Replacing Manual Operation with Bio-Automation II: Construction of a Biological Digestion Gene Circuit to Eliminate the Interference of Food Matrices in the Rapid Detection of Heavy Metals
Abstract
1. Introduction

2. Materials and Methods
2.1. Chemicals and Bacteria
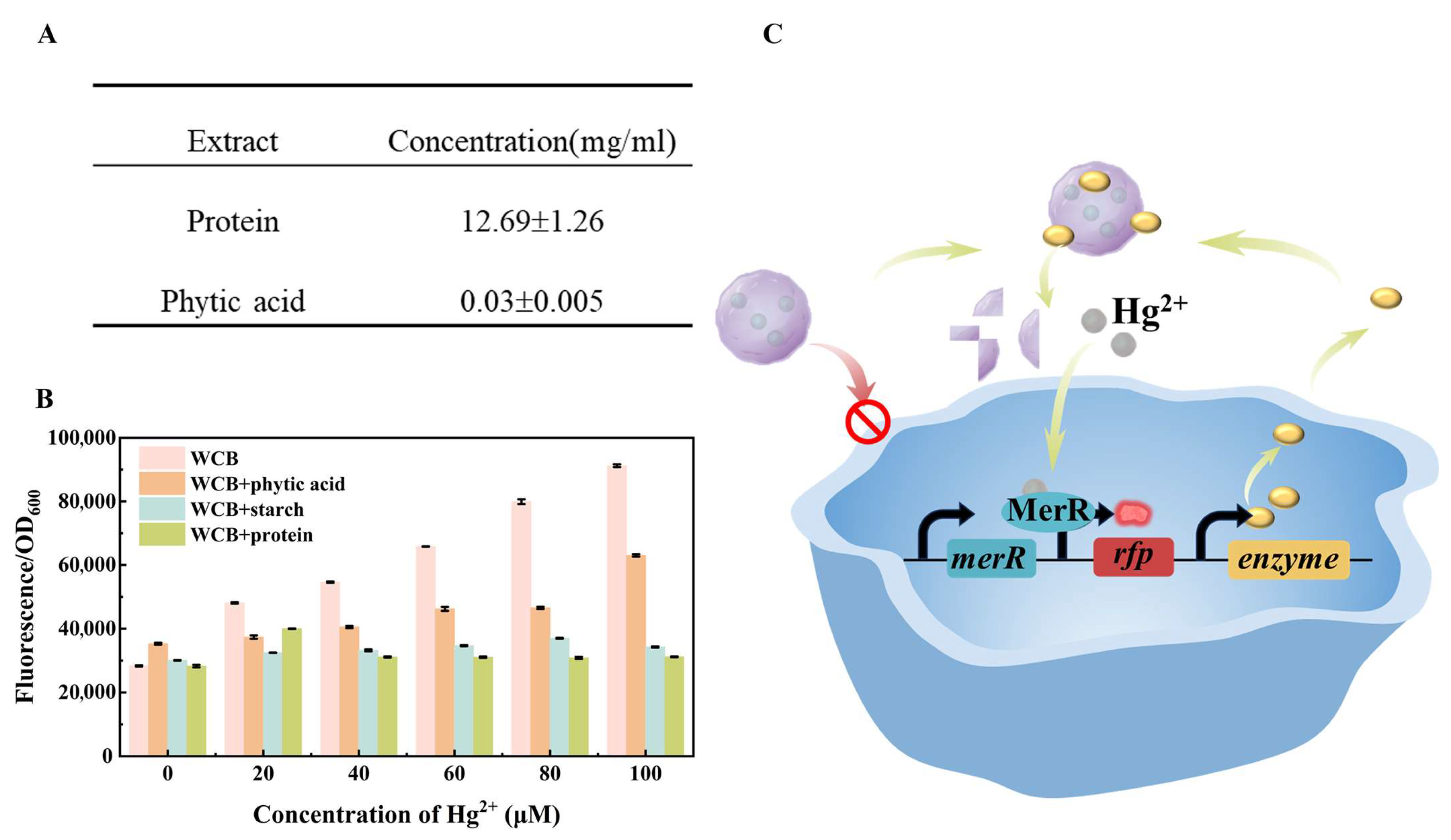
