Extraction Techniques and Modification Methods for Regulating the Structural and Functional Properties of Oleosome-Associated Proteins: A Review
Abstract
1. Introduction
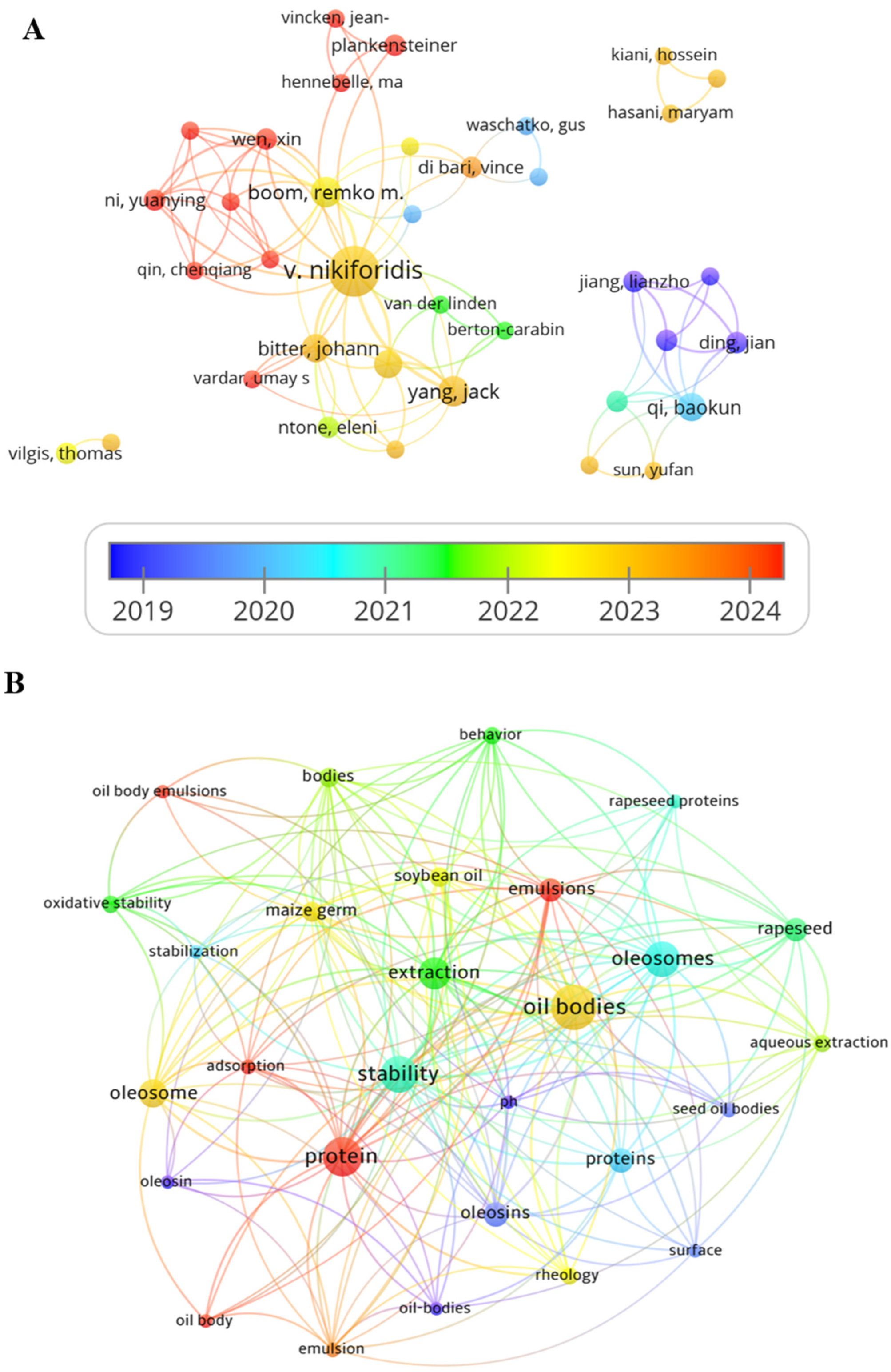
2. Current Research Status
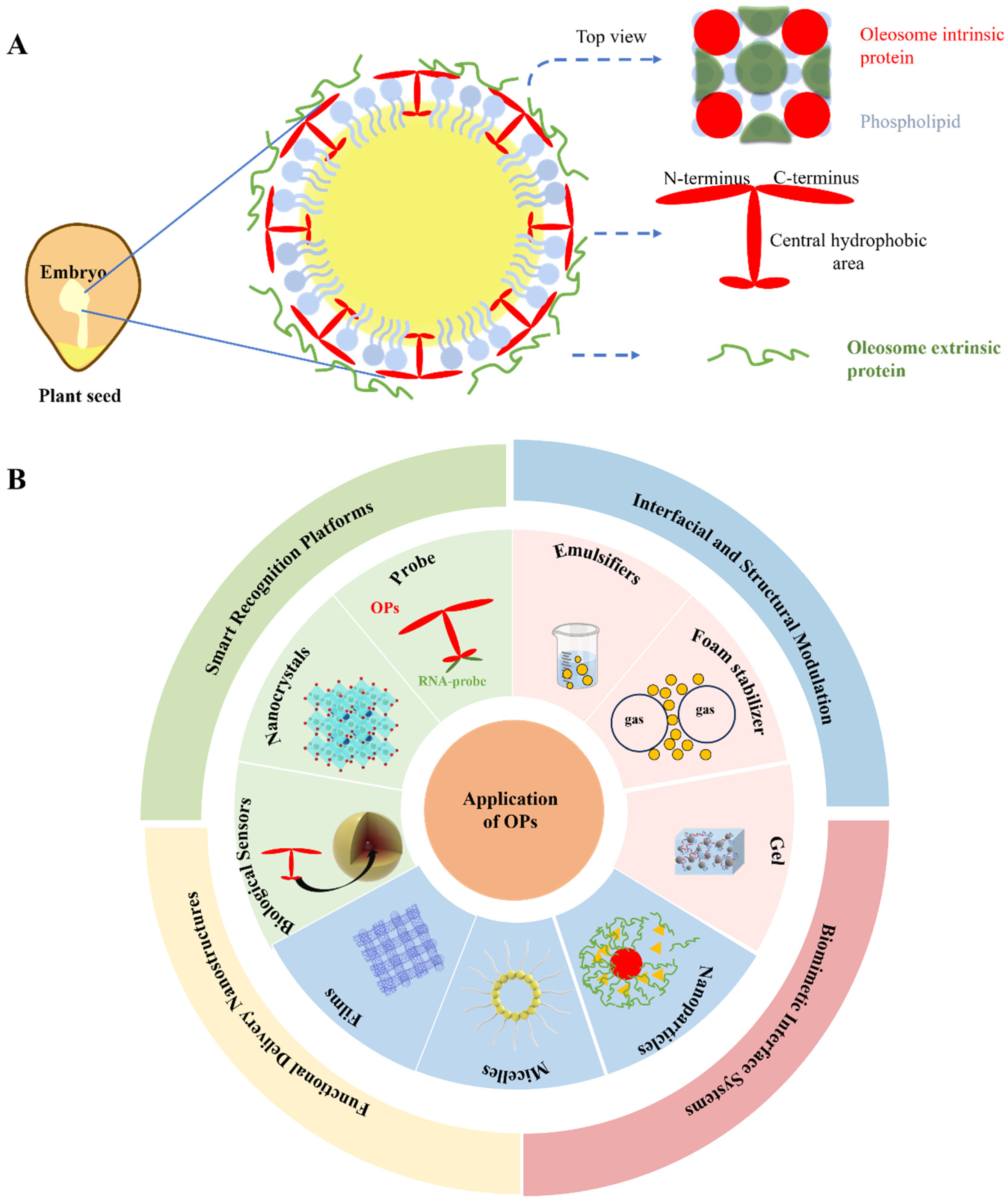
3. Structure, Composition, and Key Sources of OPs from Various Sources
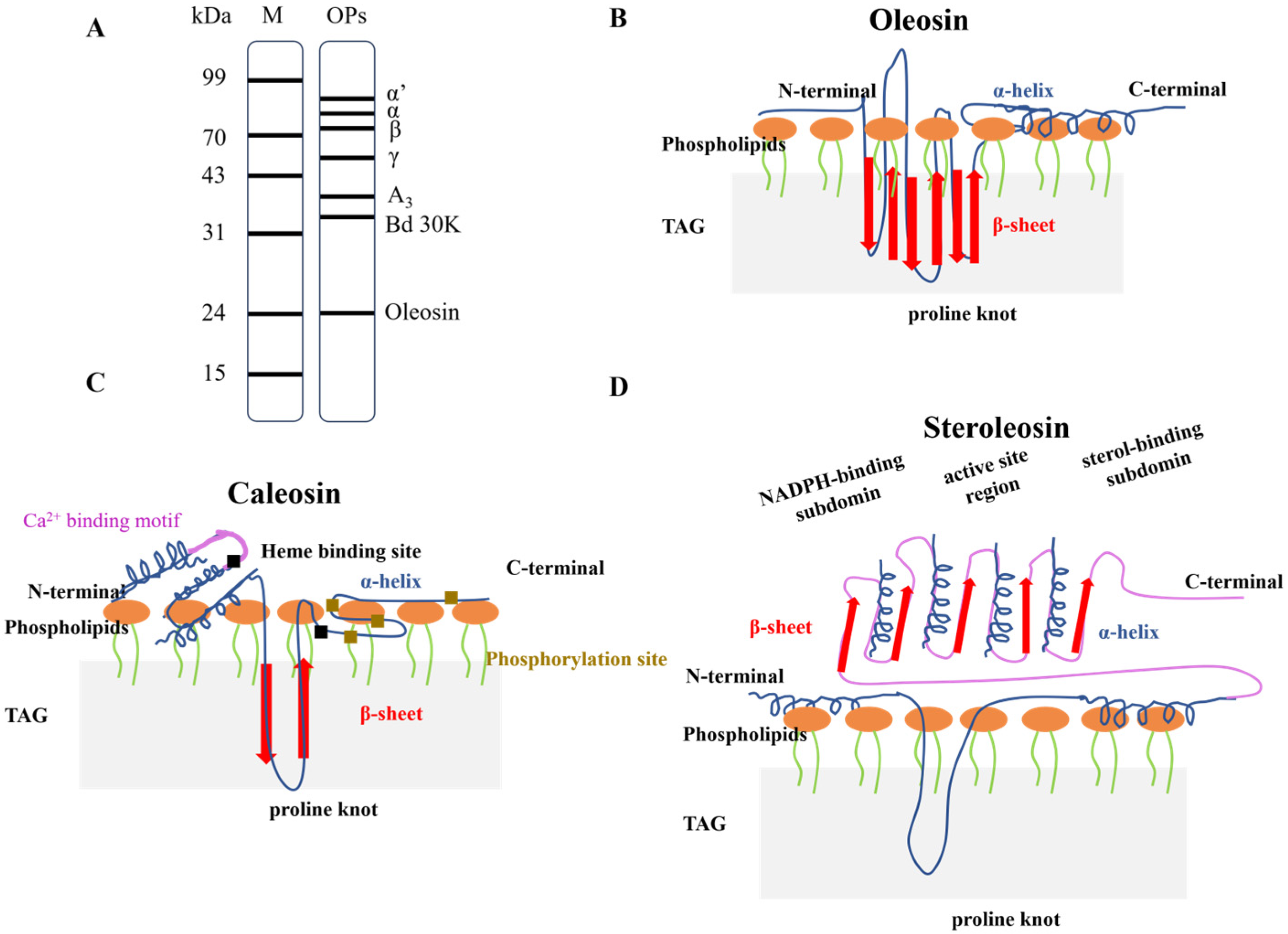
3.1. Structural Characteristics of Intrinsic Proteins: Oleosin, Caleosin, and Steroleosin
3.2. Interaction and Influence of Extrinsic Proteins on Oleosomes
3.3. Variations in OPs Composition Among Different Plant Sources
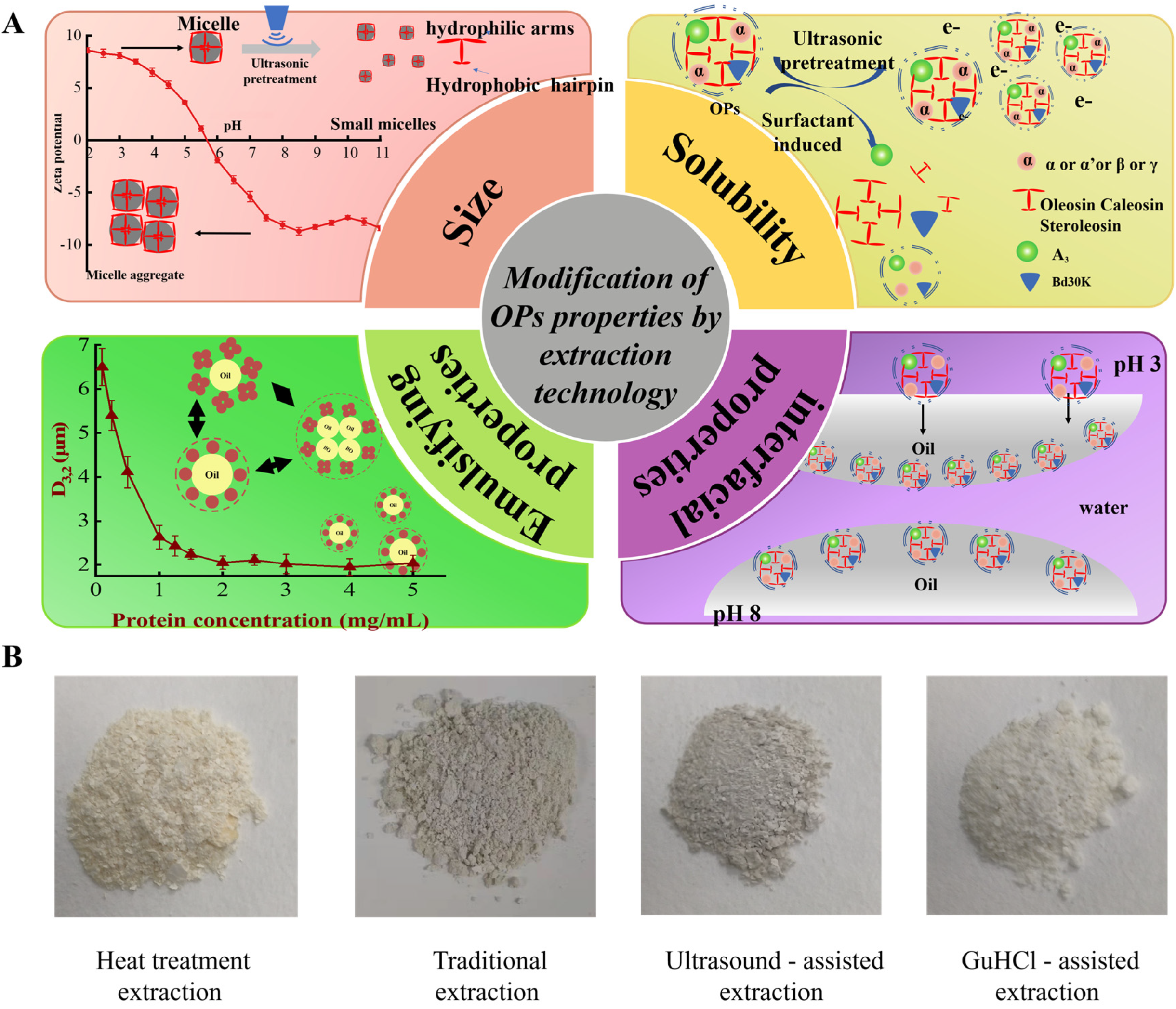
4. Modification of OPs Through Extraction Techniques
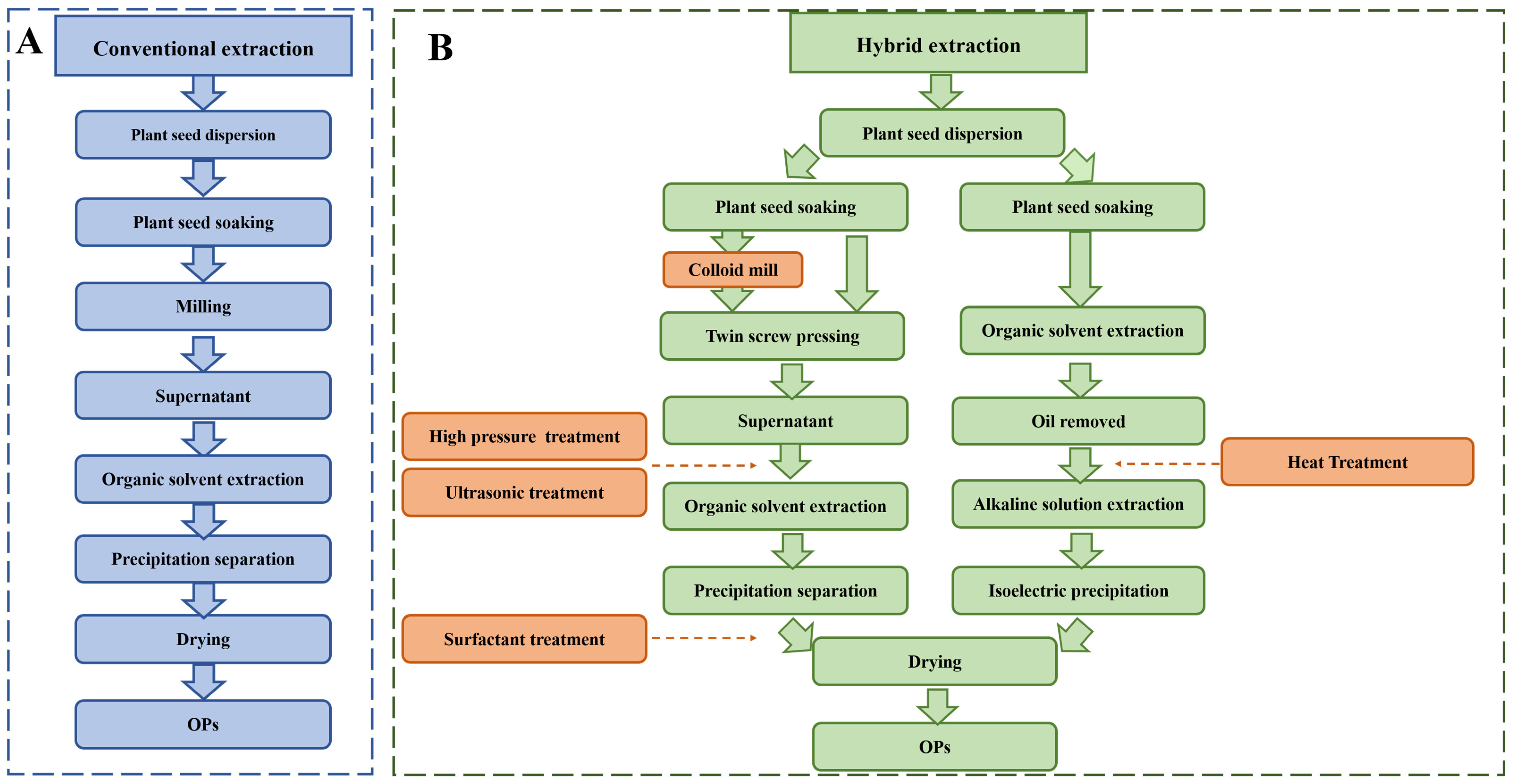
4.1. Traditional Extraction
4.1.1. Enrichment of OPs Using Chemical Reagents
4.1.2. Selection of Different Solvents for OPs Extraction
4.1.3. Separation and Recovery of OPs by Precipitation
4.2. Hybrid Extraction
5. Structural Modulation of OPs via Extraction Techniques
5.1. Molecular Composition of OPs
5.2. Secondary Structure of OPs
5.3. Tertiary Structure of OPs
6. Improving Physicochemical and Functional Properties Through Extraction Techniques
6.1. Particle Size
6.2. Solubility
6.3. Appearance and Microstructure
6.4. Emulsifying Properties of OPs
6.5. Interfacial Properties of OPs
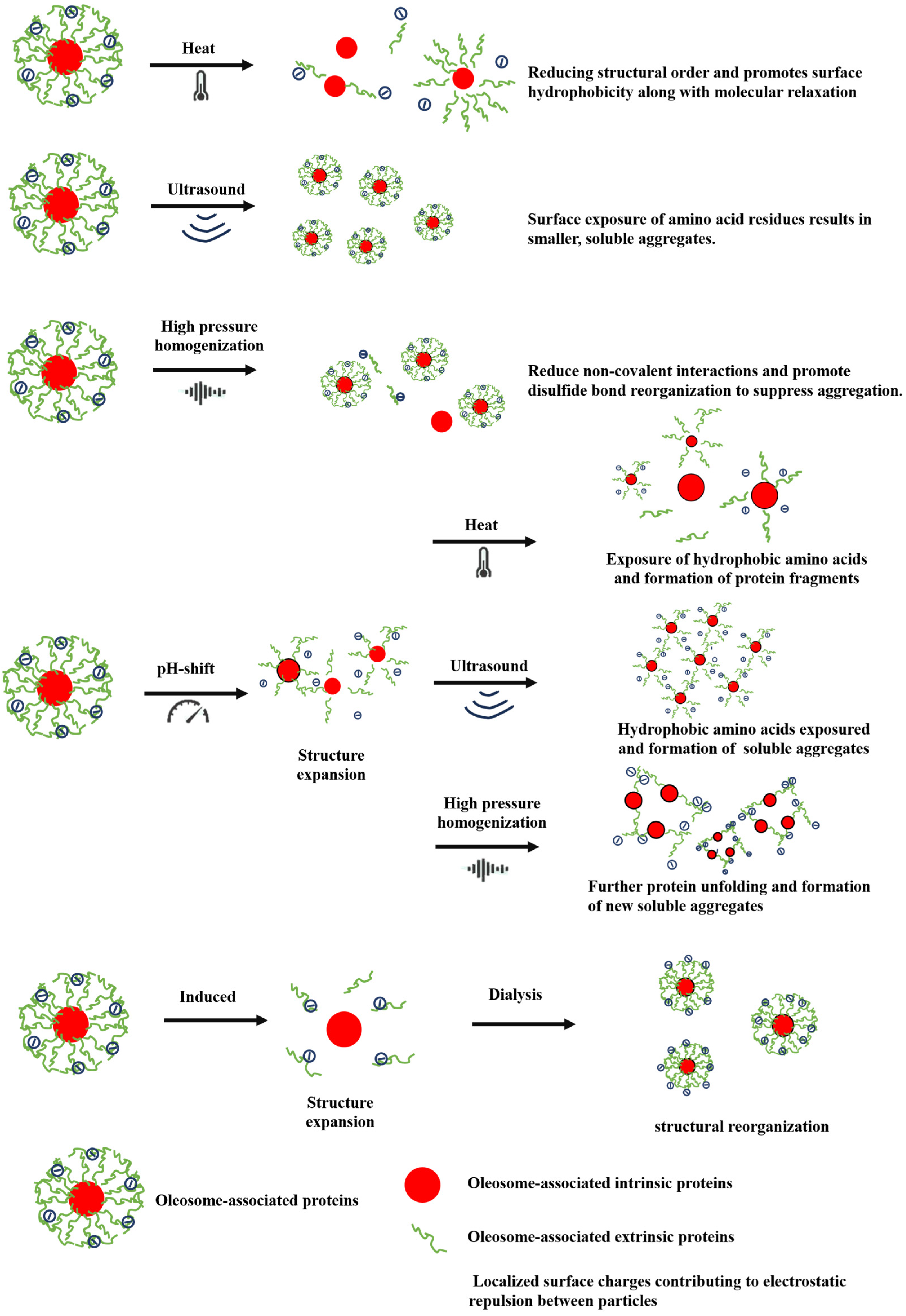
7. Post-Extraction Modification Techniques for OPs
7.1. Physical Modification
7.1.1. Heat Treatment
7.1.2. Ultrasound Treatment
7.1.3. High-Pressure Homogenization
7.2. Chemical Modification
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aliyari, A.; di Bari, V.; Ratcliffe, L.P.; Borah, P.K.; Dong, Y.; Gray, D. Characterising the concentration-dependent behaviour of heat-treated sunflower oleosomes at an air-water interface. Food Hydrocoll. 2025, 162, 110896. [Google Scholar] [CrossRef]
- Karefyllakis, D.; Van Der Goot, A.J.; Nikiforidis, C. The behaviour of sunflower oleosomes at the interfaces. Soft Matter. 2019, 15, 4639–4646. [Google Scholar] [CrossRef]
- Shi, Z.; Li, K.; Meng, Z. Recent trends in oleosomes: Extraction methods, structural characterization, and novel applications. Trends Food Sci. Technol. 2024, 151, 104621. [Google Scholar] [CrossRef]
- Abbasi, S.; Scanlon, M. Microemulsion: A novel alternative technique for edible oil extraction a mechanistic viewpoint. Crit. Rev. Food Sci. Nutr. 2023, 63, 10461–10482. [Google Scholar] [CrossRef]
- Abdullah, W.; Zhang, H. Recent advances in the composition, extraction and food applications of plant-derived oleosomes. Trends Food Sci. Technol. 2020, 106, 322–332. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, J.; Ukwatta, R.H.; Xue, F.; Xiong, X.; Li, C. Artificial oil bodies: A review on composition, properties, biotechnological applications, and improvement methods. Food Chem. X 2024, 21, 101109. [Google Scholar] [CrossRef]
- Ahmad, S.; d’Avanzo, N.; Mancuso, A.; Barone, A.; Cristiano, M.C.; Carresi, C.; Mollace, V.; Celia, C.; Fresta, M.; Paolino, D. Skin Tolerability of Oleic Acid Based Nanovesicles Designed for the Improvement of Icariin and Naproxen Percutaneous Permeation. ACS Appl. Bio Mater. 2024, 7, 7852–7860. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhong, M.; Kang, M.; Liao, Y.; Wang, Z.; Li, Y.; Qi, B. Novel core-shell nanoparticles: Encapsulation and delivery of curcumin using guanidine hydrochloride-induced oleosome-associated protein self-assembly. LWT 2023, 173, 114352. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Xie, F.; Zhong, M.; Jiang, L.; Qi, B.; Li, Y. Effects of covalent modification with epigallocatechin-3-gallate on oleosin structure and ability to stabilize artificial oil body emulsions. Food Chem. 2021, 341, 128272. [Google Scholar] [CrossRef] [PubMed]
- Honaker, L.W.; Eijffius, A.; Plankensteiner, L.; Nikiforidis, C.V.; Deshpande, S.J.S. Biosensing with Oleosin-Stabilized Liquid Crystal Droplets. Small 2024, 20, 2309053. [Google Scholar] [CrossRef]
- Plankensteiner, L.; Hennebelle, M.; Vincken, J.; Nikiforidis, C. Insights into the emulsification mechanism of the surfactant-like protein oleosin. J. Colloid Interface Sci. 2024, 657, 352–362. [Google Scholar] [CrossRef]
