Abstract
Honey adulteration has long been a nuisance in local and international trade. Sugar syrup addition and false labeling of botanical origin have created a challenge in identifying fraudulent honey supplies and products. Stable carbon isotope ratio analysis (SCIRA) has been widely employed in honey authentication. While it is effective in identifying the addition of C4 plant-derived sugars, it does not provide information related to honey’s botanical source. This research investigated the combination of SCIRA and melissopalynology to provide a more robust assessment of honey integrity and showed that PCA analysis of δ13C together with sugar profiles could further improve the decision involving addition of sugar syrups. A total of 34 beekeeper honey samples were analyzed from 7 provinces of Thailand with a focus on longan honey. Twenty-four samples passed the δ13C criteria, exhibiting δ13C of bulk honey ranging from −28.53 ± 0.19‰ to −22.89 ± 0.08‰ and δ13C of extracted protein ranging from −29.30 ± 0.07‰ to −22.76 ± 0.03‰. Pollen profiling further eliminated honey of questionable and multifloral origins, yielding only eight samples that passed both criteria of being monofloral and not being adulterated with C4-derived sugars. These included six samples of longan honey and two honey samples of other botanical origins, yielding an overall passing rate of 23.5%. Our study showed that by combining SCIRA and melissopalynology, a robust determination of honey integrity could be achieved.
1. Introduction
Honey is a natural sweetener with bioactive properties, and it has been important for local and international trade across the globe []. A vast majority is produced from flower nectar by honeybees. It provides an important source of carbohydrates as it contains at least 60% fructose and glucose, and usually less than 5% sucrose, with small amounts of pollen, proteins, enzymes, vitamins, polyphenols, and organic acid [,]. As the market grows, consumers become more aware of the value of authentic honey with verifiable botanical origins, especially monofloral honey with unique flavor. In Thailand, honey is produced from many floral sources including longan, lychee, wildflower, and many others. Longan honey is a commercially important honey for both local consumption and international trade [,]. In 2019, Thailand honey export value rose to USD 19 million with an export amount of 7900 tons []. Therefore, determining product authenticity and verifying its botanical origin are of interest and have the potential to promote value addition of honey products.
Longan honey is derived from the nectar of the Dimocarpus longan L. It is a famous monofloral honey among consumers in East and Southeast Asia []. Thailand is one of the lead producers and exporters of longan honey, with the major production area in the Chiang Mai-Lamphun valley in the northern region [,]. Important international markets include China, Taiwan, and Japan. In addition to its characteristic smell, flavor, and natural sweetness, studies have shown that it exhibits interesting antioxidant and antimicrobial properties [,,]. As a result, longan honey is often considered a premium product and is sold at a higher price than many other kinds of honey on the market. Due to its ability to achieve a higher price, the market is at risk of it being adulterated to obtain a more profitable sales margin. Although the extent of adulterated longan honey being sold or exported is not known, a previous study has demonstrated the existence of false claims regarding the authenticity of longan honey [].
An authentication issue of concern is the addition of external sugar syrup to increase the volume of honey. The syrups often used by honey producers are sugarcane-derived, causing the sucrose content to exceed the standard level [,]. Sugarcane is considered a C4 plant with the Hatch–Slack photosynthesis pathway []. C4 plants such as sugarcane and maize can be differentiated from C3 plants, which form the majority of plant species within the Plant Kingdom, by their anatomy, physiology, and stable carbon isotope ratio [,,,]. Differences in stable carbon isotope ratio, especially the δ13C value, between C3 and C4 plants stem from their physiological differences in biological isotope fractionation related to the photosynthetic pathway. C3 plants typically exhibit δ13C values ranging from −30‰ to −22‰, while C4 plants typically have δ13C values of approximately −14‰ to −10‰ []. Consequently, δ13C analysis has been widely accepted as a method to detect exogenous C4 carbon in honey [,,] and can be applied to longan honey and most other types of Thai honey from C3 botanical origin.
Botanical origin of honey is an important factor that influences buying decisions. Sources of nectar can impact on the appearance, texture, flavor, and physicochemical properties of honey []. To determine botanical origin, melissopalynological analysis is the method of choice [,]. Honey is considered monofloral when it contains a high percentage of pollen from a single source, typically greater than 45% []. It has been established that the pollen species with greater than 45% prevalence is the predominant pollen, those with 16–45% prevalence are the secondary pollen, those with 3–15.9% prevalence are important minority pollen, and those with less than 3% prevalence are the minority pollen [].
This study investigated the efficacy of combining stable carbon isotope ratio analysis and melissopalynology to authenticate honey purity from undeclared carbon sources and to verify the botanical origin of honey. It covered a range of honey types with an emphasis on longan honey from northern provinces of Thailand and including a few honey samples of other botanical origins from other parts of the country. Combustible-module cavity ringdown spectroscopy (CM-CRDS) was used to establish the method to detect C4 contamination. CM-CRDS requires a lower cost of analysis, is simpler to operate, and has been proven in various studies to have a comparable precision and stability to the standard method, elemental analyzer–isotope ratio mass spectrometry (EA-IRMS) [,], making it practical for routine honey testing, especially in smaller laboratories or for on-site testing. In addition, pollen identification and counting were used to verify the nectar sources. We proposed that the two methods can be combined to detect fraudulent honey samples and can potentially be used in the screening of suppliers for the local honey industry in Thailand.
2. Materials and Methods
2.1. Honey Samples
The authentic honey was the raw longan honey stock obtained from a honey factory in Chiang Mai province, Thailand. The sample was analyzed by Intertek Food Services GmbH, Bremen, Germany, using EA/LC-IRMS to confirm authenticity (analysis report no. 2212210813). A total of 34 honey samples from various botanical sources were obtained from beekeepers with colonies of Apis mellifera L. on a voluntary submission basis. These included 1 sample of coffee blossom honey, 2 samples of sunflower honey, 2 samples of lychee honey, 3 samples of wildflower honey, and 26 samples of longan honey. These samples mostly came from bee farms in Chiang Mai. The rest of them came from Chiang Rai, Lamphun, Phrae, Nan, Saraburi, and Chumphon province of Thailand.
2.2. Adulterants
Adulterants used in this study came from commercial supplies. They included maltose syrup, brown rice syrup, molasses, golden syrup, invert sugar, high-fructose syrup 55%, cane syrup, corn syrup, and glucose syrup. For the deliberate adulteration experiment, syrups were added to the authentic honey samples at 2%, 5%, 10%, 20%, and 50% (w/w).
2.3. Sugar Profile Determination
The sugar profiles of the authentic honey sample and deliberately adulterated honey samples were analyzed for total sugar, glucose, fructose, sucrose, and maltose using high-performance liquid chromatography according to the AOAC 980.13 method [].
2.4. Stable Carbon Isotope Analysis
Bulk honey and its protein fraction were prepared according to AOAC Method 998.12 C4 Plant Sugars in Honey []. Stable isotope ratio 13C/12C was measured using combustion module–cavity ring down spectroscopy (CM-CRDS) (Picarro G2131-I, Picarro Inc., Santa Clara, CA, USA). The carbon isotope ratios were normalized against USGS61 and USGS62 reference materials. For measurements using isotope ratio mass spectrometry (IRMS, Delta V Advantage, Thermo Fisher Scientific, Waltham, MA, USA), the isotope ratios were normalized against USGS40 and USGS41a reference materials. δ13C was calculated according to the following equation in parts per thousand (‰) with reference to Vienna Pee Dee Belemine (VPDB) standard. Triplicate measurements were performed for each sample.
δ13C = [(13C/12C)sample/(13C/12C)VPDB − 1] × 1000,
2.5. Melissopalynology
Pollen preparation and analysis were performed according to Louveaux et al., 1978 []. A total of 5 g of honey was mixed with 5 mL of sterilized distilled water and centrifuged at 6000 rpm for 30 min. The precipitate was diluted with 100 µL of distilled water and counted using hemocytometers under a microscope with 400× magnification. For each honey sample, quantitative pollen analysis was performed using three independent replicate slides. A minimum of 500 pollen grains was counted per slide, or the entire area scanned if fewer grains were present []. Pollens were identified based on comparison with reference slides made from identified plants. Their prevalence was classified according to Louveaux et al., 1978 [].
