Revealing the Impact of Pasteurization and Derivatization Chemistry on the Fatty Acid Profile of Dairy Cream: A Comparative Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Cream Material
2.2. Extraction, Purification and Identification of Selected Fatty Acids
2.2.1. Derivatization in Acid Medium
2.2.2. Derivatization in Alkaline Medium
2.3. Conditions for Chromatographic Analysis
2.4. Statistical Analysis

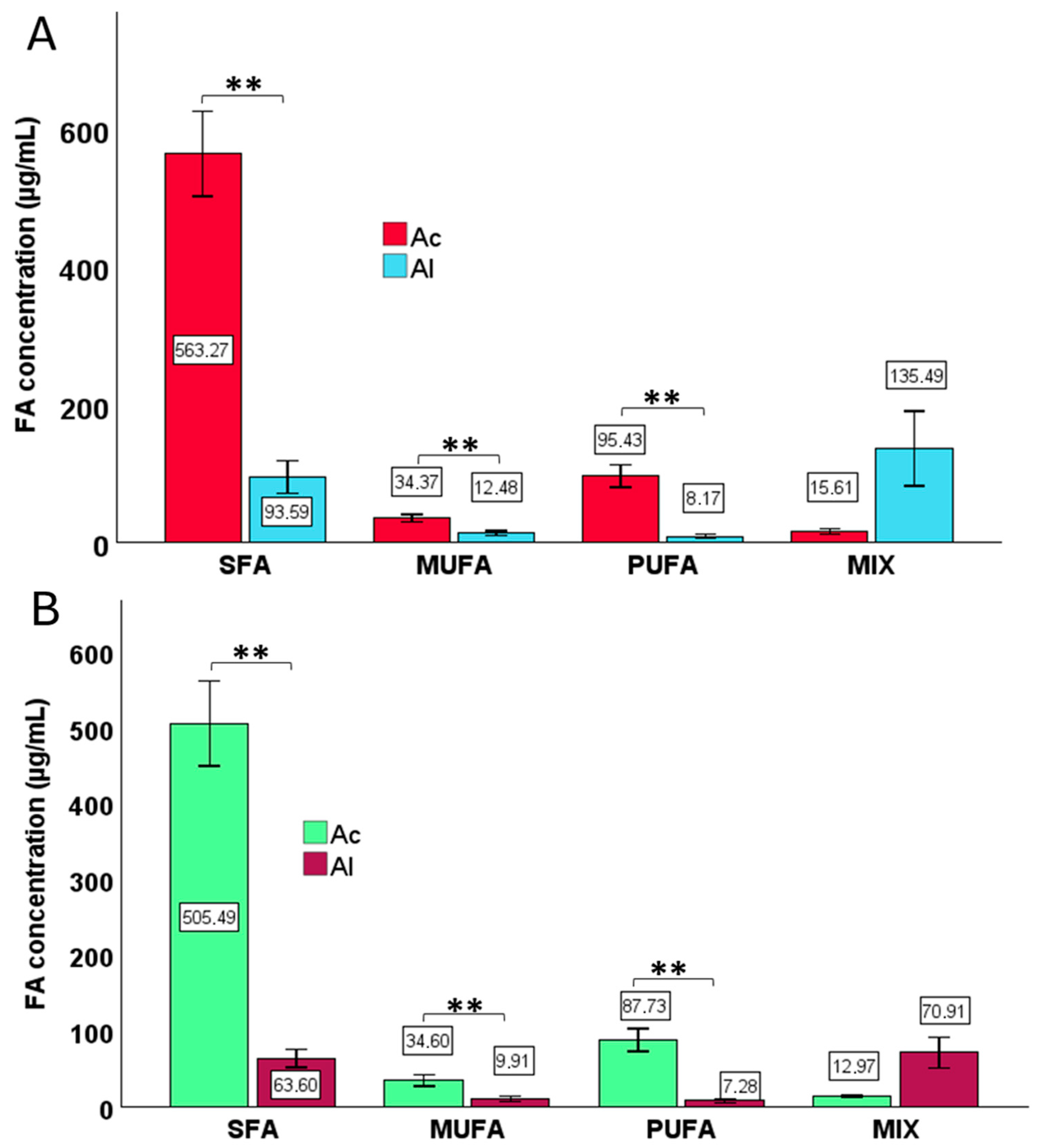
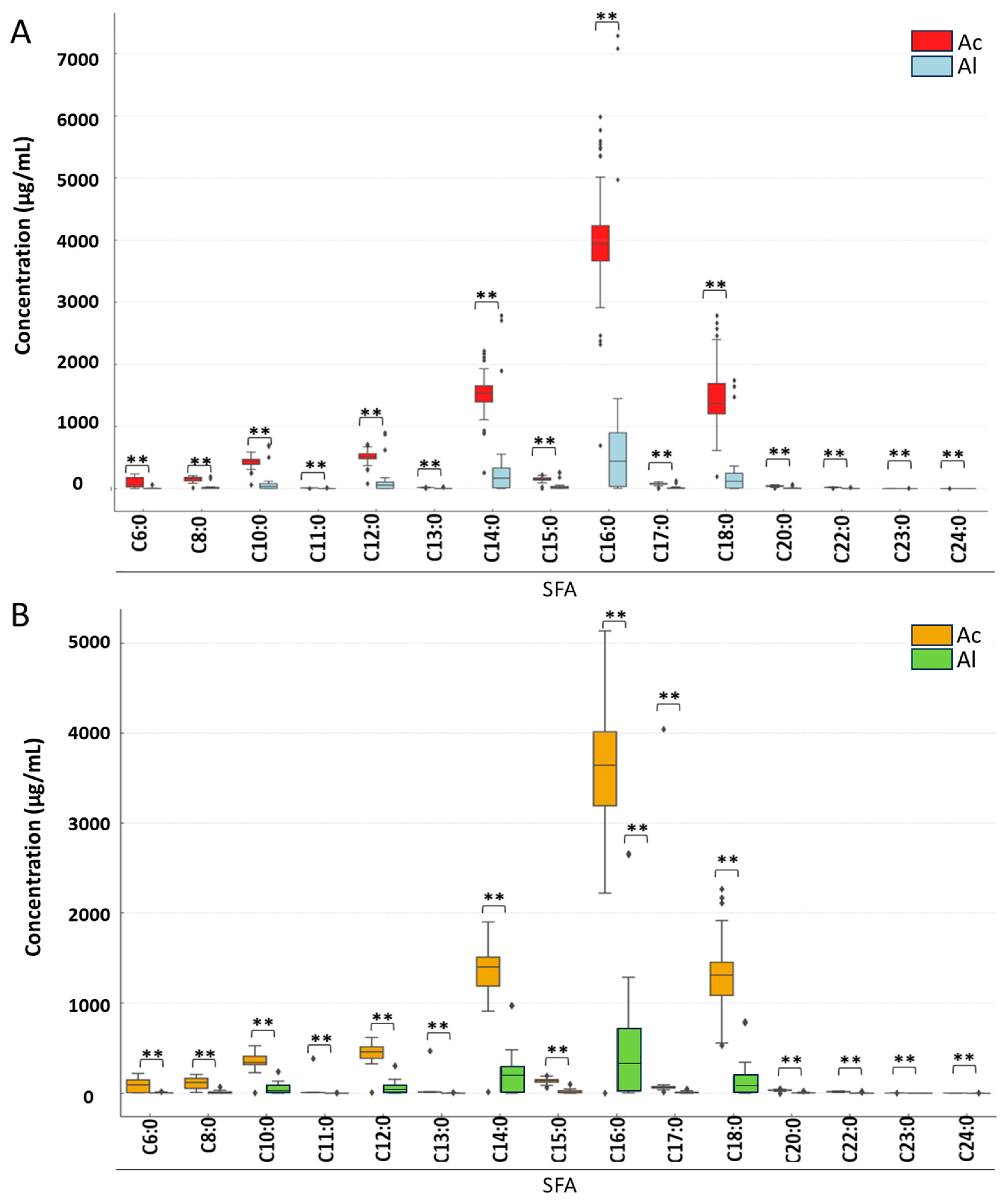

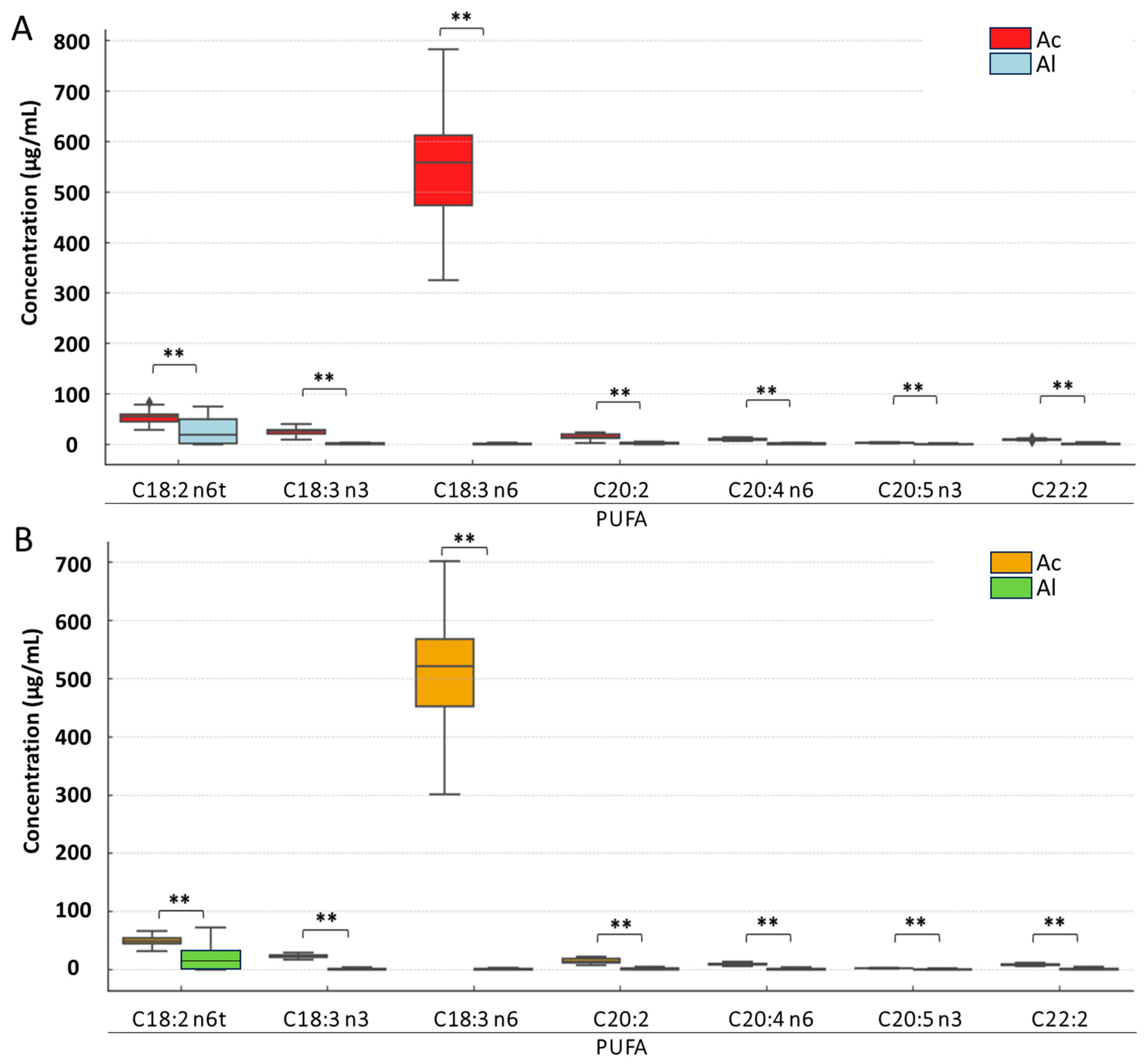
3. Results
3.1. The Effect of Derivatization and Pasteurization Methods on the Overall Concentration of Fatty Acids, Along with the Division into Specific Lipid Classes
3.2. Analysis of Detailed Changes in Individual Fatty Acid Classes Under Derivatization and Pasteurization
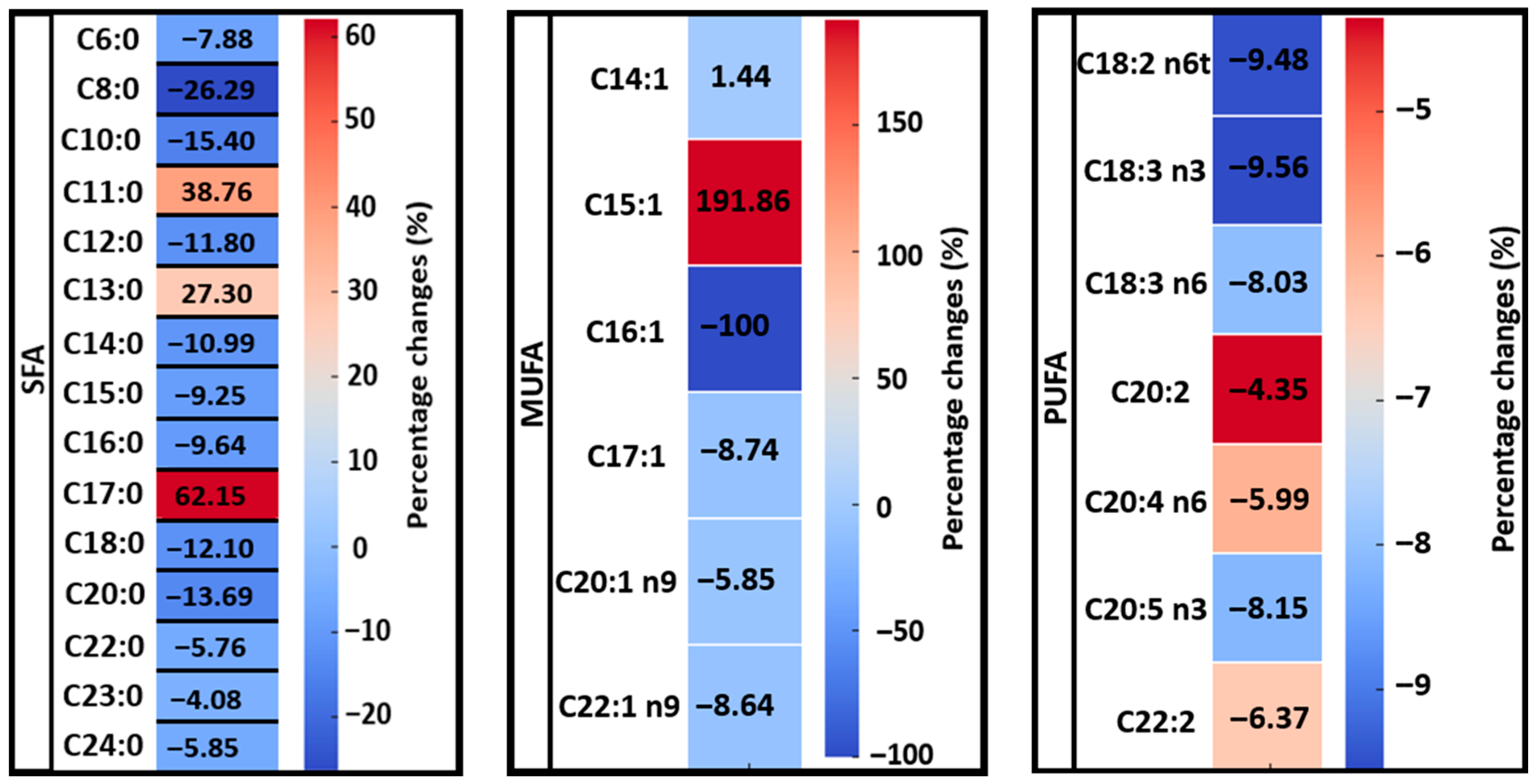
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAs | Fatty Acids |
| FAME | Fatty Acid Methyl Esters |
| GC-FID | Gas Chromatography-Flame Ionization Detector |
| SFA | Saturated Fatty Acids |
| MUFA | Monounsaturated Fatty Acids |
| PUFA | Polyunsaturated Fatty Acids |
References
- Douphrate, D.I.; Hagevoort, G.R.; Nonnenmann, M.W.; Lunner Kolstrup, C.; Reynolds, S.J.; Jakob, M.; Kinsel, M. The dairy industry: A brief description of production practices, trends, and farm characteristics around the world. J. Agromedicine 2013, 18, 187–197. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M. The Technology of Dairy Products, 2nd ed.; Early, R., Ed.; Springer: New York, NY, USA, 1993; Volume 19. [Google Scholar]
- Deosarkar, S.S.; Khedkar, C.D.; Kalyankar, S.D.; Sarode, A.R. Cream: Types of cream. In Encyclopedia of Food and Health; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 331–337. ISBN 9780123849533. [Google Scholar]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, metabolism and functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability-A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Sapozhnikova, Y.; Mol, H.G. Current issues involving screening and identification of chemical contaminants in foods by mass spectrometry. TRAC 2015, 69, 62–75. [Google Scholar] [CrossRef]
