Cu2+ Modulates Enzymatic Browning in Potato Tubers Through Amino Acid and Organic Acid Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.1.1. Preparation of Test Materials
2.1.2. Test Treatment
2.1.3. Sampling
2.2. Test Method
2.2.1. Determination of Chlorophyll Content
2.2.2. Browning Index
2.2.3. PPO Activity
2.2.4. Total Phenol Content
2.2.5. Starch Content
2.2.6. Sucrose Content
2.2.7. Soluble Sugar Content
2.2.8. POD Activity
2.2.9. PAL Activity
2.2.10. MDA Content
2.2.11. Organic Acid Content
2.2.12. Chlorogenic Acid Content
2.2.13. Amino Acid Content
2.2.14. Statistical Analysis
3. Results and Analysis
3.1. Effects of Copper on the Contents of Starch, Sucrose, and Soluble Sugar in Tubers
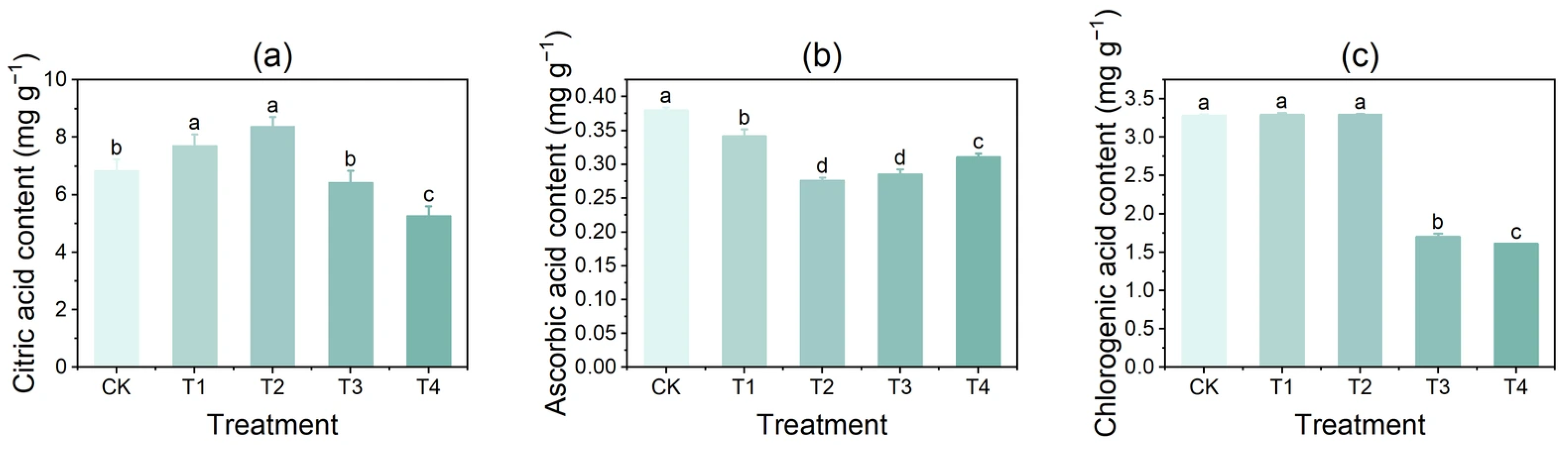
3.2. Effect of Copper on the Content of Citric Acid, Ascorbic Acid, and Chlorogenic Acid in Tubers
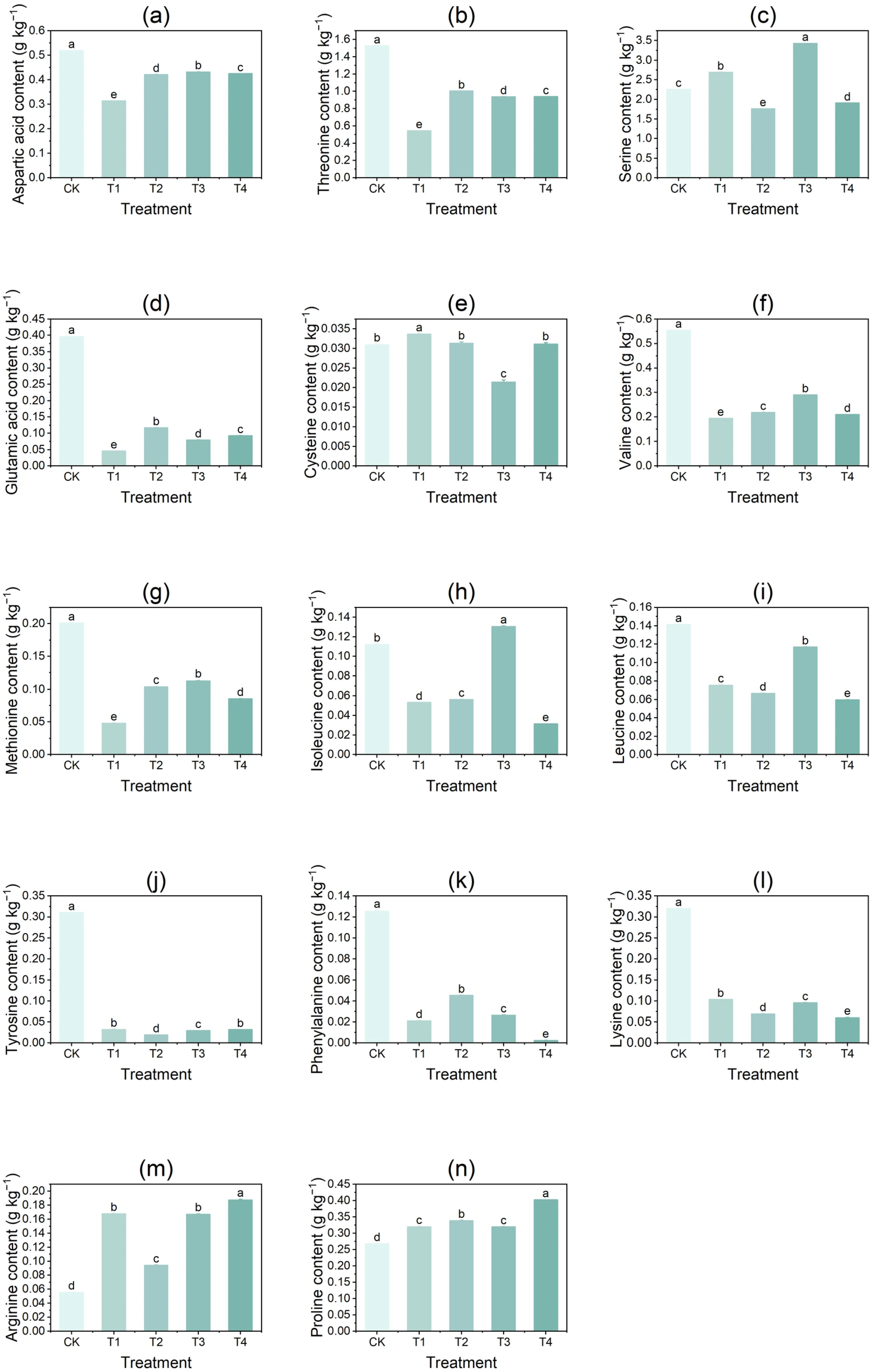
3.3. Effect of Copper on the Content of Free Amino Acids in Tubers
3.4. Effects of Copper on Potato Growth and Yield
3.5. Effect of Copper on PPO Activity and Total Phenol Content in Tubers
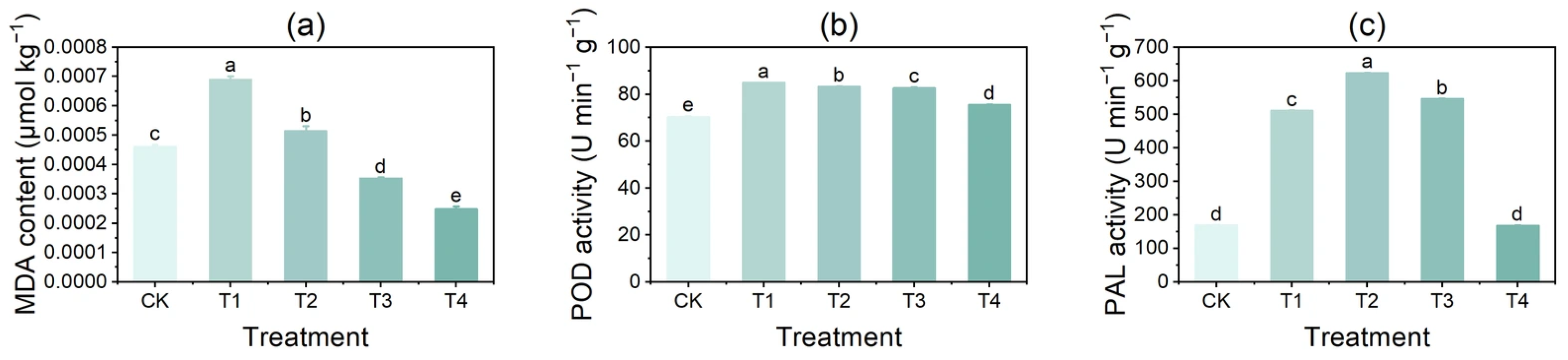
3.6. Effects of Copper on Membrane Lipid Peroxidation and Browning-Related Enzyme Activities in Potato Tubers
3.7. Effects of Copper on Color Parameters and Browning Index of Potato Tubers
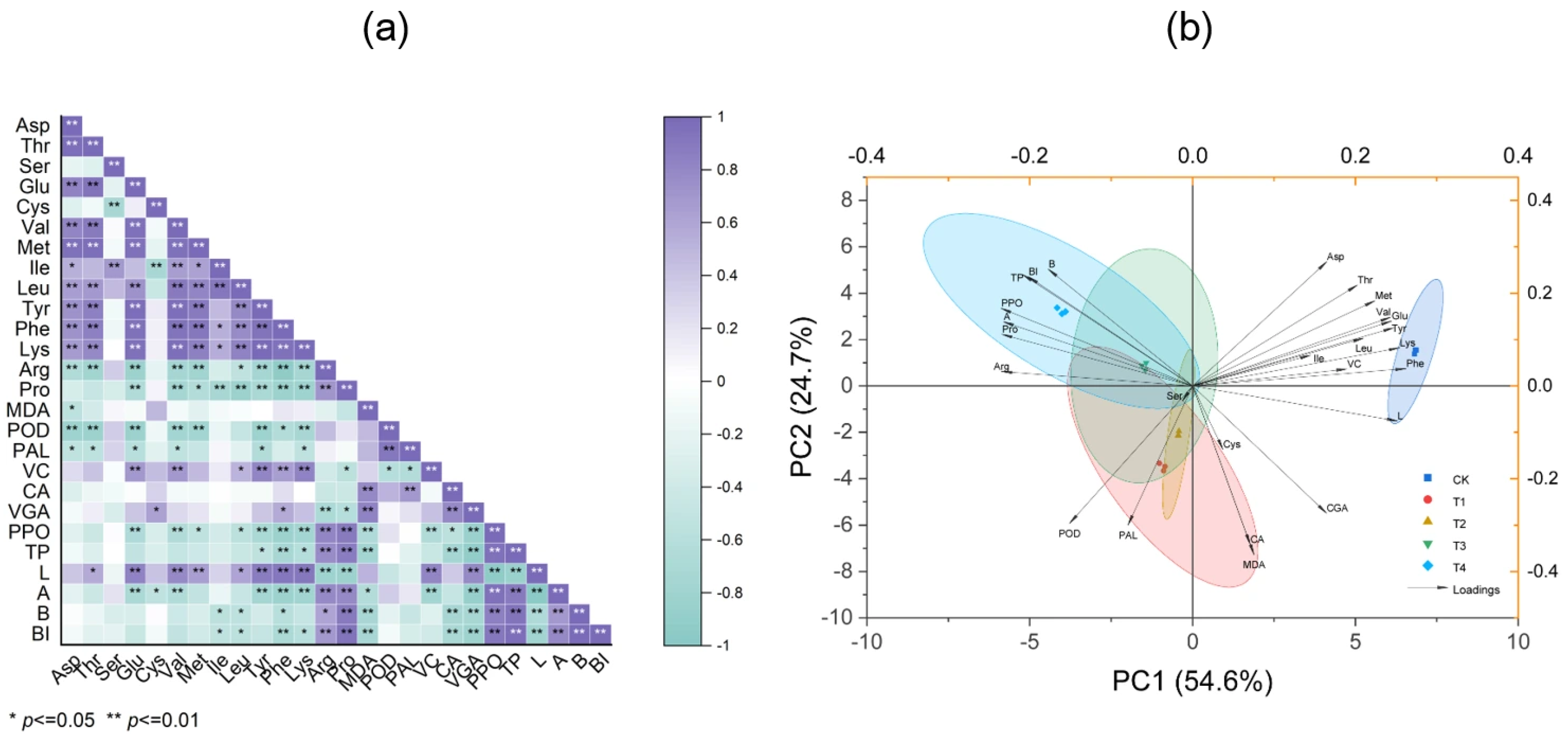
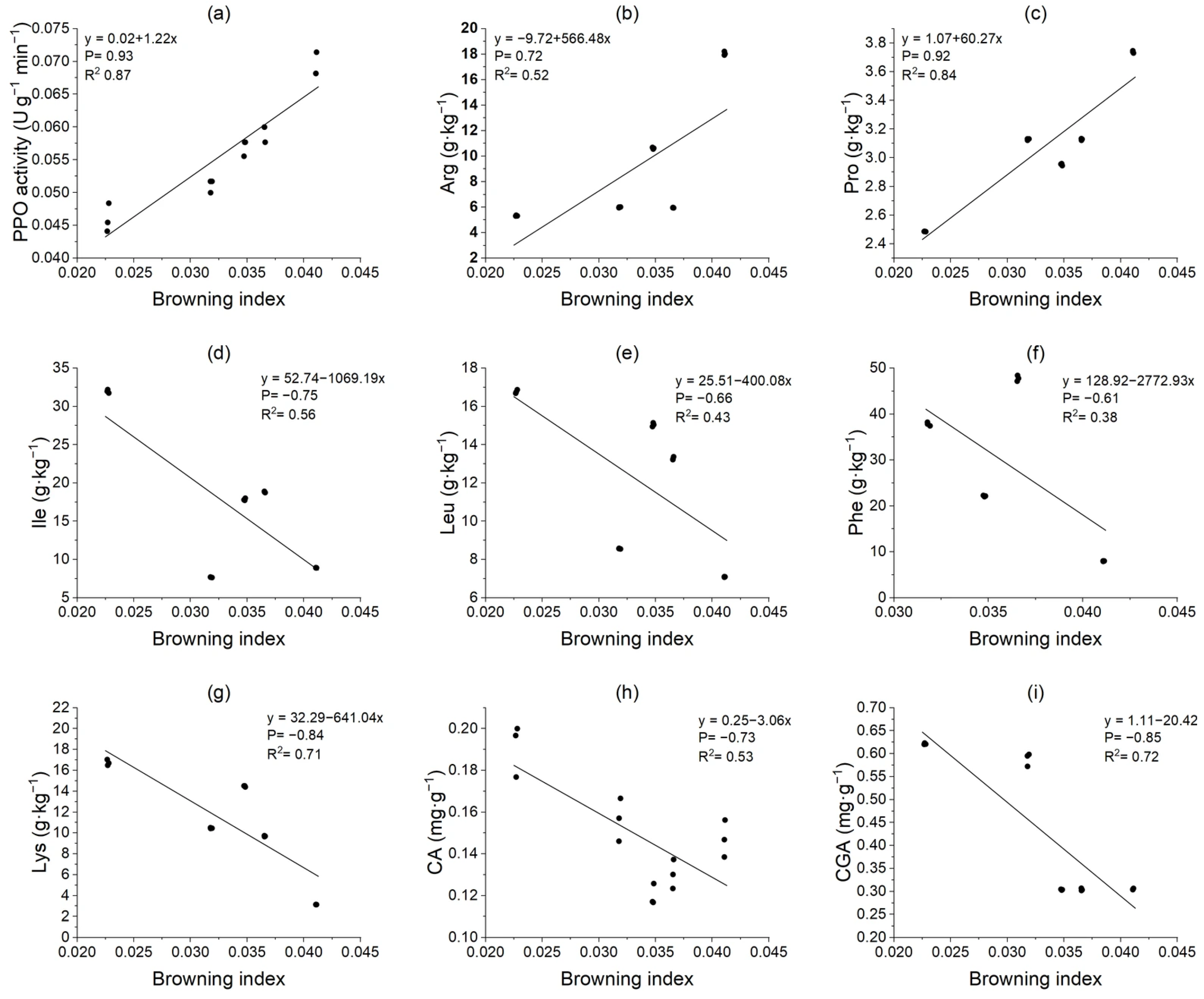
3.8. Correlation Analysis
3.9. Correlation Analysis of Potato Tuber Browning Index and Key Metabolite Activity
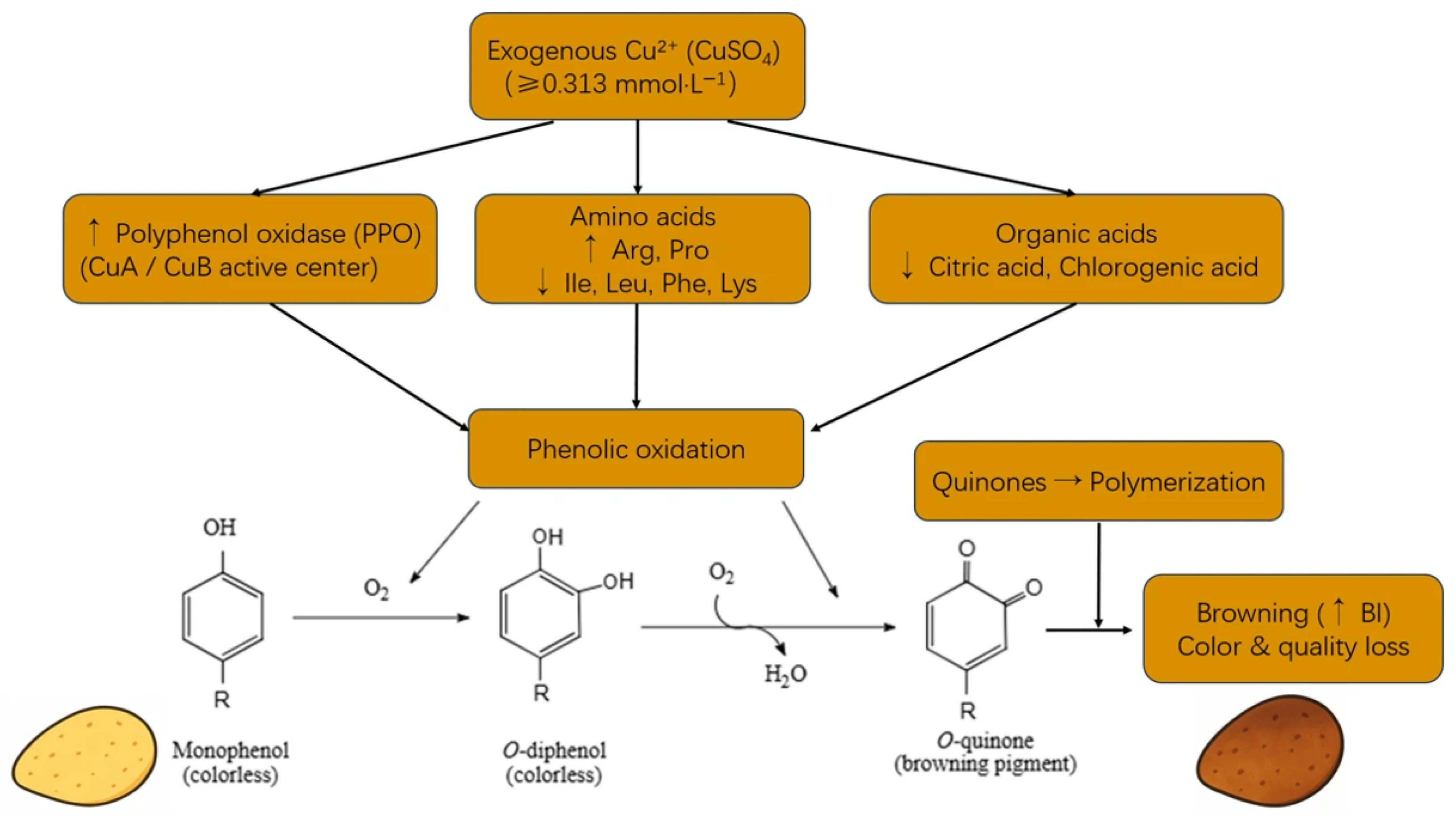
3.10. Copper-Mediated Metabolic Pathways Driving Enzymatic Browning in Potato Tubers
4. Discussion
4.1. Effects of Copper Ions on the Growth and Development of Potato Plants
4.2. Effects of Copper Ions on Potato Tuber Quality
4.3. Effects of Copper Ions on Potato Tuber Browning-Related Enzyme Activity
4.4. Regulation of Organic Acids by Copper Ions and Their Effects on Enzymatic Browning
4.5. Regulation of Amino Acids by Copper Ions and Their Effects on Enzymatic Browning
4.6. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Z.; Fan, D.; Jiang, R.; Abbasi, N.; Song, D.; Zou, G.; Wei, D.; He, P.; He, W. Improving potato productivity and mitigating nitrogen losses using enhanced-efficiency fertilizers: A global meta-analysis. Agric. Ecosyst. Environ. 2023, 348, 108416. [Google Scholar] [CrossRef]
- Sikora, M.; Świeca, M.; Franczyk, M.; Jakubczyk, A.; Bochnak, J.; Złotek, U. Biochemical Properties of Polyphenol Oxidases from Ready-to-Eat Lentil (Lens culinaris Medik.) Sprouts and Factors Affecting Their Activities: A Search for Potent Tools Limiting Enzymatic Browning. Foods 2019, 8, 154. [Google Scholar]
