Investigating the Effects of Yunnan Lufeng Aromatic Vinegar Intervention on Intestinal Microbiota, SCFAs, and Metabolites in Mice Using Multi-Omics Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
2.3. Mouse Grouping and Experimental Design
2.4. 16S rRNA Gene Sequencing
2.5. SCFA Quantification by GC–MS
2.5.1. Sample Preparation
2.5.2. GC-MS Analysis
2.6. Untargeted Metabolomics
2.6.1. Metabolite Extraction
2.6.2. Untargeted Metabolomics Measurement
2.7. Statistical Analysis
3. Results and Discussion
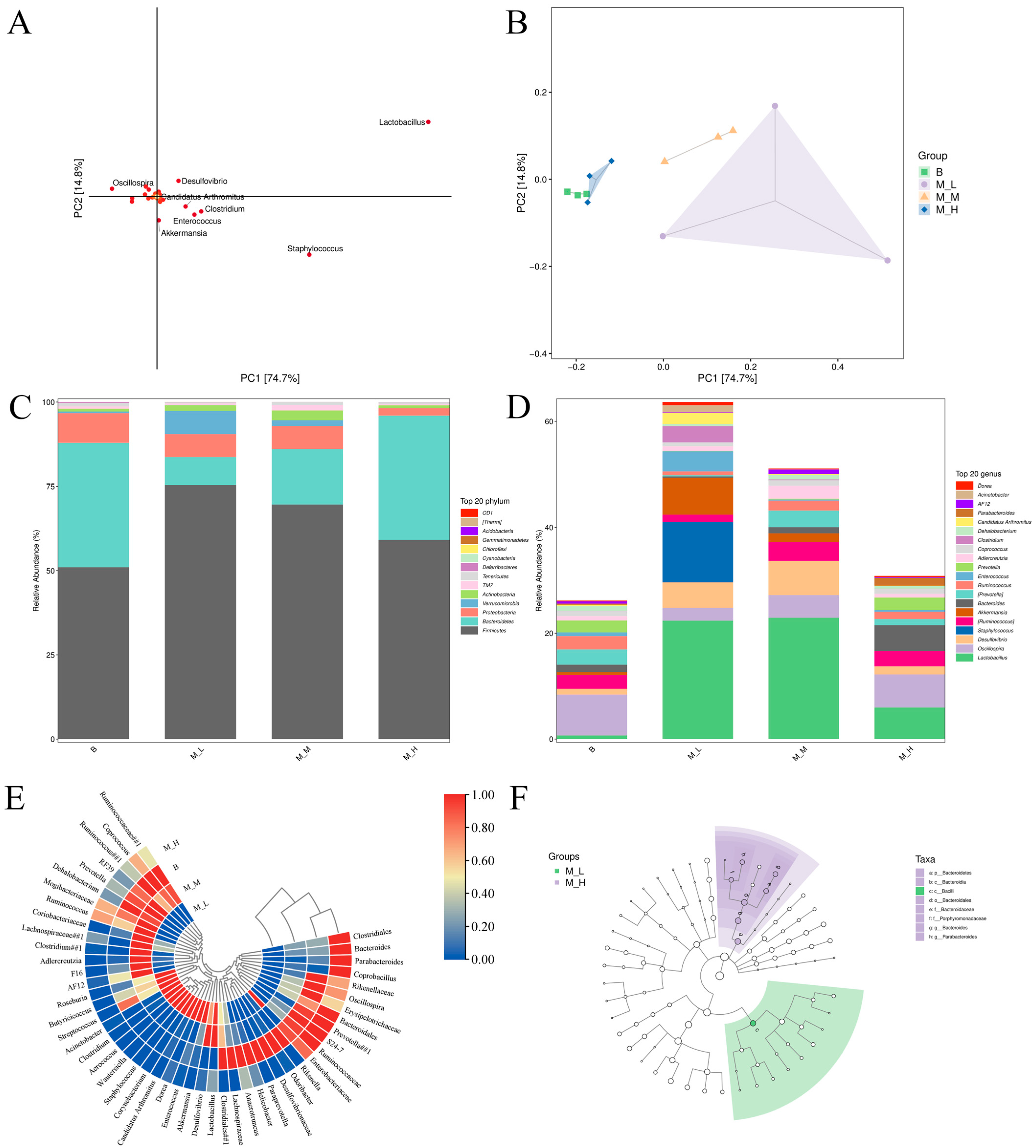
3.1. Effects of LFAV on Taxonomic Units of Intestinal Microbiota in Mice
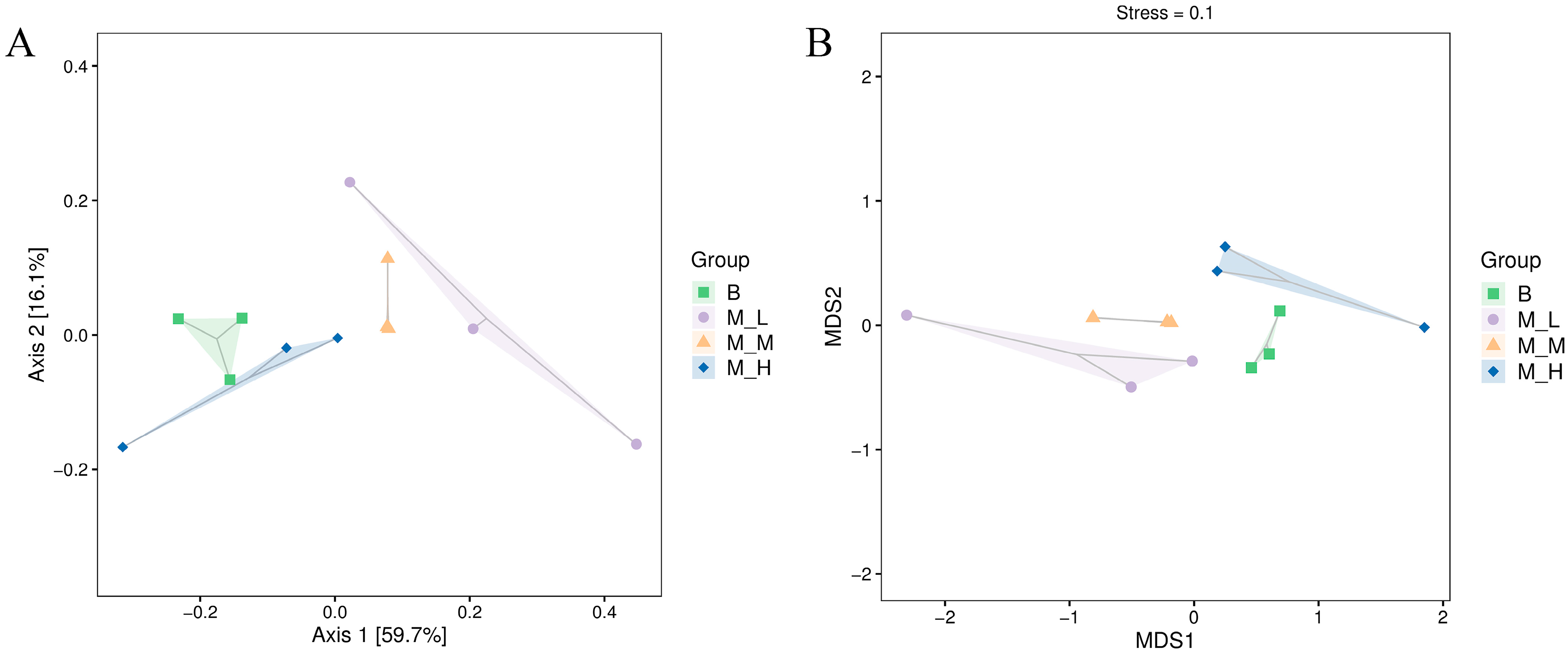
3.2. Effects of LFAV on Alpha and Beta Diversity of Intestinal Microbiota
3.3. Effects of LFAV on Gut Microbiota Composition and Taxonomic Shifts
3.4. Effects of LFAV on SCFA Profiles in Mice
3.5. Untargeted Metabolomics Analysis
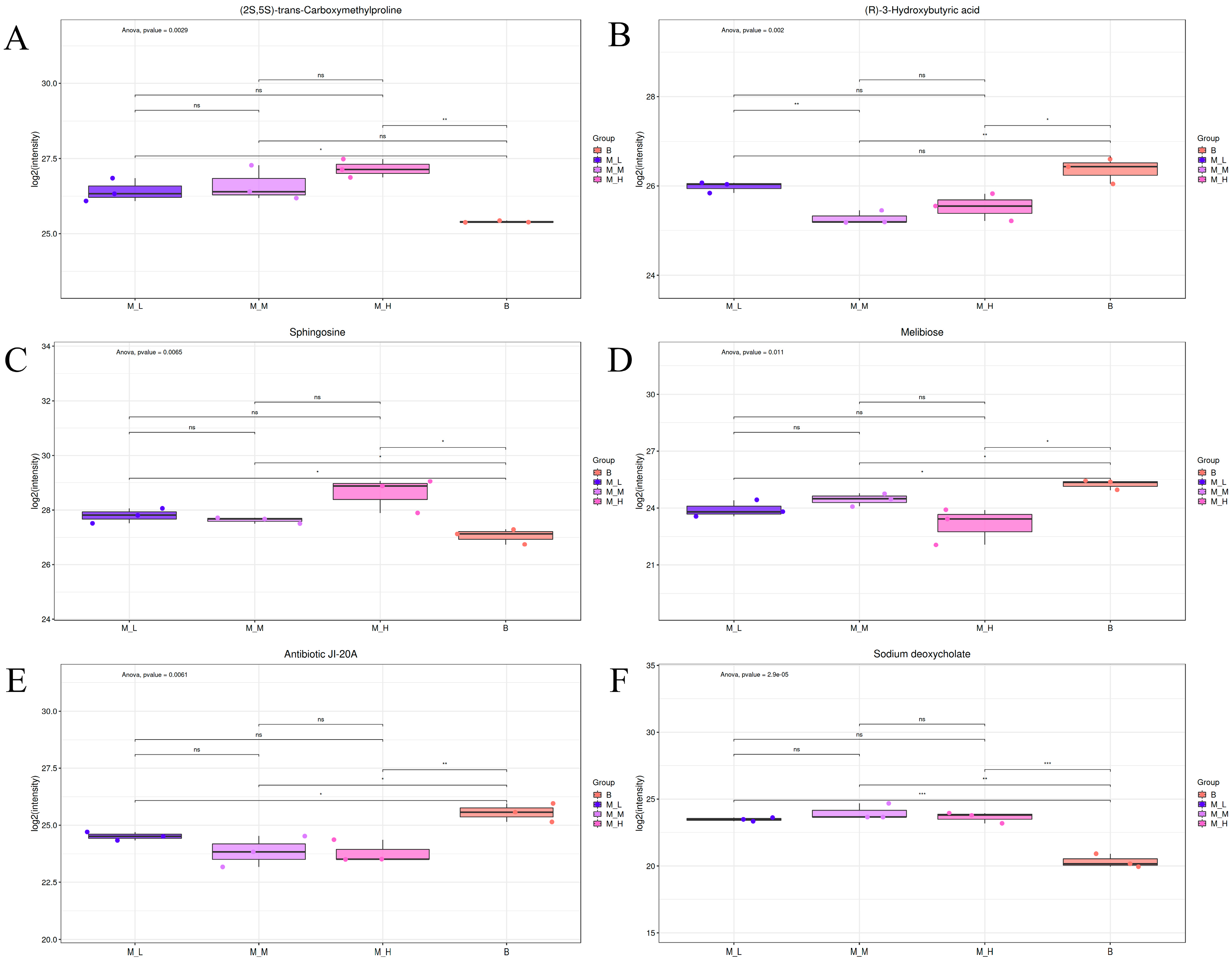
3.5.1. Differential Metabolite Profiling
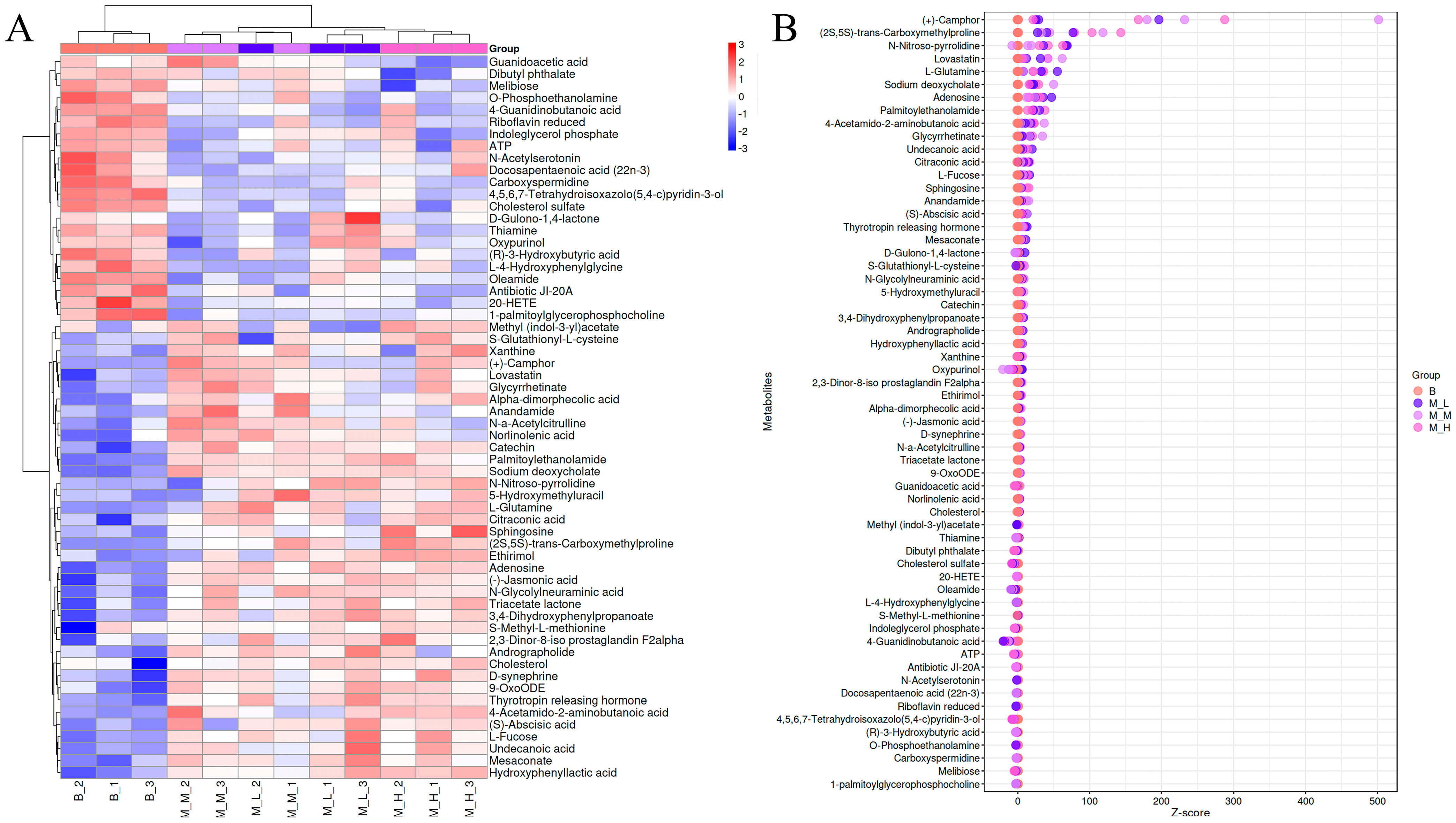
3.5.2. Clustering and Z-Score Analysis
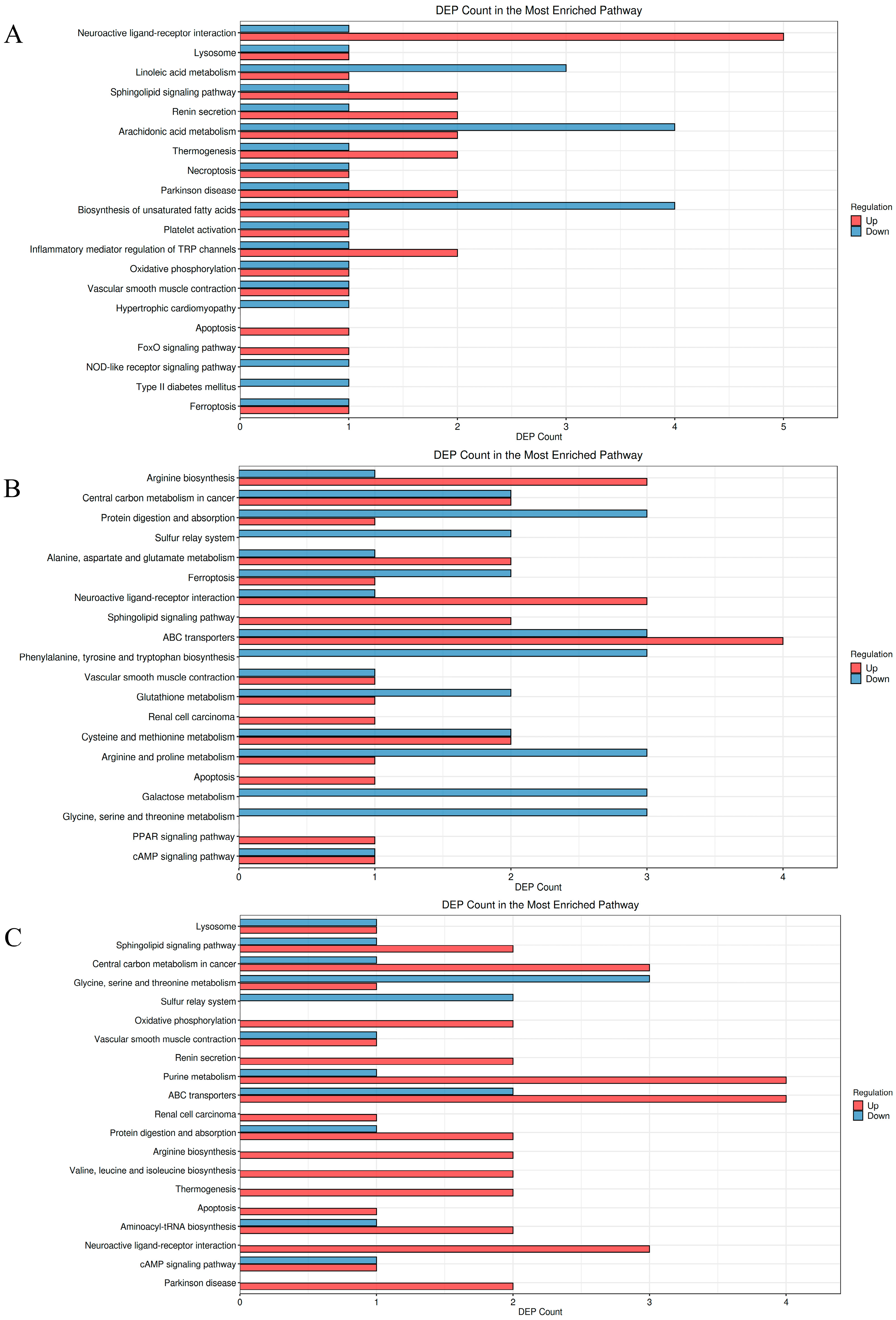
3.5.3. KEGG Pathway Enrichment Analysis
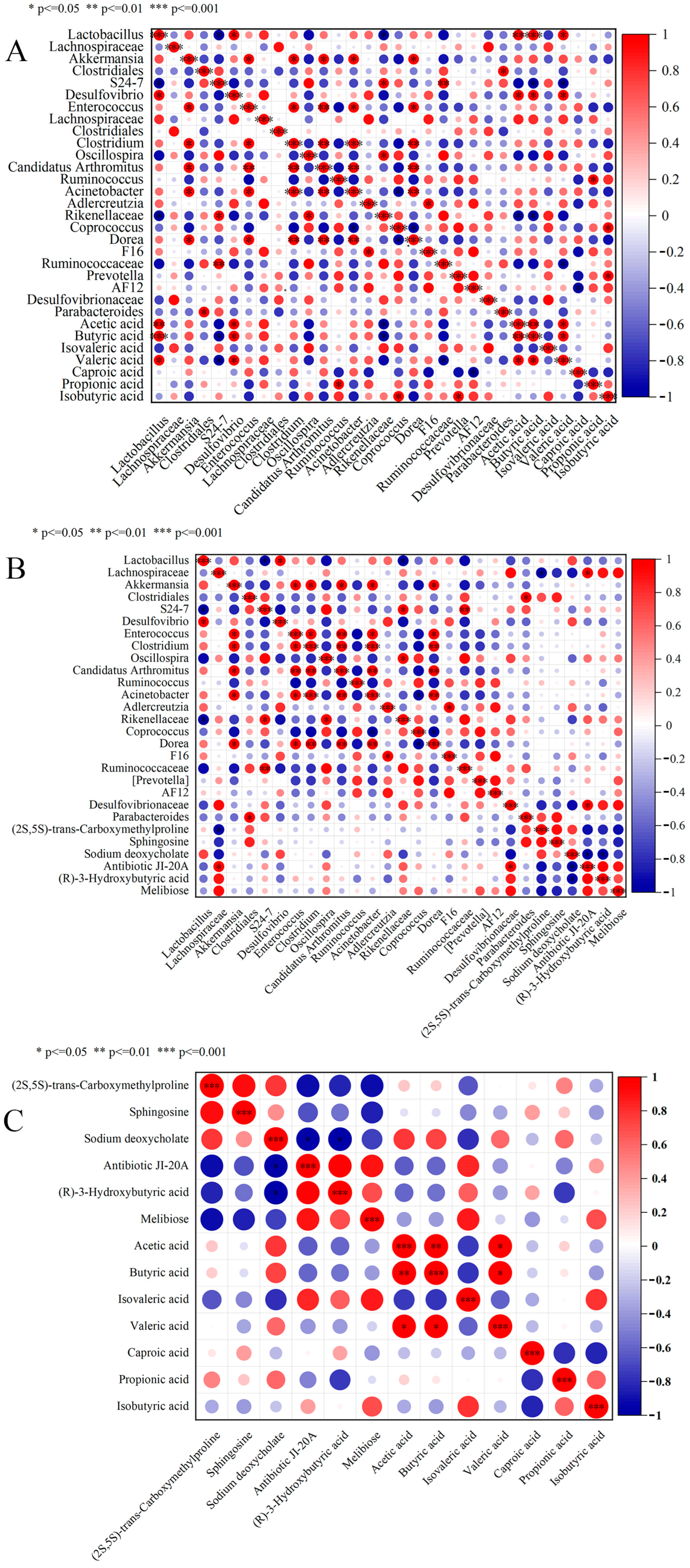
3.6. Correlation Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LFAV | Lufeng aromatic vinegar |
| SCFAs | Short-chain fatty acids |
| GC-MS | Gas chromatography–mass spectrometry |
| LC-MS | Liquid chromatography–mass spectrometry |
References
- Khalifa, S.A.M.; El-Shabasy, R.M.; Tahir, H.E.; Abo-Atya, D.M.; Saeed, A.; Abolibda, T.Z.; Guo, Z.; Zou, X.; Zhang, D.; Du, M.; et al. Vinegar—A Beneficial Food Additive: Production, Safety, Possibilities, and Applications from Ancient to Modern Times. Food Funct. 2024, 15, 10262–10282. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, U.F.; Jolly, N.P.; Chidi, B.S.; Ngongang, M.M.; Ntwampe, S.K.O. Vinegar Engineering: A Bioprocess Perspective. Food Eng. Rev. 2019, 11, 290–305. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, G.; Gu, D.; Xu, Z. Analysis of Bacterial Diversity in Lufeng Aromatic Vinegar with Different Storage Years. Shipin Kexue 2024, 45, 118–125. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Q.; An, M.; Li, G.; Kang, X.; Xu, Z. Analysis of Volatile Flavor Substances and Components ofLufeng Aromatic Vinegar Based on HS-SPME-GC-MS. China Condiment 2023, 48, 164–171. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, R.; Xu, Z.; Gu, D. Optimization of Saccharification Process of Lufeng Aromatic Vinegar by Double-Enzymatic Method. China Condiment 2024, 49, 80–88. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, N.; Zhang, C.; Lam, Y.Y.; Zhao, L. Guild-Based Analysis for Understanding Gut Microbiome in Human Health and Diseases. Genome Med. 2021, 13, 22. [Google Scholar] [CrossRef]
