Food Safety in the Age of Climate Change: The Rising Risk of Pesticide Residues and the Role of Sustainable Adsorbent Technologies
Abstract
1. Introduction
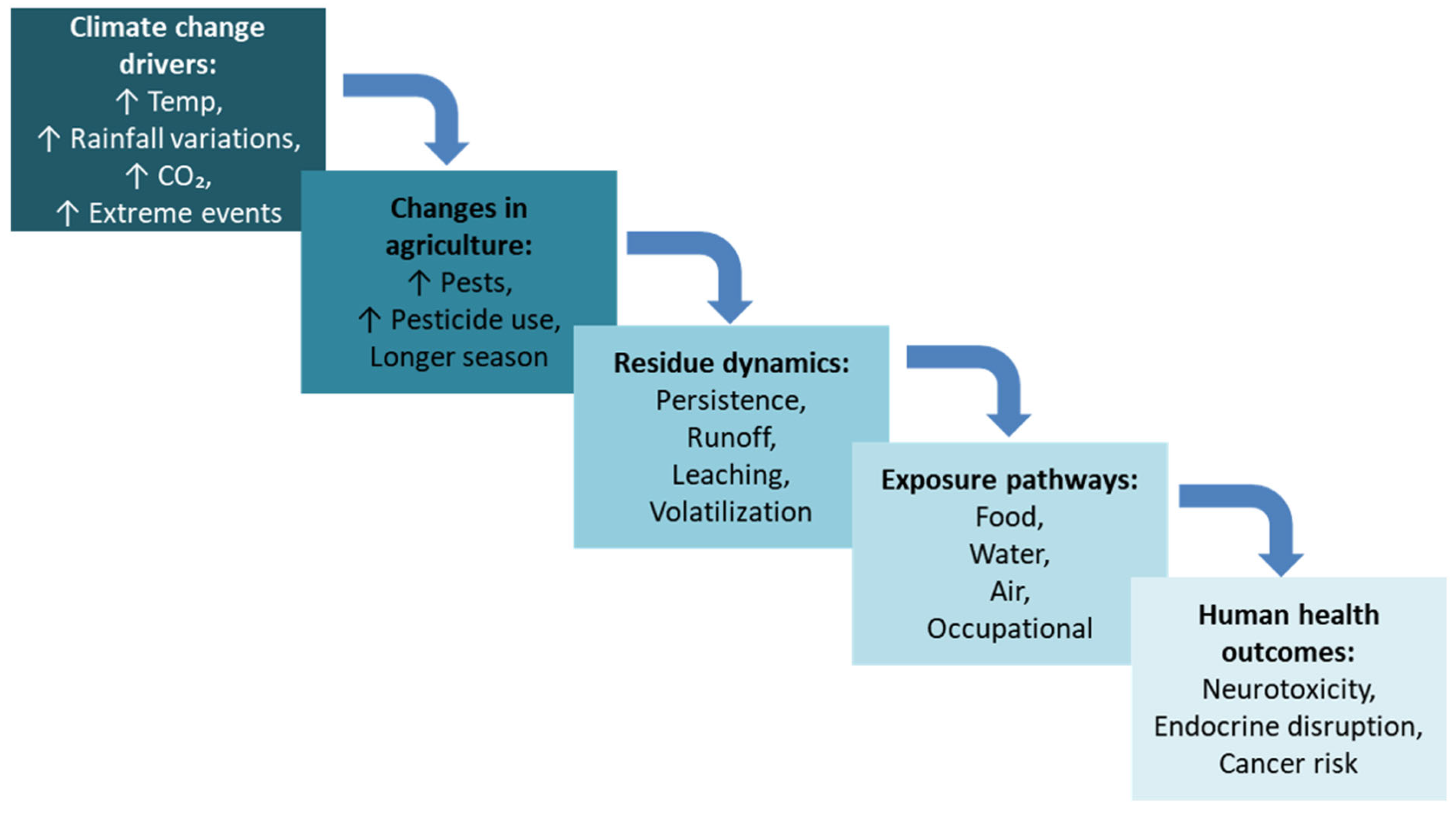
2. Climate Change and Pesticide Residue Dynamics in Food and Their Health Implications
3. Current Strategies for Pesticide Removal
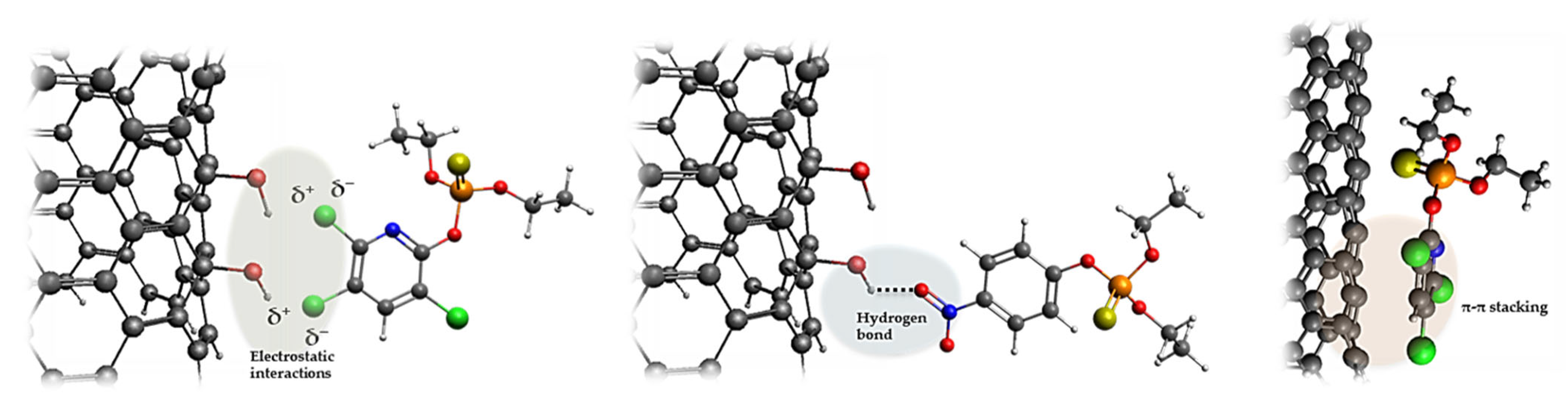
4. Sustainable Adsorbent Materials for Mitigating Pesticide Residues
5. Analytical Methods for Detecting Pesticide Residues in Food and Water
5.1. Chromatographic Techniques
5.2. Spectroscopic Methods
5.3. Rapid and Field-Deployable Methods
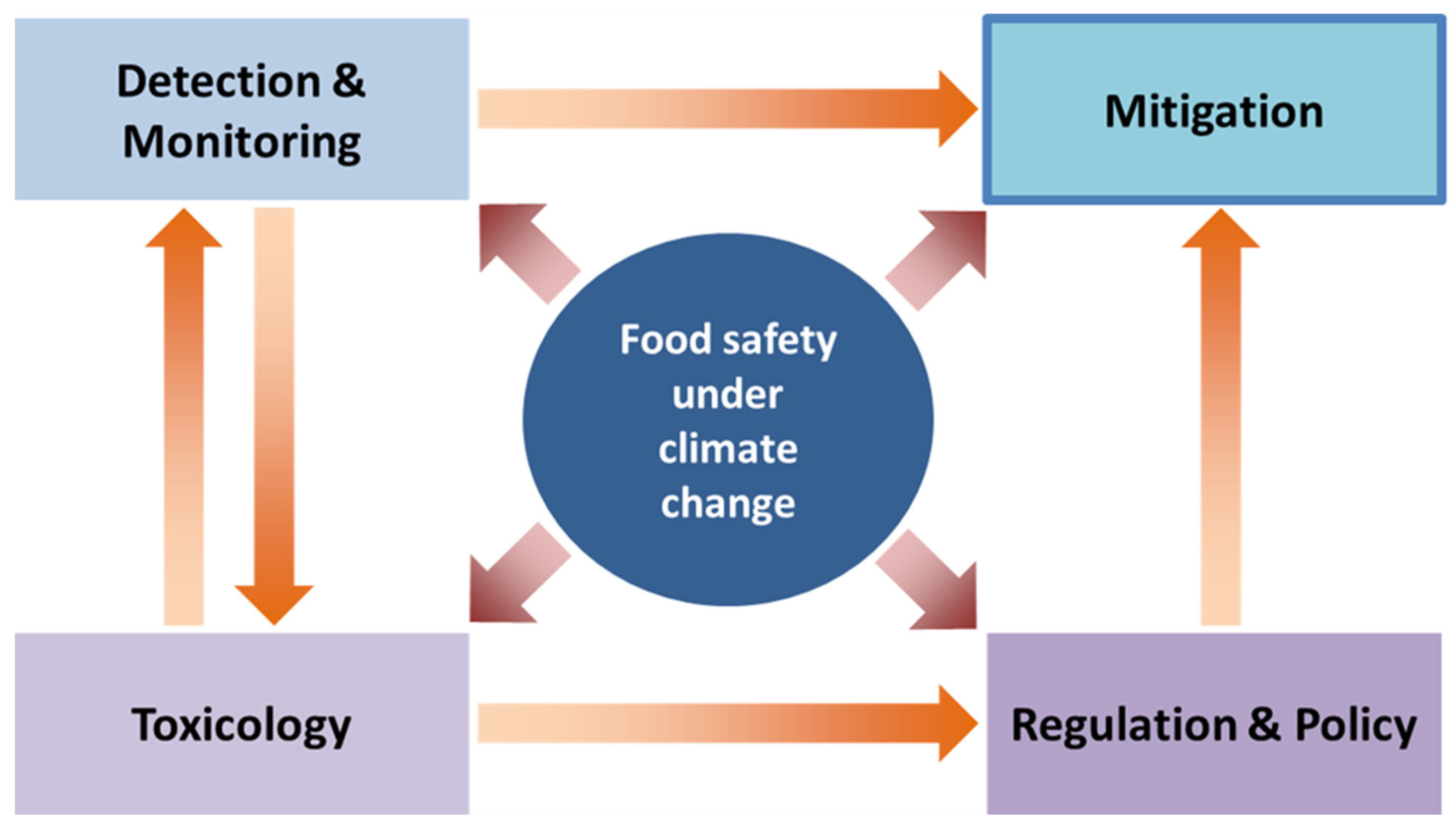
6. Future Perspectives and Research Needs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duchenne-Moutien, R.A.; Neetoo, H. Climate Change and Emerging Food Safety Issues: A Review. J. Food Prot. 2021, 84, 1884–1897. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2025, 11, 100410. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Subedi, B.; Poudel, A.; Aryal, S. The impact of climate change on insect pest biology and ecology: Implications for pest management strategies, crop production, and food security. J. Agric. Food Res. 2023, 14, 100733. [Google Scholar] [CrossRef]
- Available online: https://www.ipcc.ch/sr15/ (accessed on 31 October 2025).