- Maurer, S.; Waschatko, G.; Schach, D.; Zielbauer, B.I.; Dahl, J.; Weidner, T.; Bonn, M.; Vilgis, T.A. The role of intact oleosin for stabilization and function of oleosomes. J. Phys. Chem. B 2013, 117, 13872–13883. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, M.; Liao, Y.; Kang, M.; Qi, B.; Li, Y. Pickering emulsions stabilized by reassembled oleosome-associated protein nanoparticles for co-encapsulating hydrophobic nutrients. Food Hydrocoll. 2023, 138, 108445. [Google Scholar] [CrossRef]
- Plankensteiner, L.; Yang, J.; Bitter, J.H.; Vincken, J.-P.; Hennebelle, M.; Nikiforidis, C.V. High yield extraction of oleosins, the proteins that plants developed to stabilize oil droplets. Food Hydrocoll. 2023, 137, 108419. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Dave, A.; Singh, H. Nature-assembled structures for delivery of bioactive compounds and their potential in functional foods. Front. Chem. 2020, 8, 564021. [Google Scholar] [CrossRef]
- Karabulut, G.; Goksen, G.; Khaneghah, A. Plant-based protein modification strategies towards challenges. J. Agric. Food Res. 2024, 15, 101017. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, S.; Wang, O.; Zhao, L.; Zhao, L. A comprehensive review on walnut protein: Extraction, modification, functional properties and its potential applications. J. Agric. Food Res. 2024, 16, 101141. [Google Scholar] [CrossRef]
- Zheng, X.; Zou, B.; Zhang, J.; Cai, W.; Na, X.; Du, M.; Zhu, B.; Wu, C. Recent advances of ultrasound-assisted technology on aquatic protein processing: Extraction, modification, and freezing/thawing-induced oxidation. Trends Food Sci. Technol. 2024, 144, 104309. [Google Scholar] [CrossRef]
- Zhang, S.; McClements, D.J.; Zheng, R.; Yu, X.; Sun, Z.; Xie, B.; Chen, Y.; Deng, Q. A promising perspective to boost the utilizability of oil bodies: Moderate regulation and modification of interface. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70145. [Google Scholar] [CrossRef] [PubMed]
- Huang, A. Oil bodies and oleosins in seeds. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 177–200. [Google Scholar] [CrossRef]
- Shao, Q.; Liu, X.; Su, T.; Ma, C.; Wang, P. New insights into the role of seed oil body proteins in metabolism and plant development. Front. Plant Sci. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.D.; Huang, A.H. Oleosin KD 18 on the surface of oil bodies in maize. Genomic and cDNA sequences and the deduced protein structure. J. Biol. Chem. 1990, 265, 2238–2243. [Google Scholar] [CrossRef]
- Fujii, T. Coagulation and rheological behaviors of soy milk colloidal dispersions. Biosci. Biotechnol. Biochem. 2017, 81, 680–686. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, X.; Zhan, G.; Liu, G.; Hua, W.; Wang, H. Unusually large oilbodies are highly correlated with lower oil content in Brassica napus. Plant Cell Rep. 2009, 28, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Tai, S.; Peng, C.C.; Tzen, J. Identification of three novel unique proteins in seed oil bodies of sesame. Plant Cell Physiol. 1998, 39, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Tzen, J.T.C. Integral proteins in plant oil bodies. Int. Sch. Res. Not. 2012, 2012, 173954. [Google Scholar] [CrossRef]
- Jiang, P.; Tzen, J. Caleosin serves as the major structural protein as efficient as oleosin on the surface of seed oil bodies. Plant Signal Behav. 2010, 5, 447–449. [Google Scholar] [CrossRef]
- Asefy, Z.; Tanomand, A.; Hoseinnejhad, S.; Ceferov, Z.; Oshaghi, E.A.; Rashidi, M. Unsaturated fatty acids as a co-therapeutic agents in cancer treatment. Mol. Biol. Rep. 2021, 48, 2909–2916. [Google Scholar] [CrossRef]
- Lin, L.; Tzen, J. Two distinct steroleosins are present in seed oil bodies. Plant Physiol. Biochem. 2004, 42, 601–608. [Google Scholar] [CrossRef]
- Nikiforidis, C.V. Structure and functions of oleosomes (oil bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef]
- Zaaboul, F.; Matabaro, E.; Raza, H.; Xin, B.; Duhoranimana, E.; Cao, C.; Liu, Y.F. Validation of a simple extraction method for oil bodies isolated from peanuts. Eur. J. Lipid Sci. Technol. 2018, 120, 1700363. [Google Scholar] [CrossRef]
- Katavic, V.; Agrawal, G.K.; Hajduch, M.; Harris, S.; Thelen, J. Protein and lipid composition analysis of oil bodies from two Brassica napus cultivars. Proteomics 2006, 6, 4586–4598. [Google Scholar] [CrossRef]
