Comparative Volatile Profiles of Plain Poached (PP) and Steamed over Water (SW) Wenchang Chicken Analyzed by GC-MS, GC-IMS, and E-Nose
Abstract
1. Introduction
2. Materials and Methods
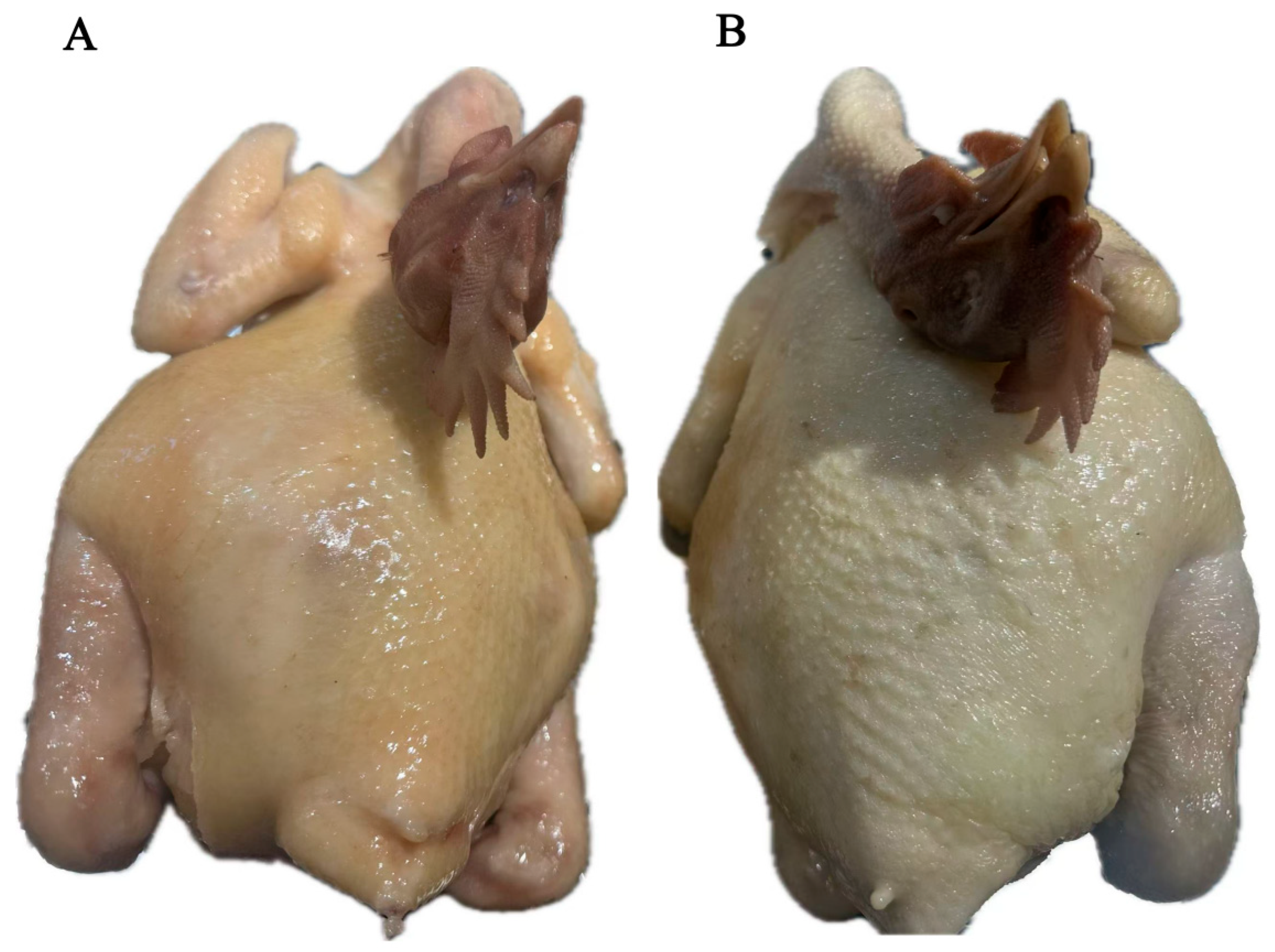
2.1. Samples
2.2. Thermal Treatment of Wenchang Chicken
2.3. Sensory Evaluation
2.4. E-Nose Analysis
2.5. Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS)
2.6. Headspace Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS)
2.7. Data Analysis
3. Results
3.1. Sensory Evaluation
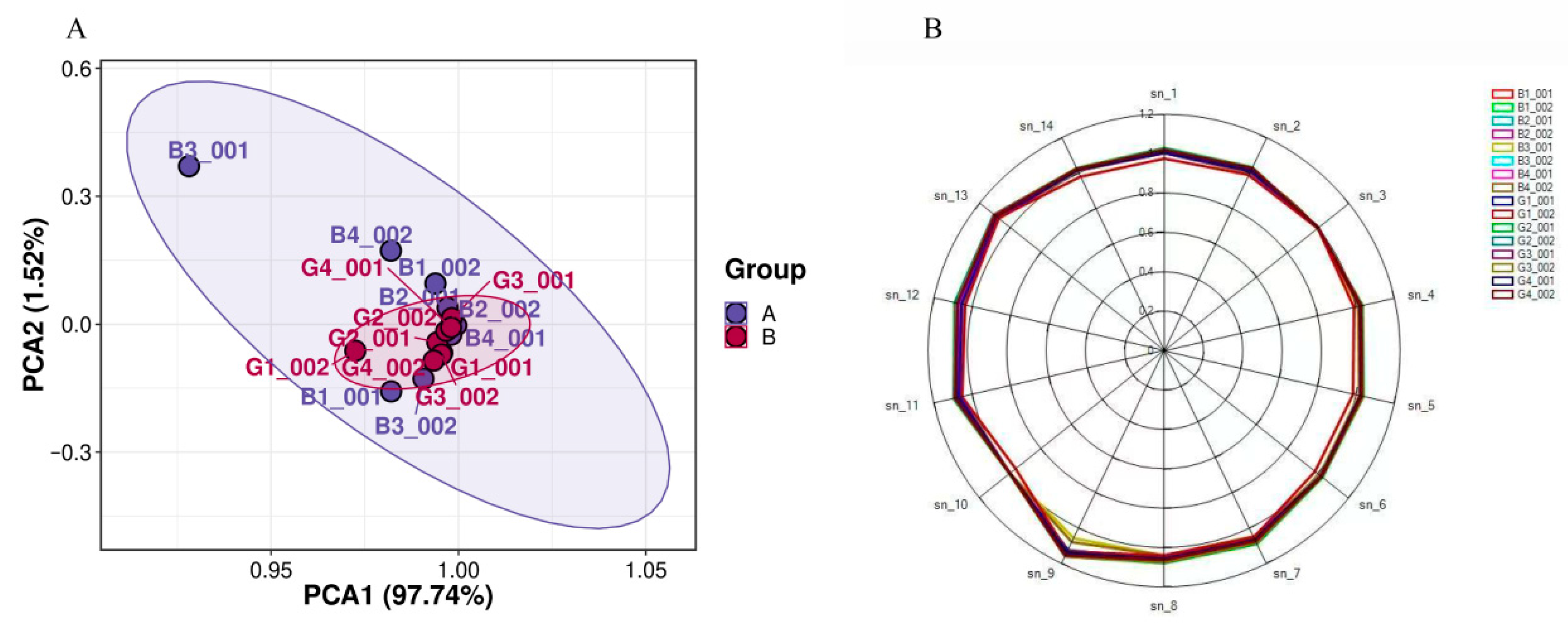
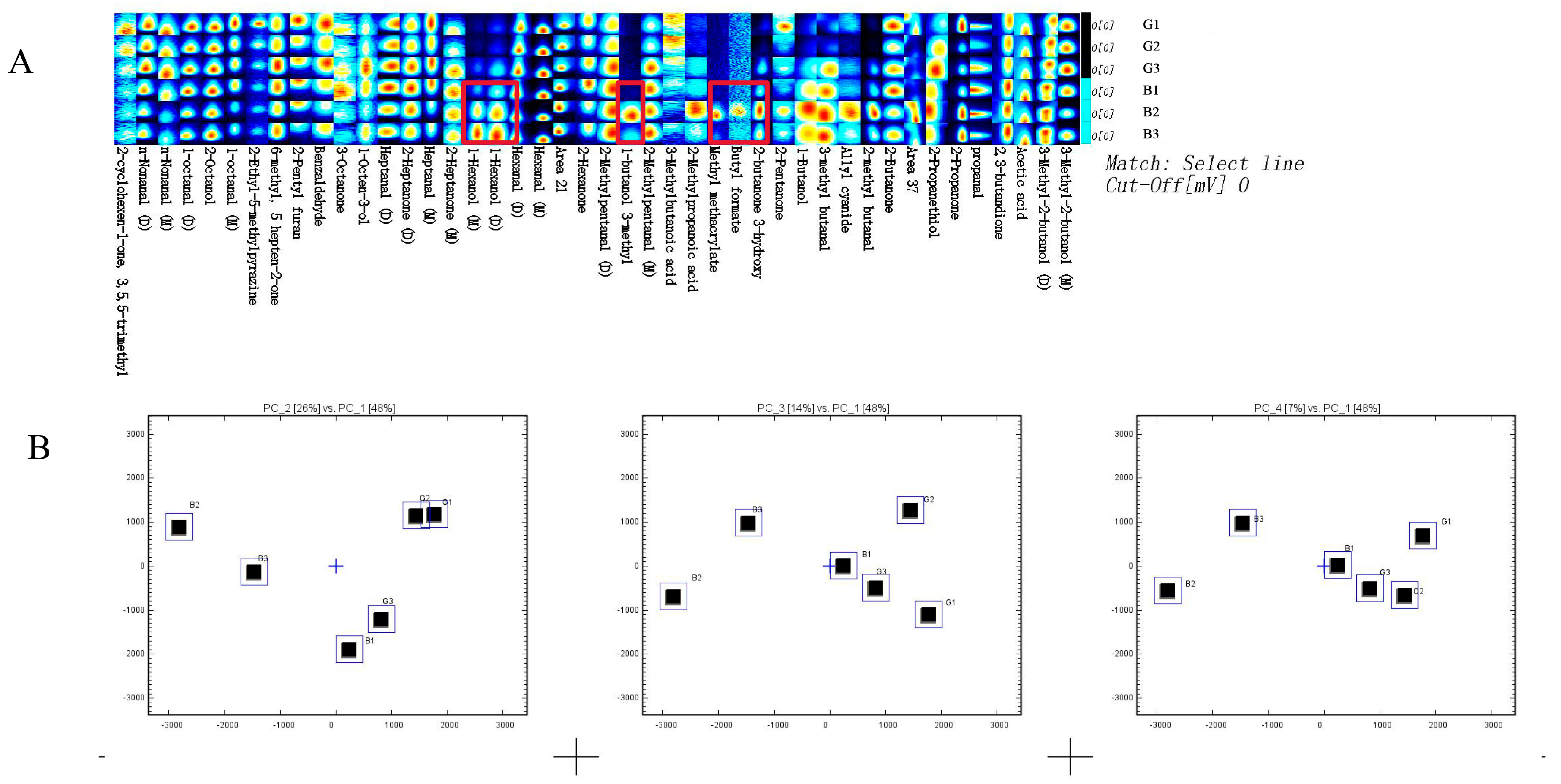
3.2. E-Nose Analysis
3.3. HS-SPME-GC-MS Analysis of VOCs in Boiled and SW Chicken
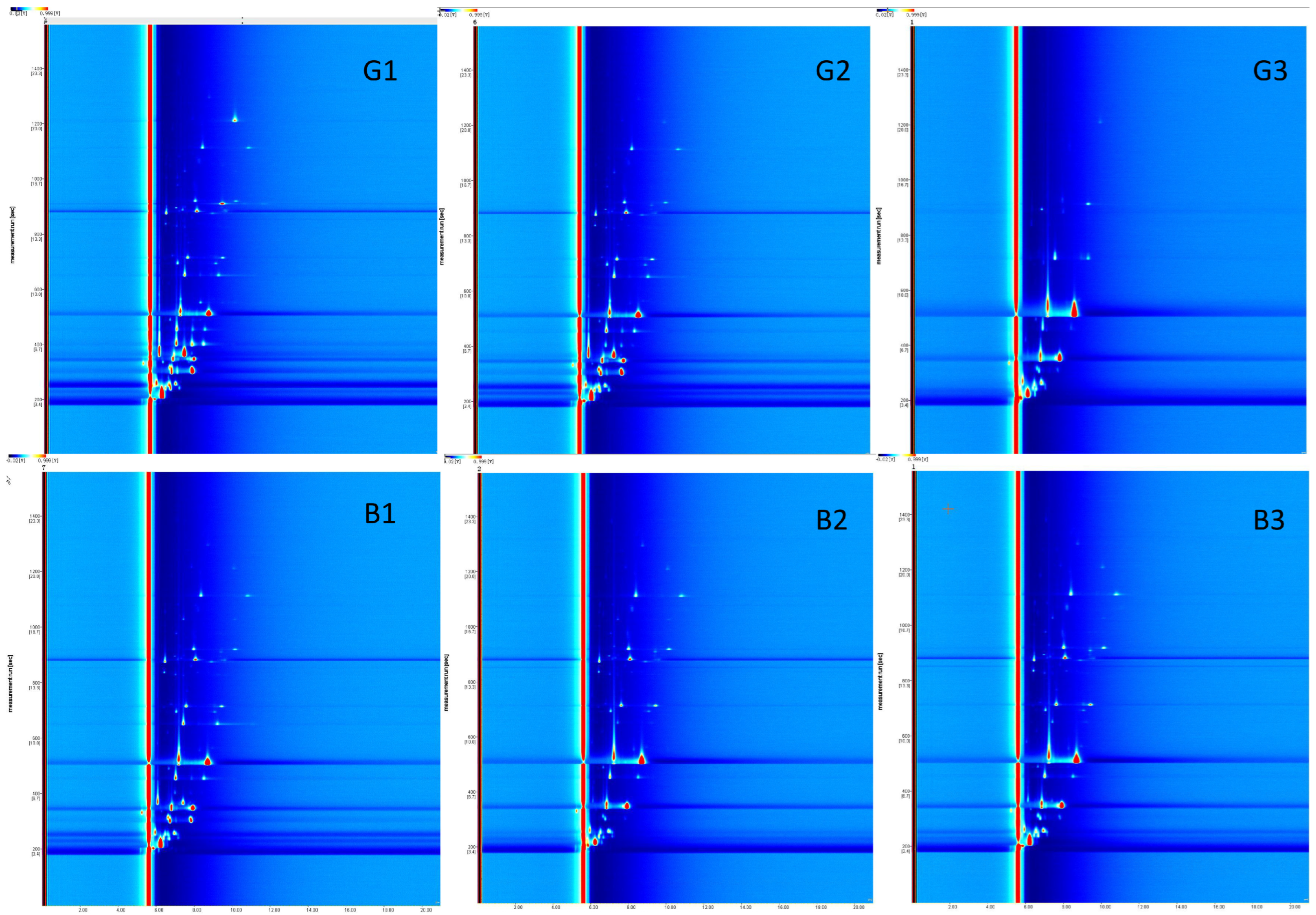
3.4. HS-GC-IMS Analysis of VOCs in Boiled and SW Chicken
3.4.1. Identification of VOCs
3.4.2. Fingerprint Profile Comparisons in Boiled and SW Chicken
3.5. Identification of Volatile Flavor Compounds by GC–MS and GC-IMS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Wang, Y.; Wang, Y.; Luo, N.; Cai, R.; Yu, Y.; Zhang, X.; Zhu, J.; Zhao, G.; Wen, J.; et al. Main lipid sources affecting key aroma volatile compounds in Chinese native chicken. Food Chem. 2025, 474, 142990. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Han, C.; Han, X.; Song, J.; Ju, J.; Zhu, H. Acetamiprid in Rizhao green tea: Residue dynamics, degradation pathways, and ecological risks via integrated experimental and computational approaches. J. Hazard. Mater. 2025, 495, 138786. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Shen, Q.; Liu, D.; Ma, L.; Wang, F.; Xiao, G. Investigation of microwave, irradiation, low-temperature plasma on flavor changes of mango powder. Food Chem. X 2025, 27, 102479. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.Q.; Tang, C.B.; Tao, T.Y.; Song, W.; Li, C.B.; Qi, J.; Ding, S.J.; Zhou, G.H. Cultured biomass enhances the flavor and nutrition characteristics of biomass/plant hybrid cultured meatballs. Food Res. Int. 2025, 214, 116627. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Abbas, F.; Wang, Z.; Yu, Y.; Yue, Y.; Li, X.; Yu, R.; Fan, Y. HS-SPME-GC-MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules 2021, 26, 5425. [Google Scholar] [CrossRef]