- Bolan, S.; Padhye, L.P.; Jasemizad, T.; Govarthanan, M.; Karmegam, N.; Wijesekara, H.; Amarasiri, D.; Hou, D.; Zhou, P.; Biswal, B.K.; et al. Impacts of climate change on the fate of contaminants through extreme weather events. Sci. Total Environ. 2024, 909, 168388. [Google Scholar] [CrossRef]
- Saber, A.N.; Malhat, F.; Cervantes-Avilés, P.; Mahmoud, M.; Watanabe, H. Validation of a small-scale portable rainfall simulator on the simultaneous transport of sediments and pesticides in agriculture soils. Pest Manag. Sci. 2025, 81, 3250–3262. [Google Scholar] [CrossRef]
- Leskovac, A.; Petrović, S. Pesticide Use and Degradation Strategies: Food Safety, Challenges and Perspectives. Foods 2023, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, C.; Khosya, R.; Thakur, K.; Mahajan, D.; Kumar, R.; Kumar, S.; Sharma, A.K. A systematic review of pesticide exposure, associated risks, and long-term human health impacts. Toxicol. Rep. 2024, 13, 101840. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, F. Scientific and Regulatory Perspectives on Chemical Risk Assessment of Pesticides in the European Union. J. Xenobiotics 2025, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Vryzas, Z.; Ramwell, C.; Sans, C. Pesticide prioritization approaches and limitations in environmental monitoring studies: From Europe to Latin America and the Caribbean. Environ. Int. 2020, 143, 105917. [Google Scholar] [CrossRef]
- Li, W.-K.; Shi, Y.-P. Recent advances of carbon materials on pesticides removal and extraction based determination from polluted water. TrAC Trends Anal. Chem. 2024, 171, 117534. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Brković, S.; Potkonjak, N.; Unterweger, C.; Pašti, I.; Lazarević-Pašti, T. Sustainable carbon materials from biowaste for the removal of organophosphorus pesticides, dyes, and antibiotics. J. Environ. Manag. 2025, 376, 124463. [Google Scholar] [CrossRef] [PubMed]
- Harabi, S.; Guiza, S.; Álvarez-Montero, A.; Gómez-Avilés, A.; Belver, C.; Rodríguez, J.J.; Bedia, J. Adsorption of 2,4-dichlorophenoxyacetic acid on activated carbons from macadamia nut shells. Environ. Res. 2024, 247, 118281. [Google Scholar] [CrossRef]
- Ashraf, B.; Mubarak, S.; Asghar, A.; Hafeez, A.; Asif, F. Recycling fruit peels into:magnetite/carbon adsorbents: A scalable solution for pyrethroid insecticide removal. Desalination Water Treat. 2025, 321, 100884. [Google Scholar] [CrossRef]
- Ogunbolude, O.K.; Oyekanmi, O.F.; Olatunji, F.T.; Idowu, C.F.; Aremu, A.E. Synthesis of Carbon-Nanomaterial from Rice Husk and Its Application as Adsorbent in Pesticide Residues Analysis. Int. J. Chem. Technol. 2025, 9, 56–68. [Google Scholar] [CrossRef]
- Zlatković, M.; Kurtić, R.; Pašti, I.A.; Tasić, T.; Milanković, V.; Potkonjak, N.; Unterweger, C.; Lazarević-Pašti, T. Application of Carbon Materials Derived from Nocino Walnut Liqueur Pomace Residue for Chlorpyrifos Removal from Water. Materials 2025, 18, 3072. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Tasić, T.; Milanković, V.; Batalović, K.; Breitenbach, S.; Unterweger, C.; Fürst, C.; Pašti, I.A.; Lazarević-Pašti, T. Application of Viscose-Based Porous Carbon Fibers in Food Processing—Malathion and Chlorpyrifos Removal. Foods 2023, 12, 2362. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, R.; Kaushal, S.; Thakur, N.; Umar, A.; Akbar, S.; Ibrahim, A.A.; Baskoutas, S. Biomass waste-derived carbon materials for sustainable remediation of polluted environment: A comprehensive review. Chemosphere 2023, 345, 140419. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Khezerlou, A.; Rezvani-Ghalhari, M.; McClements, D.J.; Varma, R.S. Advanced carbon-based nanomaterials: Application in the development of multifunctional next-generation food packaging materials. Adv. Colloid Interface Sci. 2025, 339, 103422. [Google Scholar] [CrossRef]
- Bremenkamp, I.; Sousa Gallagher, M.J. Edible Coatings for Ready-to-Eat Products: Critical Review of Recent Studies, Sustainable Packaging Perspectives, Challenges and Emerging Trends. Polymers 2025, 17, 376. [Google Scholar] [CrossRef]
- Kainth, S.; Sharma, P.; Pandey, O.P. Green sorbents from agricultural wastes: A review of sustainable adsorption materials. Appl. Surf. Sci. Adv. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Ramírez-Morales, D.; Pérez-Villanueva, M.E.; Chin-Pampillo, J.S.; Aguilar-Mora, P.; Arias-Mora, V.; Masís-Mora, M. Pesticide occurrence and water quality assessment from an agriculturally influenced Latin-American tropical region. Chemosphere 2021, 262, 127851. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Bub, S.; Petschick, L.L.; Schemmer, A.; Stehle, S.; Schulz, R. Pesticide occurrence in protected surface waters in nature conservation areas of Germany. Sci. Total Environ. 2023, 858, 160074. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Shin, W.S.; Masud, M.A.A.; Nandi, R.; Islam, T. A critical review of sustainable pesticide remediation in contaminated sites: Research challenges and mechanistic insights. Environ. Pollut. 2024, 341, 122940. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kumar, M.; Chauhan, C.; Sirohi, U.; Srivastav, A.L.; Rani, L. Strategies for mitigation of pesticides from the environment through alternative approaches: A review of recent developments and future prospects. J. Environ. Manag. 2024, 354, 120326. [Google Scholar] [CrossRef]
- Delpla, I.; Baurès, E.; Jung, A.-V.; Thomas, O. Impacts of rainfall events on runoff water quality in an agricultural environment in temperate areas. Sci. Total Environ. 2011, 409, 1683–1688. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Bahar, M.M.; Naidu, R. Diffuse soil pollution from agriculture: Impacts and remediation. Sci. Total Environ. 2025, 962, 178398. [Google Scholar] [CrossRef]
- Liu, N.; Huang, J.; Liu, X.; Wu, J.; Huang, M. Pesticide-induced metabolic disruptions in crops: A global perspective at the molecular level. Sci. Total Environ. 2024, 957, 177665. [Google Scholar] [CrossRef]
- Available online: https://www.pan-uk.org/the-cocktail-effect/ (accessed on 31 October 2025).
- Available online: https://multimedia.efsa.europa.eu/pesticides-report-2023/ (accessed on 31 October 2025).
