12, 1495. [Google Scholar] [CrossRef]
- Quaglia, N.C.; Capuozzo, F.; Ceci, E.; Cometa, S.; Di Pinto, A.; Mottola, A.; Piredda, R.; Dambrosio, A. Preliminary survey on the occurrence of microplastics in bivalve mollusks marketed in Apulian fish markets. Ital. J. Food Saf. 2023, 12, 10906. [Google Scholar] [CrossRef] [PubMed]
- Chinfak, N.; Charoenpong, C.; Sampanporn, A.; Wongpa, C.; Sompongchaiyakul, P. Microplastics in commercial bivalves from coastal areas of Thailand and health risk associated with microplastics in ingested bivalves. Mar. Pollut. Bull. 2024, 208, 116937. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Yang, D.; Zhang, D.; Li, J.; Yang, X.; Sui, H.; Wu, Y. The occurrence and exposure of microplastics in bivalves from Qingdao, China. Mar. Pollut. Bull. 2024, 207, 116880. [Google Scholar] [CrossRef]
- Expósito, N.; Barrientos-Riosalido, A.; Santini, S.; Cincinelli, A.; Alcalde, L.; Castell, V.; Nadal, M.; Sierra, J.; Rovira, J. Microplastics levels in cultured or harvested mollusks non-depurated and commercially depurated at different times. Mar. Pollut. Bull. 2025, 212, 117568. [Google Scholar] [CrossRef]
- Ribeiro, V.V.; Casado-Coy, N.; Rangel, D.F.; Sanz-Lazaro, C.; Castro, Í.B. Microplastic in bivalves of an urbanized Brazilian estuary: Human modification, population density and vegetation influence. J. Hazard. Mater. 2025, 482, 136546. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T.S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef]
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Zaki, M.R.M.; Ying, P.X.; Zainuddin, A.H.; Razak, M.R.; Aris, A.Z. Occurrence, abundance, and distribution of microplastics pollution: An evidence in surface tropical water of Klang River estuary, Malaysia. Environ. Geochem. Health 2021, 43, 3733–3748. [Google Scholar] [CrossRef]
- Xu, X.; Wong, C.Y.; Tam, N.F.; Lo, H.S.; Cheung, S.G. Microplastics in invertebrates on soft shores in Hong Kong: Influence of habitat, taxa and feeding mode. Sci. Total Environ. 2020, 715, 136999. [Google Scholar] [CrossRef]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef]
- Bonello, G.; Varrella, P.; Pane, L. First evaluation of microplastic content in benthic filter-feeders of the Gulf of La Spezia (Ligurian Sea). J. Aquat. Food Prod. Technol. 2018, 27, 284–291. [Google Scholar] [CrossRef]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.T.; Le, P.T.; Nguyen, T.N.H.; Hoang, T.Q.; Ngo, H.M.; Doan, T.O.; Le, T.P.Q.; Bui, H.T.; Bui, M.H.; Trinh, V.T.; et al. Selection of a density separation solution to study microplastics in tropical riverine sediment. Environ. Monit. Assess. 2022, 194, 65. [Google Scholar] [CrossRef]
- Minder, M.L.; Colombo, I.G.; Rountos, K.J. Baseline assessment of microplastics in commercially important marine bivalves from New York, USA. Mar. Pollut. Bull. 2023, 188, 114625. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Le Tilly, V.; César, G.; Sire, O.; Bruzaud, S. Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling. Mar. Pollut. Bull. 2017, 115, 120–129. [Google Scholar] [CrossRef]
- Dowarah, K.; Patchaiyappan, A.; Thirunavukkarasu, C.; Jayakumar, S.; Devipriya, S.P. Quantification of microplastics using Nile Red in two bivalve species Perna viridis and Meretrix meretrix from three estuaries in Pondicherry, India and microplastic uptake by local communities through bivalve diet. Mar. Pollut. Bull. 2020, 153, 110982. [Google Scholar] [CrossRef]
- Sfriso, A.A.; Tomio, Y.; Rosso, B.; Gambaro, A.; Sfriso, A.; Corami, F.; Rastelli, E.; Corinaldesi, C.; Mistri, M.; Munari, C. Microplastic accumulation in benthic invertebrates in Terra Nova bay (Ross Sea, Antarctica). Environ. Int. 2020, 137, 105587. [Google Scholar] [CrossRef]
- Ho, D.; Liu, S.; Wei, H.; Karthikeyan, K.G. The glowing potential of Nile red for microplastics Identification: Science and mechanism of fluorescence staining. Microchem. J. 2024, 197, 109708. [Google Scholar] [CrossRef]