- Yao, W.; Ma, S.; Wu, H.; Liu, D.; Liu, J.; Zhang, M. Flavor profile analysis of grilled lamb seasoned with classic salt, chili pepper, and cumin (Cuminum cyminum) through HS-SPME-GC-MS, HS-GC-IMS, E-nose techniques, and sensory evaluation on Sonit sheep. Food Chem. 2024, 454, 139514. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Liang, Z.; Liu, J.; Yang, C.; Zhai, H.; Chen, A.; Lu, Z.; Gao, Y.; Ding, X.; et al. Characterization of Flavor Compounds in Chinese Indigenous Sheep Breeds Using Gas Chromatography-Ion Mobility Spectrometry and Chemometrics. Foods 2024, 13, 2647. [Google Scholar] [CrossRef]
- Stoppelmann, F.; Chan, L.F.; Hildebrand, G.; Hermann-Ene, V.; Vetter, W.; Rigling, M.; Zhang, Y. Molecular decoding a meat-like aroma generated from Laetiporus sulphureus-mediated fermentation of onion (Allium cepa L.). Food Res. Int. 2024, 192, 114757. [Google Scholar] [CrossRef]
- DB 46/T 545-2021; Geographical Indication Product—Wenchang Chicken. Hainan Provincial Intellectual Property Bureau: Haikou, China, 2021.
- GB/T 16291.1-2012; Sensory Analysis—Selection, Training and Monitoring of Assessors—Part 1: Selection of Assessors. China Standards Press: Beijing, China, 2012.
- ISO 8586:2023; Analyse Sensorielle—Sélection et Entraînement des Sujets Sensoriels. International Organization for Standardization: Geneva, Switzerland, 2023.
- Jiang, H.; Ou, S.; Ye, J.; Qian, M.; Wen, J.; Qi, H.; Zeng, X.; Zhao, W.; Bai, W. Variation of volatile flavor substances in salt-baked chicken during processing. Food Chem. X 2024, 24, 101846. [Google Scholar] [CrossRef]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of volatile flavor compounds and characterization of aroma-active compounds of water-boiled salted duck using GC-MS-O, GC-IMS, and E-nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, Y.; Li, P.; Li, C.; Wang, Z.; Cao, J. Preparation and characterization of lauric acid-rich wax oleogel chocolates: Insights into crystallization behavior, storage stability, and bloom resistance. Food Res. Int. 2025, 204, 115886. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Yang, P.; Wang, J.; Zhang, H.; Wang, T.; Zhang, Z.; Wei, W.; Zhang, C. Heat transfer enhancement mediated by moisture diffusion improves the volatile profiles of meat stir-fried with high-temperature short-time. Meat Sci. 2025, 223, 109770. [Google Scholar] [CrossRef]
- Bichlmaier, C.; Frohlich, S.M.; Brychcy, V.; Grassl, A.; Behrens, M.; Lang, R. Contribution of mozambioside roasting products to coffee’s bitter taste. Food Chem. 2025, 469, 142547. [Google Scholar] [CrossRef] [PubMed]