- Ishii, T.; Matsumiya, K.; Nambu, Y.; Samoto, M.; Yanagisawa, M.; Matsumura, Y. Interfacial and emulsifying properties of crude and purified soybean oil bodies. Food Struct. 2017, 12, 64–72. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Zhao, L.; Kong, X.; Hua, Y. Macronutrients and micronutrients of soybean oil bodies extracted at different pH. J. Food Sci. 2014, 79, C1285–C1291. [Google Scholar] [CrossRef]
- Lan, X.; Qiang, W.; Yang, Y.; Gao, T.; Guo, J.; Du, L.; Noman, M.; Li, Y.; Li, J.; Li, H.; et al. Physicochemical stability of safflower oil body emulsions during food processing. LWT 2020, 132, 109838. [Google Scholar] [CrossRef]
- Guan, M.; Lv, D.; Chen, F.; Yao, F.; Lin, F. Recent Advances in the Extraction, Functional Characteristics, and Food Applications of Plant Oleosomes. J. Food Sci. 2025, 90, e70413. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kornet, R.; Ntone, E.; Meijers, M.G.J.; Van den Hoek, I.; Sagis, L.; Venema, P.; Meinders, M.; Berton-Carabin, C.; Nikiforidis, C.; et al. Plant protein aggregates induced by extraction and fractionation processes: Impact on techno-functional properties. Food Hydrocoll. 2024, 155, 110223. [Google Scholar] [CrossRef]
- Meza, S.; Cañizares, L.; Dannenberg, B.; Peres, B.; Rodrigues, L.; Mardade, C.; de Leon, M.; Gaioso, C.; Egea, I.; de Oliveira, M. Sustainable rice bran protein: Composition, extraction, quality properties and applications. Trends Food Sci. Technol. 2024, 145, 104355. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, R.; Xu, Z.; Jiang, L.; Sui, X. Recent advances in soy protein extraction technology. J. Am. Oil Chem. Soc. 2023, 100, 187–195. [Google Scholar] [CrossRef]
- Jin, W.; Pan, Y.; Wu, Y.; Chen, C.; Xu, W.; Peng, D.; Huang, Q. Structural and interfacial characterization of oil bodies extracted from Camellia oleifera under the neutral and alkaline condition. LWT 2021, 141, 110911. [Google Scholar] [CrossRef]
- Jin, W.; Yang, X.; Shang, W.; Wu, Y.; Guo, C.; Huang, W.; Deng, Q.; Peng, D. Assembled structure and interfacial properties of oleosome-associated proteins from Camellia oleifera as natural surface-active agents. LWT 2023, 173, 114318. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, L.; Ying, Y.; Kong, X.; Hua, Y.; Chen, Y. The characterization of soybean oil body integral oleosin isoforms and the effects of alkaline pH on them. Food Chem. 2015, 177, 288–294. [Google Scholar] [CrossRef]
- Nikiforidis, C.; Ampatzidis, C.; Lalou, S.; Scholten, E.; Karapantsios, T.; Kiosseoglou, V. Purified oleosins at air–water interfaces. Soft Matter. 2013, 9, 1354–1363. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, H.; Wang, K.; Azam, S.R.; Wang, Y.; Zhou, J.; Qu, W. Ultrasound frequency effect on soybean protein: Acoustic field simulation, extraction rate and structure. LWT 2021, 145, 111320. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Chen, Y.; Kong, X.; Hua, Y. Effects of pH on protein components of extracted oil bodies from diverse plant seeds and endogenous protease-induced oleosin hydrolysis. Food Chem. 2016, 200, 125–133. [Google Scholar] [CrossRef]
- Wijesundera, C.; Boiteau, T.; Xu, X.; Shen, Z.; Watkins, P.; Logan, A. Stabilization of fish oil-in-water emulsions with oleosin extracted from canola meal. J. Food Sci. 2013, 78, C1340–C1347. [Google Scholar] [CrossRef]
- Samoto, M.; Maebuchi, M.; Miyazaki, C.; Kugitani, H.; Kohno, M.; Hirotsuka, M.; Kito, M. Abundant proteins associated with lecithin in soy protein isolate. Food Chem. 2007, 102, 317–322. [Google Scholar] [CrossRef]
- Zhong, M.; Xie, F.; Zhang, S.; Sun, Y.; Qi, B.; Li, Y. Preparation and digestive characteristics of a novel soybean lipophilic protein-hydroxypropyl methylcellulose-calcium chloride thermosensitive emulsion gel. Food Hydrocoll. 2020, 106, 105891. [Google Scholar] [CrossRef]
- Romero-Guzmán, M.; Petris, V.; De Chirico, S.; di Bari, V.; Gray, D.; Boom, R.; Nikiforidis, C. The effect of monovalent (Na+, K+) and divalent (Ca2+, Mg2+) cations on rapeseed oleosome (oil body) extraction and stability at pH 7. Food Chem. 2020, 306, 125578. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhong, M.; Wu, L.; Huang, Y.; Li, Y.; Qi, B. Effects of ultrasound-assisted salt (NaCl) extraction method on the structural and functional properties of Oleosin. Food Chem. 2022, 372, 131238. [Google Scholar] [CrossRef] [PubMed]