2.6. Statistical Analysis
One-way ANOVA was performed to compare the means among groups of samples. Differences were considered statistically significant if p < 0.05. Homogeneity of variances was tested to satisfy ANOVA assumptions. When ANOVA indicated significant differences, Tukey’s Honestly Significant Difference (HSD) post hoc test was conducted to compare all pairs of group means while controlling the Type I error rate. Principal component analysis (PCA) was performed to classify adulterated honey. IBM SPSS (Chicago, IL, USA) Version 19.0.0 was used for both analyses.
3. Results and Discussion
3.1. δ13C of Authentic Honey
δ13C measurements of an authentic sample of longan honey obtained from Chiang Mai province, Thailand, were carried out using EA/LC-IRMS, EA-IRMS, and CM-CRDS methods (Table 1). The average δ13C of bulk honey (δ13CH) was −26.40 ± 0.13‰, and that of honey protein (δ13CP) was −25.75 ± 0.22‰. The three methods did not yield significant differences in δ13CH (p > 0.05), but significant differences in δ13CP were found among the three samples. However, δ13C values measured using CM-CRDS and EA/LC-IRMS were not statistically different. The significant differences in δ13CP among the three methods could stem from the fact that the protein content in honey is very low (~0.2–0.6%) []. Thus, protein extraction could be prone to contamination with residual sugars, organic acids, and other compounds that might co-precipitate during extraction. As a result, sufficient amounts of starting materials are needed, and proper handling of samples is crucial. In addition, proper drying of protein extract must be ensured because retained moisture can interfere with CM-CRDS measurements and affect isotopic accuracy, as water vapor absorbs in similar spectral regions as CO2 [].

Table 1.
δ13C values of a honey sample and its extracted proteins measured using EA/LC-IRMS, EA-IRMS, and CM-CRDS.
In terms of authenticity, the sample being investigated was categorized as authentic honey, as its δ13CH was within the range of δ13C for C3 plants (<−22.00‰) [,], and the difference between δ13CP and δ13CH (∆δ13CP-H) was less than 1.00‰ [,] (Table 1). These observations were in accordance with a previous report of Thai honey with δ13CH in the range of −29.1‰ to −23.1‰ [], in compliance with the AOAC guideline of −32.0‰ to −23.5‰ [,], and in similar ranges to previously reported data from other countries [,,,,].
3.2. Comparison Between CM-CRDS and EA-IRMS for δ13C Measurements
Although EA-IRMS is an official AOAC method for determining the presence of C4 sugar in honey [], advanced techniques are also available, including LC-IRMS and 1H-NMR. While EA-IRMS measures the bulk isotopic average of the sample, LC-IRMS separates the sugar composition and individually measures their isotopic characteristics. EA-IRMS application is limited to the detection of added C4 sugars with a sensitivity of approximately 7% []. It cannot detect added C3 sugars, which share the same δ13C range with most honey products. LC-IRMS shows high sensitivity and can detect both C3 and C4 sugar addition at the levels as low as 3–5% [], but it requires complex instrumentation and a highly skilled operator. On the other hand, 1H-NMR provides a chemical fingerprint of the sample, can detect C3 and C4 sugar adulteration, and can be used to determine geographical and botanical origins []. Interpretation of 1H-NMR data requires a reference database, which can influence the interpretation itself. It offers a fast and simple method of adulteration detection. The limitation lies in its high investment cost and lower sensitivity for sugar adulteration.
CM-CRDS is an alternative technique. Its principle of adulteration detection relies on δ13C determination of bulk samples, similar to EA-IRMS with similar limitations [,]. However, it requires less capital investment and does not need a highly skilled operator. Previous studies have shown that CM-CRDS is robust and can be used as an alternative to EA-IRMS [,,]. To confirm its robustness against EA-IRMS, 34 Thai honey samples were analyzed for their δ13CH and δ13CP using both CM-CRDS and EA-IRMS techniques. CM-CRDS and EA-IRMS yielded comparable results of δ13C values for both bulk honey samples and the protein extracts (Figure 1). δ13CH was found in the range of −28.80‰ to −15.65‰ using EA-IRMS and −28.53‰ to −15.64‰ using CM-CRDS. δ13CP was found in the range of −33.01‰ to −21.12‰ using EA-IRMS and −33.75‰ to −21.15‰ using CM-CRDS. Regression analysis showed that the δ13CH and δ13CP values measured using the two methods formed a linear relationship with the slope values close to 1 (R2 > 0.999) (Figure 1). The regression p values were less than 0.00001 for both techniques, indicating significant relationships (regression statistics can be found in Tables S1 and S2). Reproducibility testing of CM-CRDS was performed on a honey sample with nine separate preparations and measurements, yielding RSDs of less than 1% (Table S3). These observations were in agreement with other studies where EA-IRMS and CM-CRDS were found to be comparable [,,], supporting the robustness of CM-CRDS and indicating that it can be used as an alternative to EA-IRMS. It is to be noted that regression analyses of δ13CP data yielded a larger residual sum of squares than δ13CH data (Tables S1 and S2), indicating greater departure of data points from the predicted values, as seen in Figure 1B. This observation underlines our previous discussion regarding the reported limitation of honey protein extract preparation and interference of sample moisture in CM-CRDS measurements. Although some limitations exist, CM-CRDS results appeared robust and comparable to EA-IRMS. Therefore, it was chosen as the method of choice in subsequent analyses.
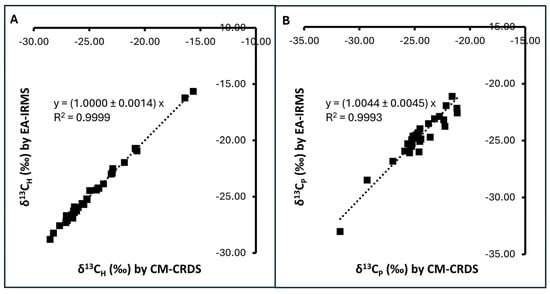
Figure 1.
CM-CRDS and EA-IRMS measurements of 34 honey samples exhibiting a linear relationship: (A) δ13C of bulk honey (δ13CH); (B) δ13C of honey protein (δ13CP). The equations show the slope ± standard error for y = ax. Regression analysis yielded p < 0.00001 for both (A) and (B), indicating significant relationships.
3.3. Deliberate Adulteration of Authentic Honey
3.3.1. δ13C of Adulterants
To determine the ability of CM-CRDS to detect added C4 sugar in honey samples, sugar syrups from C3 and C4 sources were added to authentic honey at varying concentrations. δ13C, which indicates the relative abundance of 13C in comparison to 12C, was used to identify the carbon sources of the syrups and honey samples. The photosynthetic pathway of C3 plants uses the Calvin Cycle to directly fix CO2 in the mesophyll cells to form 3-phosphoglyceric acid, a 3-carbon compound, while in C4 plants, CO2 is initially fixed in mesophyll cells to form oxaloacetate, a 4-carbon compound []. Then, oxaloacetate is converted to malate and transported into the bundle sheath cells where Calvin Cycle fixation occurs []. These physiological differences resulted in differing distributions of natural carbon isotopes between C3 and C4 plants. C3 plants exhibit δ13C in the range of −32‰ to −22‰, whereas C4 plants exhibit δ13C in the range of −16‰ to −8‰ [].