- Karademir, Y.; Goncuoglu, N.; Gokmen, V. Investigation of heat induced reactions between lipid oxidation products and amino acids in lipid rich model systems and hazelnuts. Food Funct. 2013, 4, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Sun, J.; Zhang, N.; Wang, S.; Sun, B. Effects of cooking methods on aroma formation in pork: A comprehensive review. Food Chem. X 2023, 20, 100884. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Chen, Y.L.; Huo, W.S.; Wang, J.Y.; Zhang, L.J.; Huang, J.Y.; Sun, L.C. Formation of different flavor characteristics of raw- and boiled-dried oysters. Food Chem. X 2025, 29, 102854. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, L.; Huang, F.; Wei, W.; Barbut, S.; Erasmus, S.; Zhang, C. Lipid-derived odour-active volatile compound formation pathways in Tibetan pork across different cooking methods: Insights from iron properties, lipid oxidation, and lipidomics analysis. Food Chem. 2025, 491, 145256. [Google Scholar] [CrossRef]
- Feng, S.; Li, P.; Li, Y.; Xiao, X.; Chen, X.; Leng, H.; Liang, H.; Zhou, L.; Chen, T.; Ding, C. Volatile profiles and characteristic odorants in camellia seeds with different heat pretreatments. Food Chem. 2025, 468, 142497. [Google Scholar] [CrossRef]
- Li, J.; Bai, J.; Yuan, L.; Zhou, H.; Xu, L.; Yu, C.; Hu, M.; Tu, Z.; Peng, B. Comprehensive lipidomics and flavoromics reveals the accelerated oxidation mechanism of fish oil from silver carp (Hypophthalmichthys molitrix) viscera during heating. Food Chem. 2025, 478, 143651. [Google Scholar] [CrossRef]
- Gargouri, M.; Drouet, P.; Legoy, M.D. Hydroperoxide-lyase activity in mint leaves. Volatile C6-aldehyde production from hydroperoxy-fatty acids. J. Biotechnol. 2004, 111, 59–65. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, Q.; Wang, J.; Liu, C.; Huang, F.; Huang, Y. Identification of key aroma-active compounds in sesame oil from microwaved seeds using E-nose and HS-SPME-GCxGC-TOF/MS. J. Food Biochem. 2019, 43, e12786. [Google Scholar] [CrossRef]
- He, Y.; Qin, H.; Wen, J.; Cao, W.; Yan, Y.; Sun, Y.; Yuan, P.; Sun, B.; Fan, S.; Lu, W.; et al. Characterization of Key Compounds of Organic Acids and Aroma Volatiles in Fruits of Different Actinidia argute Resources Based on High-Performance Liquid Chromatography (HPLC) and Headspace Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS). Foods 2023, 12, 3615. [Google Scholar] [CrossRef]
- Shao, X.; Wang, H.; Song, X.; Xu, N.; Cai, L.; Xu, X. Elucidating the pattern of flavor evolution during the steaming process of fermented sausages in two dimensions: Strain fermentation and steaming time. Food Chem. 2025, 480, 143945. [Google Scholar] [CrossRef]