- Balkan, T.; Kara, K.; Kızılarslan, M. Dissipation behavior and dietary risk assessment of acetamiprid, boscalid, and pyraclostrobin applied individually and in mixtures in sweet cherries. J. Food Compos. Anal. 2025, 148, 108213. [Google Scholar] [CrossRef]
- Alehashem, M.; Peters, R.; Fajana, H.O.; Eslamizad, S.; Hogan, N.; Hecker, M.; Siciliano, S.D. Herbicides and pesticides synergistically interact at low concentrations in complex mixtures. Chemosphere 2024, 353, 141431. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M. Poisoning by pesticides. Medicine 2020, 48, 214–217. [Google Scholar] [CrossRef]
- Amaral, L.; Martins, M.; Côrte-Real, M.; Outeiro, T.F.; Chaves, S.R.; Rego, A. The neurotoxicity of pesticides: Implications for Parkinson’s disease. Chemosphere 2025, 377, 144348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Sharma, P.; Pasrija, R.; Kaur, K.; Umesh, M.; Thazeem, B. Toxicity analysis of endocrine disrupting pesticides on non-target organisms: A critical analysis on toxicity mechanisms. Toxicol. Appl. Pharmacol. 2023, 474, 116623. [Google Scholar] [CrossRef]
- Chen, L.; Yan, H.; Di, S.; Guo, C.; Zhang, H.; Zhang, S.; Gold, A.; Wang, Y.; Hu, M.; Wu, D.; et al. Mapping pesticide-induced metabolic alterations in human gut bacteria. Nat. Commun. 2025, 16, 4355. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Iglesias, V.; Zúñiga-Venegas, L.; Pancetti, F.; Foerster, C.; Landeros, N.; Lucero, B.; Schwantes, D.; Cortés, S. Exposure to pesticides in Chile and its relationship with carcinogenic potential: A review. Front. Public Health 2025, 13, 1531751. [Google Scholar] [CrossRef]
- Cavalier, H.; Trasande, L.; Porta, M. Exposures to pesticides and risk of cancer: Evaluation of recent epidemiological evidence in humans and paths forward. Int. J. Cancer 2023, 152, 879–912. [Google Scholar] [CrossRef]
- Kumar, D.; Sinha, S.N.; Rajendra, S.; Sharma, K. Assessing farmer’s exposure to pesticides and the risk for non-communicable diseases: A biomonitoring study. Sci. Total Environ. 2023, 891, 164429. [Google Scholar] [CrossRef]
- Bliznashka, L.; Roy, A.; Jaacks, L.M. Pesticide exposure and child growth in low- and middle-income countries: A systematic review. Environ. Res. 2022, 215, 114230. [Google Scholar] [CrossRef]
- Stevens, D.R.; Blaauwendraad, S.M.; Bommarito, P.A.; van den Dries, M.; Trasande, L.; Spaan, S.; Pronk, A.; Tiemeier, H.; Gaillard, R.; Jaddoe, V.W.V.; et al. Gestational organophosphate pesticide exposure and childhood cardiovascular outcomes. Environ. Int. 2024, 193, 109082. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Nian, B.; Yu, C.; Maimaiti, D.; Chai, M.; Yang, X.; Zang, X.; Xu, D. Mechanisms of Neurotoxicity of Organophosphate Pesticides and Their Relation to Neurological Disorders. Neuropsychiatr. Dis. Treat. 2024, 20, 2237–2254. [Google Scholar] [CrossRef]
- Akanbi, C.A.; Rotimi, D.E.; Ojo, A.B.; Ojo, O.A. Endocrine-disrupting chemicals (EDCs) and epigenetic regulation in embryonic development: Mechanisms, impacts, and emerging trends. Toxicol. Rep. 2025, 14, 101885. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Mun, S.; Kim, H.J.; Han, S.J.; Kim, D.W.; Cho, B.-S.; Kim, A.G.; Park, D.W. Effectiveness of Different Washing Strategies on Pesticide Residue Removal: The First Comparative Study on Leafy Vegetables. Foods 2022, 11, 2916. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Barták, M.; Hájek, J.; Staňková, E.; Trnková, K. Effects of Foliar Application of a Lambda-Cyhalothrin Insecticide on Photosynthetic Characteristics of a Fodder Plant Malva moschata. Agronomy 2024, 14, 2818. [Google Scholar] [CrossRef]
- Qi, H.; Huang, Q.; Hung, Y.-C. Effectiveness of electrolyzed oxidizing water treatment in removing pesticide residues and its effect on produce quality. Food Chem. 2018, 239, 561–568. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Romero-González, R.; Garrido Frenich, A. Dissipation kinetics of fenamidone, propamocarb and their metabolites in ambient soil and water samples and unknown screening of metabolites. J. Environ. Manag. 2020, 254, 109818. [Google Scholar] [CrossRef]
- Flamminii, F.; Minetti, S.; Mollica, A.; Cichelli, A.; Cerretani, L. The Effect of Washing, Blanching and Frozen Storage on Pesticide Residue in Spinach. Foods 2023, 12, 2806. [Google Scholar] [CrossRef]
- Wu, Y.; An, Q.; Li, D.; Wu, J.; Pan, C. Comparison of Different Home/Commercial Washing Strategies for Ten Typical Pesticide Residue Removal Effects in Kumquat, Spinach and Cucumber. Int. J. Environ. Res. Public Health 2019, 16, 472. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Zhao, Q.; Liu, S.; Hu, W. Effects of Ozonated Water on Microbial Growth, Quality Retention and Pesticide Residue Removal of Fresh-cut Onions. Ozone Sci. Eng. 2020, 42, 399–407. [Google Scholar] [CrossRef]
- Karaca, H. The Effects of Ozone-Enriched Storage Atmosphere on Pesticide Residues and Physicochemical Properties of Table Grapes. Ozone Sci. Eng. 2019, 41, 404–414. [Google Scholar] [CrossRef]
- Souza, L.P.d.; Faroni, L.R.D.A.; Heleno, F.F.; Pinto, F.G.; Queiroz, M.E.L.R.d.; Prates, L.H.F. Ozone treatment for pesticide removal from carrots: Optimization by response surface methodology. Food Chem. 2018, 243, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, C.; Jiang, A.; Zhang, Y.; Zhao, Q.; Hu, W. Effects of aqueous ozone treatment on microbial growth, quality, and pesticide residue of fresh-cut cabbage. Food Sci. Nutr. 2021, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Solanki, V.; Bardhan, K.; Kansara, R.; Vyas, T.K.; Gandhi, K.; Dhakan, D.; Ali, H.M.; Siddiqui, M.H. Evaluation of Ozonation Technique for Pesticide Residue Removal in Okra and Green Chili Using GC-ECD and LC-MS/MS. Plants 2022, 11, 3202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Y.; Chen, F.; Zhang, Y.; Liao, X. Chlorine dioxide treatment for the removal of pesticide residues on fresh lettuce and in aqueous solution. Food Control. 2014, 40, 106–112. [Google Scholar] [CrossRef]
- Ong, K.C.; Cash, J.N.; Zabik, M.J.; Siddiq, M.; Jones, A.L. Chlorine and ozone washes for pesticide removal from apples and processed apple sauce. Food Chem. 1996, 55, 153–160. [Google Scholar] [CrossRef]
- Studziński, W.; Narloch, I.; Dąbrowski, Ł. Removal of Pesticides from Lemon and Vegetables Using Electrolyzed Water Kitchen Devices. Molecules 2024, 29, 5797. [Google Scholar] [CrossRef]
- Ünal Turhan, E.; Erginkaya, Z.; Korukluoğlu, M.; Konuray, G. Beneficial Biofilm Applications in Food and Agricultural Industry. In Health and Safety Aspects of Food Processing Technologies; Malik, A., Erginkaya, Z., Erten, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 445–469. [Google Scholar]
- K, S.; Anjali, P.; Akshatha, B.S.; Alex, R.; Radhakrishnan, E.K. Enhanced biodegradation of chlorpyrifos in the presence of sub-inhibitory concentration of ZnONPs by Pseudomonas sCF7b. Process Saf. Environ. Prot. 2024, 190, 256–263. [Google Scholar] [CrossRef]
- Dar, M.A.; Pandey, J.; Kaushik, G. Optimizing malathion biodegradation by Bacillus cereus via a design of experiment framework. Bioremediation J. 2025, 29, 1–19. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Liu, Y.; Li, G.; Ning, M.; Yang, W.; Zhen, J.; Yue, D.; Mu, Q.; Ding, F. Bio-enzyme cleaning agent for pesticide residues on fruits and vegetables. In CN104322490A; S.I.P.O.o.t.P.s.R.o. China, Ed.; Henan Academy of Sciences Biological Research Institute Co., Ltd.: Zhengzhou, China, 2013. [Google Scholar]