- Pulikkottil Rajan, D. Extraction, isolation, and characterization techniques of structural proteins. In Fish Structural Proteins and Its Derivatives: Functionality and Applications; Springer Nature: Singapore, 2024; pp. 37–72. [Google Scholar] [CrossRef]
- Shukla, D.; Schneider, C.P.; Trout, B.L. Molecular level insight into intra-solvent interaction effects on protein stability and aggregation. Adv. Drug Deliv. Rev. 2011, 63, 1074–1085. [Google Scholar] [CrossRef]
- Şen, A.; Acevedo-Fani, A.; Dave, A.; Ye, A.; Husny, J.; Singh, H. Plant oil bodies and their membrane components: New natural materials for food applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 256–279. [Google Scholar] [CrossRef]
- Ji, L.; Feng, W.; Chen, H.; Chu, Y.; Wong, A.; Zhu, Y.; Sinatra, G.; Bramante, F.; Carrière, F.; Stocks, F.; et al. Rapeseed oleosomes facilitate intestinal lymphatic delivery and oral bioavailability of cannabidiol. Int. J. Pharm. 2025, 668, 124947. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Farooq, S. Role of peanut oleosomes in the delivery of curcumin embedded in interpenetrating emulsion-filled gels made with whey protein and chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2025, 707, 135962. [Google Scholar] [CrossRef]
- Ma, Z.; Bitter, J.H.; Boom, R.M.; Nikiforidis, C.V. Thermal treatment improves the physical stability of hemp seed oleosomes during storage. LWT 2023, 189, 115551. [Google Scholar] [CrossRef]
- Matsumura, Y.; Sirison, J.; Ishi, T.; Matsumiya, K. Soybean lipophilic proteins—Origin and functional properties as affected by interaction with storage proteins. Curr. Opin. Colloid Interface Sci. 2017, 28, 120–128. [Google Scholar] [CrossRef]
- Yang, R.; Deng, H.; Zhao, Y.; Lin, H.; Song, Y.; Zhao, L.; Miao, W.; Zheng, B. Interface engineering of plant oil body for an innovative food ingredient: A review. Trends Food Sci. Technol. 2025, 159, 104954. [Google Scholar] [CrossRef]
- Kaur, M.; Sinha, K.; Eastmond, P.J.; Bhunia, R.K. Exploiting lipid droplet metabolic pathway to foster li-pid production: Oleosin in focus. Plant Cell Rep. 2025, 44, 12. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Pan, T.; Chen, H.; Liu, D.; Wang, W. Interfacial Effects Induced by Nanobubbles: Char-acteristics, Consequences, and Applications in Sustainable Food Processing, Commercial Quality, and Human Health. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70303. [Google Scholar] [CrossRef]
- Krolitzki, E.; Winkler, W.; Schwaminger, S.; Berensmeier, S. Bare magnetic iron oxides for binding and selective elution of lactoferrin from acid whey. Colloids Surf. A Physicochem. Eng. Asp. 2025, 716, 136654. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, Y.; Qayum, A.; Liang, Q.; Rehman, A.; Gan, R.; Ma, H.; Ren, X. Research progress in soybean lipophilic protein (LP): Extraction, structural, techno-functional properties, and high-performance food applications. Trends Food Sci. Technol. 2024, 147, 104440. [Google Scholar] [CrossRef]
- Fabre, J.; Lacroux, E.; Cerny, M.; Vaca-Medina, G.; Cassen, A.; Merah, O.; Valentin, R.; Mouloungui, Z.J.O. Oil body extraction from oleo-proteaginous seeds and conservation of valuable native compounds. OCL 2023, 30, 26. [Google Scholar] [CrossRef]
- Islam, F.; Saeed, F.; Afzaal, M.; Ahmad, A.; Hussain, M.; Khalid, M.A.; Saevan, S.A.; Khashroum, A.O. Applications of green technologies-based approaches for food safety enhancement: A comprehensive review. Food Sci. Nutr. 2022, 10, 2855–2867. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, L.; Wu, Y.; Ding, X.; Wang, W.; Zhang, D.; Zhao, L. Effect of Heat Treatment on the Digestive Characteristics of Different Soybean Oil Body Emulsions. Foods 2023, 12, 2942. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Shan, Z.; Jia, W.; Li, M.; Wen, X.; Ni, Y. Comparative analysis of twin-screw pressing and blending methods for walnut oleosome extraction: Yield, physical stability, and functionalities. J. Food Eng. 2025, 386, 112292. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, Y.; Zhu, Y.; Shen, W.; Jin, W.; Peng, D.; Huang, Q. Assembly of oleosin during efficient extraction: Altering the sequence of defatting solvents. Food Chem. X 2024, 25, 102022. [Google Scholar] [CrossRef]
- Kumari, S.; Memba, L.J.; Dahuja, A.; Vinutha, T.; Saha, S.; Sachdev, A. Elucidation of the role of oleosin in off-flavour generation in soymeal through supercritical CO2 and biotic elicitor treatments. Food Chem. 2016, 205, 264–271. [Google Scholar] [CrossRef]