- Górska, A. Special Issue on Application of Instrumental Methods for Food and Food By-Products Analysis. Appl. Sci. 2022, 12, 3888. [Google Scholar] [CrossRef]
- Di Paolo, M.; Pelizzola, V.; De Luca, L.; Casalino, L.; Polizzi, G.; Povolo, M.; Marrone, R. Effect of Technological Process and Temperature on Phospholipids in Buffalo Milk, Whey and Buttermilk. Foods 2025, 14, 2756. [Google Scholar] [CrossRef]
- Xu, M.L.; Gao, Y.; Wang, X.; Han, X.X.; Zhao, B. Comprehensive strategy for sample preparation for the analysis of food contaminants and residues by GC–MS/MS: A review of recent research trends. Foods 2021, 10, 2473. [Google Scholar] [CrossRef]
- Nguyen, B.; Thurbide, K.B. Flameless Operating Mode for Improved Multiple Flame Photometric Detection in Gas Chromatography. Chromatographia 2024, 87, 363–373. [Google Scholar] [CrossRef]
- Engewald, W.; Dettmer-Wilde, K.; Rotzsche, H. Columns and stationary phases. In Practical Gas Chromatography: A Comprehensive Reference; Springer: Berlin/Heidelberg, Germany, 2014; pp. 59–116. [Google Scholar]
- Kloos, D.; Lingeman, H.; Mayboroda, O.A.; Deelder, A.M.; Niessen, W.M.A.; Giera, M. Analysis of biologically-active, endogenous carboxylic acids based on chromatography-mass spectrometry. TrAC Trends Anal. Chem. 2014, 61, 17–28. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Reglero, G.; Ibañez, E. Recent trends in the advanced analysis of bioactive fatty acids. J. Pharm. Biomed. Anal. 2010, 51, 305–326. [Google Scholar] [CrossRef]
- Liu, K.S. Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. JAOCS 1994, 71, 1179–1187. [Google Scholar] [CrossRef]