- Zzaman, W.; Bhat, R.; Yang, T.A.; Easa, A.M. Influences of superheated steam roasting on changes in sugar, amino acid and flavour active components of cocoa bean (Theobroma cacao). J. Sci. Food Agric. 2017, 97, 4429–4437. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Lin, Z.; Li, Y.; Chen, F.; Liu, S.; Li, C. Effects of different cooking methods on volatile flavor compounds of chicken breast. J. Food Biochem. 2021, 45, e13770. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Mui, L.; Aldridge, E.; McKinney, J.; Hewson, L.; Fisk, I.D. The progression of lipid oxidation, beta-carotenes degradation and sensory perception of batch-fried sliced sweet potato crisps during storage. Food Funct. 2021, 12, 4535–4543. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Guo, S.; Rao, H.; Lan, B.; Zheng, B.; Zhang, N. Characterization of Volatile Flavor Compounds and Aroma Active Components in Button Mushroom (Agaricus bisporus) across Various Cooking Methods. Foods 2024, 13, 685. [Google Scholar] [CrossRef]
- Ge, L.; Wu, Y.; Zou, W.; Mao, X.; Wang, Y.; Du, J.; Zhao, H.; Zhu, C. Analysis of the trend of volatile compounds by HS-SPME-GC-MS and the main factors affecting the formation of rancid odor during the oxidation process of infant nutrition package. J. Food Sci. Technol. 2022, 59, 3367–3378. [Google Scholar] [CrossRef]
- Lan, H.; Luo, X.; Xu, D.; Cui, K.; Li, L.; Li, C.; Qi, H.; Liu, Q. Analysis of flavor formation and metabolite changes during production of Double-Layer Steamed Milk Custard made from buffalo milk. PLoS ONE 2025, 20, e0331277. [Google Scholar] [CrossRef]
- Sun, M.; Ma, J.; Cai, Z.; Yan, J.; Ma, R.; Yu, M.; Xie, Y.; Shen, Z. Integrating Sensory Evaluation, Electronic Nose, and Metabolomics to Characterize Aroma in Peach and Nectarine Varieties. Foods 2025, 14, 3087. [Google Scholar] [CrossRef]
- Aghili, N.S.; Rasekh, M.; Karami, H.; Edriss, O.; Wilson, A.D.; Ramos, J. Aromatic Fingerprints: VOC Analysis with E-Nose and GC-MS for Rapid Detection of Adulteration in Sesame Oil. Sensors 2023, 23, 6294. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Chen, P.; Zeng, X.; Bai, W.; Zhang, Y.; Sun, B. Enhancing beef flavor profiles via Maillard reaction of gamma-Glutamylated beef protein hydrolysates and xylose. Food Chem. 2025, 476, 143313. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Ahn, D.U.; Nam, K.C.; Jo, C. Flavour chemistry of chicken meat: A review. Asian-Australas. J. Anim. Sci. 2013, 26, 732–742. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