2.2. Extraction of Matrix Ingredients from Different Foods
2.3. Bioinformatics Analysis of Microbial Matrix Lyase Genes
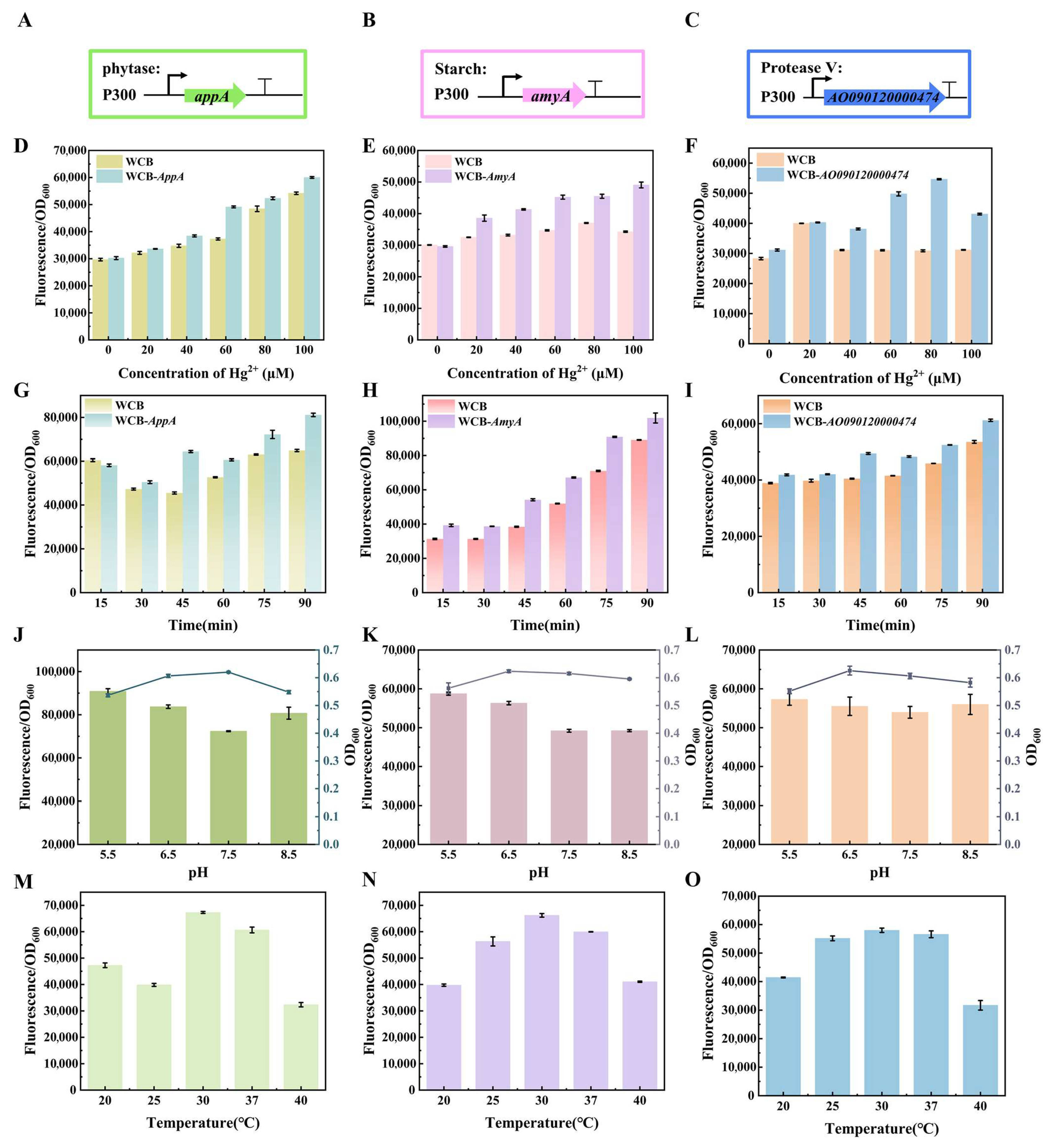
2.4. Construction of a Matrix Digestion Whole-Cell Biosensor
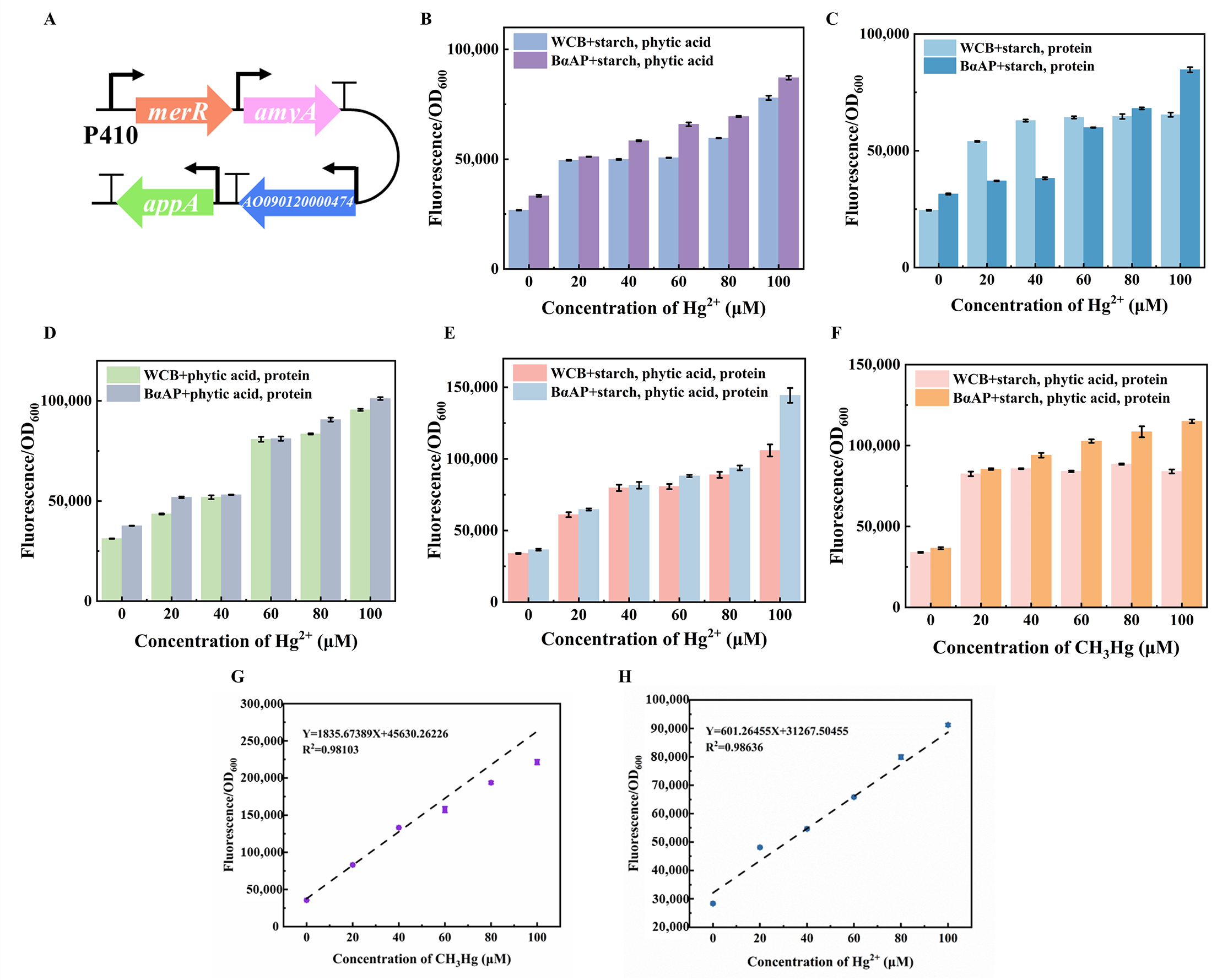
2.5. Construction of a Heavy Metal Pollutant Bio-Digestion Pathway