- Perumpuli, B.; Dilrukshi, N. Vinegar: A Functional Ingredient for Human Health. IFRJ 2022, 29, 959–974. [Google Scholar] [CrossRef]
- Wei, J.; Qing, Y.; Zhou, H.; Liu, J.; Qi, C.; Gao, J. 16S rRNA Gene Amplicon Sequencing of Gut Microbiota in Gestational Diabetes Mellitus and Their Correlation with Disease Risk Factors. J. Endocrinol. Investig. 2022, 45, 279–289. [Google Scholar] [CrossRef]
- Zhuge, A.; Li, S.; Lou, P.; Wu, W.; Wang, K.; Yuan, Y.; Xia, J.; Li, B.; Li, L. Longitudinal 16s rRNA Sequencing Reveals Relationships among Alterations of Gut Microbiota and Nonalcoholic Fatty Liver Disease Progression in Mice. Microbiol. Spectr. 2022, 10, e00047-22. [Google Scholar] [CrossRef]
- Wang, L.-Y.; He, L.-H.; Xu, L.-J.; Li, S.-B. Short-Chain Fatty Acids: Bridges between Diet, Gut Microbiota, and Health. J. Gastroenterol. Hepatol. 2024, 39, 1728–1736. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory Role of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Cell Commun. Signal 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Lee, Y.; Chae, W.; Cho, J.-Y. An Improved Method to Quantify Short-Chain Fatty Acids in Biological Samples Using Gas Chromatography–Mass Spectrometry. Metabolites 2022, 12, 525. [Google Scholar] [CrossRef]
- Czarnowski, P.; Mikula, M.; Ostrowski, J.; Żeber-Lubecka, N. Gas Chromatography–Mass Spectrometry-Based Analyses of Fecal Short-Chain Fatty Acids (SFCAs): A Summary Review and Own Experience. Biomedicines 2024, 12, 1904. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent Advances in the Application of Metabolomics for Food Safety Control and Food Quality Analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469. [Google Scholar] [CrossRef]
- Utpott, M.; Rodrigues, E.; de Rios, A.O.; Mercali, G.D.; Flôres, S.H. Metabolomics: An Analytical Technique for Food Processing Evaluation. Food Chem. 2022, 366, 130685. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Zheng, X.; Huang, Q.; Farag, M.A.; Zhu, R.; Zhao, C. Emerging Applications of Metabolomics in Food Science and Future Trends. Food Chem. X 2022, 16, 100500. [Google Scholar] [CrossRef]