- Zhu, Y.; Du, X.; Zheng, J.; Wang, T.; You, X.; Liu, H.; Liu, X. The effect of ultrasonic on reducing anti-browning minimum effective concentration of purslane extract on fresh-cut potato slices during storage. Food Chem. 2021, 343, 128401. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xie, W.; Sun, Y.; Shi, Q.; Xing, X.; Wang, Q.; Li, Q. Calcium chloride connects potato greening and enzymatic browning through salicylic acid. Food Chem. Mol. Sci. 2024, 9, 100229. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Meng, Z.; Dong, T.; Fan, X.; Wang, Q. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, B.; Xing, S.; Chen, Y.; Liao, Q.; Mo, J.; Chen, Y.; Li, Q.; Sun, H. Medicinal prospects of targeting tyrosinase: A feature review. Curr. Med. Chem. 2023, 30, 2638–2671. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Zeng, L.; Suo, H.; Li, C.; Shan, J.; Liu, J.; Luo, H.; Li, X.; Xiong, X. Characteristics and differences of polyphenol oxidase, peroxidase activities and polyphenol content in different potato (Solanum tuberosum) tubers. Appl. Ecol. Environ. Res. 2020, 18, 8443–8457. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Lu, Y.; Yang, Q.; Li, Y.; Deng, X.; Liu, Y.; Du, X.; Qiao, L.; Zheng, J. Effect of high oxygen pretreatment of whole tuber on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 301, 125287. [Google Scholar] [CrossRef]
- Gao, H.; Zeng, Q.; Ren, Z.; Li, P.; Xu, X. Effect of exogenous γ-aminobutyric acid treatment on the enzymatic browning of fresh-cut potato during storage. J. Food Sci. Technol. 2018, 55, 5035–5044. [Google Scholar] [CrossRef]
- Song, Z.; Qiao, J.; Tian, D.; Dai, M.; Guan, Q.; He, Y.; Liu, P.; Shi, J. Glutamic acid can prevent the browning of fresh-cut potatoes by inhibiting PPO activity and regulating amino acid metabolism. LWT 2023, 180, 114735. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Yan, H.; Malik, A.U.; Dong, T.; Wang, Q. Pre-cut NaCl solution treatment effectively inhibited the browning of fresh-cut potato by influencing polyphenol oxidase activity and several free amino acids contents. Postharvest Biol. Technol. 2021, 178, 111543. [Google Scholar]
- Meng, Z.; Dong, T.; Malik, A.U.; Zhang, S.; Wang, Q. Harvest maturity affects the browning of fresh-cut potatoes by influencing contents of amino acids. Postharvest Biol. Technol. 2021, 173, 111404. [Google Scholar] [CrossRef]
- Xu, X.; Liu, P.; Dong, T.; Zhang, S.; Wang, Q. Short-term warming inhibits polyphenol oxidase activity and influences free amino acid accumulation and de novo synthesis of tyrosine in fresh-cut potato. Postharvest Biol. Technol. 2022, 186, 111830. [Google Scholar] [CrossRef]