- Jaiswal, S.; Dhingra, I.; Joshi, A.; Kodgire, P. Efficacious bioremediation of pesticide-contaminated water using immobilized organophosphorus acid anhydrolase–FL variant (OPAA-FL) enzyme in a lab-scale bioreactor. J. Water Process Eng. 2025, 71, 107357. [Google Scholar] [CrossRef]
- Mitrović, T.; Lazović, S.; Nastasijević, B.; Pašti, I.A.; Vasić, V.; Lazarević-Pašti, T. Non-thermal plasma needle as an effective tool in dimethoate removal from water. J. Environ. Manag. 2019, 246, 63–70. [Google Scholar] [CrossRef]
- Flores Takahashi, J.S.; Schwantes, D.; Gonçalves, A.C., Jr.; Fuentealba, D.; Valdés Gómez, H.; Piccioli, A.d.F.B. Efficiency of combined techniques in pesticide residues removal from food: A systematic review. Food Prod. Process. Nutr. 2025, 7, 31. [Google Scholar] [CrossRef]
- Zhang, B.H.; Xu, X.; Lu, H.; Wang, L.; Yang, Q. Removal of phoxim, chlorothalonil and Cr3+ from vegetable using bubble flow. J. Food Eng. 2021, 291, 110217. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Lee, D.-Y.; Oh, K.-Y.; Jeong, D.-K.; Lee, D.-Y.; Kim, J.-H. Photochemical advanced oxidative process treatment effect on the pesticide residues reduction and quality changes in dried red peppers. Sci. Rep. 2023, 13, 4444. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Z.; Malik, A.U.; Asi, M.R.; Anwar, R.; Inam Ur Raheem, M. Sonolytic-ozonation technology for sanitizing microbial contaminants and pesticide residues from spinach (Spinacia oleracea L.) leaves, at household level. Environ. Sci. Pollut. Res. 2021, 28, 52913–52924. [Google Scholar] [CrossRef] [PubMed]
- Gkika, D.A.; Tolkou, A.K.; Katsoyiannis, I.A.; Kyzas, G.Z. The adsorption-desorption-regeneration pathway to a circular economy: The role of waste-derived adsorbents on chromium removal. Sep. Purif. Technol. 2025, 368, 132996. [Google Scholar] [CrossRef]
- Kumar, M.; Dash, S.; Mahlknecht, J.; Kolok, A.; Dogra, S.; Kuroda, K.; Tobino, T.; Mora, A.; Kazmi, A.A.; Singh, R.; et al. Understanding the pathways, pollution and potential solutions pertaining to pesticides: Circular engineering for persistent chemicals. Curr. Opin. Environ. Sci. Health 2025, 46, 100638. [Google Scholar] [CrossRef]
- Ahmad, M.; Riaz, U.; Iqbal, S.; Ahmad, J.; Rasheed, H.; Al-Farraj, A.S.F.; Al-Wabel, M.I. Adsorptive Removal of Atrazine from Contaminated Water Using Low-Cost Carbonaceous Materials: A Review. Front. Mater. 2022, 9, 909534. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, L.; Cheng, Z.; Shi, T.; Ma, X.; Li, Q.X.; Hua, R. Highly Efficient Adsorption Characteristics and Mechanism of Nutshell Biochars for Aromatic Organophosphorus Insecticides. Agronomy 2023, 13, 543. [Google Scholar] [CrossRef]
- George, L.-Y.; Ma, L.; Zhang, W.; Yao, G. Parametric modelling and analysis to optimize adsorption of Atrazine by MgO/Fe3O4-synthesized porous carbons in water environment. Environ. Sci. Eur. 2023, 35, 21. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Huang, W.-D.; Guo, J.-J.; Yang, Y.; Tao, R.; Feng, X. Use of Fe-Impregnated Biochar To Efficiently Sorb Chlorpyrifos, Reduce Uptake by Allium fistulosum L., and Enhance Microbial Community Diversity. J. Agric. Food Chem. 2017, 65, 5238–5243. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.F.; Dias Ferreira, G.M. Biochars as Adsorbents of Pesticides: Laboratory-Scale Performances and Real-World Contexts, Challenges, and Prospects. ACS EST Water 2024, 4, 4264–4282. [Google Scholar] [CrossRef]
- Raul, P.K.; Thakuria, A.; Das, B.; Devi, R.R.; Tiwari, G.; Yellappa, C.; Kamboj, D.V. Carbon Nanostructures As Antibacterials and Active Food-Packaging Materials: A Review. ACS Omega 2022, 7, 11555–11559. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F.; Donato, F.F.; Kemmerich, M.; Zanella, R.; Camargo, E.R.; Avila, L.A.d. Efficiency of home water filters on pesticide removal from drinking water. Environ. Pollut. 2024, 341, 122936. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, N.; Purakayastha, T.J. Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci. Total Environ. 2017, 577, 376–385. [Google Scholar] [CrossRef]
- Ioannidou, O.A.; Zabaniotou, A.A.; Stavropoulos, G.G.; Islam, M.A.; Albanis, T.A. Preparation of activated carbons from agricultural residues for pesticide adsorption. Chemosphere 2010, 80, 1328–1336. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Brković, S.; Potkonjak, N.; Unterweger, C.; Bajuk-Bogdanović, D.; Pašti, I.; Lazarević-Pašti, T. Spent coffee grounds-derived carbon material as an effective adsorbent for removing multiple contaminants from wastewater: A comprehensive kinetic, isotherm, and thermodynamic study. J. Water Process Eng. 2024, 63, 105507. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Brković, S.; Potkonjak, N.; Unterweger, C.; Pašti, I.; Lazarević-Pašti, T. The adsorption of chlorpyrifos and malathion under environmentally relevant conditions using biowaste carbon materials. J. Hazard. Mater. 2024, 480, 135940. [Google Scholar] [CrossRef]
- Katnić, Đ.B.; Porobić, S.J.; Vujčić, I.; Kojić, M.M.; Lazarević-Pašti, T.; Milanković, V.; Marinović-Cincović, M.; Živojinović, D.Z. Irradiated fig pomace pyrochar as a promising and sustainable sterilized sorbent for water pollutant removal. Radiat. Phys. Chem. 2024, 214, 111277. [Google Scholar] [CrossRef]
- Katnić, Đ.; Porobić, S.J.; Lazarević-Pašti, T.; Kojić, M.; Tasić, T.; Marinović-Cincović, M.; Živojinović, D. Sterilized plum pomace biochar as a low-cost effective sorbent of environmental contaminants. J. Water Process Eng. 2023, 56, 104487. [Google Scholar] [CrossRef]
- Lago, A.; Silva, B.; Tavares, T. Biowaste valorization on pharmaceuticals and pesticides abatement in aqueous environments. Sustain. Mater. Technol. 2024, 39, e00792. [Google Scholar] [CrossRef]
- Binh, Q.A.; Nguyen, V.-H.; Kajitvichyanukul, P. Influence of pyrolysis conditions of modified corn cob bio-waste sorbents on adsorption mechanism of atrazine in contaminated water. Environ. Technol. Innov. 2022, 26, 102381. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, B.; Igalavithana, A.D.; Ok, Y.S.; Yang, X.; Lombi, E.; Bolan, N. Mechanistic insights of 2,4-D sorption onto biochar: Influence of feedstock materials and biochar properties. Bioresour. Technol. 2017, 246, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Baharum, N.A.; Nasir, H.M.; Ishak, M.Y.; Isa, N.M.; Hassan, M.A.; Aris, A.Z. Highly efficient removal of diazinon pesticide from aqueous solutions by using coconut shell-modified biochar. Arab. J. Chem. 2020, 13, 6106–6121. [Google Scholar] [CrossRef]
- Vimal, V.; Patel, M.; Mohan, D. Aqueous carbofuran removal using slow pyrolyzed sugarcane bagasse biochar: Equilibrium and fixed-bed studies. RSC Adv. 2019, 9, 26338–26350. [Google Scholar] [CrossRef]
- Salman, J.M. Batch Study for Insecticide Carbofuran Adsorption onto Palm-Oil-Fronds-Activated Carbon. J. Chem. 2013, 2013, 630371. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, C.; Tian, C.; Yu, X.; Liu, J.; Li, Q.; Wang, S. Sustainable biochar derived from waste lotus seedpod for efficient adsorption of residual carbamate pesticides. Heliyon 2025, 11, e42741. [Google Scholar] [CrossRef]