In this study, C3 and C4-derived syrups were used in the deliberate adulteration experiment. Commercial syrups derived from rice, barley, and cassava represented adulterants from C3 sugar sources, while those derived from sugarcane and corn represented adulterants from C4 sugar sources. Their δ13C values, as shown in Table 2, corresponded well to their source of origins except for maltose syrup. δ13C values of cane syrup, golden syrup, and molasses ranged from −13.23 ± 0.10‰ to −12.78 ± 0.29‰, which corresponded well to the previously reported range of −14‰ to −10‰ for sugarcane-derived syrup []. The δ13C value of corn syrup was −11.76 ± 0.09‰, which was slightly lower than the previously reported range of −11.5‰ to −9.7‰ [,] but still within the C4 δ13C values []. The δ13C value of rice syrup was −28.29 ± 0.20‰, which was slightly lower than the previously reported range of −28‰ to −22‰ [,] but still corresponded to the C3 δ13C values []. High-fructose syrup and glucose syrup derived from cassava showed δ13C values of −26.92 ± 0.32‰ and −27.20 ± 0.21‰, respectively, which were within the previously reported range of −33‰ to −24‰ []. Invert sugar derived from sugar beet exhibited δ13C values of −27.69 ± 0.29‰, which corresponded well to the previously reported range of −30‰ to −24‰ for sugar beet []. The slight discrepancies that were found for corn syrup and rice syrups could be due to differences in geographical origins and agricultural practices, which are known to influence δ13C values [,,,,]. On the other hand, maltose syrup, which was labeled as deriving from rice and barley, showed a δ13C value of −18.83 ± 0.21‰ which lied in between the C3 and C4 δ13C ranges, suggesting a mixture of C3 and C4 carbon sources. Rice syrup δ13C values were previously reported in the range of −28‰ to −22‰ [], and barley malt extract δ13C values were reported to be below −24.3‰ [], well within the range of δ13C for C3 plants. Therefore, the deviation of the δ13C of maltose syrup in this study would indicate the presence of undeclared C4 carbon in this syrup sample, which can be found in some commercial products [].

Table 2.
δ13C values of sugar syrups used as adulterants and the deduced C3/C4 plant sources.
3.3.2. Sugar Profile of Authentic Honey
Like most honey, the sugar profile of the authentic longan honey used in this study was primarily dominated by reducing sugar, namely fructose and glucose. Its total sugar content was 73.6% by weight, comprising 37.4% fructose, 29.7% glucose, 4.0% maltose, and 2.5% sucrose. These observations were close to the reported range of honey sugar profiles from other botanical sources. Waworuntu (2024) [] reported the total sugar content and reducing sugar content of honey from four botanical sources to be in the range of 78.40–80.30% and 64.4–65.02%, respectively. Fructose content may range from 35 to over 40%, glucose content from a little below 30% to close to 40%, while maltose and sucrose contents are typically below 5% [,,].
3.3.3. Sugar Levels of Deliberately Adulterated Honey
The additions of syrups as external sugar sources at 5%, 10%, 20%, and 50% may or may not significantly change the sugar profile of longan honey detected using HPLC (Figure 2). Cane syrup, golden syrup, and molasses from sugarcane contained high levels of sucrose. When added at 10%, it caused the sucrose level in the mixture to rise above 5% (Figure 2A,C,D), which is the maximum level allowed by the CODEX Standard for honey [], while the reducing sugar levels were still above 60%. High-fructose syrup and invert sugar even added at 50% by weight still kept the sucrose level below 5% and the reducing sugar above 60% (Figure 2F,G). On the other hand, the addition of corn syrup, maltose syrup, rice syrup, and glucose syrup at increasing levels caused the reducing sugar contents in the honey mix to decrease and the maltose level to increase (Figure 2B,E,H,I). At 10%, their addition led to a rise of maltose levels to above 5%. As a result, sugar content can be used to detect external sugar, with some limitations. It can detect the addition of cane-derived sugar when the sucrose level rises above 5% and glucose/maltose-based sugar sources when maltose becomes higher than 5%. However, it cannot detect adulteration at a lower level or adulteration with fructose-based syrup or invert sugar.
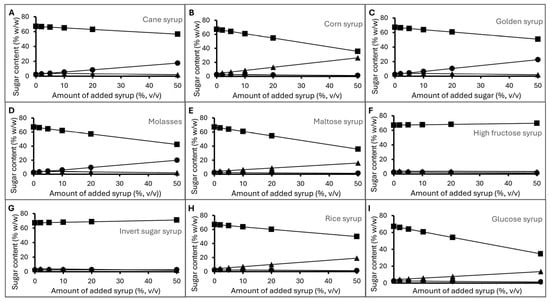
Figure 2.
Sugar content of honey samples intentionally adulterated with (A) cane syrup, (B) corn syrup, (C) golden syrup, (D) molasses, (E) maltose syrup, (F) high-fructose syrup, (G) invert sugar syrup, (H) rice syrup, (I) glucose syrup. Markers represent reducing sugar (fructose and glucose) content (square), sucrose content (circle), and maltose content (triangle).
3.3.4. δ13C of Deliberately Adulterated Honey
Figure 3 shows the changes in the δ13C of longan honey deliberately adulterated with various sources of external sugar. The longan honey without any added sugar syrup showed a δ13C value of −26.26 ± 0.09‰ measured using CM-CRDS technique in this study. This value was close to −26.43‰, the δ13C value measured by EA/LC-IRMS (Table 1), and in the same range as previously reported values for pure longan honey from Taiwan and Thailand (−26.57‰to −24.53‰) []. Rises in δ13CH were observed when they were adulterated with syrup that came from a C4 source including sugarcane and corn (cane syrup, corn syrup, golden syrup, molasses: Figure 3A–D), as these sugar sources exhibited significantly higher δ13C values than authentic longan honey (see discussion above). Interestingly, samples adulterated with maltose syrup also showed a rise in δ13CH (Figure 3E). As mentioned earlier, this sample of maltose syrup showed a δ13C value that indicated the presence of both C3 and C4 sugar sources. As a result, its addition into longan honey would cause the observed increases in the δ13CH of the mixture. On the other hand, the addition of syrup from a C3 carbon source, including cassava-derived high-fructose and glucose syrups, sugar beet invert sugar, and rice syrup, did not result in a significant rise in δ13CH (Figure 3F–I).
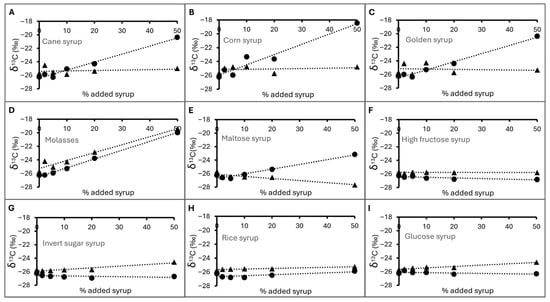
Figure 3.
ẟ13C of longan honey samples deliberately adulterated with (A) cane syrup, (B) corn syrup, (C) golden syrup, (D) molasses, (E) maltose syrup, (F) high-fructose syrup, (G) invert sugar syrup, (H) rice syrup, (I) glucose syrup. Markers represent δ13CH (circles) and δ13CP (triangle). Dotted lines indicated the measurement trends only.
To determine honey adulteration, δ13C values of honey protein (δ13CP) are also determined to use as an internal comparison for the δ13C value of bulk honey samples (δ13CH). Honey contains proteins in small quantities comprising several enzymes and pollen proteins [], which will influence its δ13CP and δ13CH values. As the majority of flowering plants utilize the C3 photosynthetic pathway, the δ13C of honey and its proteins are often within the range of C3 plants. The addition of external C4 sugars will affect δ13CH and theoretically will not affect δ13CP. Therefore, δ13CP is used as an internal reference. Departures of δ13CH from δ13CP will indicate the presence of an external C4 carbon source. The use of δ13CP as an internal reference has been widely accepted and utilized commercially [].
Extracted protein from longan honey had δ13CP values of −25.68 ± 0.08‰, measured using the CM-CRDS technique, and −25.57‰ measured using EA/LC IRMS (Table 1). δ13CP from deliberately adulterated honey samples mostly remained statistically unchanged, showing an average value of −25.4 ± 0.8‰. This observation pertained to honey samples adulterated with cane syrup, corn syrup, golden syrup, maltose syrup, high-fructose syrup, invert sugar syrup, and glucose syrup. This is due to the fact that sugar syrups are normally made of sugar and water as the main ingredients without a protein source. As a result, all proteins obtained from the extraction process had a honey origin. On the other hand, extracted proteins from longan honey adulterated with molasses showed a rise in δ13CP. Although molasses was not supposed to contain any proteins, a rise and high correlations between δ13CH and δ13CP of the adulterated samples were observed. It was possible that part of molasses sugars might have coprecipitated with the proteins, causing the contamination of molasses carbon in the protein fraction. Interestingly, the δ13CP of molasses-adulterated honey eventually rose above the C3 δ13C upper range of −22.00‰. This observation strongly supports the molasses–honey protein coprecipitation hypothesis and might indicate a limitation of the technique.