- Fang, C.; Zheng, R.; Chen, H.; Hong, F.; Lin, L.; Lin, H.; Guo, H.; Bailey, C.; Segner, H.; Mu, J.; et al. Comparison of microplastic contamination in fish and bivalves from two major cities in Fujian province, China and the implications for human health. Aquaculture 2019, 512, 734322. [Google Scholar] [CrossRef]
- Aramendia, J.; García-Velasco, N.; Amigo, J.M.; Izagirre, U.; Seifert, A.; Soto, M.; Castro, K. Evidence of internalized microplastics in mussel tissues detected by volumetric Raman imaging. Sci. Total Environ. 2024, 914, 169960. [Google Scholar] [CrossRef]
- Giarratano, E.; Trovant, B.; Hernández-Moresino, R.D. Asian clam Corbicula fluminea as potential biomonitor of microplastics and metal (oid) s in a Patagonian River. Mar. Environ. Res. 2024, 198, 106548. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total Environ. 2021, 782, 146830. [Google Scholar] [CrossRef]
- Ríos, M.F.; Hernández-Moresino, R.D.; Galván, D.E. Assessing urban microplastic pollution in a benthic habitat of Patagonia Argentina. Mar. Pollut. Bull. 2020, 159, 111491. [Google Scholar] [CrossRef]
- Baudrimont, M.; Arini, A.; Guégan, C.; Venel, Z.; Gigault, J.; Pedrono, B.; Prunier, J.; Maurice, L.; Ter Halle, A.; Feurtet-Mazel, A. Ecotoxicity of polyethylene nanoplastics from the North Atlantic oceanic gyre on freshwater and marine organisms (microalgae and filter-feeding bivalves). Environ. Sci. Pollut. Res. 2020, 27, 3746–3755. [Google Scholar] [CrossRef]
- Fabbri, D.; Rombolà, A.G.; Vassura, I.; Torri, C.; Franzellitti, S.; Capolupo, M.; Fabbri, E. Off-line analytical pyrolysis GC–MS to study the accumulation of polystyrene microparticles in exposed mussels. J. Anal. Appl. Pyrolysis 2020, 149, 104836. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellov, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Lusher, A.Á.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. In Analysis of Nanoplastics and Microplastics in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 119–148. [Google Scholar]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Y.; Xu, P.; Lim, L.S.; Cheong, K.-L.; Wang, Y.; Tan, K. Microplastic pollution in commercially important edible bivalves: A comprehensive review. Food Chem. 2024, 23, 101647. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Fischer, E.K. Various digestion protocols within microplastic sample processing—Evaluating the resistance of different synthetic polymers and the efficiency of biogenic organic matter destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global production of marine bivalves. Trends and challenges. In Goods and Services of Marine Bivalves; Smaal, A., Ferreira, J., Grant, J., Petersen, J., Strand, Ø., Eds.; Springer: Cham, Switzerland, 2018; pp. 7–26. [Google Scholar] [CrossRef]
- Yaghubi, E.; Carboni, S.; Snipe, R.M.; Shaw, C.S.; Fyfe, J.J.; Smith, C.M.; Kaur, G.; Tan, S.Y.; Hamilton, D.L. Farmed mussels: A nutritive protein source, rich in omega-3 fatty acids, with a low environmental footprint. Nutrients 2021, 13, 1124. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Tiago, G.A.; Mariquito, A.; Martins-Dias, S.; Marques, A.C. The problem of polyethylene waste–recent attempts for its mitigation. Sci. Total Environ. 2023, 892, 164629. [Google Scholar] [CrossRef]
- Corniuk, R.N.; Shaw, K.R.; McWhirter, A.; Lynch, H.W., IV; Royer, S.J.; Lynch, J.M. Polymer identification of floating derelict fishing gear from O’ahu, Hawai’i. Mar. Pollut. Bull. 2023, 196, 115570. [Google Scholar] [CrossRef] [PubMed]
- Astute Analytica. Global Polyamide Market to Surpass Valuation of US$69.52 Billion by 2033. GlobeNewswire. 12 February 2025. Available online: https://www.globenewswire.com/news-release/2025/02/12/3024942/0/en/Global-Polyamide-Market-to-Surpass-Valuation-of-US-69-52-Billion-By-2033-Astute-Analytica.html (accessed on 23 September 2025).