- Ponnuchamy, M.; Kapoor, A.; Senthil Kumar, P.; Vo, D.-V.N.; Balakrishnan, A.; Mariam Jacob, M.; Sivaraman, P. Sustainable adsorbents for the removal of pesticides from water: A review. Environ. Chem. Lett. 2021, 19, 2425–2463. [Google Scholar] [CrossRef]
- Lee, G.; Kim, C.; Park, C.; Ryu, B.-G.; Hong, H.-J. High-carbon-content biochar from chemical manufacturing plant sludge for effective removal of ciprofloxacin from aqueous media. Chemosphere 2024, 364, 143118. [Google Scholar] [CrossRef]
- Ulrich, B.A.; Im, E.A.; Werner, D.; Higgins, C.P. Biochar and Activated Carbon for Enhanced Trace Organic Contaminant Retention in Stormwater Infiltration Systems. Environ. Sci. Technol. 2015, 49, 6222–6230. [Google Scholar] [CrossRef]
- Kujawska, J. Content of Heavy Metals in Various Biochar and Assessment Environmental Risk. J. Ecol. Eng. 2023, 24, 287–295. [Google Scholar] [CrossRef]
- Li, Q.; Qi, Y.; Gao, C. Chemical regeneration of spent powdered activated carbon used in decolorization of sodium salicylate for the pharmaceutical industry. J. Clean. Prod. 2015, 86, 424–431. [Google Scholar] [CrossRef]
- Faheem, M.; Azher Hassan, M.; Du, J.; Wang, B. Harnessing potential of smart and conventional spent adsorbents: Global practices and sustainable approaches through regeneration and tailored recycling. Sep. Purif. Technol. 2025, 354, 128907. [Google Scholar] [CrossRef]
- Al Hattab, M.T.; Ghaly, A.E. Disposal and Treatment Methods for Pesticide Containing Wastewaters: Critical Review and Comparative Analysis. J. Environ. Prot. 2012, 3, 431–453. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Khan, M.S.; Othmani, A.; Khanday, W.A.; Gökkuş, Ö.; Osagie, C.; Ahmaruzzaman, M.; Mishra, S.R.; et al. Sustainable remediation technologies for removal of pesticides as organic micro-pollutants from water environments: A review. Appl. Surf. Sci. Adv. 2024, 19, 100558. [Google Scholar] [CrossRef]
- Prodhan, M.D.H.; Alam, S.N.; Jalal Uddin, M. Analytical Methods in Measuring Pesticides in Foods. In Pesticide Residue in Foods: Sources, Management, and Control; Khan, M.S., Rahman, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 135–145. [Google Scholar]
- Thach, T.D.; Nguyen, L.K.T.; Nguyen, T.D.N.; Nguyen, T.M.T.; Dang, V.S.; Mai, D.T.; Le, V.D.; Dang, C.H.; Le, Q.N.N.; Nguyen, P.T.V.; et al. Novel sulfonylurea fluorescence probes for antiproliferative activity, detection and imaging of fluoride ions in living cells. Microchem. J. 2024, 207, 111760. [Google Scholar] [CrossRef]
- Han, Y.; Tian, Y.; Li, Q.; Yao, T.; Yao, J.; Zhang, Z.; Wu, L. Advances in Detection Technologies for Pesticide Residues and Heavy Metals in Rice: A Comprehensive Review of Spectroscopy, Chromatography, and Biosensors. Foods 2025, 14, 1070. [Google Scholar] [CrossRef]
- Pszczolińska, K.; Barchańska, H.; Lalek, D. Comprehensive multiresidue chromatographic methods for monitoring pesticides in agricultural areas and corresponding plant protection zones. Environ. Pollut. 2024, 344, 123422. [Google Scholar] [CrossRef]
- Wang, M.; Niu, Y.; Peng, H.; Zhang, P.; Bu, Q.; Song, X.; Yuan, S. Nanomaterial-Enabled Spectroscopic Sensing: Building a New Paradigm for Precision Detection of Pesticide Residues. Nanomaterials 2025, 15, 1634. [Google Scholar] [CrossRef]
- Feng, L.; Yue, X.; Li, J.; Zhao, F.; Yu, X.; Yang, K. Research Advances in Nanosensor for Pesticide Detection in Agricultural Products. Nanomaterials 2025, 15, 1132. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kumari, A.; Kumar, A.; Arya, V.; Chauhan, A.; Upadhyay, N.K.; Guleria, I.; Amarowicz, R.; Kumar, D.; Kuca, K. Biosensors for detection of pesticide residue, mycotoxins and heavy metals in fruits and vegetables: A concise review. Microchem. J. 2024, 205, 111292. [Google Scholar] [CrossRef]
- Xu, L.; Abd El-Aty, A.M.; Eun, J.-B.; Shim, J.-H.; Zhao, J.; Lei, X.; Gao, S.; She, Y.; Jin, F.; Wang, J.; et al. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.P. 2.29–Column-Switching Sample Preparation. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 649–676. [Google Scholar]
- Lodha, L.; Sharma, N.; Viswas, A.; Khinchi, M. A review on chromatography techniques. Asian J. Pharm. Res. Dev. 2017, 5, 1–8. [Google Scholar]
- Kang, S.-M.; Won, J.-H.; Han, J.-E.; Kim, J.-H.; Kim, K.-H.; Jeong, H.-I.; Sung, S.-H. Chromatographic Method for Monitoring of Pesticide Residues and Risk Assessment for Herbal Decoctions Used in Traditional Korean Medicine Clinics. Molecules 2023, 28, 3343. [Google Scholar] [CrossRef]
- Batool, A.; AlMasoud, N.; Nazar, Z.; Ullah, H.; Sajid, M.; Alomar, T.S.; Ali Khan, M.; Muhammad Asif, H.; Iram, S.; Ullah, L.; et al. Synthesis of Polyoxometalates-Ionic Liquids@Fe3O4@SiO2 composites for the extraction of atrazine and deltamethrin pesticides residues from food samples and their determination by HPLC. Microchem. J. 2024, 200, 110312. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, X.; Zhang, Y.; Han, J.; Jing, X.; Wu, J. Determination of triazine herbicides from water, tea, and juice samples using magnetic dispersive micro-solid phase extraction and magnetic dispersive liquid-liquid microextraction with HPLC. Food Chem. 2025, 468, 142430. [Google Scholar] [CrossRef]
- Hao, L.; Liu, X.; Wang, J.; Wang, C.; Wu, Q.; Wang, Z. Use of ZIF-8-derived nanoporous carbon as the adsorbent for the solid phase extraction of carbamate pesticides prior to high-performance liquid chromatographic analysis. Talanta 2015, 142, 104–109. [Google Scholar] [CrossRef]
- Jiao, C.; Li, M.; Ma, R.; Wang, C.; Wu, Q.; Wang, Z. Preparation of a Co-doped hierarchically porous carbon from Co/Zn-ZIF: An efficient adsorbent for the extraction of trizine herbicides from environment water and white gourd samples. Talanta 2016, 152, 321–328. [Google Scholar] [CrossRef]
- Nasrollahpour, A.; Moradi, S.E. A Simple Vortex-Assisted Magnetic Dispersive Solid Phase Microextraction System for Preconcentration and Separation of Triazine Herbicides from Environmental Water and Vegetable Samples Using Fe3O4@MIL-100(Fe) Sorbent. J. AOAC Int. 2019, 101, 1639–1646. [Google Scholar] [CrossRef]
- Senosy, I.A.; Guo, H.-M.; Ouyang, M.-N.; Lu, Z.-H.; Yang, Z.-H.; Li, J.-H. Magnetic solid-phase extraction based on nano-zeolite imidazolate framework-8-functionalized magnetic graphene oxide for the quantification of residual fungicides in water, honey and fruit juices. Food Chem. 2020, 325, 126944. [Google Scholar] [CrossRef]
- Liang, L.; Wang, X.; Sun, Y.; Ma, P.; Li, X.; Piao, H.; Jiang, Y.; Song, D. Magnetic solid-phase extraction of triazine herbicides from rice using metal-organic framework MIL-101(Cr) functionalized magnetic particles. Talanta 2018, 179, 512–519. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, R.; Li, D.; Sun, P.; Su, P.; Yang, Y. Preparation of iron-based MIL-101 functionalized polydopamine@Fe3O4 magnetic composites for extracting sulfonylurea herbicides from environmental water and vegetable samples. J. Sep. Sci. 2018, 41, 2046–2055. [Google Scholar] [CrossRef]