2.6. Mercury and Methylmercury Detection Procedures
2.7. Impact of Environmental Factors on Whole-Cell Biosensors
2.8. Biosensor-Based Analysis of Mercury and Methylmercury Chelation State in Fish, Rice, and Black Beans
2.9. Statistics Analysis
3. Results and Discussion
3.1. The Effect of Food Matrix on the Accuracy of Whole-Cell Biosensor for Mercury Detection
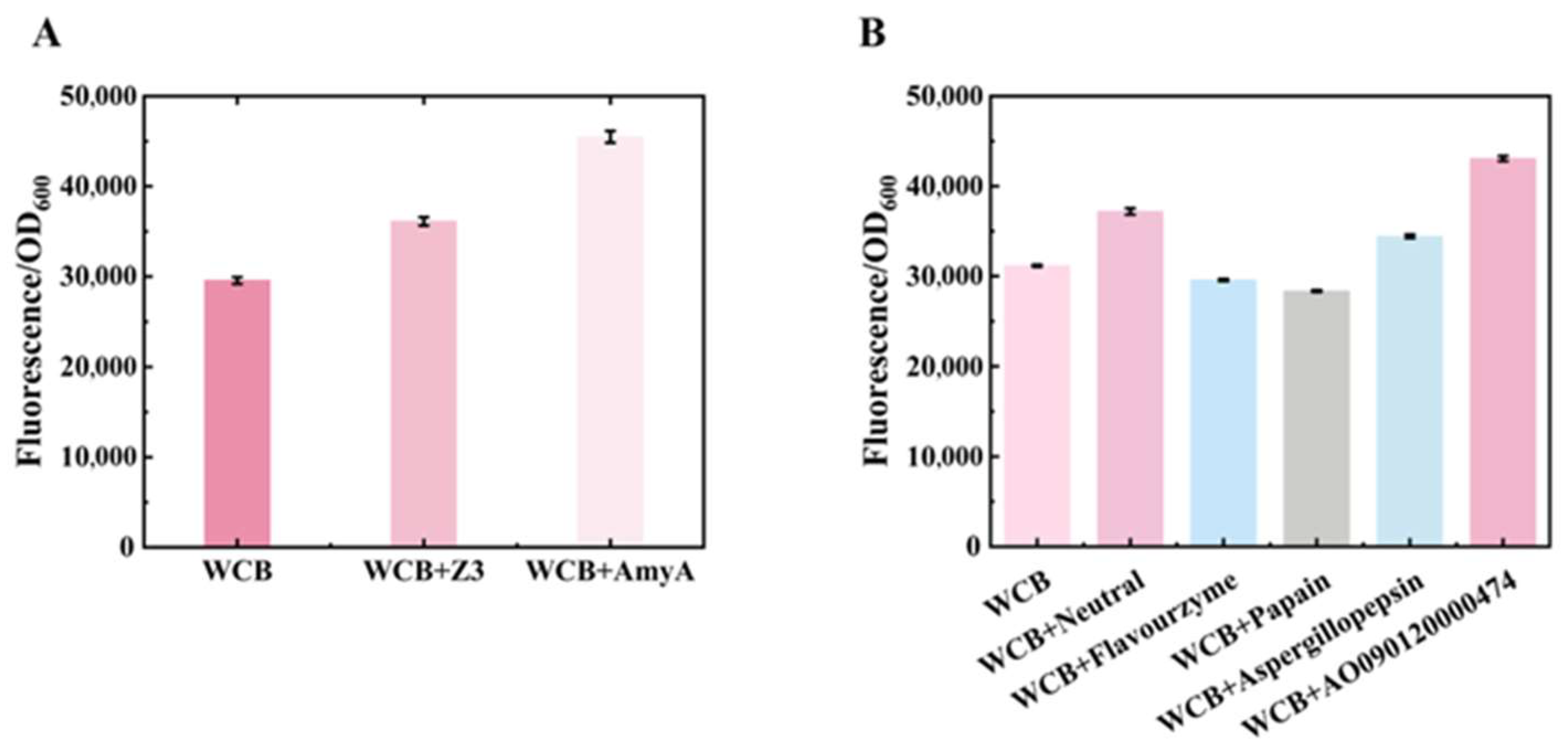
3.2. Screening of Enzymes for the Digestion of Food Matrix Components and Their Impact on Whole Cell Biosensor Performance
3.3. Construction of the Biological Digestion Gene Circuit