- Amores, G.; Virto, M. Total and free fatty acids analysis in milk and dairy fat. Separations 2019, 6, 14. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.Z.; Qing, J.P.; Li, H.J.; Xiao, W. Classification and quantification analysis of peach kernel from different origins with near-infrared diffuse reflection spectroscopy. Pharmacogn. Mag. 2014, 10, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Handbook of Chromatography; Mangold, H.K., Ed.; CRC Press: Boca Raton, FL, USA, 1984; Volume 1, pp. 33–46. [Google Scholar]
- Martínez, B.; Miranda, J.M.; Franco, C.M.; Cepeda, A.; Rodríguez, J.L. Development of a simple method for the quantitative determination of fatty acids in milk with special emphasis on long-chain fatty acids. CYTA-J. Food 2012, 10, 27–35. [Google Scholar] [CrossRef]
- Wells, R.J. Recent advances in non-silylation derivatization techniques for gas chromatography. J. Chromatogr. A 1999, 843, 1–18. [Google Scholar] [CrossRef]
- Orata, F. Derivatization reactions and reagents for gas chromatography analysis. Adv. Gas Chromatogr.-Prog. Agric. Biomed. Ind. Appl. 2012, 91, 83–108. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Nouri, N.; Khorram, P. Derivatization and microextraction methods for determination of organic compounds by gas chromatography. TRAC 2014, 55, 14–23. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Combined extraction and microextraction techniques: Recent trends and future perspectives. TRAC 2018, 103, 74–86. [Google Scholar] [CrossRef]
- Machado, F.; Duarte, R.V.; Pinto, C.A.; Casal, S.; Lopes-da-Silva, J.A.; Saraiva, J.A. High Pressure and Pasteurization Effects on Dairy Cream. Foods 2023, 12, 3640. [Google Scholar] [CrossRef]
- Decimo, M.; Ordónez, J.A.; Brasca, M.; Cabeza, M.C. Fatty acids released from cream by psychrotrophs isolated from bovine raw milk. Int. J. Dairy Technol. 2006, 70, 339–344. [Google Scholar] [CrossRef]
- Rutkowska, J.; Bialek, M.; Adamska, A.; Zbikowska, A. Differentiation of geographical origin of cream products in Poland according to their fatty acid profile. Food Chem. 2015, 178, 26–31. [Google Scholar] [CrossRef]