| Sample | Evaluation Dimension | Specific Indicators | SW Chicken | PP Chicken | p-Values |
|---|---|---|---|---|---|
| Breast Muscle | Color | Brightness of skin color | 4 ± 0.19 | 3.59 ± 0.17 | 0.05 |
| Uniformity of muscle color | 3.78 ± 0.22 | 3.41 ± 0.23 | 0.11 | ||
| Taste | Fresh and savory chicken flavor | 3.83 ± 0.25 | 3.6 ± 0.26 | 0.33 | |
| Uniformity of seasoning penetration | 3.5 ± 0.13 | 3.38 ± 0.13 | 0.28 | ||
| Persistence of aftertaste | 3.33 ± 0.21 | 3.4 ± 0.26 | 0.75 | ||
| Sweetness perception | 3.18 ± 0.17 | 3.11 ± 0.11 | 0.56 | ||
| Saltiness penetration | 3.29 ± 0.13 | 3.15 ± 0.06 | 0.15 | ||
| Tenderness | Crispness/elasticity of chicken skin | 3.33 ± 0.23 | 3.48 ± 0.26 | 0.50 | |
| Ease of chewing chicken muscle fibers | 3.15 ± 0.06 | 3.33 ± 0.01 | 0.01 | ||
| Juice retention rate | 3.26 ± 0.26 | 3.15 ± 0.06 | 0.50 |
| Indicator | Dorsal Skin | p-Values | ||
|---|---|---|---|---|
| PP Chicken | SW Chicken | |||
| Skin color | Brightness value | 50.88 ± 5.35 | 61.05 ± 4.37 | 4.0 × 10−6 |
| Redness value | 1.64 ± 0.8 | 1.3 ± 0.72 | 0.23 | |
| Yellowness value | 17.21 ± 1.56 | 12.56 ± 1.76 | 2.5 × 10−8 | |
| Cas | Compound Name | Molecular Formula | Estimated Concentration (μg/kg) | |||
|---|---|---|---|---|---|---|
| PP Chicken | SW Chicken | p-Values | Food Change | |||
| 111-71-7 | Heptanal | C7H14O | 44.87 ± 30.45 | 24.18 ± 8.33 | 0.36 | 0.86 |
| 139185-79-8 | Z,Z-2,5-Pentadecadien-1-ol | C15H28O | 0.04 ± 0.01 | 0.28 ± 0.12 | 0.08 | −0.85 |
| 13019-20-0 | 3-Heptanone, 2-methyl- | C8H16O | 270.67 ± 0 | 270.67 ± 0 | 0.00 | 0.00 |
| 10544-96-4 | Octadecane, 6-methyl- | C19H40 | 2.14 ± 1.1 | 0.6 ± 0.94 | 0.14 | 2.54 |
| 110-13-4 | 2,5-Hexanedione | C6H10O2 | 24.63 ± 32.42 | 43.68 ± 30.06 | 0.50 | −0.44 |
| 124-13-0 | Octanal | C8H16O | 21.01 ± 10.63 | 21.4 ± 9.33 | 0.96 | −0.02 |
| 18409-17-1 | 2-Octen-1-ol, (E)- | C8H16O | 0.91 ± 1.54 | 1.23 ± 1.81 | 0.81 | −0.26 |
| 116465-18-0 | 2-Trifluoroacetoxytridecane | C15H27F3O2 | 0.12 ± 0.16 | 0.97 ± 1.06 | 0.30 | −0.88 |
| 14905-56-7 | Tetradecane, 2,6,10-trimethyl- | C17H36 | 0.96 ± 0.39 | 1.68 ± 0.82 | 0.27 | −0.43 |
| 124-19-6 | Nonanal | C9H18O | 40.8 ± 17.78 | 45.31 ± 27.63 | 0.83 | −0.10 |
| 112-40-3 | Dodecane | C12H26 | 3.15 ± 0.3 | 3.71 ± 2.57 | 0.74 | −0.15 |
| 112-31-2 | Decanal | C10H20O | 4.64 ± 0.88 | 4.62 ± 2.89 | 0.99 | 0.00 |