3.4. Integration of the Biological Digestion Gene Circuit with Methylmercury Whole-Cell Biosensor
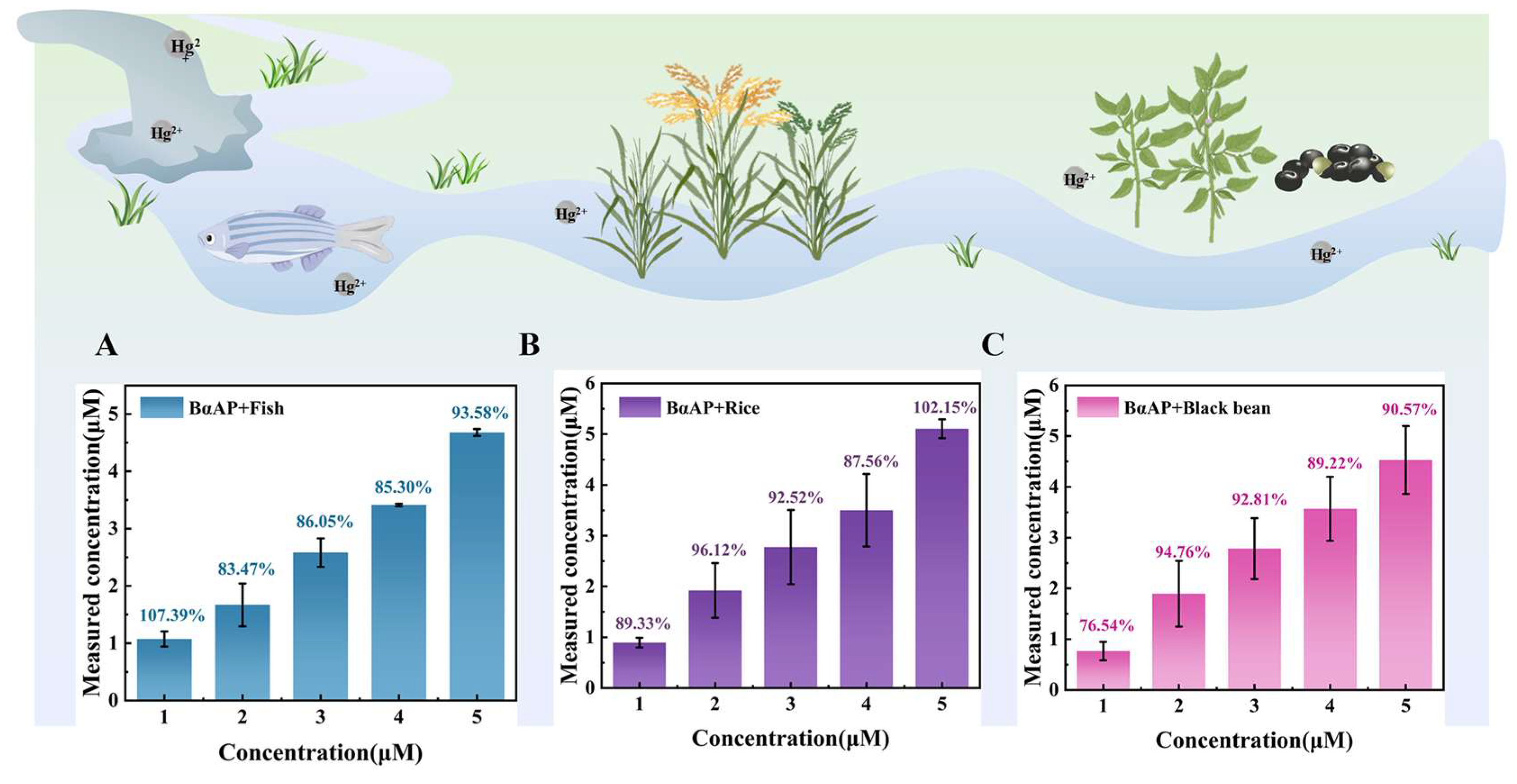
3.5. Application of the Mercury Whole-Cell Biosensor with Digestion Function in Real Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Mercury. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 22 April 2025).