Differences between δ13CH and δ13CP can be used to determine adulteration by C4 sugar sources. White and Winters (1989) [] proposed to use differences of 1‰ or more as the criteria for honey adulterated with C4 sugar. Considering this, our experiments could potentially detect the addition of corn syrup at 5%; golden syrup, invert sugar syrup, and molasses at 10%; cane syrup and maltose syrup at 20%; and high-fructose syrup and glucose at 50% and above. The technique appeared not to detect the addition of rice syrup reliably.
3.3.5. Chemometric Analysis of Deliberately Adulterated Honey
Principal component analysis (PCA) has been used in a few studies to determine honey adulteration [,]. In this study, PCA was applied to five factors related to honey properties, including δ13CH, δ13CP, and amounts of fructose, glucose, and maltose in honey samples. Only these three sugars were considered together with δ13CH, δ13CP because the Kaiser–Meyer–Olkin Measure of Sampling Adequacy indicated better suitability for factor analysis (0.69) compared to including sucrose (0.53). Bartlett’s Test of Sphericity was significant (chi-square = 126.18, p < 0.001), indicating a strong relationship among the five variables. The first two eigenvalues (PC1 and PC2) equaled 2.94 and 1.24, which represented 83.6% of the total variability (Figure 4, Table S4). Component 1 (PC1) comprised the fructose level, the glucose level, and the maltose level, while Component 2 (PC2) comprised δ13CH and δ13CP. The PCA score plot (Figure 4) placed the authentic longan honey in quadrant II (positive PC1 and negative PC2).
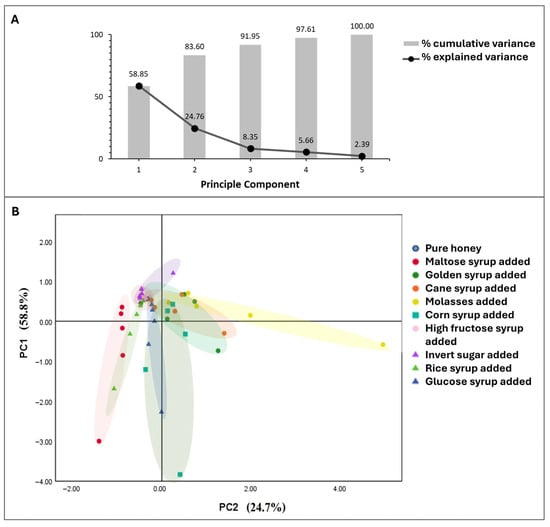
Figure 4.
Principal component analysis showing (A) explained variance for each principal component and cumulative variance with each additional component, (B) PCA score plot of honey samples deliberately adulterated with maltose syrup, golden syrup, cane syrup, molasses, corn syrup, high-fructose corn syrup, invert sugar, rice syrup, and glucose syrup.
Greater than 10% adulteration caused most samples to be placed outside the authentic honey quadrant. According to Figure 4, the PCA plot shows that honey samples with ≥10% sugar adulteration by C4-plant-derived adulterants like cane syrup, molasses, corn syrup, and golden syrup could be easily distinguished. However, a limitation of PCA application remained, as it was unable to display the adulteration of honey with C3-plant-derived adulterants like high-fructose corn syrup and invert sugar at concentrations lower than 20%. Samples adulterated at 20% or lower with high-fructose corn syrup and invert sugar still fell within the “authentic honey” region.
The PCA plot in Figure 4 reveals distinct distribution trends for different adulterants in the honey samples, with clear separation of C4 sugar-adulterated samples and partial overlap for C3 sugar-adulterated samples. Adding different adulterants showed unique isotopic and sugar profile signatures, which aided in the identification of adulteration patterns. Considering different clusters along PC1 which comprised sugar levels of glucose, fructose, and maltose, adulterating honey with more corn syrup, maltose syrup, glucose syrup, or rice syrup led to a large change in individual sugar content presented in the samples (cluster widely spread along PC1 in the lower left quadrant with higher negative PC1 values compared to other adulterants).
Different distribution trends were found for PC2. Including molasses in honey samples led to a significant shift of δ13C toward less negative values compared to pure honey (cluster appeared widely along PC2 in the upper-right quadrant with much higher positive PC2 values than other adulterants). Adding more than 10% of cane syrup or golden syrup also caused an obvious reduction in the δ13C values of the honey samples; however, to a smaller extent than molasses (a moderate spread along PC2). Glucose syrup, rice syrup, high-fructose syrup, invert sugar, and maltose showed a low influence on the shift in δ13C values (narrow cluster spread along PC2). Clusters of samples with <10% rice syrup or glucose syrup fell within the same quadrant (upper-left) as pure honey, and the values remained closer to the authentic region, making the identification ambiguous.
3.4. Authentication of Thai Honey
3.4.1. ẟ13C of Thai Honey
Thirty-four honey samples were collected from Chiang Mai, Chiang Rai, Chumphon, Lamphun, Nan, Phrae, and Saraburi provinces, Thailand, on a voluntary submission basis by beekeepers. However, the campaign was targeted to longan honey, as it was the focus of this study. The obtained samples consisted of 26 samples of longan honey, 2 samples of lychee honey, 3 samples of wildflower honey, 2 samples of sunflower honey, and 1 sample of coffee blossom honey. Most samples were from northern provinces, except for the sunflower honey samples, which were from the central region, and the coffee blossom honey, which was from Southern Thailand (Figure 5). Northern Thailand is an important production region of longan fruits (Dimocarpus longan L.) for internal consumption, exporting over 80% of Thai longan fruits [], which are highly dependent on bees for pollination. Apiculture and longan honey production are gaining in economic importance, and premium longan honey is in high demand for export. In this study, only a few samples of wildflower, lychee, sunflower, and coffee blossom honey were included. Therefore, they were not considered good representatives of their kind. The purpose of their inclusion in this study was to provide a more generalized view of the Thai honey landscape in addition to the repertoire of longan honey.
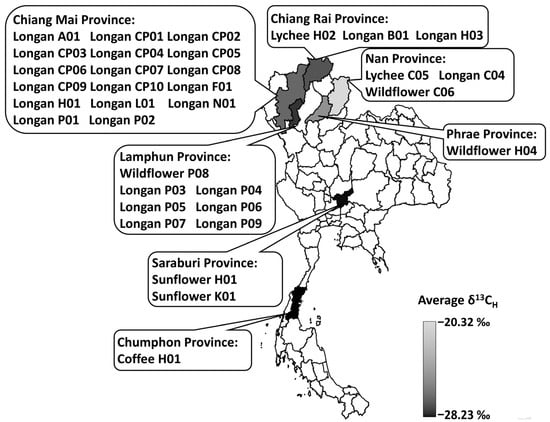
Figure 5.
Map of the provincial origins of honey samples and the average δ13CH. Thirty-four samples were collected from Chiang Mai, Chiang Rai, Chumphon, Lamphun, Nan, Phrae and Saraburi, Thailand. Color intensities represent the levels of provincial-averaged δ13CH.
Bulk honey and honey proteins were examined for their ẟ13C values. A range of −28.53‰ to −15.64‰ was observed for δ13CH and −31.75‰ to −21.15 to‰ for δ13CP (Table 3 and Figure 5). The δ13CH values of longan honey were found in similar ranges as other honey samples: −27.68‰ to −16.40‰ for longan honey and −28.53‰ to −15.64‰ for other honeys. When Grubb’s test was applied to all 34 honey samples (Table 3), the δ13CH of Wildflower C06 at −15.64‰ was identified as an outlier (p < 0.05). When applied to the 26 longan honey samples, the δ13CH of Longan CP04 at −16.40‰ was identified as an outlier (p < 0.05). By eliminating the outliers, the δ13CH range of the longan honey became −27.68‰ to −20.80‰. When comparing this range to the δ13CH range of C3 plants, it was apparent that a few of longan honey and other honey samples contained C4 carbon sources.