- Liu, P.; Xu, N.; Liu, R.; Liu, J.; Peng, Y.; Wang, Q. Exogenous proline treatment inhibiting enzymatic browning of fresh-cut potatoes during cold storage. Postharvest Biol. Technol. 2022, 184, 111754. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, T.; Malik, A.U.; Wang, Q. Exogenous isoleucine can confer browning resistance on fresh-cut potato by suppressing polyphenol oxidase activity and improving the antioxidant capacity. Postharvest Biol. Technol. 2022, 184, 111772. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Q.; Liu, P.; Shi, J.; Wang, Q. Aspartic acid can effectively prevent the enzymatic browning of potato by regulating the generation and transformation of brown product. Postharvest Biol. Technol. 2020, 166, 111209. [Google Scholar] [CrossRef]
- Wen, Y.T.; Liang, Y.Q.; Chai, W.M.; Wei, Q.M.; Yu, Z.Y.; Wang, L.J. Effect of ascorbic acid on tyrosinase and its anti-browning activity in fresh-cut fuji apple. J. Food Biochem. 2021, 45, e13995. [Google Scholar]
- Tsouvaltzis, P.; Brecht, J.K. Inhibition of enzymatic browning of fresh-cut potato by immersion in citric acid is not solely due to pH reduction of the solution. J. Food Process. Preserv. 2017, 41, e12829. [Google Scholar]
- You, W.; Wang, C.; Zhang, J.; Ru, X.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. Exogenous chlorogenic acid inhibits quality deterioration in fresh-cut potato slices. Food Chem. 2024, 446, 138866. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Wang, G.; Tang, J.; Yao, C.; Li, P.; Song, Q.; Wang, C. Inhibitory effect of chlorogenic acid on polyphenol oxidase and browning of fresh-cut potatoes. Postharvest Biol. Technol. 2020, 168, 111282. [Google Scholar] [CrossRef]
- Sendovski, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Du, C.; Yu, H.; Zhao, X.; Zhang, X.; Wang, X. Purification and characterization of polyphenol oxidase (PPO) from water yam (Dioscorea alata). CyTA-J. Food 2019, 17, 676–684. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.; Saleh, M.A. The role of various amino acids in enzymatic browning process in potato tubers, and identifying the browning products. Food Chem. 2016, 192, 879–885. [Google Scholar] [CrossRef]
- Gao, L.Y.; Tang, M.Q.; Huang, J.H.; Xu, Z.R. Research progress of copper chaperone in plant. Guizhou Agric. Sci. 2017, 45, 9–15. [Google Scholar]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and molecular mechanisms of plant responses to copper stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Obiero, C.O.; Milroy, S.P.; Bell, R.W. Photosynthetic and respiratory response of potato leaves of different ages during and after an episode of high temperature. J. Agron. Crop Sci. 2020, 206, 352–362. [Google Scholar] [CrossRef]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of chlorophyll degradation and color loss in heated broccoli juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef]