- Spiegel, S.J. New mercury pollution threats: A global health caution. Lancet 2017, 390, 226–227. [Google Scholar] [CrossRef]
- Guo, Y.; Hui, C.-y.; Liu, L.; Chen, M.-p.; Huang, H.-y. Development of a bioavailable Hg(II) sensing system based on MerR-regulated visual pigment biosynthesis. Sci. Rep. 2021, 11, 13516. [Google Scholar] [CrossRef]
- Hui, C.-y.; Guo, Y.; Li, L.-m.; Liu, L.; Chen, Y.-t.; Yi, J.; Zhang, N.-x. Indigoidine biosynthesis triggered by the heavy metal-responsive transcription regulator: A visual whole-cell biosensor. Appl. Microbiol. Biotechnol. 2021, 105, 6087–6102. [Google Scholar] [CrossRef]
- Zhang, N.X.; Guo, Y.; Li, H.; Yang, X.Q.; Gao, C.X.; Hui, C.Y. Versatile artificial mer operons in Escherichia coli towards whole cell biosensing and adsorption of mercury. PLoS ONE 2021, 16, e0252190. [Google Scholar] [CrossRef] [PubMed]
- Weiping, W.; Ni, L.I. Safety Risk of Heavy Metal Pollution and Its Control of Rice. J. Food Sci. Technol. 2016, 34, 12–20. [Google Scholar]
- Limin, C.A.O.; Ting, Z.; Nasha, M.I. Issues and Countermeasures of Heavy Metals and Toxins in Shellfish. J. Food Sci. Technol. 2015, 33, 12–17. [Google Scholar]
- Cohen, J.T.; Bellinger, D.C.; Shaywitz, B.A. A Quantitative Analysis of Prenatal Methyl Mercury Exposure and Cognitive Development. Am. J. Prev. Med. 2005, 29, 353–353.e324. [Google Scholar] [CrossRef]
- Ashraf, W.; Mian, A. Levels of mercury and arsenic contamination in popular fish and shrimp brands consumed in Saudi Arabia. Bull. Chem. Soc. Ethiop. 2019, 33, 573. [Google Scholar] [CrossRef]
- Londonio, A.; Morzán, E.; Smichowski, P. Determination of toxic and potentially toxic elements in rice and rice-based products by inductively coupled plasma-mass spectrometry. Food Chem. 2019, 284, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Su, H.; Guo, M.; Liu, H. Advances in Synthetic-Biology-Based Whole-Cell Biosensors: Principles, Genetic Modules, and Applications in Food Safety. Int. J. Mol. Sci. 2023, 24, 7989. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, T.-Y.; Woo, M.-A. Trends in sensor development toward next-generation point-of-care testing for mercury. Biosens. Bioelectron. 2021, 183, 113228. [Google Scholar] [CrossRef]
- Şahin, S.; Caglayan, M.O.; Üstündağ, Z. A review on nanostructure-based mercury (II) detection and monitoring focusing on aptamer and oligonucleotide biosensors. Talanta 2020, 220, 121437. [Google Scholar] [CrossRef]
- Clarkson, T.W. Factors involved in heavy metal poisoning. Fed. Proc. 1977, 36, 1634–1639. [Google Scholar]
- Yánez-Jácome, G.S.; Romero-Estévez, D.; Navarrete, H.; Simbaña-Farinango, K.; Vélez-Terreros, P.Y. Optimization of a Digestion Method to Determine Total Mercury in Fish Tissue by Cold Vapor Atomic Fluorescence Spectrophotometry. Methods Protoc. 2020, 3, 45. [Google Scholar] [CrossRef]
- Guo, M.; Chen, X.; Chen, S.; Su, H.; Liu, H.; Xie, G.; Sun, B. Replacing manual operation with bio-automation: A high-throughput evolution strategy to construct an integrated whole-cell biosensor for the simultaneous detection of methylmercury and mercury ions without manual sample digestion. J. Hazard. Mater. 2024, 465, 133492. [Google Scholar] [CrossRef]