Table 3.
δ13C values and classification results of honey samples from various botanical sources.
When comparing the δ13CH ranges of longan and other honey samples to that of C3 plants (−32‰ to −22‰ [,,]), it was apparent that a few longan honey samples, as well as a few other honey samples, contained C4 carbon sources. To determine the authenticity of the honey samples, first, their δ13CH values were considered in comparison with the upper limit of the δ13C value of C3 plants. Acceptable samples must exhibit a δ13C value not exceeding −22.00‰ (Table 3). This caused three longan honey samples (CP04 CP07 and N01) to be rejected, as well as wildflower honey C06 and lychee honey C05. Next, δ13C differences between the protein fraction and bulk honey (δ13CP–δ13CH or ∆δ13CP-H) were applied, with acceptable samples having ∆δ13CP-H greater than −1.00‰ []. This criterion was successfully used in other previous reports including Kamdee et al. (2023) []. When applied to the honey samples in this study, two additional longan honey samples (CP01 and P02) as well as wildflower H04 and coffee H01 were rejected.
A discrepancy was observed for sample Longan N01; its δ13CH was −21.84 ± 0.04‰ (above −22.00‰), but its ∆δ13CP-H value was −0.53 (above −1.00‰). We rejected this sample, as its δ13CH suggested the presence of C4 sugars. The passing ∆δ13CP-H value was caused by a rather high δ13CH of −22.37 ± 0.16‰. An explanation could be carried-over sugars during protein extract preparation or that the bee colony was also fed with cane sugars in addition to natural foraging, resulting in a small difference between δ13CP and δ13CH. Another discrepancy was observed for Longan C04, where its δ13CH was −24.36 ± 0.15‰ (below the criterion of −22.00‰), but its δ13CP was −21.18 ± 0.13‰ (above the criterion of −22.00‰), and its ∆δ13CP-H was 3.18 (above the criterion of −1.00‰). Using the criteria described above, this sample would appear to pass as acceptable. However, its δ13CP made the decision ambiguous. This unexpectedly high δ13CP could not be explained easily and might demonstrate limitations of protein extract preparation and/or CM-CRDS measurements, as discussed above. Therefore, it was decided to reject this sample.
At this point, 20 out of 26 longan honey samples (76.9%) passed the δ13C criteria. The overall passing rate for the 34 honey samples was 24 out of 34, or 70.6%. The δ13CH range of acceptable honey samples was −28.53 ± 0.19‰ to −22.89 ± 0.08‰, with δ13CP of −29.30 ± 0.07‰ to −22.76 ± 0.03‰. Acceptable longan honeys exhibited a δ13CH range of −27.68 ± 0.05‰ to −22.89 ± 0.08‰ and δ13CP of −25.90± 0.14‰ to −21.18 ± 0.13‰. Other kinds of honey exhibited a δ13CH range of −28.53 ± 0.19‰ to −26.00 ± 0.05‰ and δ13CP of −29.30± 0.07‰ to −24.52 ± 0.05‰ (Figure 6). The observed ranges, especially of δ13CH, were almost in the same range as that previously reported for Thai honey, which ranged from to −29.1‰ to −23.1‰ [].
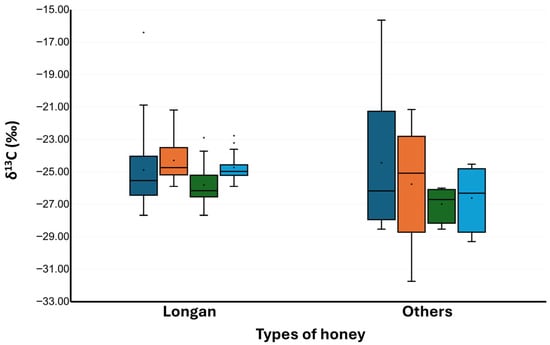
Figure 6.
ẟ13CH and ẟ13CP of longan honey and other types of honey. Dark blue bars represent ẟ13CH of collected samples. Orange bars represent ẟ13CP of collected samples. Green bars represent ẟ13CH of samples that passed the ẟ13C criteria. Light blue bars represent ẟ13CP of samples that passed the ẟ13C criteria. Mean values are shown as black dots within the bar graphs; median values are shown as black horizontal lines within bar graphs.
3.4.2. Pollen Composition of Thai Honey
The 34 honey samples were examined for their pollen composition to verify the botanical origin. All of them carried pollen from multiple plant species, with at least 12 pollen species identified in this study (Table 4). The six most prevalent pollens were of Coffea genus, Dimocarpus longan Lour., Helianthus annuus, Litchi chinensis, Mimosa pudica L., and Mimosa pigra L. (Figure 7). Pollens were classified based on their prevalence in honey as predominant (>45%), secondary (16–45%), minor (3–15.9%), and including (<3%) according to the method proposed by Louveaux et al. (1978) []. Thirty samples contained pollen that corresponded with the claimed plant origin as either the predominant or the secondary pollen (>16% prevalence), indicating their association with the claimed origin. When considering the longan honey group, 4 of 26 samples (longan A01, CP09, F01, N01) failed to carry Dimocarpus longan Lour. pollen at a significant level (>16%), indicating false claims of origin. For the other 22 longan samples, Dimocarpus longan Lour. pollen was present at 18–66% (Table 4). Other pollens found in the longan honey samples included those of Mimosa pudica, Mimosa pigra, Leucaena leucocephala L., Salicaceae, and Bidens pilosa L., with Mimosa pudica pollen found in all samples except for one (longan CP10). Mimosa pudica is a weed species that can be found across Thailand, including in longan orchards. Bees foraging in longan orchards would also be exposed to Mimosa pudica L. weeds, hence their presence at significant levels in the pollen content of most longan honey samples.

Table 4.
Pollen composition of 34 honey samples showing percentages of predominant, secondary, and minor pollen.
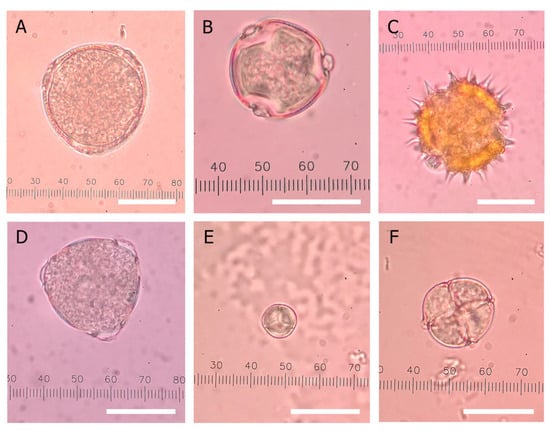
Figure 7.
Examples of the major pollen found in this study. Pollen grains of (A) genus Coffea, the coffee genus, (B) Dimocarpus longan Lour., the longan tree, (C) Helianthus annuus, the sunflower plant, (D) Litchi chinensis, the lychee tree, (E) Mimosa pudica L., the sensitive plant, and (F) Mimosa pigra L., the catclaw mimosa plant. Each white scale bar corresponds to 20 μm in length.
In Thailand, the longan flowering season typically occurs once per year, from February to April, depending on regional climatic conditions and orchard management []. During the late flowering or dry season, nectar availability can be substantially reduced, leading beekeepers to supplement colonies with sucrose syrup to maintain bee health and productivity [,]. This practice can result in honey with diluted floral markers and lower pollen representation from the primary source plant, even when the apiaries are located within longan orchards. In areas where longan orchards are interspersed with other crops (e.g., common flowering weeds like Mimosa pudica L.), bees will prioritize the most rewarding nectar source. When longan nectar is scarce, bees will forage widely, collecting both nectar and pollen from alternative sources. This mixed foraging pattern directly results in a lower proportion of Dimocarpus longan L. pollen in the final product. Moreover, variations in pollen content may also arise from differences in foraging behavior, local vegetation diversity, and post-harvest blending practices.