- Sun, B.; Jia, X.; Zhou, Y.; Wang, H.; Chen, Y.; Zhang, W.; Zhang, G.; Xu, B. Study of Shanxi Aged Vinegar by Non-Targeted Metabolomics Techniques and Antioxidant Activity Characteristics. Food Biosci. 2024, 58, 103757. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut Microbiota-Derived Metabolites as Key Actors in Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Xia, T.; Kang, C.; Qiang, X.; Zhang, X.; Li, S.; Liang, K.; Wang, Y.; Wang, J.; Cao, H.; Wang, M. Beneficial Effect of Vinegar Consumption Associated with Regulating Gut Microbiome and Metabolome. Curr. Res. Food Sci. 2024, 8, 100566. [Google Scholar] [CrossRef]
- Cui, N.; Huang, X.; Kong, Z.; Huang, Y.; Huang, Q.; Yang, S.; Zhang, L.; Xu, C.; Zhang, X.; Cui, Y. Newcastle Disease Virus Infection Interferes with the Formation of Intestinal Microflora in Newly Hatched Specific-Pathogen-Free Chicks. Front. Microbiol. 2018, 9, 900. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; You, Y.; Yin, M.; Ren, C.; Zhan, J.; Huang, W. A Fast and Accurate Way to Determine Short Chain Fatty Acids in Mouse Feces Based on GC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1099, 73–82. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Zhu, M.-J. A Sensitive GC/MS Detection Method for Analyzing Microbial Metabolites Short Chain Fatty Acids in Fecal and Serum Samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R.; O’Sullivan, J.F.; Friesen, M.; Becker, C.E.; Zhang, X.; Liu, P.; Wakabayashi, Y.; Morningstar, J.E.; Shi, X.; Choi, J.; et al. Induced Pluripotent Stem Cell Differentiation Enables Functional Validation of GWAS Variants in Metabolic Disease. Cell Stem Cell 2017, 20, 547–557.e7. [Google Scholar] [CrossRef] [PubMed]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; HUSERMET Consortium; et al. Development of a Bobust and Repeatable UPLC−MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global Metabolic Profiling of Animal and Human Tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Procházková, N.; Laursen, M.F.; La Barbera, G.; Tsekitsidi, E.; Jørgensen, M.S.; Rasmussen, M.A.; Raes, J.; Licht, T.R.; Dragsted, L.O.; Roager, H.M. Gut Physiology and Environment Explain Variations in Human Gut Microbiome Composition and Metabolism. Nat. Microbiol. 