- Hu, L.; Su, H.; Chen, S.; Chen, X.; Guo, M.; Liu, H.; Yang, H.; Sun, B. Lab in a cell: A bioautomated and biointegrated whole-cell biosensing platform for food hazards analysis. Trends Food Sci. Technol. 2024, 148, 104489. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Du, R.; Liu, Y.; Chi, J.; He, X.; Huang, K.; Luo, Y.; Xu, W. A test strip platform based on a whole-cell microbial biosensor for simultaneous on-site detection of total inorganic mercury pollutants in cosmetics without the need for predigestion. Biosens. Bioelectron. 2020, 150, 111899. [Google Scholar] [CrossRef]
- Shi, N.; Ruan, C.; Li, Z.; Zhang, D. Extraction Technology of Phytic Acid from Black Bean. Food Ind. 2021, 42, 1–4. [Google Scholar]
- Na, Y.; Jungun, L.; Hee, L.S.; Pawan, K.; Hak, K.J.; Patel, R. Removal of heavy metals by polysaccharide: A review. Polym.-Plast. Technol. Mater. 2020, 59, 1770–1790. [Google Scholar] [CrossRef]
- Rupa, S.A.; Patwary, M.A.M.; Matin, M.M.; Ghann, W.E.; Uddin, J.; Kazi, M. Interaction of mercury species with proteins: Towards possible mechanism of mercurial toxicology. Toxicol. Res. 2023, 12, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Eissa, F.; Younes, A. Fish contamination: Analysis of the EU RASFF notifications over the last 23 years. Food Control 2024, 161, 110404. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, C.; Zhang, Q.; Li, Y.; Chen, Z.; Li, M. Concentrations of cadmium, lead, mercury and arsenic in Chinese market milled rice and associated population health risk. Food Control 2010, 21, 1757–1763. [Google Scholar] [CrossRef]
- Khandare, A.L.; Validandi, V.; Jamalpur, R.P.; Dheeravath, S.; Kurella, S.; Chauhan, A.; Boiroju, N.k.; Thingnganing, L. Potential Health Risks Associated with the Heavy Metal Content in Commonly Consumed Food from Prakasam District of Andhra Pradesh, India. Biol. Trace Elem. Res. 2022, 200, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, S.; Chen, S.; Su, H.; Hu, L.; Qi, X.; Guo, M. Replacing Manual Operation with Bio-Automation II: Construction of a Biological Digestion Gene Circuit to Eliminate the Interference of Food Matrices in the Rapid Detection of Heavy Metals. Foods 2025, 14, 3798. https://doi.org/10.3390/foods14213798
Xia S, Chen S, Su H, Hu L, Qi X, Guo M. Replacing Manual Operation with Bio-Automation II: Construction of a Biological Digestion Gene Circuit to Eliminate the Interference of Food Matrices in the Rapid Detection of Heavy Metals. Foods. 2025; 14(21):3798. https://doi.org/10.3390/foods14213798
Chicago/Turabian StyleXia, Shiqi, Shijing Chen, Hongfei Su, Liangshu Hu, Xiaozhe Qi, and Mingzhang Guo. 2025. "Replacing Manual Operation with Bio-Automation II: Construction of a Biological Digestion Gene Circuit to Eliminate the Interference of Food Matrices in the Rapid Detection of Heavy Metals" Foods 14, no. 21: 3798. https://doi.org/10.3390/foods14213798
APA StyleXia, S., Chen, S., Su, H., Hu, L., Qi, X., & Guo, M. (2025). Replacing Manual Operation with Bio-Automation II: Construction of a Biological Digestion Gene Circuit to Eliminate the Interference of Food Matrices in the Rapid Detection of Heavy Metals. Foods, 14(21), 3798. https://doi.org/10.3390/foods14213798