- Fang, T.; Yao, J.; Duan, Y.; Zhong, Y.; Zhao, Y.; Lin, Q. Phytic acid treatment inhibits browning and lignification to promote the quality of fresh-cut apples during storage. Foods 2022, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pina, S.; Duch-Calabuig, A.; David, E.R.D.A.; Ros-Lis, J.V.; Amorós, P.; Argüelles, Á.; Andrés, A. Bioactive compounds and enzymatic browning inhibition in cloudy apple juice by a new magnetic UVM-7-SH mesoporous material. Food Res. Int. 2022, 162, 112073. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, F.; Cai, W.; Peng, B.; Ning, M.; Shan, C.; Yang, X. Chitosan treatment reduces softening and chilling injury in cold-stored Hami melon by regulating starch and sucrose metabolism. Front. Plant Sci. 2022, 13, 1096017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, J.; Mao, L.; Li, Q.; Wang, L.; Lin, Q. Low temperature reduces potato wound formation by inhibiting phenylpropanoid metabolism and fatty acid biosynthesis. Front. Plant Sci. 2023, 13, 1109953. [Google Scholar] [CrossRef]
- He, Y.; Chen, R.; Yang, Y.; Liang, G.; Zhang, H.; Deng, X.; Xi, R. Sugar metabolism and transcriptome analysis reveal key sugar transporters during Camellia oleifera fruit development. Int. J. Mol. Sci. 2022, 23, 822. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, L.; Pang, L.; Chen, X.; Jia, X.; Li, X. Ultrasound treatment inhibits browning and improves antioxidant capacity of fresh-cut sweet potato during cold storage. RSC Adv. 2020, 10, 9193–9202. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Wang, Q.; Dong, T. Diacetyl inhibits the browning of fresh-cut stem lettuce by regulating the metabolism of phenylpropane and antioxidant ability. Foods 2023, 12, 740. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, Y.; Li, A.; Peng, M.; Wang, T.; Kou, R.; Kang, J.; Liu, X. The Super Anti-Browning Effect of High-Oxygen Pretreatment Combined with Cod Peptides on Fresh-Cut Potatoes During Storage. Foods 2025, 14, 1564. [Google Scholar] [CrossRef]
- Ikegaya, A.; Ohba, S.; Nakajima, T.; Toyoizumi, T.; Ito, S.; Arai, E. Practical long-term storage of strawberries in refrigerated containers at ice temperature. Food Sci. Nutr. 2020, 8, 5138–5148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xu, D.; Liu, C.; Chen, C.; Tian, M.; Jiang, A. Ascorbic acid treatment inhibits wound healing of fresh-cut potato strips by controlling phenylpropanoid metabolism. Postharvest Biol. Technol. 2021, 181, 111644. [Google Scholar] [CrossRef]