| 150-86-7 | Phytol | C20H40O | 0.47 ± 0.35 | 0.56 ± 0.62 | 0.84 | −0.16 |
| 540-97-6 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 20.52 ± 9.11 | 20.11 ± 9.01 | 0.96 | 0.02 |
| 107-50-6 | Cycloheptasiloxane, tetradecamethyl- | C14H42O7Si7 | 3.68 ± 0.56 | 9.46 ± 12.22 | 0.41 | −0.61 |
| 330455-64-6 | Thymol, TBDMS derivative | C16H28OSi | 0.1 ± 0.14 | 0.04 ± 0.04 | 0.54 | 1.43 |
| 1894-68-4 | 2-Trifluoroacetoxydodecane | C14H25F3O2 | 0.24 ± 0.24 | 0.16 ± 0.2 | 0.69 | 0.49 |
| 66-25-1 | Hexanal | C6H12O | 660.81 ± 369.67 | 911 ± 389.99 | 0.47 | −0.27 |
| 3391-86-4 | 1-Octen-3-ol | C8H16O | 92.25 ± 91.31 | 49.28 ± 27.85 | 0.51 | 0.87 |
| 1940-18-7 | Cyclohexanol, 1-ethyl- | C8H16O | 3.04 ± 2.61 | 1.62 ± 1.58 | 0.48 | 0.87 |
| 2548-87-0 | 2-Octenal, (E)- | C8H14O | 4.64 ± 4.53 | 5.28 ± 4.29 | 0.87 | −0.12 |
| 541-02-6 | Cyclopentasiloxane, decamethyl- | C10H30O5Si5 | 14.87 ± 1.05 | 29.83 ± 10.44 | 0.13 | −0.50 |
| 195194-80-0 | 2-Piperidinone, N-[4-bromo-n-butyl]- | C9H16BrNO | 0.08 ± 0.07 | 0.21 ± 0.24 | 0.38 | −0.61 |
| 2425-77-6 | 1-Decanol, 2-hexyl- | C16H34O | 0.95 ± 0.77 | 0.8 ± 0.74 | 0.82 | 0.18 |
| 630-08-0 | Carbon monoxide | CO | 0.14 ± 0.06 | 0.13 ± 0.04 | 0.74 | 0.12 |
| 13019-16-4 | 2-Octenal, 2-butyl- | C12H22O | 6.21 ± 5.77 | 6.7 ± 7.93 | 0.93 | −0.07 |
| 19780-11-1 | 2-Dodecen-1-yl(-)succinic anhydride | C16H26O3 | 1.1 ± 0.89 | 8.35 ± 9.47 | 0.32 | −0.87 |
| 1654-86-0 | Decanoic acid, decyl ester | C20H40O2 | 8.48 ± 6.83 | 4.08 ± 3.11 | 0.31 | 1.08 |
| 25144-04-1 | Cyclopentanol, 2-methyl-, trans- | C6H12O | 0.11 ± 0.11 | 0.14 ± 0.17 | 0.74 | −0.26 |
| 28023-80-5 | N-Isopentyl-N-nitroso-pentylamine | C10H22N2O | 7.54 ± 7.18 | 2.64 ± 3.65 | 0.36 | 1.86 |
| 2122-26-1 | Aspidospermidin-17-ol | C23H30N2O5 | 0.34 ± 0.56 | 0.41 ± 0.19 | 0.84 | −0.16 |
| 3555-45-1 | Silicic acid, | C10H28O4Si3 | 0.02 ± 0.02 | 0.03 ± 0.02 | 0.50 | −0.37 |
| 18030-67-6 | 3-Ethoxy-1, | C11H32O4Si4 | 0.02 ± 0.01 | 0.03 ± 0.04 | 0.66 | −0.31 |
| 19095-23-9 | Heptasiloxane | C14H44O6Si7 | 0.17 ± 0.17 | 0.06 ± 0.05 | 0.37 | 2.05 |
| 150304-08-8 | 4-Hydroxy-2,2′,4′ | C12H6Cl4O | 0.05 ± 0.05 | 0.07 ± 0.04 | 0.57 | −0.29 |
| 105037-97-6 | Azetidine-2-one | C10H19NO | 0.11 ± 0.02 | 0.65 ± 0.6 | 0.17 | −0.83 |
| 29812-79-1 | Hydroxylamine, | C10H23NO | 1.31 ± 1.39 | 2.57 ± 2.15 | 0.45 | −0.49 |
| 10537-47-0 | Propanedinitrile | C18H22N2O | 1.06 ± 1.03 | 0.65 ± 0.91 | 0.63 | 0.63 |
| 56797-40-1 | 7-Hexadecenal, (Z)- | C16H30O | 0.5 ± 0.68 | 0.24 ± 0.4 | 0.60 | 1.12 |