- De Chirico, S.; di Bari, V.; Foster, T.; Gray, D. Enhancing the recovery of oilseed rape seed oil bodies (oleosomes) using bicarbonate-based soaking and grinding media. Food Chem. 2018, 241, 419–426. [Google Scholar] [CrossRef]
- De Chirico, S.; di Bari, V.; Romero Guzmán, M.J.; Nikiforidis, C.; Foster, T.; Gray, D. Assessment of rapeseed oil body (oleosome) lipolytic activity as an effective predictor of emulsion purity and stability. Food Chem. 2020, 316, 126355. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, M.; Xie, F.; Sun, Y.; Zhang, S.; Qi, B. The effect of pH on the stabilization and digestive characteristics of soybean lipophilic protein oil-in-water emulsions with hypromellose. Food Chem. 2020, 309, 125579. [Google Scholar] [CrossRef]
- Qin, C.; Han, M.; Fu, R.; Mei, Y.; Wen, X.; Ni, Y.; Boom, R.; Nikiforidis, C. Influence of extraction pH and homogenization on soybean oleosome emulsion stability. LWT 2024, 203, 116404. [Google Scholar] [CrossRef]
- Qin, C.; Fu, R.; Mei, Y.; Wen, X.; Ni, Y.; Boom, R.; Nikiforidis, C. Combining colloid milling and twin screw pressing for oleosome extraction. J. Food Eng. 2024, 368, 111908. [Google Scholar] [CrossRef]
- Romero-Guzmán, M.; Jung, L.; Kyriakopoulou, K.; Boom, R.; Nikiforidis, C. Efficient single-step rapeseed oleosome extraction using twin-screw press. J. Food Eng. 2020, 276, 109890. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Janssen, P.H.; Dickhoff, B.H. The impact of material chemistry and morphology on attrition behavior of excipients during high shear blending. Powder Technol. 2023, 427, 118694. [Google Scholar] [CrossRef]
- Li, Q.; Lih, T.; Clark, D.; Chen, L.; Schnaubelt, M.; Zhang, H. Sonication-assisted protein extraction improves proteomic detection of membrane-bound and DNA-binding proteins from tumor tissues. Nat. Protoc. 2025, 20, 2083–2099. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochemistry 2023, 101, 106646. [Google Scholar] [CrossRef]
- Khalid, S.; Chaudhary, K.; Aziz, H.; Amin, S.; Sipra, H.M.; Ansar, S.; Rasheed, H.; Naeem, M.; Onyeaka, H. Trends in extracting protein from microalgae Spirulina platensis, using innovative extraction techniques: Mechanisms, potentials, and limitations. Crit. Rev. Food Sci. Nutr. 2025, 65, 4293–4309. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Agarwal, D.; Logan, A.; Buckow, R. Oleosome extraction: Challenges, innovations, and opportunities for industrial applications. J. Food Eng. 2025, 404, 112780. [Google Scholar] [CrossRef]
- Qi, B.; Ding, J.; Wang, Z.; Li, Y.; Ma, C.; Chen, F.; Sui, X.; Jiang, L. Deciphering the characteristics of soybean oleosome-associated protein in maintaining the stability of oleosomes as affected by pH. Food Res. Int. 2017, 100, 551–557. [Google Scholar] [CrossRef]
- Shao, F.; Zhang, Y.; Wan, X.; Duan, Y.; Cai, M.; Hu, K.; Zhang, H. Regulation in protein hydrophobicity via whey protein-zein self-assembly for improving the techno-functional properties of protein. Food Chem. 2025, 463, 141174. [Google Scholar] [CrossRef]
- Pan, Y.; Jin, W.; Huang, Q. Structure, assembly and application of novel peanut oil body protein extracts nanoparticles. Food Chem. 2022, 367, 130678. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, Y.; Sun, Y.; Song, H.; Zhang, S.; Qi, B.; Li, Y. Sodium Dodecyl Sulfate-Dependent Disassembly and Reassembly of Soybean Lipophilic Protein Nanoparticles: An Environmentally Friendly Nanocarrier for Resveratrol. J. Agric. Food Chem. 2022, 70, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, L.; Li, L.; Chi, H.; Teng, F. High-pressure homogenization assisted pH-shifting modified soybean lipophilic protein interacting with chitosan hydrochloride: Double emulsion construction, physicochemical properties, stability, and in vitro digestion analysis. Food Hydrocoll. 2024, 160, 110834. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, Y.; Song, H.; Liao, Y.; Qi, B.; Li, Y. Dithiothreitol-induced reassembly of soybean lipophilic protein as a carrier for resveratrol: Preparation, structural characterization, and functional properties. Food Chem. 2023, 399, 133964. [Google Scholar] [CrossRef]
- Tian, Y.; Lv, X.; Oh, D.H.; Kassem, J.M.; Salama, M.; Fu, X. Emulsifying properties of egg proteins: Influencing factors, modification techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70004. [Google Scholar] [CrossRef]