- Dong, T.; Cao, Y.; Li, G.; Zhu, Z.; Zhang, S.; Jiang, C.Z.; Wang, Q. A novel aspartic protease inhibitor inhibits the enzymatic browning of potatoes. Postharvest Biol. Technol. 2021, 172, 111353. [Google Scholar] [CrossRef]
- Wang, Z.C.; Chen, M.X.; Yang, Y.X.; Fang, X.; Liu, Z.J.; Wang, L.Y.; Ge, M.Q.; Zhang, C.; Fang, J.G.; Shangguan, L.F. Effects of copper stress on plant growth and advances in the mechanisms of plant tolerance research. J. Plant Nutr. Fertil. 2021, 27, 1849–1863. [Google Scholar]
- Ke, S.S. Effects of different copper levels on photosynthetic parameters and active oxygen metabolism of Amaranthus tricolor seedlings. J. Plant Nutr. Fertil. 2008, 41, 1317–1325. [Google Scholar]
- Mediouni, C.; Benzarti, O.; Tray, B.; Ghorbel, M.H.; Jemal, F. Cadmium and Copper Toxicity for Tomato Seedlings. Agron. Sustain. Dev. 2006, 26, 227–232. [Google Scholar] [CrossRef]
- Fu, H.; Shi, Q.Q.; Zhong, M.; Liu, Y. Effect of copper sulphate stress during germination on the growth of mung bean and the intracellular density change response. Plant Physiol. Commun. 2016, 52, 1891–1900. [Google Scholar]
- Li, Z.W.; Chen, S.M.; Wang, F.Z.; Zhang, H.Y.; Zhang, L.; Xu, Z.C. Mechanism research advances in plant abiotic stress resistance regulated by hydrogen sulfide. J. Agric. Sci. Technol. 2020, 22, 24–32. [Google Scholar]
- Du, J.; Huang, Z.; Li, Y.; Ren, X.; Zhou, C.; Liu, R.; Zhang, P.; Lei, G.; Lyu, J.; Li, J. Copper exerts cytotoxicity through inhibition of iron-sulfur cluster biogenesis on ISCA1/ISCA2/ISCU assembly proteins. Free Radic. Biol. Med. 2023, 204, 359–373. [Google Scholar] [CrossRef]
- Hu, W.; Song, X.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J. Changes in electron transport, superoxide dismutase and ascorbate peroxidase isoenzymes in chloroplasts and mitochondria of cucumber leaves as influenced by chilling. Photosynthetica 2008, 46, 581–588. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, H.; Duan, D.; Li, H.; Lei, T.; Wang, M.; Zhao, H.; Zhao, Y. The influence of duckweed species diversity on ecophysiological tolerance to copper exposure. Aquat. Toxicol. 2015, 164, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.F.d.; Alves, P.B.; Ferreira, T.C.; Santos, B.S.D.; Cozin, B.B.; Souza, R.P.D.; Camargos, L.S. Effects of iron and copper on emergence and physiology of Canavalia ensiformis (L.) DC. Rev. Caatinga 2024, 38, e12686. [Google Scholar] [CrossRef]
- Wenbin, Y.; Liang, F.; Hanbo, Y. Effect of Pb2+ on Germination and Seedling Growth and Physiological Characters of Codonopsis pilosula of Gansu Chinese Herbal Medicine. Acta Agric. Boreali-occident. Sin. 2012, 21, 142–148. [Google Scholar]