| 111-87-5 | 1-Octanol | C8H18O | 6.45 ± 5.35 | 4.69 ± 5.05 | 0.74 | 0.38 |
| 55162-61-3 | Tetracontane, 3,5,24-trimethyl- | C43H88 | 2.55 ± 4.1 | 1.13 ± 1.15 | 0.56 | 1.26 |
| 3891-98-3 | Dodecane, 2,6,10-trimethyl- | C15H32 | 6.49 ± 6.56 | 4.06 ± 6.01 | 0.66 | 0.60 |
| 110-62-3 | Pentanal | C5H10O | 58.22 ± 39.61 | 31.67 ± 10.5 | 0.37 | 0.84 |
| 55334-42-4 | Dodecane, 1,2-dibromo- | C12H24Br2 | 0.8 ± 0.73 | 6.1 ± 4.42 | 0.17 | −0.87 |
| 74708-73-9 | 1,4-Methanobenzocyclodecene, | C15H22 | 3.47 ± 0.66 | 12.1 ± 9.27 | 0.25 | −0.71 |
| 10552-94-0 | 1H-Pyrrole, 2,5-dihydro-1-nitroso- | C4H6N2O | 0.06 ± 0.02 | 14.75 ± 29.21 | 0.39 | −1.00 |
| 1883-13-2 | Dodecanoic acid, 3-hydroxy- | C12H24O3 | 0.2 ± 0.26 | 0.13 ± 0.11 | 0.73 | 0.49 |
| 19095-24-0 | Octasiloxane, | C16H50O7Si8 | 0.25 ± 0.19 | 0.14 ± 0.1 | 0.42 | 0.83 |
| 87867-97-8 | 3-Butoxy-1, | C13H36O4Si4 | 0.05 ± 0.05 | 0.04 ± 0.03 | 0.86 | 0.21 |
| 15399-43-6 | Olean-12-ene-3,15,16,21,22,28-hexol, | C30H50O6 | 0.04 ± 0.03 | 0.08 ± 0.05 | 0.27 | −0.53 |
| 103577-45-3 | Lansoprazole | C16H14F3N3O2S | 0.11 ± 0.09 | 0.11 ± 0.08 | 0.97 | 0.02 |
| 5638-09-5 | Cyclopentane, (4-octyldodecyl)- | C25H50 | 1.32 ± 1.16 | 5.27 ± 8.67 | 0.51 | −0.75 |
| 948-60-7 | Pterin-6-carboxylic acid | C7H5N5O3 | 0.05 ± 0.05 | 0.05 ± 0.05 | 0.84 | 0.22 |
| 122-16-7 | Sulfanitran | C14H13N3O5S | 37.68 ± 65.15 | 73.11 ± 112.55 | 0.67 | −0.48 |
| 13463-39-3 | Nickel tetracarbonyl | C4NiO4 | 8.42 ± 14.49 | 1.5 ± 2.41 | 0.50 | 4.63 |
| 995-82-4 | Hexasiloxane | C12H38O5Si6 | 0.12 ± 0.13 | 0.02 ± 0.03 | 0.32 | 4.17 |
| 25144-05-2 | Cyclopentanol | C6H12O | 0.03 ± 0.02 | 0.36 ± 0.58 | 0.43 | −0.91 |
| 626-33-5 | 4-Heptanone | C8H16O | 0.13 ± 0.03 | 0.29 ± 0.46 | 0.62 | −0.54 |
| 624-42-0 | 3-Heptanone | C8H16O | 0.01 ± 0.01 | 0.11 ± 0.04 | 0.03 | −0.86 |
| 7325-84-0 | Silane, trichlorodocosyl- | C22H45Cl3Si | 0 | 0.27 ± 0.22 | 0.00 | #DIV/0! |
| 52132-58-8 | Acetic acid, chloro- | C18H35ClO2 | 1.54 ± 0.07 | 1.01 ± 0.89 | 0.41 | 0.52 |
| 1560-96-9 | Tridecane | C14H30 | 1.83 ± 0.32 | 10.31 ± 11.02 | 0.31 | −0.82 |
| 7225-68-5 | Dodecane | C25H48 | 0.54 ± 0.48 | 9.85 ± 14.18 | 0.37 | −0.95 |
| 59426-46-9 | 2,5-Furandione | C16H26O3 | 0.5 ± 0.26 | 1.47 ± 0.28 | 0.04 | −0.66 |
| 3892-00-0 | Pentadecane | C18H38 | 1.62 ± 0.39 | 12.74 ± 14.65 | 0.32 | −0.87 |
| 25360-09-2 | tert-Hexadecanethiol | C16H34S | 2.49 ± 2.57 | 6.52 ± 6.2 | 0.39 | −0.62 |