- Ostermann, A.I.; Müller, M.; Willenberg, I.; Schebb, N.H. Determining the fatty acid composition in plasma and tissues as fatty acid methyl esters using gas chromatography—A comparison of different derivatization and extraction procedures. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.K.; Fellner, V.; Dugan, M.E.; Sauer, F.D.; Mossoba, M.M.; Yurawecz, M.P. Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids 1997, 32, 1219–1228. [Google Scholar] [CrossRef]
- Liu, Z.; Ezernieks, V.; Rochfort, S.; Cocks, B. Comparison of methylation methods for fatty acid analysis of milk fat. Food Chem. 2018, 261, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Comparison and validation of 2 analytical methods for the determination of free fatty acids in dairy products by gas chromatography with flame ionization detection. J. Dairy Sci. 2016, 99, 5047–5063. [Google Scholar] [CrossRef] [PubMed]
- Antolín, E.M.; Delange, D.M.; Canavaciolo, V.G. Evaluation of five methods for derivatization and GC determination of a mixture of very long chain fatty acids (C24:0–C36:0). J. Pharm. Biomed. Anal. 2008, 46, 194–199. [Google Scholar] [CrossRef]
- Salimon, J.; Omar, T.A.; Salih, N. An accurate and reliable method for identification and quantification of fatty acids and trans fatty acids in food fats samples using gas chromatography. Arab. J. Chem. 2017, 10, 1875–1882. [Google Scholar] [CrossRef]
- Ferrara, D.; Beccaria, M.; Cordero, C.E.; Purcaro, G. Comprehensive comparison of fatty acid methyl ester profile in different food matrices using microwave-assisted extraction and derivatization methods and comprehensive two-dimensional gas chromatography coupled with flame ionization detection. Adv. Sample Prep. 2024, 11, 100124. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Wang, Y. Production of high-concentration n-caproic acid from lactate through fermentation using a newly isolated Ruminococcaceae bacterium CPB6. Biotechnol. Biofuels 2017, 10, 102. [Google Scholar] [CrossRef]
- Siqi, Y.; Ziyang, J.; Ayaz, A.; Chengjun, W.; Jun, L. Caproic Acid-Producing Bacteria in Chinese Baijiu Brewing. Front. Microbiol. 2022, 13, 883142. [Google Scholar] [CrossRef]