- Liu, J.; Liu, Q.; An, L.; Wang, M.; Yang, Q.; Zhu, B.; Ding, J.; Ye, C.; Xu, Y. Microfiber pollution in the earth system. Rev. Environ. Contam. Toxicol. 2022, 260, 13. [Google Scholar] [CrossRef]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic fibers—Underestimated threat to aquatic organisms? Sci. Total Environ. 2021, 798, 149256. [Google Scholar] [CrossRef]
- Athey, S.N.; Erdle, L.M. Are we underestimating anthropogenic microfiber pollution? Environ. Toxicol. Chem. 2022, 41, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Kohutiar, M.; Kakošová, L.; Krbata, M.; Janík, R.; Fekiač, J.J.; Breznická, A.; Eckert, M.; Mikuš, P.; Timárová, Ľ. Comprehensive review: Technological approaches, properties, and applications of pure and reinforced polyamide 6 (PA6) and polyamide 12 (PA12) composite materials. Polymers 2025, 17, 442. [Google Scholar] [CrossRef]
- Bürkle GmbH. Chemische Beständigkeit von Kunststoffen (Version 3.8). 2017. Available online: https://www.buerkle.de/de/chemische-bestaendigkeit (accessed on 15 September 2025).
- Koutny, M.; Lemaire, J.; Delort, A.M. Biodegradation of polyethylene films with prooxidant additives. Chemosphere 2006, 64, 1243–1252. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef]
- De-la-Torre, G.E.; Mendoza-Castilla, L.; Laura, R.P. Microplastic contamination in market bivalve Argopecten purpuratus from Lima, Peru. Manglar 2019, 16, 85–89. [Google Scholar] [CrossRef]
- Truchet, D.M.; Lopez, A.F.; Ardusso, M.G.; Rimondino, G.N.; Buzzi, N.S.; Malanca, F.E.; Spetter, C.V.; Severini, M.F. Microplastics in bivalves, water and sediments from a touristic sandy beach of Argentina. Mar. Pollut. Bull. 2021, 173, 113023. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in marine zooplankton. Environ. Sci. Technol. 2014, 48, 1638–1645. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef] [PubMed]
- Vieira, K.S.; Neto, J.A.B.; Crapez, M.A.C.; Gaylarde, C.; da Silva Pierri, B.; Saldaña-Serrano, M.; Dias Bainy, A.C.; Nogueira, D.J.; Fonseca, E.M. Occurrence of microplastics and heavy metals accumulation in native oysters Crassostrea gasar in the Paranaguá estuarine system, Brazil. Mar. Pollut. Bull. 2021, 166, 112225. [Google Scholar] [CrossRef] [PubMed]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017, 36, 947–951. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene, and poly(propylene). Macromol. Chem. Phys. 2001, 202, 1055–1063. [Google Scholar] [CrossRef]
- Hagelskjær, O.; Crézé, A.; Le Roux, G.; Sonke, J.E. Investigating the correlation between morphological features of microplastics (5–500 µm) and their analytical recovery. Microplast. Nanoplast. 2023, 3, 22. [Google Scholar] [CrossRef]
- Lee, H.B.; Lee, K.S.; Kim, S.J.; Choi, B.I.; Go, B.R.; Rhu, C.J.; Han, T.H. Effect of chemical agents on the morphology and chemical structures of microplastics. Polymers 2022, 14, 4353. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.S.; Wu, Y.S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Adedapo, O.A.; Olowoyo, J.O. The effect of significant acid and alkaline environments on microplastic integrity. Environ. Sci. Pollut. Res. 2024, 31, 12345–12356. [Google Scholar] [CrossRef]