When the rest of honey samples are considered, samples of lychee honey, sunflower honey, and coffee blossom honey all had their corresponding pollen species present at greater than 16%, indicating their association with the claimed botanical origin (Table 4). Litchi chinensis pollen was present at 25–26% in lychee honey samples, while Helianthus annuus was present at 37–88% in sunflower honey samples. The single coffee blossom honey sample in this study contained pollens of Coffea genus at 93% prevalence. On the other hand, the wildflower honey samples in our study contained pollen from a few plant species. Interestingly, Mimosa pudica L. showed a high prevalence in these samples, being found at 61–81%. Other pollens found came from Dimocarpus longan L., Bidens pilosa L., and the Fabaceae family. This observation confirmed the above discussion that Mimosa pudica L. is one of the prevalent weed species in Thailand.
To be considered as monofloral honey, the criterion of the predominant pollen was applied, where pollen species of the claimed origin must be present at levels greater than 45%. Considering this, only 8 of the 26 longan samples could pass as monofloral longan honey; these were Longan C04, CP02, CP03, CP04, CP10, L01, P06, P09. These constituted 30.7% of the longan honey samples in this study. This low percentage might indicate that longan pollen was not abundantly present in the nectar, and a correction factor should be applied to account for the low pollen recovery rate. This correction factor is known as the pollen coefficient in melissopalynology. The pollen coefficient for Dimocarpus longan L. has not been reported previously. However, a study by Tangtragoon et al. [] reported greater than 70% prevalence of Dimocarpus longan L. pollen found in 13 out of 19 monofloral longan honey samples. Thus, pollen abundance in the nectar might not be an issue for Dimocarpus longan L., and a pollen coefficient might not be needed. Nonetheless, a formal study to confirm this observation should be conducted.
When the monofloral honey criterion was applied to the rest of the honey samples in this study, it was found that Sunflower Honey K01 and Coffee Blossom Honey H01 could be identified as monofloral honey, having the corresponding pollen at greater than 45% prevalence (88% and 93%, respectively). The three wildflower honey samples contained Mimosa pudica L. as the predominant pollen and could be labeled as Mimosa pudica monofloral honey. However, Mimosa pudica L. is a weed species and has not gained recognition as a bee-foraging species, nor its honey as a monofloral honey. Therefore, “wildflower honey” labelling would likely be more appropriate commercially.
3.4.3. Authentication Consideration
Many studies of honey authentication considered only a single aspect of authentication, being C4 sugar adulteration, geographical origin, or botanical origin. This study took both C4 sugar adulteration and botanical claim into consideration. δ13C analysis was first applied to eliminate samples with possible C4 sugar addition. Then, melissopalynology was applied to keep only monofloral honey samples.
Of the 34 honey samples, 24 samples were accepted based on their δ13C values as samples without C4 sugar adulteration (Table 5). Of these, eight samples could be considered monofloral honey with correct botanical claim. A correlation between the two sets of criteria was not observed, as they are independent technically. The samples that “passed” were six longan honey samples (CP02, CP03, CP10, L01, P06 and P09), one sunflower honey sample (K01), and one wildflower honey sample (P08) (Table 5), although Mimosa pudica honey was labeled as wildflower honey, as discussed above. Considering each set of criteria separately, the passing rate for δ13C analysis was 24 out of 34, or 70.6%, and for melissopalynology, it was 13 out of 34, or 38.2%. A low passing rate under melissopalynology criteria might come from the beekeepers’ practice of naming their honey according to the major crops in orchards where they place their bee colonies. Since pollen analysis is not routinely practiced, honey products were not subjected to proper quality control regarding pollen composition. However, for premium monofloral honey products, especially monofloral longan honey, it is recommended that melissopalynology is performed to ensure quality and proper claim of the botanical origin.

Table 5.
Authentication consideration of 34 honey samples.
Implementing δ13C and melissopalynology criteria together resulted in a more stringent consideration than either set of criteria alone. Together, they yielded a passing rate of 8 out of 34, or 23.5% (Table 5). When the longan honey samples were compared with the rest of the honey samples, a passing rate of 23.1% was observed for longan honey, and a 25.0% passing rate was observed for the other kinds of honey. It appeared that the final passing rates did not differ greatly between longan honey and the other honey, as well as among the longan honey group, the other honey group, and the entire sample set of honey together. It should be noted that other kinds of honey besides longan honey were underrepresented in this study. The comparison of their passing rates must be interpreted with caution.
4. Conclusions
Stable carbon isotope analysis measured using the CM-CRDS method can be effectively used to determine possible contamination of C4 sugar in honey samples. PCA analysis of δ13C together with the sugar profile information could potentially identify the addition of some C3 and C4 sugar sources. The authentic longan honey used in this study showed δ13C values of −26.26‰ and −25.68‰ for bulk honey and honey protein, respectively, using the CM-CRDS measurement technique. These δ13C values were in the same range as previously reported for Thai honey [] and in a similar range reported for Thai and Taiwanese authentic longan honey [].
In this study, we have demonstrated that δ13C measurements and melissopalynology can be implemented on the same set of honey samples to create a more stringent set of criteria for honey authentication. The proposed criteria were (1) δ13CH not exceeding −22.00‰ based on the δ13C range of C3 plants, (2) Δδ13CP-H of at least −1.00‰ using δ13CP as the internal reference for δ13CH measurements, (3) predominant pollen greater than 45% to ensure the predominant source of nectar, and (4) the predominant pollen species must match the claimed botanical origin of the honey sample. The first two criteria were applied to eliminate samples with possible C4 sugar adulteration. The latter two criteria were used to verify the botanical claim and monofloral honey consideration.
The above criteria set was applied to 34 beekeepers’ honey samples, comprising 26 samples of longan honey and 8 samples of other kinds of honey including wildflower honey, lychee honey, sunflower honey, and coffee blossom honey. δ13C measurements yielded a passing rate of 24 out of 34, or 70.6%, and melissopalynology yielded a passing rate of 13 out of 34, or 38.2%. When implemented together, the passing rate was reduced to 8 out of 34, or 23.5%, indicating increased stringency. When longan honey and the group of other kinds of honey were considered separately, similar passing rates were obtained. The passing rate for longan honey samples was 23.1%, and that of the group of other honey was 25.0%. Although other honey types were underrepresented in this study, it was demonstrated that the proposed criteria set could be applied to the longan honey and could likely be generalized and applied to other kinds of honey as well.
Longan honey is an economically important honey of Northern Thailand. Criteria that could ensure high-quality or premium-quality products can help to create value addition and allow these products to enter certain niche markets abroad. Of course, honey quality cannot depend only on δ13C and melissopalynology criteria; other criteria such as physicochemical properties and sensory quality must also be considered. Nonetheless, δ13C measurements and melissopalynology will certainly play an important role in creating high- or premium-quality longan honey products in Thailand, which can lead to an economically sustained apicultural practice among northern beekeepers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14223850/s1, Table S1: Linear regression statistics based on δ13CH values from CM-CRDS (y) and from EA-IRMS (x) assuming y = ax; Table S2: Linear regression statistics based on δ13CP values from CM-CRDS (y) and from EA-IRMS (x) assuming y = ax; Table S3: Reproducibility of δ13C measurements by CM-CRDS. δ13CH and δ13CP of a honey sample designated ‘Sample C’ was measured in nine separate preparations using CM-CRDS; Table S4: Factor loading results obtained from the principal component analysis (PCA) of deliberately adulterated honey samples.