2024, 9, 3210–3225. [Google Scholar] [CrossRef]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol Metabolites from Colonic Microbiota Exert Anti-Inflammatory Activity on Different Inflammation Models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H. Evaluation of the Therapeutic Effect and Dose-Effect of Bifidobacterium Breve on the Primary Clostridioides Difficile Infected Mice. Appl. Microbiol. Biotechnol. 2021, 105, 9243–9260. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, Z.; Zhao, Y.; Kang, C.; Zhang, X.; Tian, Y.; Yu, J.; Cao, H.; Wang, M. The Anti-Diabetic Activity of Polyphenols-Rich Vinegar Extract in Mice via Regulating Gut Microbiota and Liver Inflammation. Food Chem. 2022, 393, 133443. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Yue, Y.; Guan, Q.; Ren, Y.; Guo, L.; Fan, Y.; Lu, Z.; Shi, J.; Xu, Z. Cereal Vinegar Sediment Alleviates Spontaneous Ulcerative Colitis in Il-10 Deficient Mice. Mol. Nutr. Food Res. 2021, 65, 2001227. [Google Scholar] [CrossRef]
- Leal Maske, B.; Murawski de Mello, A.F.; da Silva Vale, A.; Prado Martin, J.G.; de Oliveira Soares, D.L.; De Dea Lindner, J.; Soccol, C.R.; de Melo Pereira, G.V. Exploring Diversity and Functional Traits of Lactic Acid Bacteria in Traditional Vinegar Fermentation: A Review. Int. J. Food Microbiol. 2024, 412, 110550. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, X.; Xiao, Y.; Sheng, Q.; Tu, L.; Chen, F.; Yan, Y.; Zheng, Y.; Wang, M. Interaction of Acetic Acid Bacteria and Lactic Acid Bacteria in Multispecies Solid-State Fermentation of Traditional Chinese Cereal Vinegar. Front. Microbiol. 2022, 13, 964855. [Google Scholar] [CrossRef]
- Cai, F.; Huang, M.; Liu, W.; Wan, X.; Qiu, K.; Xu, X. Dietary Addition of Compound Organic Acids Improves the Growth Performance, Carcass Trait, and Body Health of Broilers. Front. Nutr. 2025, 12, 1536606. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Liu, B.; Wang, F.; Lu, Z.; Jin, M.; Wang, Y. Maternal Consumption of a Fermented Diet Protects Offspring against Intestinal Inflammation by Regulating the Gut Microbiota. Gut Microbes 2022, 14, 2057779. [Google Scholar] [CrossRef]
- Peng, J.; Li, X.; Zheng, L.; Duan, L.; Gao, Z.; Hu, D.; Li, J.; Li, X.; Shen, X.; Xiao, H. Ban-Lan-Gen Granule Alleviates Dextran Sulfate Sodium-Induced Chronic Relapsing Colitis in Mice via Regulating Gut Microbiota and Restoring Gut SCFA Derived-GLP-1 Production. J. Inflamm. Res. 2022, 15, 1457–1470. [Google Scholar] [CrossRef]