- Enenche, D.E.; Davidson, C.M.; Liggat, J.J. Towards a consensus method for the isolation of microplastics from freshwater sediments. Environments 2024, 11, 146. [Google Scholar] [CrossRef]
- McIlwraith, H.K.; Lindeque, P.K.; Tolhurst, T.J.; Cole, M. Positive controls with representative materials are essential for the advancement of microplastics research. Microplast. Nanoplast. 2025, 5, 9. [Google Scholar] [CrossRef]
- Brown, H.; Williams, I.D. Optimising H2O2 digestion and quantifying microplastics in sediment and pacific oyster (Crassostrea gigas) samples. Mar. Pollut. Bull. 2025, 220, 118353. [Google Scholar] [CrossRef]
- Al-Azzawi, M.S.M.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of sample preparation methods for microplastic analysis in wastewater matrices—Reproducibility and standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Gao, Y.; Li, X.; Gong, Y. Study on the extraction method of microplastic system in textile wastewater. Polymers 2023, 15, 1394. [Google Scholar] [CrossRef] [PubMed]
- Pfohl, P.; Roth, C.; Meyer, L.; Heinemeyer, U.; Gruendling, T.; Lang, C.; Nestle, N.; Hofmann, T.; Wohlleben, W.; Jessl, S. Microplastic extraction protocols can impact the polymer structure. Microplastics 2021, 1, 8. [Google Scholar] [CrossRef]
- Martínez-Francés, E.; Morales-Caselles, C.; Bayo, J.; García-Vargas, M.; González-Sálamo, J.; Hernández-Sánchez, C.; Herrera, A.; Izquierdo, C.; López-Rubio, A.; Luzardo, O.P.; et al. Innovative reference materials for method validation in microplastic analysis: A step toward harmonisation and interlaboratory comparability. J. Hazard. Mater. 2023, 452, 131171. [Google Scholar] [CrossRef]
- Mattsson, K.; Ekstrand, E.; Granberg, M.; Hassellöv, M.; Magnusson, K. Comparison of pre-treatment methods and heavy density liquids to optimize microplastic extraction from natural marine sediments. Sci. Rep. 2022, 12, 15459. [Google Scholar] [CrossRef]
- Rani, M.; Ducoli, S.; Depero, L.E.; Prica, M.; Tubić, A.; Ademovic, Z.; Morrison, L.; Federici, S. A complete guide to extraction methods of microplastics from complex environmental matrices. Molecules 2023, 28, 5710. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C.; Herring, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. NOAA Technical Memorandum NOS-OR&R-48. 2015. Available online: https://repository.library.noaa.gov/view/noaa/10296 (accessed on 15 September 2025).
- Schütze, B.; Thomas, D.; Kraft, M.; Brunotte, J.; Kreuzig, R. Comparison of different salt solutions for density separation of conventional and biodegradable microplastic from solid sample matrices. Environ. Sci. Pollut. Res. 2022, 29, 81452–81467. [Google Scholar] [CrossRef]