- Fishbein, L. Trace and ultra trace elements in nutrition: An overview. Toxicol. Environ. Chem. 1987, 14, 73–99. [Google Scholar] [CrossRef]
- Xu, S.; Song, Z.; Wang, Q.G. Screening of Polyphenol Oxidase Interacting Proteins in Potato Tuber. Food Sci. 2023, 48, 8–16. [Google Scholar]
- Wang, L.P.; Yin, J.; Yue, P.X.; Chen, X.; Li, H.Y.; Li, X.H. Effect of Different Metal Ions on Polyphenol Oxidase from Eggplant Fruit. Food Sci. 2011, 32, 244–247. [Google Scholar]
- Li, F.; Wu, X.; Liu, H.; Liu, M.; Yue, Z.; Wu, Z.; Liu, L.; Li, F. Copper depletion strongly enhances ferroptosis via mitochondrial perturbation and reduction in antioxidative mechanisms. Antioxidants 2022, 11, 2084. [Google Scholar] [CrossRef]
- Buapet, P.; Mohammadi, N.S.; Pernice, M.; Kumar, M.; Kuzhiumparambil, U.; Ralph, P.J. Excess copper promotes photoinhibition and modulates the expression of antioxidant-related genes in Zostera muelleri. Aquat. Toxicol. 2019, 207, 91–100. [Google Scholar] [CrossRef]
- Meacham-Hensold, K.; Cavanagh, A.P.; Sorensen, P.; South, P.F.; Fowler, J.; Boyd, R.; Jeong, J.; Burgess, S.; Stutz, S.; Dilger, R.N. Shortcutting photorespiration protects potato photosynthesis and tuber yield against heatwave stress. Glob. Change Biol. 2024, 30, e17595. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Li, X.; Ren, W.B.; Deng, Y.; Wang, Y.M.; Liu, Y.; Si, H.J.; Zhang, L.B. Effects of High-Energy Carbon Ion Beam and X-Ray Irradiation on Biological Effects of Rice Seedlings. J. Nucl. Agric. Sci. 2022, 36, 1897–1906. [Google Scholar]
- Cantos, E.; Tudela, J.A.; Gil, M.I.; Espín, J.C. Phenolic compounds and related enzymes are not rate-limiting in browning development of fresh-cut potatoes. J. Agric. Food Chem. 2002, 50, 3015–3023. [Google Scholar] [CrossRef]
- Chen, W.N.; Li, X.; Wang, W.Y.; Bi, J.F. Research Progress on the Mechanism and Regulation of Enzymatic Browning in Peach Fruit. Food Sci. 2024, 45, 290–298. [Google Scholar]
- Wang, T.Q.; Xu, X.Q.; Xu, M.F. The Effects of Different Concentrations of Gibberellins on Cut Leaves of Asparagus cochinchinensis. J. Trop. Biol. 2016, 7, 64–69. [Google Scholar]
- Li, X.; Luo, S.; Shen, J.; Li, C.; Kadeer, W.; Chen, L.; Li, X.; Jiang, Y.; Tang, Y. Synergistic Anti-Browning Effects of Short-Term High Oxygen Pre-Stimulation and Supercooled Storage on Fresh-Cut Potatoes by Regulating Polyphenol Biosynthesis and Membrane Lipid Oxidation. Postharvest Biol. Technol. 2025, 219, 113257. [Google Scholar] [CrossRef]
- Ren, L.P.; Song, W.J.; Li, Y.; Sun, T.X.; Djimeli, J.R.S.; Yang, X.S.; Zhang, W.Y. Cloning and expression analysis of Ipomoea batatas (L.) Lam. gene IbPAL. Plant Sci. J. 2020, 38, 360–368. [Google Scholar]