- Abdoul-Aziz, S.K.A.; Zhang, Y.; Wang, J. Milk Odd and Branched Chain Fatty Acids in Dairy Cows: A Review on Dietary Factors and Its Consequences on Human Health. Animals 2021, 11, 3210. [Google Scholar] [CrossRef] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826. [Google Scholar] [CrossRef] [PubMed]
- Tormási, J.; Abrankó, L. Lipid digestibility of sour cream and its analogue during in vitro digestion simulation and co-consumption with cooked pasta. Int. J. Food Sci. Technol. 2023, 58, 6330–6341. [Google Scholar] [CrossRef]
- Yilmaz-Ersan, L. Fatty acid composition of cream fermented by probiotic bacteria. Dairy/Mljekarstvo 2013, 63, 132–139. [Google Scholar]
- Moltó-puigmartí, C.; Permanyer, M.; Isabel, A.; López-sabater, M.C. Effects of pasteurisation and high-pressure processing on vitamin C, tocopherols and fatty acids in mature human milk. Food Chem. 2011, 124, 697–702. [Google Scholar] [CrossRef]
- German, J.B.; Dillard, C.J.; Ward, R.E. Bioactive components in milk. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 653–658. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- FAO/WHO Codex Alimentarius. FAO/WHO Codex Alimentarius STANDARD FOR CREAM AND PREPARED CREAMS CXS 288–1976 Formerly CODEX STAN A-9-1976; Adopted in 1976. Revised in 2003, 2008. Amended in 2010. GSFA Online Food Category 01.04.04. Milk and Dairy Products in Human Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Michalski, M.C.; Genot, C.; Gayet, C.; Lopez, C.; Fine, F.; Joffre, F.; Vendeuvre, J.L.; Bouvier, J.; Chardigny, J.M.; Raynal-Ljutovac, K.; et al. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog. Lipid Res. 2013, 52, 354–373. [Google Scholar] [CrossRef]
- Ye, A.; Singh, H.; Taylor, M.W.; Anema, S. Interactions of whey proteins with milk fat globule membrane proteins during heat treatment of whole milk. Le Lait 2004, 84, 269–283. [Google Scholar] [CrossRef]