- Tuttle, E.; Stubbins, A. An optimized acidic digestion for the isolation of microplastics from biota-rich samples and cellulose acetate matrices. Environ. Pollut. 2023, 322, 121198. [Google Scholar] [CrossRef]
- Fraissinet, S.; Pennetta, A.; Rossi, S.; De Benedetto, G.E.; Malitesta, C. Optimization of a new multi-reagent procedure for quantitative mussel digestion in microplastic analysis. Mar. Pollut. Bull. 2021, 173, 112931. [Google Scholar] [CrossRef]
- Gulizia, A.M.; Brodie, E.; Daumuller, R.; Bloom, S.B.; Corbett, T.; Santana, M.M.; Motti, G.A.; Vamvounis, G. Evaluating the effect of chemical digestion treatments on polystyrene microplastics: Recommended updates to chemical digestion protocols. Macromol. Chem. Phys. 2022, 223, 2100485. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Rocha-Santos, T.; Prata, J.C.; Duarte, A.C.; Girão, A.V.; Lopes, P.; Cristovão, T.; da Costa, J.P. A straightforward method for microplastic extraction from organic-rich freshwater samples. Sci. Total Environ. 2022, 815, 152941. [Google Scholar] [CrossRef]
- Binda, G.; Kalčíková, G.; Allan, I.J.; Hurley, R.; Rødland, E.; Spanu, D.; Nizzetto, L. Microplastic aging processes: Environmental relevance and analytical implications. TrAC Trends Anal. Chem. 2024, 172, 117566. [Google Scholar] [CrossRef]
- Larrea, G.; Elustondo, D.; Durán, A. Extraction methods of microplastics in environmental matrices. Molecules 2025, 30, 3178. [Google Scholar] [CrossRef] [PubMed]
- Way, C.; Hudson, M.D.; Williams, I.D.; Langley, G.J. Evidence of underestimation in microplastic research: A meta-analysis of recovery rate studies. Sci. Total Environ. 2022, 805, 150227. [Google Scholar] [CrossRef]
- Tuuri, E.M.; Gascooke, J.R.; Leterme, S.C. Efficacy of chemical digestion methods to reveal undamaged microplastics from planktonic samples. Sci. Total Environ. 2024, 947, 174279. [Google Scholar] [CrossRef] [PubMed]
- Prihandari, R.; Karnpanit, W.; Kittibunchakul, S.; Kemsawasd, V. Development of optimal digesting conditions for microplastic analysis in dried seaweed Gracilaria fisheri. Foods 2021, 10, 2118. [Google Scholar] [CrossRef]
- Lam, T.W.L.; Tsui, Y.C.J.; Cheng, Y.L.; Ma, A.T.H.; Fok, L. Microplastic contamination in edible clams from popular recreational clam-digging sites in Hong Kong and implications for human health. Sci. Total Environ. 2023, 875, 162576. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
| Temperature | 10% KOH | 30% H2O2 |
|---|---|---|
| Room Temperature | 40 ± 0.58 | 68 ± 6.56 |
| 30 °C | 42 ± 2.65 | 72 ± 2.08 |
| 40 °C | 40 | 68 ± 6.66 |
| 50 °C | 78 ± 0.58 | 70 ± 1.53 |
| 60 °C | 58 ± 1.73 | 73 ± 7.09 |
| 70 °C | 38 ± 4.58 | 73 ± 7.51 |
| 10% KOH | 30% H2O2 | ||||
|---|---|---|---|---|---|
| Temperature | Filtration Level | Fragments Recovered | Fibres Recovered | Fragments Recovered | Fibres Recovered |
| Room Temperature | 50 mL | 40 | 0 | 36 ± 2.12 | 32 ± 7.07 |
| 100 mL | 0 | 0 | 0 | 0 | |
| 150 mL | 0 | 0 | 0 | 0 | |
| 30 °C | 50 mL | 39 ± 2.12 | 3 ± 1.42 | 40 | 32 ± 2.83 |
| 100 mL | 0 | 0 | 0 | 0 | |
| 150 mL | 0 | 1 ± 0.71 | 0 | 0 | |