- Liu, G.; Huang, X.; Lu, M.; Li, L.; Li, T.; Xu, D. Facile synthesis of magnetic zinc metal-organic framework for extraction of nitrogen-containing heterocyclic fungicides from lettuce vegetable samples. J. Sep. Sci. 2019, 42, 1451–1458. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, P.; Li, X.; Piao, H.; Li, D.; Sun, Y.; Wang, X.; Song, D. Application of metal-organic framework MIL-101(Cr) to microextraction in packed syringe for determination of triazine herbicides in corn samples by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1574, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Su, P.; Deng, Y.; Yang, Y. Self-assembled magnetic nanoparticle supported zeolitic imidazolate framework-8: An efficient adsorbent for the enrichment of triazine herbicides from fruit, vegetables, and water. J. Sep. Sci. 2017, 40, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Piao, H.; Qin, Z.; Li, X.; Ma, P.; Sun, Y.; Wang, X.; Song, D. One-step synthesized magnetic MIL-101(Cr) for effective extraction of triazine herbicides from rice prior to determination by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2019, 42, 2900–2908. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Cai, Z.; Dong, J.; Wang, S.; Wang, Y.; Kang, H.; Chen, X. Preparation of porous zinc ferrite/carbon as a magnetic-assisted dispersive miniaturized solid phase extraction sorbent and its application. J. Chromatogr. A 2018, 1567, 73–80. [Google Scholar] [CrossRef]
- Li, D.; He, M.; Chen, B.; Hu, B. Metal organic frameworks-derived magnetic nanoporous carbon for preconcentration of organophosphorus pesticides from fruit samples followed by gas chromatography-flame photometric detection. J. Chromatogr. A 2019, 1583, 19–27. [Google Scholar] [CrossRef]
- Amiri, A.; Tayebee, R.; Abdar, A.; Narenji Sani, F. Synthesis of a zinc-based metal-organic framework with histamine as an organic linker for the dispersive solid-phase extraction of organophosphorus pesticides in water and fruit juice samples. J. Chromatogr. A 2019, 1597, 39–45. [Google Scholar] [CrossRef]
- Xia, J.; Xu, W.; Li, Y.; Li, G.; Wang, X.; Xiong, Y.; Min, S. Quality control of pesticide using infrared spectroscopic coupled with fingerprint analysis. Infrared Phys. Technol. 2022, 122, 104052. [Google Scholar] [CrossRef]
- dos Santos, G.F.S.; Paganoto, G.T.; Cosme, L.C.; Prado, A.R.; Cassini, S.T.A.; Guimarães, M.C.C.; de Oliveira, J.P. Surface-enhanced Raman spectroscopy as a tool for food and environmental monitoring of pesticides: Recent trends and perspectives. Nanoscale Adv. 2025, 7, 7061–7085. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Q.; Meng, G.; Wang, X.; Hu, X.; Han, F.; Lei, Y. Silver nanoparticle-assembled micro-bowl arrays for sensitive SERS detection of pesticide residue. Nanotechnology 2020, 31, 205303. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Zhu, W.; Li, P.; Yuan, H.; Zhang, X.; Zheng, L.; Zhao, J.; Huang, L.; Han, P. Dynamic surface-enhanced Raman spectroscopy for the detection of acephate residue in rice by using gold nanorods modified with cysteamine and multivariant methods. Food Chem. 2020, 310, 125855. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Wang, L.; Xu, L.; Li, J.; Zhang, X.; Chen, H. Rapid quantitative determination of chlorpyrifos pesticide residues in tomatoes by surface-enhanced Raman spectroscopy. Eur. Food Res. Technol. 2020, 246, 239–251. [Google Scholar] [CrossRef]
- Asgari, S.; Wu, G.; Aghvami, S.A.; Zhang, Y.; Lin, M. Optimisation using the finite element method of a filter-based microfluidic SERS sensor for detection of multiple pesticides in strawberry. Food Addit. Contam. Part A 2021, 38, 646–658. [Google Scholar] [CrossRef]
- Balaji, R.; Vengudusamy, R.; Chen, S.-M.; Chen, T.-W.; Liu, X.; Khan, M.R.; Alothman, Z.A.; Ajmal Ali, M.; Wabaidur, S.M. High-performance SERS detection of pesticides using BiOCl-BiOBr@Pt/Au hybrid nanostructures on styrofoams as 3D functional substrate. Microchim. Acta 2020, 187, 580. [Google Scholar] [CrossRef]
- Zhao, B.; Feng, S.; Hu, Y.; Wang, S.; Lu, X. Rapid determination of atrazine in apple juice using molecularly imprinted polymers coupled with gold nanoparticles-colorimetric/SERS dual chemosensor. Food Chem. 2019, 276, 366–375. [Google Scholar] [CrossRef]
- Yande, L.; Yuxiang, Z.; Haiyang, W.; Bing, Y. Detection of pesticides on navel orange skin by surface-enhanced Raman spectroscopy coupled with Ag nanostructures. Int. J. Agric. Biol. Eng. 2016, 9, 179–185. [Google Scholar] [CrossRef]
- Wu, S.; Li, D.; Gao, Z.; Wang, J. Controlled etching of gold nanorods by the Au(III)-CTAB complex, and its application to semi-quantitative visual determination of organophosphorus pesticides. Microchim. Acta 2017, 184, 4383–4391. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Fiber optic profenofos sensor based on surface plasmon resonance technique and molecular imprinting. Biosens. Bioelectron. 2016, 79, 150–157. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, R.; Liu, Y.; Xiang, D.; Liu, Y.; Gui, W.; Li, M.; Zhu, G. A non-competitive surface plasmon resonance immunosensor for rapid detection of triazophos residue in environmental and agricultural samples. Sci. Total Environ. 2018, 613–614, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dou, X.; Zhao, X.; Zhang, L.; Luo, J.; Xing, X.; Yang, M. A gold/Fe3O4 nanocomposite for use in a surface plasmon resonance immunosensor for carbendazim. Microchim. Acta 2019, 186, 313. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dou, X.; Zhang, L.; Zhao, X.; Luo, J.; Yang, M. Oriented assembly of surface plasmon resonance biosensor through staphylococcal protein A for the chlorpyrifos detection. Anal. Bioanal. Chem. 2019, 411, 6057–6066. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Z.; Ding, G.; Li, J.; Vasylieva, N.; Li, Q.X.; Li, D.; Gee, S.J.; Hammock, B.D.; Xu, T. Development of a one-step immunoassay for triazophos using camel single-domain antibody–alkaline phosphatase fusion protein. Anal. Bioanal. Chem. 2019, 411, 1287–1295. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Wu, S.; Wang, Z.; Ding, G.; Hao, X.; Li, Q.X.; Li, J.; Gee, S.J.; Hammock, B.D.; et al. Development of an immunoassay for the detection of carbaryl in cereals based on a camelid variable heavy-chain antibody domain. J. Sci. Food Agric. 2019, 99, 4383–4390. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Wang, Y.; Dong, J.-X.; Yang, J.-Y.; Zhang, Y.-Q.; Wang, F.; Si, R.; Xu, Z.-L.; Wang, H.; Xiao, Z.-L.; et al. Development of a Simple Pretreatment Immunoassay Based on an Organic Solvent-Tolerant Nanobody for the Detection of Carbofuran in Vegetable and Fruit Samples. Biomolecules 2019, 9, 576. [Google Scholar] [CrossRef]
- Liu, R.; Liang, X.; Xiang, D.; Guo, Y.; Liu, Y.; Zhu, G. Expression and Functional Properties of an Anti-Triazophos High-Affinity Single-Chain Variable Fragment Antibody with Specific Lambda Light Chain. Int. J. Mol. Sci. 2016, 17, 823. [Google Scholar] [CrossRef]
- Xu, B.; Wang, K.; Vasylieva, N.; Zhou, H.; Xue, X.; Wang, B.; Li, Q.X.; Hammock, B.D.; Xu, T. Development of a nanobody-based ELISA for the detection of the insecticides cyantraniliprole and chlorantraniliprole in soil and the vegetable bok choy. Anal. Bioanal. Chem. 2021, 413, 2503–2511. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Xu, Z.-L.; Wang, F.; Cai, J.; Dong, J.-X.; Zhang, J.-R.; Si, R.; Wang, C.-L.; Wang, Y.; Shen, Y.-D.; et al. Isolation of Bactrian Camel Single Domain Antibody for Parathion and Development of One-Step dc-FEIA Method Using VHH-Alkaline Phosphatase Fusion Protein. Anal. Chem. 2018, 90, 12886–12892. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Q.; Zhang, Z.; Ding, X.; Jiang, J.; Zhang, W.; Li, P. Rapid, on-site and quantitative paper-based immunoassay platform for concurrent determination of pesticide residues and mycotoxins. Anal. Chim. Acta 2019, 1078, 142–150. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Park, S.J.; Kwon, C.; Noh, H. Development of Colorimetric Paper Sensor for Pesticide Detection Using Competitive-inhibiting Reaction. BioChip J. 2018, 12, 326–331. [Google Scholar] [CrossRef]