- Zhao, H.; Bai, H.; Deng, F.; Zhong, R.; Liu, L.; Chen, L.; Zhang, H. Chemically Protected Sodium Butyrate Improves Growth Performance and Early Development and Function of Small Intestine in Broilers as One Effective Substitute for Antibiotics. Antibiotics 2022, 11, 132. [Google Scholar] [CrossRef]
- Zhai, S.; Zhu, L.; Qin, S.; Li, L. Effect of Lactulose Intervention on Gut Microbiota and Short Chain Fatty Acid Composition of C57BL/6J Mice. Microbiologyopen 2018, 7, e00612. [Google Scholar] [CrossRef]
- González Ariza, A.; Arando Arbulu, A.; León Jurado, J.M.; Navas González, F.J.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Discriminant Canonical Tool for Differential Biometric Characterization of Multivariety Endangered Hen Breeds. Animals 2021, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, B.; Yang, J.; Zhou, J.; Xu, Y. Linear Discriminant Analysis. Nat. Rev. Methods Primers 2024, 4, 70. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Q.; Li, T.; Lu, L.; Wang, F.; Zhang, H.; Liu, Z.; Ma, H.; Zhu, Q.; Wang, J.; et al. Lactobacillus Plantarum-Derived Indole-3-Lactic Acid Ameliorates Colorectal Tumorigenesis via Epigenetic Regulation of CD8+ T Cell Immunity. Cell Metab. 2023, 35, 943–960.e9. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Jensen, P.Ø.; Nielsen, A.K.; Johansen, H.K.; Homøe, P.; Høiby, N.; Givskov, M.; Kirketerp-Møller, K. Antibiofilm Properties of Acetic Acid. Adv. Wound Care 2015, 4, 363–372. [Google Scholar] [CrossRef]
- Cao, L.; Wu, Y.; Liu, K.-Y.; Qi, N.-X.; Zhang, J.; Tie, S.-S.; Li, X.; Tian, P.-P.; Gu, S.-B. Cornus Officinalis Vinegar Alters the Gut Microbiota, Regulating Lipid Droplet Changes in Nonalcoholic Fatty Liver Disease Model Mice. Food Med. Homol. 2024, 1, 9420002. [Google Scholar] [CrossRef]
- Li, D.; Tang, W.; Wang, Y.; Gao, Q.; Zhang, H.; Zhang, Y.; Wang, Y.; Yang, Y.; Zhou, Y.; Zhang, Y.; et al. An Overview of Traditional Chinese Medicine Affecting Gut Microbiota in Obesity. Front. Endocrinol. 2023, 14, 1149751. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Zhang, R.; Li, Y.; Li, X.; Ding, Y.; Feng, Y.; Zhang, X.; Yang, Y.; Su, J.; et al. Prebiotic Characteristics of Degraded Polysaccharides from Acanthopanax Senticosus Polysaccharide on Broilers Gut Microbiota Based on in Vitro Digestion and Fecal Fermentation. Poult. Sci. 2024, 103, 103807. [Google Scholar] [CrossRef]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Galindo-Prieto, B.; Trygg, J.; Geladi, P. A New Approach for Variable Influence on Projection (VIP) in O2PLS Models. Chemom. Intell. Lab. Syst. 2017, 160, 110–124. [Google Scholar] [CrossRef]