Author Contributions
Conceptualization, K.J., T.D., C.S. (Chainarong Sinpoo) and K.B.; methodology, K.J., K.B., C.S. (Chainarong Sinpoo), S.-a.M., K.K. and T.D.; validation, K.J., S.-a.M., S.N., R.F. and J.E.; formal analysis, K.J., S.-a.M., K.K., C.S. (Chainarong Sinpoo), T.D., W.T. and K.B.; investigation, S.N., C.S. (Chainarong Sinpoo), C.S. (Chakrit Saengkorakot), R.F., N.U., P.P. and W.T.; resources, C.S. (Chainarong Sinpoo), P.P., N.U., C.S. (Chakrit Saengkorakot) and T.D.; data curation, K.J., K.B., C.S. (Chainarong Sinpoo) and K.K.; writing—original draft preparation, K.B., C.S. (Chainarong Sinpoo) and K.J.; writing—review and editing, K.B., C.S. (Chainarong Sinpoo) and K.J.; visualization, K.B.; supervision, K.J., T.D., J.E., K.K. and K.B.; project administration, K.B.; funding acquisition, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Thailand’s National Science, Research and Innovation Fund, Fundamental Fund grant number 192660 through the Thailand Institute of Nuclear Technology (Public Organization), and partially supported by Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors deeply thank Somjit Lungbubpha and the Strategy and Budget Section, Thailand Institute of Nuclear Technology (Public Organization) for coordinating the distribution of funds among the participating research institutes, as well as Rugipas Yongsawa for providing the flower and pollen sources used for pollen identification.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| SCIRA | Stable carbon isotope ratio analysis |
| EA/LC-IRMS | Elemental analyzer/liquid chromatography–isotope ratio mass spectrometer |
| EA-IRMS | Elemental analyzer–isotope ratio mass spectrometer |
| CM-CRDS | Combustion module–cavity ring down spectroscopy |
| δ13CH | δ13C of bulk honey |
| δ13CP | δ13C of honey protein fraction |
| δ13CP-H | δ13C of protein fraction minus δ13C of bulk honey |
| HPLC | High-performance liquid chromatography |
References
- Powrel, P.J.; Sharma, S. Dynamics of Global Honey Trade: A Longitudinal Analysis from 1961 to 2022. Arch. Curr. Res. Int. 2024, 24, 92–103. [Google Scholar] [CrossRef]
- Terrab, A.; González, A.G.; Díez, M.J.; Heredia, F.J. Characterisation of Moroccan Unifloral Honeys Using Multivariate Analysis. Eur. Food Res. Technol. 2003, 218, 88–95. [Google Scholar] [CrossRef]
- Alcivar-Saldaña, J.J.; Rodriguez-Monroy, M.A.; Carrillo-Miranda, L.; Canales-Martinez, M.M. Botanical Origin and Biological Properties of Honey and Propolis from Cuautitlan, State of Mexico, Mexico. Antioxidants 2024, 13, 874. [Google Scholar] [CrossRef]
- Bunket, A.; Na Lampang, K.; Intanon, M.; Chaisowwong, W. Knowledge, Attitudes, and Practices of Beekeepers Regarding Good Agricultural Practice in Northern Thailand. Vet. Integr. Sci. 2026, 24, e2026021. [Google Scholar]
- Kafle, L.; Mabuza, T.Z. An Analysis of Longan Honey from Taiwan and Thailand Using Flow Cytometry and Physicochemical Analysis. Foods 2024, 13, 3772. [Google Scholar] [CrossRef] [PubMed]
- Disayathanoowat, T.; Judprasong, K.; Boonsirichai, K.; Kamdee, K.; Sinpoo, C.; Phokasem, P.; Takewwong, T.; Segsarnviriya, C.; Esor, J.; Fungklin, R.; et al. The Standard of Thai Honey; Chiang Mai University: Chiang Mai, Thailand, 2025; p. 8. [Google Scholar]
- Wongsiri, S.; Thapa, R.; Kongpitak, P. Longan: A Major Honey Plant in Thailand. Bee World 1998, 79, 23–28. [Google Scholar] [CrossRef]
- Narjes, M.E.; Lippert, C. Regional Differences in Farmers’ Preferences for a Native Bee Conservation Policy: The Case of Farming Communities in Northern and Eastern Thailand. PLoS ONE 2021, 16, e0251206. [Google Scholar] [CrossRef] [PubMed]
- Wanjai, C.; Sringarm, K.; Santasup, C.; Pak-Uthai, S.; Chantawannakul, P. Physicochemical and Microbiological Properties of Longan, Bitter Bush, Sunflower and Litchi Honeys Produced by Apis mellifera in Northern Thailand. J. Apic. Res. 2012, 51, 36–44. [Google Scholar] [CrossRef]
- Chaikham, P.; Prangthip, P. Alteration of Antioxidative Properties of Longan Flower-Honey after High Pressure, Ultra-Sonic and Thermal Processing. Food Biosci. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Tangtragoon, T.; Kawaree, R.; Posingtong, R. Pollen Analysis and Chemical Characterization of Longan Honey in Northern Thailand. J. Agric. Res. Ext. 2017, 34, 37–47. [Google Scholar]
- Pichai, P.; Budkrajang, P. Quality of Honey Produced in Chiang Mai, Lamphun and Chiang Rai Provinces. Bull. Dept. Med. Sci. 2022, 64, 181–196. [Google Scholar]
- Ministry of Public Health. Notification of Ministry of Public Health (No. 211) B.E. 2543 (2000) Re: Honey. R. Thai Gov. Gaz. 2001, 118, 96–98. [Google Scholar]
- Hatch, M.D.; Slack, C.R. Photosynthesis by Sugar-Cane Leaves. A New Carboxylation Reaction and the Pathway of Sugar Formation. Biochem. J. 1966, 101, 103–111. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon Isotopes in Photosynthesis: Fractionation Techniques May Reveal New Aspects of Carbon Dynamics in Plants. BioScience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Sage, R.F. The Evolution of C4 Photosynthesis. New Phytol. 2004, 161, 341–370. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Winkler, F.J.; Schmidt, H.L. Einsatzmöglichkeiten der 13C-Isotopen-Massenspektrometrie in der Lebensmitteluntersuchung. Z. Lebensm. Unters. Forsch. 1980, 171, 85–94. [Google Scholar] [CrossRef]
- AOAC International. Official Method 991.41. C4 Plant Sugars in Honey. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- AOAC International. Official Method 978.17. Corn and Cane Sugar Products in Honey. In Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- AOAC International. Official Method 998.12. C4 Plant Sugars in Honey. In Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Vîjan, L.E.; Mazilu, I.C.; Enache, C.; Enache, S.; Topală, C.M. Botanical Origin Influence on Some Honey Physicochemical Characteristics and Antioxidant Properties. Foods 2023, 12, 2134. [Google Scholar] [CrossRef]
- von der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–154. [Google Scholar] [CrossRef]
- Berman, E.; Gupta, M.; Gabrielli, C.; Garland, T.; McDonnell, J.J. High-Frequency Field Deployable Isotope Analyzer for Hydrological Applications. Water Resour. Res. 2009, 45, W10201. [Google Scholar] [CrossRef]
- Chesson, L.A.; Bowen, G.J.; Ehleringer, J.R. Analysis of the Hydrogen and Oxygen Stable Isotope Ratios of Beverage Waters without Prior Water Extraction Using Isotope Ratio Infrared Spectroscopy. Rapid Commun. Mass Spectrom. 2010, 24, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Method 980.13. Fructose, Glucose, Lactose, Maltose and Sucrose in Milk Chocolate. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- White, J.W., Jr.; Winters, K. Honey Protein as Internal Standard for Stable Carbon Isotope Ratio Detection of Adulteration of Honey. J. Assoc. Off. Anal. Chem. 1989, 72, 907–911. [Google Scholar] [CrossRef]