- Wang, T.; Lin, L.U.; Ou, J.I.E.; Chen, M.I.N.; Yan, W. The inhibitory effects of varying water activity, pH, and nisin content on Staphylococcus aureus growth and enterotoxin A production in whipping cream. J. Food Saf. 2016, 37, e12280. [Google Scholar] [CrossRef]
- Dash, K.K.; Fayaz, U.; Dar, A.H.; Shams, R.; Manzoor, S.; Sundarsingh, A.; Khan, S.A. A comprehensive review on heat treatments and related impact on the quality and microbial safety of milk and milk-based products. Food Chem. Adv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Moghaddam, N.R.; Jafari, S.M. Pasteurization in the food industry. In Thermal Processing of Food Products by Steam and Hot Water; Woodhead Publishing: Cambridge, UK, 2023; pp. 247–273. [Google Scholar] [CrossRef]
- Hogan, E.; Kelly, A.L.; Sun, D.W. High Pressure Processing of Foods. An Overview. In Emerging Technologies for Food Processing; Academic Press: Cambridge, MA, USA, 2005; Volume 1, ISBN 9780126767575. [Google Scholar]
- Donsì, G.; Ferrari, G.; Maresca, P. Rheological Properties of High Pressure Milk Cream. Procedia Food Sci. 2011, 1, 862–868. [Google Scholar] [CrossRef]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993, 57, 715S–725S. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, M.; Kowalczyk, J.; Chojecki, G. An improved method for derivatization of fatty acids for liquid chromatography. J. Anim. Feed Sci. 2001, 10, 369–375. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Niedzwiedzka, K.M.; Wasowska, I. A highly efficient method for derivatization of fatty acids for high performance liquid chromatography. J. Anim. Feed Sci. 2002, 11, 517–526. [Google Scholar] [CrossRef]
- Sutherland, K. Derivatisation using m-(trifluoromethyl)phenyltrimethylammonium hydroxide of organic materials in artworks for analysis by gas chromatography–mass spectrometry: Unusual reaction products with alcohols. J. Chromatogr. A 2007, 1149, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Topolewska, A.; Czarnowska, K.; Haliński, Ł.P.; Stepnowski, P. Evaluation