- Sorensen, J.L.; Sleeman, M.C.; Schofield, C.J. Synthesis of Deuterium Labelled L- and D-Glutamate Semialdehydes and Their Evaluation as Substrates for Carboxymethylproline Synthase (CarB)—Implications for Carbapenem Biosynthesis. Chem. Commun. 2005, 9, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shama, A.; Zhang, Y.; Chen, B.; Bu, Y.; Chen, P.-A.; Lin, C.; Liu, J.; Zheng, J.; Li, Z.; et al. Gut Microbiota and Plasma Metabolites in Pregnant Mothers and Infant Atopic Dermatitis: A Multi-Omics Study. World Allergy Organ. J. 2025, 18, 101017. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, H.; Du, K.; Wang, S.; Li, M.; Ma, C.; Liu, F.; Dong, Y.; Fu, C. Exploring the Mechanism of Taohong Siwu Decoction on the Treatment of Blood Deficiency and Blood Stasis Syndrome by Gut Microbiota Combined with Metabolomics. Chin. Med. 2023, 18, 44. [Google Scholar] [CrossRef]
- Latifi-Navid, H.; Barzegar Behrooz, A.; Jamehdor, S.; Davari, M.; Latifinavid, M.; Zolfaghari, N.; Piroozmand, S.; Taghizadeh, S.; Bourbour, M.; Shemshaki, G.; et al. Construction of an Exudative Age-Related Macular Degeneration Diagnostic and Therapeutic Molecular Network Using Multi-Layer Network Analysis, a Fuzzy Logic Model, and Deep Learning Techniques: Are Retinal and Brain Neurodegenerative Disorders Related? Pharmaceuticals 2023, 16, 1555. [Google Scholar] [CrossRef]
- Tran, U.; Boyle, T.; Shupp, J.W.; Hammamieh, R.; Jett, M. Staphylococcal Enterotoxin B Initiates Protein Kinase C Translocation and Eicosanoid Metabolism While Inhibiting Thrombin-Induced Aggregation in Human Platelets. Mol. Cell Biochem. 2006, 288, 171–178. [Google Scholar] [CrossRef]
- Monteiro, L.M.; Klider, L.M.; Marques, A.A.M.; Farago, P.V.; Emiliano, J.; Souza, R.I.C.; Dos Santos, A.C.; Dos Santos, V.L.P.; Wang, M.; Cassemiro, N.S.; et al. The Cardiorenal Effects of Piper Amalago Are Mediated by the Nitric Oxide/Cyclic Guanosine Monophosphate Pathway and the Voltage-Dependent Potassium Channels. Pharmaceuticals 2023, 16, 1630. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, L.; Zhang, Y.; Liu, Z.; Li, W.; Chen, S.; Li, G. The Identification of Key Genes and Pathways in Hepatocellular Carcinoma by Bioinformatics Analysis of High-Throughput Data. Med. Oncol. 2017, 34, 101. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Liu, Y.; Ma, Y.; Zhang, R.; Zhang, Y.; Fan, C.; Qu, L.; Ning, Z. DNA Methylome and Transcriptome Identified Key Genes and Pathways Involved in Speckled Eggshell Formation in Aged Laying Hens. BMC Genom. 2023, 24, 31. [Google Scholar] [CrossRef]