- Busa, L.S.A.; Mohammadi, S.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis. Micromachines 2016, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Tripathi, P.; Kumar, N.; Nara, S. Gold nanorods as peroxidase mimetics and its application for colorimetric biosensing of malathion. Sens. Actuators B Chem. 2016, 231, 584–592. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, P.; Kumar, N.; Nara, S. Colorimetric sensing of malathion using palladium-gold bimetallic nanozyme. Biosens. Bioelectron. 2017, 92, 280–286. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, D.; Wu, J.; Liu, C.; Chen, J. A target-induced and equipment-free biosensor for amplified visual detection of pesticide acetamiprid with high sensitivity and selectivity. Anal. Methods 2019, 11, 1168–1173. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Q.; Li, H.; Ouyang, Q.; Zhao, J. Fabricating a novel label-free aptasensor for acetamiprid by fluorescence resonance energy transfer between NH2-NaYF4: Yb, Ho@SiO2 and Au nanoparticles. Biosens. Bioelectron. 2016, 80, 398–404. [Google Scholar] [CrossRef]
- Lu, X.; Fan, Z. RecJf exonuclease-assisted fluorescent self-assembly aptasensor for supersensitive detection of pesticides in food. J. Lumin. 2020, 226, 117469. [Google Scholar] [CrossRef]
- Bagheri, N.; Khataee, A.; Hassanzadeh, J.; Habibi, B. Sensitive biosensing of organophosphate pesticides using enzyme mimics of magnetic ZIF-8. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 209, 118–125. [Google Scholar] [CrossRef]
- Tong, X.; Cai, G.; Xie, L.; Wang, T.; Zhu, Y.; Peng, Y.; Tong, C.; Shi, S.; Guo, Y. Threaded 3D microfluidic paper analytical device-based ratiometric fluorescent sensor for background-free and visual detection of organophosphorus pesticides. Biosens. Bioelectron. 2023, 222, 114981. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Huangfu, C.; Zhu, M.; Li, C.; Feng, L. The BODIPY-based chemosensor for the fluorometric determination of organochlorine pesticide dicofol. Food Chem. 2022, 370, 131033. [Google Scholar] [CrossRef]
- He, L.; Jiang, Z.W.; Li, W.; Li, C.M.; Huang, C.Z.; Li, Y.F. In Situ Synthesis of Gold Nanoparticles/Metal–Organic Gels Hybrids with Excellent Peroxidase-Like Activity for Sensitive Chemiluminescence Detection of Organophosphorus Pesticides. ACS Appl. Mater. Interfaces 2018, 10, 28868–28876. [Google Scholar] [CrossRef]
- He, Y.; Xu, B.; Li, W.; Yu, H. Silver Nanoparticle-Based Chemiluminescent Sensor Array for Pesticide Discrimination. J. Agric. Food Chem. 2015, 63, 2930–2934. [Google Scholar] [CrossRef]
- Cahyadi, E.R.; Hidayati, N.; Zahra, N.; Arif, C. Integrating Circular Economy Principles into Agri-Food Supply Chain Management: A Systematic Literature Review. Sustainability 2024, 16, 7165. [Google Scholar] [CrossRef]
- Mihajlović, I.; Hgeig, A.; Novaković, M.; Gvoić, V.; Ubavin, D.; Petrović, M.; Kurniawan, T.A. Valorizing Date Seeds into Biochar for Pesticide Removal: A Sustainable Approach to Agro-Waste-Based Wastewater Treatment. Sustainability 2025, 17, 5129. [Google Scholar] [CrossRef]
- Goumas, G.; Vlachothanasi, E.N.; Fradelos, E.C.; Mouliou, D.S. Biosensors, Artificial Intelligence Biosensors, False Results and Novel Future Perspectives. Diagnostics 2025, 15, 1037. [Google Scholar] [CrossRef]

| Agro-Industrial Precursor | Synthesis Conditions | Pesticide | Adsorption Capacity (mg/g) | Specific Surface Area (m2/g) | Reference |
|---|---|---|---|---|---|
| Rice straw | Dried (60 °C, 24 h, <10% moisture), cut (<5 cm), pyrolyzed at 600 °C (N2, 3 °C min−1, 1 h); ground, sieved (60 BSS), stored in PTFE. | Atrazine | 838.1 | 220.2 | [80] |
| Imidacloprid | 852.3 | ||||
| Olive kernel | Dried, milled (1 mm), oven-dried (105 °C, 24 h); pyrolyzed at 800 °C (N2, 27 °C min−1, 1 h); steam-activated (800 °C, 30 min, 0.5 bar). | Bromopropylate | 16.88 | 600 | [81] |
| Corn cobs | 26.64 | 630 | |||
| Rapeseed stalks | 106.29 | 490 | |||
| Soya stalks | 59.44 | 570 | |||
| Spent coffee grounds | Washed, dried (RT, 24 h), carbonized at 900 °C (N2, 5 °C min−1, 1 h); impregnated with H3PO4 (1:2), re-carbonized (900 °C, 1 h); washed (NaOH, water, EtOH), dried, stored | Malathion | 92.0 | 846 | [82] |
| Chlorpirifos | 259 | ||||
| Spent coffee grounds | Washed, dried (RT, 24 h), carbonized at 400 °C (N2, 100 L h−1, 1 h). activation: chemical (KOH or H3PO4, 1 mol dm−3, 2:1), physical (CO2), or combined activation. | Malathion | 11.2 | 6 | [83] |
| Chlorpirifos | 16.1 | ||||
| Fig pomace | Dried, pyrolyzed at 500 °C (N2, 5 °C min−1, 2 h); acidified (HCl, pH 5.0), dried (60 °C, 24 h); modified by γ-irradiation (Co60, 50 kGy, 8 kGy h−1). | Malathion | 0.625 | - | [84] |
| Chlorpyrifos | 0.495 | ||||
| Plum pomace | Dried, pyrolyzed at 500 °C (N2, 5 °C min−1, 2 h); acidified (HCl, pH 5.0), dried (60 °C, 24 h); modified by γ-irradiation (Co60, 50 kGy, 8 kGy h−1). | Malathion | 1.067 | - | [85] |
| Chlorpyrifos | 0.219 | ||||
| Pine bark | Ground (1–2 mm), washed, dried (30 °C, 24 h). Modified with NaOH (2.5 mol dm−3), HNO3 (1 mol dm−3), or H2O2 (1 mol dm−3) (180 rpm, 24 h, 25 °C); washed, dried (50 °C, 48 h). HTC hydrochar: 220 °C, 12 h, 25% solid slurry. | Atrazine | 0.522 | - | [86] |
| Corn cob | Sun-dried (1 week), oven-dried (105 °C, 24 h), pyrolyzed 400–600 °C (O2-limited, 3 °C min−1, 2–6 h). | Atrazine | 19.58 | 303.36 | [87] |
| Tea waste | Air-dried, ground (<1 mm), pyrolyzed at 700 °C (N2, 7 °C min−1, 2 h). With steam activation (5 mL min−1 steam, last 45 min). | 2,4-Dichlorophenoxy acetic acid | 58.8 | 576 | [88] |
| Coconut shell | Slow pyrolysis at 700 °C (7 °C min−1, 2 h). Modified with H3PO4 (1 mol dm−3, BC3) or NaOH (1 mol dm−3, BC4); washed, dried (80 °C, 24 h), sieved <1 mm. | Diazinon | 10.33 | 508 | [89] |
| Sugarcane bagasse | Sun-dried (1 week), cut, pyrolyzed at 500 °C (10 °C min−1, 30 min, O2-limited). | Carbofuran | 18.9 | 148.23 | [90] |
| Palm Oil Fronds | Dried, carbonized at 700 °C (10 °C min−1, 2 h, N2 150 cm3 min−1), impregnated with KOH (2.75:1), activated at 850 °C (N2/CO2, 1 h); washed (0.1 mol dm−3 HCl, H2O). | Carbofuran | 164 | 1237.13 | [91] |
| Lotus seedpod | Dried (85 °C, 24 h), sieved (<0.38 mm), impregnated with 85% H3PO4 (1:1–1:12, 24 h), pyrolyzed at 500–900 °C (10 °C min−1, 30–240 min); washed to neutral pH, dried (85 °C, 24 h), ground (<0.38 mm). | Metolcarb | 12.6537 | 728.23 | [92] |
| Isoprocarb | 12.9326 | ||||
| Pirimicarb | 18.4554 | ||||
| Methiocarb | 19.2862 | ||||
| Carbaryl | 19.3357 | ||||
| Nocino walnut | Walnut pomace dried (90 °C, 2 h; RT, 10 d), carbonized at 900 °C (N2, 5 °C min−1, 1 h). One sample was further treated with CO2 (100 L h−1, 1 h). | Chlorpyrifos | 45.2 | 737 | [17] |
| Method | Advantages | Limitations |
|---|---|---|