- Rehman, A.; Liang, Q.; Karim, A.; Assadpour, E.; Jafari, S.M.; Rasheed, H.; Virk, M.; Qayyum, A.; Suleria, H.; Ren, X. Pickering high internal phase emulsions stabilized by biopolymeric particles: From production to high-performance applications. Food Hydrocoll. 2024, 150, 109751. [Google Scholar] [CrossRef]
- Yang, J.; Plankensteiner, L.; de Groot, A.; Hennebelle, M.; Sagis, L.; Nikiforidis, C. The role of oleosins and phosphatidylcholines on the membrane mechanics of oleosomes. J. Colloid Interface Sci. 2025, 678, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Byanju, B.; Lamsal, B.P. Protein, lipid, and chitin fractions from insects: Method of extraction, functional properties, and potential applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 6415–6431. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, M.; Mujumdar, A.S.; Semenov, G.; Luo, Z. Technological advances in protein extraction, structure improvement and assembly, digestibility and bioavailability of plant-based foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 11556–11574. [Google Scholar] [CrossRef]
- Sun, Y.; Jia, S.; Hou, Y.; Cheng, S.; Tan, M.; Zhu, B.; Wang, H. Novel thyme essential oil-loaded biodegradable emulsion film based on soybean lipophilic proteins for salmon preservation. Food Hydrocoll. 2025, 160, 110790. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Zhang, S.; Zhong, M.; Zhao, C.; Xie, F.; Qi, B. Stability and in vitro simulated release characteristics of ultrasonically modified soybean lipophilic protein emulsion. Food Funct. 2020, 11, 3800–3810. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Hou, Y.; Cheng, S.; Tan, M.; Zhu, B.; Wang, H. Enhanced stability and antibacterial efficacy of edible oleogels-in-water high internal phase emulsions prepared from soybean lipophilic protein. Food Hydrocoll. 2024, 154, 110058. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, Y.; Sun, Y.; Fang, L.; Wang, Q.; Qi, B.; Li, Y. Soy lipophilic protein self-assembled by pH-shift combined with heat treatment: Structure, hydrophobic resveratrol encapsulation, emulsification, and digestion. Food Chem. 2022, 394, 133514. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, T.; Li, L.; Chi, H.; Teng, F. Modification of soybean lipophilic protein based on pH-shifting and high-pressure homogenization: Focus on structure, physicochemical properties and delivery vehicle. Food Chem. 2025, 463, 141001. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, X.; Li, L.; Xie, T.; Teng, F. Co-encapsulation of vitamin E and quercetin by soybean lipophilic proteins based on pH-shifting and ultrasonication: Focus on interaction mechanisms, structural and physicochemical properties. Food Chem. 2024, 460, 140608. [Google Scholar] [CrossRef]
- Wang, S.; Miao, S.; Sun, D. Modifying structural and techno-functional properties of quinoa proteins through extraction techniques and modification methods. Trends Food Sci. Technol. 2024, 143, 104285. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, Y.; Song, H.; Wang, S.; Qi, B.; Li, X.; Li, Y. Ethanol as a switch to induce soybean lipophilic protein self-assembly and resveratrol delivery. Food Chem. X 2023, 18, 100698. [Google Scholar] [CrossRef]
- Wu, D.; Wang, H.; Guo, X.; Zhang, Z.; Gao, Z.; Gao, S.; Liu, Z.; Rao, S.; Meng, X. Insight into the mechanism of enhancing myofibrillar protein gel hardness by ultrasonic treatment combined with insoluble dietary fiber from oat. LWT 2023, 178, 114539. [Google Scholar] [CrossRef]
| Solvent | Extraction Conditions | Protein Content | Structural Changes | Functional Property Improvements | References |
|---|---|---|---|---|---|
| Organic Reagent Extraction | |||||
| Acetone, Ether | v/v, 1/1 | NA | Changes in intermolecular components, alteration in protein secondary structure | Increased denaturation temperature | [40] |
| Chloroform, Methanol | v/v, 2/1 | NA | Changes in intermolecular components, alteration in protein secondary structure | Increased diffusion rate at air–water interface | [41] |
| Methanol, Hexane, Ethanol | v/v/v, 2/2/1 | 87.1% | Molecular weight changes | Significant improvement in interfacial properties | [14] |
| Methanol, Chloroform | v/v, 95/5 | NA | Changes in molecular weight, surface hydrophobicity | Reduced solubility, weakened intermolecular electrostatic repulsion | [42] |