- Forid, M.S.; Rahman, M.A.; Aluwi, M.F.F.M.; Uddin, M.N.; Roy, T.G.; Mohanta, M.C.; Huq, A.M.; Amiruddin Zakaria, Z. Pharmacoinformatics and UPLC-QTOF/ESI-MS-Based Phytochemical Screening of Combretum Indicum against Oxidative Stress and Alloxan-Induced Diabetes in Long-Evans Rats. Molecules 2021, 26, 4634. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, L.-M.; Zhuang, Z.-X.; Wang, X.-R.; Fu, Q.; Huang, H.-B.; Huang, L.-X.; Qin, Y.-X.; Yan, Q.-P. Transcriptomic and Metabolomic Insights into the Role of the flgK Gene in the Pathogenicity of Pseudomonas Plecoglossicida to Orange-Spotted Grouper (Epinephelus Coioides). Zool. Res. 2022, 43, 952–965. [Google Scholar] [CrossRef]
- Wang, J.; Dou, B.; Zheng, L.; Cao, W.; Dong, P.; Chen, Y.; Zeng, X.; Wen, Y.; Pan, W.; Ma, J.; et al. The Metabolic Chemical Reporter Ac46AzGal Could Incorporate Intracellular Protein Modification in the Form of UDP-6AzGlc Mediated by OGT and Enzymes in the Leloir Pathway. Front. Chem. 2021, 9, 708306. [Google Scholar] [CrossRef] [PubMed]
- Priya, K.L.; Mahendra, J.; Mahendra, L.; Kanakamedala, A.; Alsharif, K.F.; Mugri, M.H.; Varadarajan, S.; Alamoudi, A.; Hassan, A.A.-H.A.-A.; Alnfiai, M.M.; et al. Salivary Biomarkers in Periodontitis Post Scaling and Root Planing. J. Clin. Med. 2022, 11, 7142. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-B.; Damonte, V.M.; Chen, C.; Wang, G.X.; Kebschull, J.M.; Yamaguchi, H.; Bian, W.-J.; Purmann, C.; Pattni, R.; Urban, A.E.; et al. Hyperexcitable Arousal Circuits Drive Sleep Instability during Aging. Science 2022, 375, eabh3021. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; La, S.; Guo, Z.; Liu, B.; Gao, Z.; Farooq, U.; Wang, Z.; Zhu, X.; Cui, Y.; et al. Microbial Short-Chain Fatty Acids: A Bridge between Dietary Fibers and Poultry Gut Health—A Review. Anim. Biosci. 2022, 35, 1461. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Liang, Y.; Li, X.; Wang, Y.; Li, J. Sishen Wan Treats Ulcerative Colitis in Rats by Regulating Gut Microbiota and Restoring the Treg/Th17 Balance. Evid. Based Complement. Altern. Med. 2022, 2022, 1432816. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The Interplay between Gut Microbiota, Short-Chain Fatty Acids, and Implications for Host Health and Disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, N.; Lu, M.; Wang, Q.; Zhao, C.; Wang, J.; Zhou, M.; Xu, Y. Effects of Electroacupuncture on Urinary Metabolome and Microbiota in Presenilin1/2 Conditional Double Knockout Mice. Front. Microbiol. 2022, 13, 1047121. [Google Scholar] [CrossRef]