of four derivatization methods for the analysis of fatty acids from green leafy vegetables by gas chromatography. J. Chromatogr. B 2015, 990, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Unger, A.L.; Bourne, D.E.; Walsh, H.; Kraft, J. Fatty acid content of retail cow’s milk in the northeastern United States-What’s in it for the consumer? J. Agric. Food Chem. 2020, 68, 4268–4276. [Google Scholar] [CrossRef]
- Fuke, G.; Nornberg, J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1–7. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Rajion, M.A.; Meng, G.Y.; Boo, L.J.; Ebrahimi, M.; Royan, M.; Sahebi, M.; Azizi, P.; Abiri, R.; Jahromi, M.F. Conjugated linoleic acid: A potent fatty acid linked to animal and human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2737–2748. [Google Scholar] [CrossRef]
- Den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Taormina, V.M.; Unger, A.L.; Kraft, J. Full-fat dairy products and cardiometabolic health outcomes: Does the dairy-fat matrix matter? Front. Nutr. 2024, 11, 1386257. [Google Scholar] [CrossRef]
- Khademi, F.; Naghizadeh Raeisi, S.; Younesi, M.; Motamedzadegan, A.; Rabiei, K.; Shojaei, M.; Rokni, H.; Falsafi, M. Effect of probiotic bacteria on physicochemical, microbiological, textural, sensory properties and fatty acid profile of sour cream. Food Chem. Toxicol. 2022, 166, 113244. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Fan, M.; Zheng, A.R.; Wei, C.K.; Liu, D.H.; Thaku, K.; Wei, Z.J. Characterization of a fermented dairy, sour cream: Lipolysis and the release profile of flavor compounds. Food Chem. 2023, 423, 136299. [Google Scholar] [CrossRef] [PubMed]
- Savatinova, M.; Ivanova, M. Functional dairy products enriched with omega-3 fatty acids. Food Sci. Appl. Biotechnol. 2024, 7, 1–13. [Google Scholar] [CrossRef]