| 5057-99-8 | 1,2-Cyclopentanediol | C5H10O2 | 0.09 ± 0.11 | 7.45 ± 7.14 | 0.22 | −0.99 |
| 112-86-7 | Erucic acid | C22H42O2 | 1.13 ± 1.35 | 0.89 ± 0.89 | 0.85 | 0.28 |
| 10584-64-2 | D-Homo-24-nor-17-oxachola-20,22-diene-3,7,16-trione, 14,15:21,23-diepoxy-4,4,8-trimethyl- | C26H32O6 | 0.04 ± 0.05 | 0.07 ± 0.04 | 0.54 | −0.42 |
| 56769-23-4 | trans-3,4-Epoxynonane | C9H18O | 0.06 ± 0.07 | 0.05 ± 0.04 | 0.85 | 0.24 |
| 1428-33-7 | 3-(1,1,2,2-Tetrafluoroethoxy)prop-1-ene | C5H6F4O | 0 | 0.24 ± 0.33 | 0.00 | N/A |
| 29783-26-4 | 4-Cyclopentene-1,3-diol, cis- | C5H8O2 | 0 | 8.13 ± 5.3 | 0.00 | N/A |
| 1068-57-1 | Acetic acid, hydrazide | C2H6N2O | 0 | 29.63 ± 51.28 | 0.00 | N/A |
| 60609-53-2 | 8-Hexadecenal, 14-methyl-, (Z)- | C17H32O | 0 | 1.46 ± 0.89 | 0.00 | N/A |
| 55255-85-1 | Cyclopentane, 1,1′-[3-(2-cyclopentylethyl)-1,5-pentanediyl]bis- | C22H40 | 0 | 1.05 ± 1.47 | 0.00 | N/A |
| 6982-39-4 | trans-2-Aminocyclohexanol | C6H13NO | 0 | 3.55 ± 5.12 | 0.00 | N/A |
| 38520-31-9 | Oxiraneundecanoic acid, 3-pentyl-, methyl ester, trans- | C19H36O3 | 0.24 ± 0.23 | 0 | 0.00 | N/A |
| 29804-22-6 | Disparlure | C19H38O | 1.28 ± 0.98 | 0 | 0.00 | N/A |
| 143-08-8 | 1-Nonanol | C9H20O | 0 | 0.02 ± 0.01 | 0.00 | N/A |
| 7045-79-6 | Oxetane, 2-methyl-4-propyl- | C7H14O | 0 | 0.06 ± 0.04 | 0.00 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Zheng, X.; Xu, T.; Chen, M.; Chen, S.; Zhang, D.; Cai, B.; Gu, L. Comparative Volatile Profiles of Plain Poached (PP) and Steamed over Water (SW) Wenchang Chicken Analyzed by GC-MS, GC-IMS, and E-Nose. Foods 2025, 14, 3778. https://doi.org/10.3390/foods14213778
Jiang Q, Zheng X, Xu T, Chen M, Chen S, Zhang D, Cai B, Gu L. Comparative Volatile Profiles of Plain Poached (PP) and Steamed over Water (SW) Wenchang Chicken Analyzed by GC-MS, GC-IMS, and E-Nose. Foods. 2025; 14(21):3778. https://doi.org/10.3390/foods14213778
Chicago/Turabian StyleJiang, Qicheng, Xinli Zheng, Tieshan Xu, Meiling Chen, Shihao Chen, Dexiang Zhang, Bolin Cai, and Lihong Gu. 2025. "Comparative Volatile Profiles of Plain Poached (PP) and Steamed over Water (SW) Wenchang Chicken Analyzed by GC-MS, GC-IMS, and E-Nose" Foods 14, no. 21: 3778. https://doi.org/10.3390/foods14213778
APA StyleJiang, Q., Zheng, X., Xu, T., Chen, M., Chen, S., Zhang, D., Cai, B., & Gu, L. (2025). Comparative Volatile Profiles of Plain Poached (PP) and Steamed over Water (SW) Wenchang Chicken Analyzed by GC-MS, GC-IMS, and E-Nose. Foods, 14(21), 3778. https://doi.org/10.3390/foods14213778