| Group | Acetic Acid (μg/g) | Butyric Acid (μg/g) | Isovaleric Acid (μg/g) | Valeric Acid (μg/g) | Caproic Acid (μg/g) | Propionic Acid (μg/g) | Isobutyric Acid (μg/g) |
|---|---|---|---|---|---|---|---|
| B | 2230.16 ± 159.22 d | 1044.04 ± 22.53 b | 19.79 ± 8.30 b | 78.43 ± 2.51 c | 1.14 ± 0.28 ab | 558.69 ± 43.03 ab | 44.90 ± 0.68 b |
| M-L | 2561.23 ± 251.09 b | 1786.51 ± 148.50 a | 30.95 ± 4.68 a | 84.59 ± 2.16 ab | 1.54 ± 0.43 a | 476.11 ± 93.66 b | 36.19 ± 4.56 c |
| M-M | 3118.86 ± 83.93 a | 1759.54 ± 61.66 a | 35.95 ± 1.18 a | 86.98 ± 1.63 a | 0.91 ± 0.29 b | 642.74 ± 32.72 a | 66.83 ± 4.31 a |
| M-H | 3248.97 ± 146.79 a | 1223.56 ± 102.29 b | 28.87 ± 0.49 ab | 80.99 ± 1.11 bc | 1.01 ± 0.17 ab | 486.02 ± 84.93 b | 40.39 ± 2.62 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zhao, R.; Xiao, Z.; Li, Y.; Yang, J.; Jiang, S.; Xu, S.; Xu, Z.; Gu, D. Investigating the Effects of Yunnan Lufeng Aromatic Vinegar Intervention on Intestinal Microbiota, SCFAs, and Metabolites in Mice Using Multi-Omics Techniques. Foods 2025, 14, 3747. https://doi.org/10.3390/foods14213747
Chen H, Zhao R, Xiao Z, Li Y, Yang J, Jiang S, Xu S, Xu Z, Gu D. Investigating the Effects of Yunnan Lufeng Aromatic Vinegar Intervention on Intestinal Microbiota, SCFAs, and Metabolites in Mice Using Multi-Omics Techniques. Foods. 2025; 14(21):3747. https://doi.org/10.3390/foods14213747
Chicago/Turabian StyleChen, Hongqin, Ruihuan Zhao, Zhichao Xiao, Yang Li, Junran Yang, Shuaihan Jiang, Sisi Xu, Zhiqiang Xu, and Dahai Gu. 2025. "Investigating the Effects of Yunnan Lufeng Aromatic Vinegar Intervention on Intestinal Microbiota, SCFAs, and Metabolites in Mice Using Multi-Omics Techniques" Foods 14, no. 21: 3747. https://doi.org/10.3390/foods14213747
APA StyleChen, H., Zhao, R., Xiao, Z., Li, Y., Yang, J., Jiang, S., Xu, S., Xu, Z., & Gu, D. (2025). Investigating the Effects of Yunnan Lufeng Aromatic Vinegar Intervention on Intestinal Microbiota, SCFAs, and Metabolites in Mice Using Multi-Omics Techniques. Foods, 14(21), 3747. https://doi.org/10.3390/foods14213747