| 40 °C | 50 mL | 40 | 0 | 39 ± 1.42 | 27 ± 7.07 |
| 100 mL | 0 | 0 | 0 | 0 | |
| 150 mL | 0 | 0 | 0 | 2 ± 3.54 | |
| 50 °C | 50 mL | 38 | 38 ± 2.12 | 40 | 28 |
| 100 mL | 1 ± 1.41 | 2 ± 2.83 | 0 | 1 ± 0.71 | |
| 150 mL | 0 | 1 ± 1.42 | 0 | 2 ± 1.41 | |
| 60 °C | 50 mL | 34 ± 1.41 | 18 ± 3.54 | 36 ± 5.66 | 29 ± 2.83 |
| 100 mL | 2 ± 2.12 | 1 ± 1.42 | 4 ± 0.71 | 4 ± 4.95 | |
| 150 mL | 0 | 5 ± 3.54 | 0 | 1 ± 2.12 | |
| 70 °C | 50 mL | 30 ± 7.07 | 2 ± 1.42 | 34 ± 8.49 | 36 ± 5.66 |
| 100 mL | 3 ± 3.54 | 1 ± 1.42 | 0 | 0 | |
| 150 mL | 0 | 2 ± 1.42 | 2 ± 2.12 | 1 ± 1.41 | |
| Minimum MP Dimensions (mm) | Maximum MP Dimensions (mm) | ||||||
|---|---|---|---|---|---|---|---|
| °C | 50 mL | 100 mL | 150 mL | 50 mL | 100 mL | 150 mL | |
| 10% KOH | RT | 0.52 ± 0.98 | - | - | 2.75 ± 0.03 | - | - |
| 30 °C | 0.74 ± 0.53 | - | 1.54 ± 2.06 | 4.95 ± 1.49 | - | 2.36 ± 2.06 | |
| 40 °C | 0.79 ± 0.09 | - | - | 5 ± 0.72 | - | - | |
| 50 °C | 0.96 ± 0.42 | 0.24 ± 0.72 | 1.34 ± 0.13 | 4.62 ± 1.25 | 2.99 ± 0.63 | 1.85 ± 0.36 | |
| 60 °C | 0.75 ± 0.06 | 0.89 ± 1.2 | 0.79 ± 0.86 | 4.08 ± 1.01 | 3.92 ± 1.98 | 3.84 ± 0.83 | |
| 70 °C | 0.53 ± 0.58 | 0.72 ± 1.31 | 0.69 ± 0.88 | 2.93 ± 0.37 | 3.69 ± 1.32 | 3.65 ± 1.7 | |
| 30% H2O2 | RT | 0.91 ± 0.46 | - | - | 4.72 ± 0.57 | - | - |
| 30 °C | 0.87 ± 0.15 | - | - | 5 ± 1.84 | - | - | |
| 40 °C | 1.27 ± 0.2 | - | 2.75 ± 1.78 | 3.44 ± 0.4 | - | 5 ± 1.78 | |
| 50 °C | 0.8 ± 0.39 | 1.01 ± 0.53 | 0.87 ± 0.17 | 4.36 ± 0.8 | 1.01 ± 0.71 | 1.88 ± 0.47 | |
| 60 °C | 0.67 ± 0.11 | 0.53 ± 0.97 | 1.15 ± 0.18 | 4.34 ± 1.43 | 1.92 ± 0.06 | 2.18 ± 0.55 | |
| 70 °C | 0.68 ± 0.39 | - | 0.59 ± 0.15 | 3.45 ± 0.5 | - | 3.21 ± 1.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capuozzo, F.; Quaglia, N.C.; Di Pinto, A.; De Rosa, M.; Ioanna, F.; Dambrosio, A. Evaluation of Digestion Methods in Microplastic Recovery from Mussels (Mytilus galloprovincialis) for a Standardised Microplastic Isolation Protocol. Foods 2025, 14, 3853. https://doi.org/10.3390/foods14223853
Capuozzo F, Quaglia NC, Di Pinto A, De Rosa M, Ioanna F, Dambrosio A. Evaluation of Digestion Methods in Microplastic Recovery from Mussels (Mytilus galloprovincialis) for a Standardised Microplastic Isolation Protocol. Foods. 2025; 14(22):3853. https://doi.org/10.3390/foods14223853
Chicago/Turabian StyleCapuozzo, Flavia, Nicoletta Cristiana Quaglia, Angela Di Pinto, Michele De Rosa, Federica Ioanna, and Angela Dambrosio. 2025. "Evaluation of Digestion Methods in Microplastic Recovery from Mussels (Mytilus galloprovincialis) for a Standardised Microplastic Isolation Protocol" Foods 14, no. 22: 3853. https://doi.org/10.3390/foods14223853
APA StyleCapuozzo, F., Quaglia, N. C., Di Pinto, A., De Rosa, M., Ioanna, F., & Dambrosio, A. (2025). Evaluation of Digestion Methods in Microplastic Recovery from Mussels (Mytilus galloprovincialis) for a Standardised Microplastic Isolation Protocol. Foods, 14(22), 3853. https://doi.org/10.3390/foods14223853