- De Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus plantarum—Survival, functional and potential probiotic properties in the human intestinal tract. I. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.; Thakur, R.C. Vitamins fortification and its consequences on food production and quality. In Microbial Vitamins and Carotenoids in Food Biotechnology; Academic Press: Cambridge, MA, USA, 2024; pp. 179–203. [Google Scholar] [CrossRef]
- Dyrda-Terniuk, T.; Railean, V.; Florkiewicz, A.B.; Walczak-Skierska, J.; Kolankowski, M.; Rudnicka, J.; Białczak, D.; Pomastowski, P. The impact of Lactiplantibacillus plantarum on the cream composition: Insight into changes of vitamin D3 content and fatty acid composition. Int. Dairy J. 2025, 161, 106118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florkiewicz, A.B.; Gużewska, G.; Arendowska, I.; Ludwiczak, A.; Rudnicka, J.; Szultka-Młyńska, M.; Ligor, T.; Pomastowski, P.P. Revealing the Impact of Pasteurization and Derivatization Chemistry on the Fatty Acid Profile of Dairy Cream: A Comparative Approach. Foods 2025, 14, 3815. https://doi.org/10.3390/foods14223815
Florkiewicz AB, Gużewska G, Arendowska I, Ludwiczak A, Rudnicka J, Szultka-Młyńska M, Ligor T, Pomastowski PP. Revealing the Impact of Pasteurization and Derivatization Chemistry on the Fatty Acid Profile of Dairy Cream: A Comparative Approach. Foods. 2025; 14(22):3815. https://doi.org/10.3390/foods14223815
Chicago/Turabian StyleFlorkiewicz, Aleksandra Bogumiła, Gaja Gużewska, Izabela Arendowska, Agnieszka Ludwiczak, Joanna Rudnicka, Małgorzata Szultka-Młyńska, Tomasz Ligor, and Paweł Piotr Pomastowski. 2025. "Revealing the Impact of Pasteurization and Derivatization Chemistry on the Fatty Acid Profile of Dairy Cream: A Comparative Approach" Foods 14, no. 22: 3815. https://doi.org/10.3390/foods14223815
APA StyleFlorkiewicz, A. B., Gużewska, G., Arendowska, I., Ludwiczak, A., Rudnicka, J., Szultka-Młyńska, M., Ligor, T., & Pomastowski, P. P. (2025). Revealing the Impact of Pasteurization and Derivatization Chemistry on the Fatty Acid Profile of Dairy Cream: A Comparative Approach. Foods, 14(22), 3815. https://doi.org/10.3390/foods14223815