- Sun, J.; Yan, X.W.; Le, M.W.; Rao, Y.L.; Yan, T.X.; Ye, Y.Y.; Zhou, H.Y. Physiological response mechanism of drought stress in different drought-tolerance genotypes of sesame during flowering period. Sci. Agric. Sin. 2019, 52, 1215–1226. [Google Scholar]
- Zhu, L.; Hu, W.; Murtaza, A.; Iqbal, A.; Li, J.; Zhang, J.; Li, J.; Kong, M.; Xu, X.; Pan, S. Eugenol treatment delays the flesh browning of fresh-cut water chestnut (Eleocharis tuberosa) through regulating the metabolisms of phenolics and reactive oxygen-species. Food Chem. X 2022, 14, 100307. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, Cross-linking and Reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.R.; He, X.X.; Hu, J. Research on the browning inhibitors in the processing of potato. China Condiment 2017, 42, 57–60. [Google Scholar]
- Yan, Z.; Li, X.; Dong, H.; Li, L.L.; Li, Y.X.; Yang, W.T.; Chen, G.G. Optimization of the production process and quality evaluation of mulberry-purple potato compound freeze-dried fruit blocks. Trans. Chin. Soc. Agric. Eng. 2024, 40, 276–285. [Google Scholar]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef]
- Sikora, M.; Świeca, M. Effect of ascorbic acid postharvest treatment on enzymatic browning, phenolics and antioxidant capacity of stored mung bean sprouts. Food Chem. 2018, 239, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Ottaway, P.B. Stability of vitamins in food. In The Technology of Vitamins in Food; Springer: Boston, MA, USA, 1993; pp. 90–113. [Google Scholar]
- Li, J.; Xu, Y.C.; Huang, M.; Yang, Y.; Cao, J.K.; Jiang, W.B. Characterization of Polyphenol Oxidase from Yali Pear. Food Sci. 2013, 34, 154. [Google Scholar]
- Furrer, A.; Cladis, D.P.; Kurilich, A.; Manoharan, R.; Ferruzzi, M.G. Changes in phenolic content of commercial potato varieties through industrial processing and fresh preparation. Food Chem. 2017, 218, 47–55. [Google Scholar] [CrossRef]
- Peng, Y.P.; Zhang, J.Y.; Su, Y.Y.; Sun, L.Q.; Liu, X.H.; Tang, X.Q.; Liao, Z.M.; Wang, K.C. Effects of spraying copper and borax fertilizer on growth physiology of Lonicera japonica Thunb. and quality of Lonicerae japonicae flos. J. Nanjing Agric. Univ. 2023, 46, 33–41. [Google Scholar]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- Morgil, H. A Potential Common Pyrroline-5-Carboxylate Synthetase (P5CS) Gene As a Stress Marker for Qrt-Pcr in Legume Plants. OKÜ Fen Bil. Ens. Dergisi 2025, 8, 739–753. [Google Scholar] [CrossRef]
- Adams, J.; Brown, H. Discoloration in raw and processed fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2007, 47, 319–333. [Google Scholar]