- Chen, H.; Karion, A.; Rella, C.W.; Winderlich, J.; Gerbig, C.; Filges, A.; Newberger, T.; Sweeney, C.; Tans, P.P. Accurate Measurements of Carbon Monoxide in Humid Air Using the Cavity Ring-Down Spectroscopy (CRDS) Technique. Atmos. Meas. Tech. 2013, 6, 1031–1040. [Google Scholar] [CrossRef]
- Kamdee, K.; Naksuriyawong, S.; Uapoonphol, N.; Fungklin, R.; Esor, J.; Permnamtip, V.; Meepho, S.; Judprasong, K. Evaluation of Honey Authenticity in Thailand by Analysis of Carbon Stable Isotope Ratio Using Elemental Analyser Coupled to Isotope Ratio Mass Spectrometry and Cavity Ring-Down Spectrometry. Int. J. Food Sci. Technol. 2023, 58, 2458–2464. [Google Scholar] [CrossRef]
- El Hawari, K.; Al Iskandarani, M.; Jaber, F.; Ezzeddine, R.; Ziller, L.; Perini, M.; Bontempo, L.; Pellegrini, M.; Camin, F. Evaluation of Honey Authenticity in Lebanon by Analysis of Carbon Stable Isotope Ratio Using Elemental Analyzer and Liquid Chromatography Coupled to Isotope Ratio Mass Spectrometry. J. Mass Spectrom. 2021, 56, e4730. [Google Scholar] [CrossRef] [PubMed]
- Mantha, M.; Urban, J.R.; Mark, W.A.; Chernyshev, A.; Kubachka, K.M. Direct Comparison of Cavity Ring Down Spectrometry and Isotope Ratio Mass Spectrometry for Detection of Sugar Adulteration in Honey Samples. J. AOAC Int. 2018, 101, 1857–1863. [Google Scholar] [CrossRef]
- Elflein, L.; Raezke, K.P. Improved Detection of Honey Adulteration by Measuring Differences between 13C/12C Stable Carbon Isotope Ratios of Protein and Sugar Compounds with a Combination of Elemental Analyzer-Isotope Ratio Mass Spectrometry and Liquid Chromatography-Isotope Ratio Mass Spectrometry (δ13C-EA/LC-IRMS). Apidologie 2008, 39, 574–587. [Google Scholar]
- Dua, A.; Bahman, S.; Kelly, S.; Dogra, S.; Sharma, K. Authentication of Indian Honey Based on Carbon Stable Isotope Ratio Analysis—Verification of Indian Regulatory Criteria. Foods 2025, 14, 1289. [Google Scholar] [CrossRef]
- Schievano, E.; Piana, L.; Tessari, M. Automatic NMR-Based Protocol for Assessment of Honey Authenticity. Food Chem. 2023, 420, 136094. [Google Scholar] [CrossRef]
- Meepho, S.; Kamdee, K.; Saengkorakot, C.; Thapprathum, P.; Judprasong, K. Comparison between Isotope Ratio Mass Spectrometry (IRMS) and Cavity Ring-Down Spectroscopy (CRDS) for Analysing the Carbon Isotope Ratio and Detection of Adulteration in Coconut Water. Int. J. Food Sci. Technol. 2021, 56, 6611–6617. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, D.G.; Jung, C. A Comparative Study on the Two Different Methods IRMS and CRDS for Estimation of δ13C (‰) of Honey Samples. J. Apic. 2018, 33, 99–105. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Li, Y.; Wang, Z. Systematic Comparison of C3 and C4 Plants Based on Metabolic Network Analysis. BMC Syst. Biol. 2012, 6, S9. [Google Scholar] [CrossRef]
- Bräutigam, A.; Gowik, U. Photorespiration Connects C3 and C4 Photosynthesis. J. Exp. Bot. 2016, 67, 2953–2962. [Google Scholar] [CrossRef]
- White, J.W., Jr.; Doner, L.W. Mass Spectrometric Detection of High-Fructose Corn Syrup in Honey by Use of 13C/12C Ratio: Collaborative Study. J. Assoc. Off. Anal. Chem. 1978, 61, 746–750. [Google Scholar]
- Rossmann, A. Determination of Stable Isotope Ratios in Food Analysis. Food Rev. Int. 2001, 17, 347–381. [Google Scholar] [CrossRef]
- Padovan, G.J.; De Jong, D.; Rodrigues, L.P.; Marchini, J.S. Detection of Adulteration of Commercial Honey Samples by the 13C/12C Isotopic Ratio. Food Chem. 2003, 82, 633–636. [Google Scholar] [CrossRef]
- Desrochers, J.; Brye, K.R.; Pollock, E.D. Long-Term Agricultural Practice Effects on Carbon and Nitrogen Isotopes of Soil Organic Matter Fractions. Agrosyst. Geosci. Environ. 2022, 5, e20232. [Google Scholar] [CrossRef]
- Ma, X.; Gong, L.; Yang, Y.; Ding, Z.; Li, X. Mineralization and Fixed Stable Carbon Isotopic Characteristics of Organic Carbon in Cotton Fields with Different Continuous Cropping Years. Agronomy 2023, 13, 804. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wang, G.; Yang, H.; Hong, L.; Xu, J.; Wang, H. Can Stable Carbon Isotope Fingerprints Be Competent for Geographic Traceability of Rice? Food Chem. 2024, 455, 139819. [Google Scholar] [CrossRef]
- Thomatou, A.-A.; Mazarakioti, E.C.; Zotos, A.; Kokkotos, E.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Stable Isotope Ratio Analysis for the Geographic Origin Discrimination of Greek Beans “Gigantes-Elefantes” (Phaseolus coccineus L.). Foods 2024, 13, 2107. [Google Scholar] [CrossRef]
- Güçlü, H.; Yücel, P.; Oktar, O.; Yazıcı, N.; Ocak, S.B. Carbon Isotope Ratios in Honey and Proteins: A Novel Method for Regional Differentiation. J. Food Compos. Anal. 2025, 144, 107676. [Google Scholar] [CrossRef]
- Camin, F.; Boner, M.; Bontempo, L.; Fauhl-Hassek, C.; Kelly, S.D.; Riedl, J.; Rossmann, A. Stable Isotope Techniques for Verifying the Declared Geographical Origin of Food in Legal Cases. Trends Food Sci. Technol. 2017, 61, 176–187. [Google Scholar] [CrossRef]
- Peterson, D.M.; Budde, A.D.; Henson, C.A.; Jones, B.L. Detecting Corn Syrup in Barley Malt Extracts. Cereal Chem. 2001, 78, 349–353. [Google Scholar] [CrossRef]
- Waworuntu, J.S. Sugar Content Composition of Various Types of Honey Produced by Apis Mellifera L.: A Review. J. Penelit. Pendidik. IPA 2024, 10, 98–106. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Malaysian Honeys Produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef]
- Mongi, R.J.; Ruhembe, C.C. Sugar Profile and Sensory Properties of Honey from Different Geographical Zones and Botanical Origins in Tanzania. Heliyon 2024, 10, e38094. [Google Scholar] [CrossRef] [PubMed]
- Zulkiflee, N.S.; Zoolfakar, A.S.; Rani, R.A.; Aryani, D.; Zolkapli, M. Detection and Classification of Honey Adulteration Combined with Multivariate Analysis. Int. J. Integr. Eng. 2022, 14, 262–272. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Standard for Honey (CXS 12-1981); FAO/WHO: Rome, Italy, 2022.
- Chen, C.-T.; Chen, B.-Y.; Nai, Y.-S.; Chang, Y.-M.; Chen, K.-H.; Chen, Y.-W. Novel Inspection of Sugar Residue and Origin in Honey Based on the 13C/12C Isotopic Ratio and Protein Content. J. Food Drug Anal. 2019, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Won, S.-R.; Lee, D.-C.; Ko, S.H.; Kim, J.-W.; Rhee, H.-I. Honey Major Protein Characterization and Its Application to Adulteration Detection. Food Res. Int. 2008, 41, 952–956. [Google Scholar] [CrossRef]
- Narjes, M.E.; Lippert, C. Longan Fruit Farmers’ Demand for Policies Aimed at Conserving Native Pollinating Bees in Northern Thailand. Ecosyst. Serv. 2016, 18, 58–67. [Google Scholar] [CrossRef]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical Profiles of Honey Produced from Monofloral Longan, Rubber, and Sunflower in Northern Thailand. Chiang Mai Univ. J. Nat. Sci. 2016, 15, 115–126. [Google Scholar]
- Suwannapong, G.; Benbow, M.E.; Nieh, J.C. Biology of Thai Honeybees: Natural History and Threats. Insect. Soc. 2011, 58, 495–506. [Google Scholar]
- Rattanawannee, A.; Chanchao, C.; Wongsiri, S. Melissopalynological Analysis and Floral Preferences of Honeybees in Thailand. Palynology 2017, 41, 339–353. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).