| Methanol, Chloroform, Water | v/v/v, 4/2/1 | 74.6% | Changes in protein secondary structure, molecular weight | Changes in emulsifying properties, particle size, water/oil absorption capacity | [8] |
| Methanol, Chloroform, and 1% NaCl | v/v/v, 4/2/1 | 75.3% | Molecular weight changes | Changes in particle size, digestion properties, interfacial properties | [43] |
| Acetone, Methanol, Chloroform | Samples first mixed with equal volumes of acetone, then with a 2:1 (v/v) mixture of methanol and chloroform | NA | Changes in protein secondary structure, molecular weight | Emulsifying properties | [44] |
| Ether | Samples mixed with equal volumes of ether | NA | Molecular weight changes | NA | [45] |
| Alkaline Extraction | |||||
| Sodium Hydroxide Solution | Plant seed samples soaked in NaOH solution overnight (40%, w/w), pH adjusted to 12.0 | NA | Molecular weight changes | NA | [46] |
| Sodium Hydroxide Solution with Na2SO3 | Plant seeds soaked in 2 M NaOH (1:8, w/v) overnight, pH 12.0, then treated with Na2SO3, heated at 55 °C, and pH adjusted to 5.5 | 76.4% | Molecular weight, surface hydrophobicity, disulfide bond content | Changes in solubility, particle size, gel properties, water/oil absorption capacity | [47,48] |
| Solvent | Extraction Conditions | Protein Content | Structural Improvements | Functional Property Enhancements | References |
|---|---|---|---|---|---|
| Urea, acetone, petroleum ether | Centrifuge to collect the cream layer, wash with 0.8 to 6.4 M urea to remove impurities, and recover OPs by treatment with a cold acetone/petroleum ether mixture at equal volumes. | 86% | Changes in protein secondary structure and surface hydrophobicity. | Changes in interfacial properties and assembly behavior. | [67] |
| Diethyl ether, methanol, chloroform, jasmonic acid | Extract the aqueous phase and interfacial layer with ether, then add 1 mL of chloroform/methanol (2:1, v/v) and treat with 5 μM jasmonic acid | NA | Changes in protein tertiary structure. | Protein activity modification and reduction in off-flavor production | [68] |
| n-Hexane, acetone, GuHCl | Centrifuge the mixture of n-hexane and acetone (threefold volume), then dialyze the OPs with 5 M GuHCl. | 75.4% | Molecular weight, surface hydrophobicity, and changes in protein secondary structure | Solubility, particle size, and changes in emulsifying properties | [8] |
| Acetone, diethyl ether, urea | Centrifuge the mixture of cold acetone and ether (equal volume), then dialyze the OPs with 8 M urea | NA | Changes in molecular weight, with extrinsic proteins in OPs completely removed. | Particle size decreased, and intermolecular electrostatic repulsion weakened. | [38] |
| Grinding medium-pH combined treatment | Grind the seeds using NaHCO3 buffer (pH 9.5), and recover OPs by organic solvent precipitation. | 8.5% | Changes in molecular weight | Changes in intermolecular electrostatic repulsion and increased solubility | [69] |
| Soaking-salt combined treatment | Soak plant seeds in salt solutions of different pH values, enrich by grinding, and recover OPs using organic solvent precipitation | 9.2% | Surface hydrophobicity was altered. | Changes in intermolecular electrostatic repulsion | [70] |
| Heat-pH combined treatment | Defatted soybean meal is subjected to dry heat treatment at 70 °C for 2 h, then stirred at pH 8.0 for 1 h followed by centrifugation. The supernatant is adjusted to pH 5.0, heated at 55 °C for 15 min, and then 50 mM NaCl solution (pH 5.5) is added | 71.6% | Molecular weight, surface hydrophobicity, and protein secondary structure were altered. | Interfacial properties and solubility were altered. | [71] |
| Ultrasound-assisted salt hybrid extraction | A 0.1 M NaCl solution is added to the plant seed slurry, followed by ultrasound-assisted extraction. After enrichment, OPs are recovered using an organic solvent precipitation method. | 78.3% | Changes in molecular weight | Particle size, digestibility, and interfacial properties were altered. | [50] |
| Twin-screw-assisted pH hybrid extraction | Plant seeds are soaked under different pH conditions, followed by twin-screw pressing-assisted extraction, and finally OPs are recovered using an organic solvent precipitation method. | 3.2% | Molecular weight was altered. | Solubility was altered. | [69] |