- Han, Z.; Dou, H.T.; Ma, Y.Q.; Zhu, P.P.; Li, S.; Gong, L. Research progress in evaluation criteria and research methodology of orange juice browning. Sci. Technol. Food Ind. 2015, 36, 360–365. [Google Scholar]
- Erihemu; Zhang, Q.; Cui, N.; Wu, J.H.; Gao, G. Inhibition of PPO Activity in Fresh-Cut Potato by L-cysteine and Vacuum Impregnation. China Food Addit. 2019, 30, 47–55. [Google Scholar]


| Treatment | CuSO4 (mmol·L−1) |
|---|---|
| CK | 0 |
| T1 | 0.078 |
| T2 | 0.157 |
| T3 | 0.235 |
| T4 | 0.313 |
| Variety | Treatment | Plant Height/cm | Stem Thick/mm | Number of Tubers per Plant/n | Yield per Plant/g | Chl a + b (mg·g−1) |
|---|---|---|---|---|---|---|
| Xisen No. 6 | CK | 32.93 ± 0.71 d | 2.20 ± 0.10 d | 1.40 ± 0.10 b | 10.10 ± 0.07 e | 3.49 ± 0.09 a |
| T1 | 34.37 ± 0.67 bc | 3.70 ± 0.10 b | 1.57 ± 0.15 ab | 13.47 ± 0.01 c | 3.51 ± 0.18 a | |
| T2 | 36.20 ± 0.92 a | 3.97 ± 0.12 a | 2.41 ± 0.11 ab | 21.93 ± 0.02 a | 3.63 ± 0.13 a | |
| T3 | 35.43 ± 0.78 ab | 3.80 ± 0.10 ab | 2.50 ± 0.10 a | 12.53 ± 0.01 d | 2.88 ± 0.16 b | |
| T4 | 33.53 ± 0.21 cd | 3.37 ± 0.12 c | 1.57 ± 0.15 ab | 16.33 ± 0.07 b | 2.54 ± 0.07 c |
| Treatment | L* | a* | b* | BI |
|---|---|---|---|---|
| CK | 66.63 ± 0.09 a | 1.45 ± 0.02 d | 13.97 ± 0.02 e | 24.34 ± 0.02 e |
| T1 | 63.75 ± 0.02 b | 2.05 ± 0.03 b | 14.52 ± 0.06 d | 27.36 ± 0.03 d |
| T2 | 62.91 ± 0.04 c | 2.35 ± 0.02 c | 14.83 ± 0.04 c | 28.75 ± 0.05 c |
| T3 | 61.46 ± 0.03 d | 3.05 ± 0.03 a | 15.34 ± 0.04 b | 31.43 ± 0.08 b |
| T4 | 61.05 ± 0.02 e | 3.07 ± 0.02 a | 20.83 ± 0.05 a | 44.02 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Li, J.; Guo, R.; Cheng, L.; Sa, G.; Yuan, J.; Yang, H.; Liu, J.; Yu, B. Cu2+ Modulates Enzymatic Browning in Potato Tubers Through Amino Acid and Organic Acid Metabolism. Foods 2025, 14, 3816. https://doi.org/10.3390/foods14223816
Feng S, Li J, Guo R, Cheng L, Sa G, Yuan J, Yang H, Liu J, Yu B. Cu2+ Modulates Enzymatic Browning in Potato Tubers Through Amino Acid and Organic Acid Metabolism. Foods. 2025; 14(22):3816. https://doi.org/10.3390/foods14223816
Chicago/Turabian StyleFeng, Shulei, Jinli Li, Rong Guo, Lixiang Cheng, Gang Sa, Jianlong Yuan, Hongyu Yang, Juan Liu, and Bin Yu. 2025. "Cu2+ Modulates Enzymatic Browning in Potato Tubers Through Amino Acid and Organic Acid Metabolism" Foods 14, no. 22: 3816. https://doi.org/10.3390/foods14223816
APA StyleFeng, S., Li, J., Guo, R., Cheng, L., Sa, G., Yuan, J., Yang, H., Liu, J., & Yu, B. (2025). Cu2+ Modulates Enzymatic Browning in Potato Tubers Through Amino Acid and Organic Acid Metabolism. Foods, 14(22), 3816. https://doi.org/10.3390/foods14223816