| Chromatographic techniques (GC–MS, LC–MS/MS) | Very high sensitivity and selectivity Capable of multi-residue analysis Widely accepted for regulatory compliance | Expensive instrumentation Requires trained personnel Time-consuming sample preparation Limited applicability in field settings |
| Spectroscopic methods (IR, Raman, UV-Vis) | Rapid and non-destructive Minimal sample preparation Suitable for high-throughput screening | Lower selectivity compared to chromatography Susceptible to matrix interferences Limited sensitivity for trace-level detection |
| Rapid/field methods (biosensors, immunoassays, test strips) | Portable and user-friendly Quick results (minutes) Useful for on-site monitoring in agriculture and food supply chains | Lower accuracy and reproducibility Limited detection range Stability issues under variable environmental conditions Often requires calibration |
| Pesticide | Type of Food | Method for Detection | Linearity Range | LOD | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| Atrazine and deltamethrin | Tomatoes, cucumbers, and brinjal | HPLC-UV-Vis | 0.01–100.0 µg/L and 0.05–100 µg/L | 0.01–0.05 µg/L | 0.03 and 0.15 µg/L | 80.9–98.6 | [112] |
| Triazine | Water, tea, and juice samples | HPLC-DAD | 1 –100 μg/L | 0.3 μg/L | 1 μg/L | 80.1–90.6 | [113] |
| Metolcarb, isoprocarb, and diethofencarb | Cabbage | HPLC-VWD | 0.5 –100 ng/g | 0.25–0.1 ng/g | - | 92.4–99.6 | [114] |
| Cyanazine, prometon, propazine, and prometryn | White gourd | HPLC-DAD | 0.5–100 ng/g | 0.1–0.2 ng/g | 4.6–5.7 ng/g | 80.3–120.6 | [115] |
| Triazine | Potato, carrot, and lettuce | HPLC-DAD | 0.0061–70 ng/mL | 2.0–5.3 ng/mL | - | 97.5–103.0 | [116] |
| Epoxiconazole, flusilazole, tebuconazole, and triadimefon | Honey, mango, grape, and orange juice | HPLC-DAD | 1–1000 µg/L | 0.014–0.109 µg/L | 0.047–0.365 µg/L | - | [117] |
| Triazine | Rice | HPLC-UV-Vis | - | 0.010–0.080 µg/kg | - | 83.9–103.5 | [118] |
| Sulfometuron methyl, bensulfuron methyl, pyrazosulfuron ethyl, and chlorimuron ethyl | Pakchoi, spinach, and celery | HPLC-PDA | 1 –150 μg/L | 0.12–0.34 µg/L | - | 87.1–108.9 | [119] |
| Epoxiconazole, fenbuconazole, difenoconazole, thiabendazole, and pyraclostrobin | Lettuce | HPLC–MS | 1.0–500 μg/L | 0.25–1.0 µg/L | - | 78.5–87.3 | [120] |
| Triazine | Corn | HPLC–MS/MS | 2.0–200.0 ng/g | 0.01–0.12 ng/g | 0.04–0.35 ng/g | 73.37–107.37 | [121] |
| Atrazine and prometryn | Pakchoi, lettuce, apple, pear, and strawberry | HPLC-PDA | 0.5–200 ng/mL | 0.18–0.72 ng/mL | - | 88.0–101.9 | [122] |
| Triazine | Rice | LC-MS | 0.10 and 20 ng/g | 1.08–18.10 pg/g | 3.60–60.20 pg/g | 79.3–116.7 | [123] |
| Chlordane, heptachlor, lindane, and aldrin | Pepper | GC-ECD | 0.05–100 ng/g | 0.005–0.3 ng/g | 0.017–0.1 ng/g | 86.1–109.4 | [124] |
| Phorate, diazinon, ethion, malathion, and fenthion | Apple, grape, pear, tomato, and green jujube | GC-FPD | 0.05–100 μg/L | 0.018 –0.045 µg/L | 3–6 μg/L | 84–116 | [125] |
| Profenofos, phosalone, fenitrothion, and fenthion | Fruit juice | GC-FID | 0.1 –100 ng/mL | 0.03–0.21 ng/mL | - | 91.9–99.5 | [126] |
| Pesticide | Type of Food | Method for Detection | Linearity Range | LOD | Recovery (%) | Reference |
|---|---|---|---|---|---|---|
| Methyl parathion | Chinese cabbage | SERS | - | 1.26 µg/kg | - | [129] |
| Acephate | Rice samples | SERS | 100.2–0.5 mg/L | 0.5 mg/L | - | [130] |
| Chlorpyrifos | Tomato | SERS | 10−3–10−9 mol/L | 10−9 mol/L | - | [131] |
| Malathion | Strawberry | SERS | - | 100 µg/kg | 90–122 | [132] |
| Cypermethrin | Kiwi | SERS | - | 10−10 M | - | [133] |
| Atrazine | Apple juice | SERS | - | 0.0012 mg/L | 93 | [134] |
| Phosmet, chlorpyrifos, and carbaryl | Orange, apple, and tomato | SERS | 5–30 mg/L | 2.94–6.66 µg/kg | - | [135] |
| Parathion | Cabbage washing solutions | SPR | 0.01–1.84 mg/L | 1.2 µg/L | 86–114 | [136] |
| Profenofos | Water | SPR | 10−4–10−1 µg/L | 2.5 × 10−6 µg/L | - | [137] |
| Triazophos | Cabbage, cucumber, and apple | SPR | 0.98–8.29 ng/mL | 0.096 ng/mL | 84–109 | [138] |
| Carbendazim | Medlar | SPR | 0.05–150 ng/mL | 0.44 ng/mL | 102.4–115.0 | [139] |
| Chlorpyrifos | Maize, apple, cabbage, and medlar | SPR | 0.25–50.0 ng/mL | 0.056 ng/mL | 86.9–119.2 | [140] |
| Pesticide | Type of Food | Method of Detection | LOD | IC50 | Reference |
|---|---|---|---|---|---|
| Triazophos | Water and apple | ELISA | / | 6.6 ng/mL | [141] |
| Carbaryl | Rice, maize, and wheat | ELISA | 0.3 ng/mL | 5.4 ng/mL | [142] |
| Carbofuran | Chinese cabbage, cucumber, and orange | Ic-ELISA | 0.65 ng/mL | 7.2 ng/mL | [143] |
| Triazophos | Water | Ic-ELISA | / | 1.73 ng/mL | [144] |
| Cyantraniliprole and chlorantraniliprole | Bok choy | ELISA | 1.2 ng/mL | 1.5 ng/mL | [145] |
| Parathion | Chinese cabbage, cucumber, and lettuce | FEA | 0.2 ng/mL | 1.6 ng/mL | [146] |
| Carbaryl and carbofuran | Corn | paper-based immunoassay | 0.02 and 60.2 ng/mL | 0.8 ng/mL and 217.6 ng/mL | [147] |
| Pesticide | Type of Food | Method for Detection | Linearity Range | LOD | Recovery (%) | Reference |
|---|---|---|---|---|---|---|
| Chlorpyrifos | Vegetable | Colorimetric sensor | 0–25 mg/kg | 8.6 mg/kg | - | [148] |
| Methyl-paraoxon and chlorpyrifos-oxon | Vegetable | Colorimetric sensor | 0.1–0.9 µg/mL | 5.3–18 ng/mL | 95 | [149] |
| Malathion | Tap water | Colorimetric biosensor | / | 1.78 µg/mL | 103 | [150] |
| Malathion | Water | Colorimetric biosensor | / | 60 ng/mL | 80–106 | [151] |
| Acetamiprid | Chinese cabbage, tomato, eggplant, and cucumber | Colorimetric biosensor | / | 10 pM | 87–105 | [152] |
| Acetamiprid | Tea | Fluorescence biosensor | 50–100 nM | 3.2 nM | 97.57–102.25 | [153] |
| Acetamiprid | Honey and orange juice | Fluorescence biosensor | 5 nM–1.2 μM | 3 nM | 94.9–104.2 | [154] |
| Diazinon | Water and fruit juices | Fluorescence biosensor | 0.5–500 nM | 0.2 nM | 95.07–101.90 | [155] |
| Dichlorvos | Tomato and spinach | Fluorescent sensor | 2.5–120 μg/L | 1.0 μg/L | 94.0–106.0 | [156] |
| Dicofol | Tea | Fluorometric chemosensor | 0–10 mg/L | 200 μg/L | 72.2–97.5 | [157] |
| Ethoprophos | Tap water | Chemiluminescent biosensor | 5–800 nM | 1 nM | 95.5–106.5 | [158] |
| Dimethoate, dipterex, carbofuran, chlorpyrifos, and carbaryl | Water | Chemiluminescent biosensor | / | 24 µg/mL | - | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarević-Pašti, T.; Tasić, T.; Milanković, V.; Pašti, I.A. Food Safety in the Age of Climate Change: The Rising Risk of Pesticide Residues and the Role of Sustainable Adsorbent Technologies. Foods 2025, 14, 3797. https://doi.org/10.3390/foods14213797
Lazarević-Pašti T, Tasić T, Milanković V, Pašti IA. Food Safety in the Age of Climate Change: The Rising Risk of Pesticide Residues and the Role of Sustainable Adsorbent Technologies. Foods. 2025; 14(21):3797. https://doi.org/10.3390/foods14213797
Chicago/Turabian StyleLazarević-Pašti, Tamara, Tamara Tasić, Vedran Milanković, and Igor A. Pašti. 2025. "Food Safety in the Age of Climate Change: The Rising Risk of Pesticide Residues and the Role of Sustainable Adsorbent Technologies" Foods 14, no. 21: 3797. https://doi.org/10.3390/foods14213797
APA StyleLazarević-Pašti, T., Tasić, T., Milanković, V., & Pašti, I. A. (2025). Food Safety in the Age of Climate Change: The Rising Risk of Pesticide Residues and the Role of Sustainable Adsorbent Technologies. Foods, 14(21), 3797. https://doi.org/10.3390/foods14213797