| High pressure homogenization assisted pH combined extraction | Plant seeds are soaked under different pH conditions, followed by high-pressure homogenization-assisted extraction. After enrichment, OPs are recovered using an organic solvent precipitation method | 34.2% | Molecular weight was altered. | Intermolecular electrostatic repulsion was altered. | [72] |
| Colloid mill-twin screw combined extraction | Plant seeds are soaked under different pH conditions, followed by twin-screw extrusion combined with colloid milling for assisted extraction. After enrichment, OPs are recovered using an organic solvent precipitation method | 21.2% | Molecular weight was altered. | Intermolecular electrostatic repulsion was altered. | [73] |
| Modification Method | Structural Alteration | Functional Property Changes | References |
|---|---|---|---|
| Physical Modification | |||
| Thermal treatment | Changes in secondary structure and surface hydrophobicity | Improves thermal stability, antioxidant activity, and film-forming properties | [91] |
| Ultrasonic treatment | Changes in secondary structure and surface hydrophobicity | Improves solubility, emulsification, storage stability, gelation; enhances heat sensitivity and biocompatibility | [10,18,92] |
| High-pressure homogenization | Alters surface hydrophobicity; weakens non-covalent interactions between proteins and promotes disulfide bond reorganization | Improves solubility and emulsification; enhances stability and bioavailability of vitamin B12 as an encapsulant | [73] |
| Chemical Modification | |||
| pH shifting | Changes in secondary and tertiary structure and surface hydrophobicity | Improves solubility, emulsification, and antioxidant activity | [93,94] |
| pH-Thermal treatment | Changes in secondary and tertiary structure and surface hydrophobicity | Enhances interfacial properties and emulsification for Pickering emulsions; enables high internal phase emulsions | [91,94] |
| pH-High pressure homogenization | Changes in secondary and tertiary structure and surface hydrophobicity | Improves stability and bioavailability of vitamin B12 as an encapsulant | [95] |
| pH-Ultrasonic treatment | Changes in secondary structure and surface hydrophobicity | Improves solubility, loading capacity, stability, and bioavailability of vitamin E and quercetin as an encapsulant | [96] |
| OPs-GuHCl | Changes in secondary and tertiary structure and surface hydrophobicity | Improves solubility, emulsification, and foaming; enhances solubility and bioavailability of curcumin | [8] |
| OPs-SDS | Changes in secondary and tertiary structure and surface hydrophobicity | Improves solubility and emulsification; enhances solubility, loading rate, and bioavailability of resveratrol as an encapsulant | [83] |
| OPs-DTT | Changes in secondary and tertiary structure and surface hydrophobicity | Enhances solubility, loading rate, and bioavailability of resveratrol as an encapsulant | [85] |
| OPs-Ethanol | Changes in secondary and tertiary structure and surface hydrophobicity | Causes aggregation and reduces solubility | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhong, M.; Virk, M.S.; Liu, Q.; Liang, Q.; Ma, H.; Ren, X. Extraction Techniques and Modification Methods for Regulating the Structural and Functional Properties of Oleosome-Associated Proteins: A Review. Foods 2025, 14, 3849. https://doi.org/10.3390/foods14223849
Sun Y, Zhong M, Virk MS, Liu Q, Liang Q, Ma H, Ren X. Extraction Techniques and Modification Methods for Regulating the Structural and Functional Properties of Oleosome-Associated Proteins: A Review. Foods. 2025; 14(22):3849. https://doi.org/10.3390/foods14223849
Chicago/Turabian StyleSun, Yufan, Mingming Zhong, Muhammad Safiullah Virk, Qin Liu, Qiufang Liang, Haile Ma, and Xiaofeng Ren. 2025. "Extraction Techniques and Modification Methods for Regulating the Structural and Functional Properties of Oleosome-Associated Proteins: A Review" Foods 14, no. 22: 3849. https://doi.org/10.3390/foods14223849
APA StyleSun, Y., Zhong, M., Virk, M. S., Liu, Q., Liang, Q., Ma, H., & Ren, X. (2025). Extraction Techniques and Modification Methods for Regulating the Structural and Functional Properties of Oleosome-Associated Proteins: A Review. Foods, 14(22), 3849. https://doi.org/10.3390/foods14223849








