Exploring Ultrasound and Microwave-Assisted Accelerated Aging of Jerez Vinegar: Impacts on Phenolic, Volatile, Colorimetric, and Sensory Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Vinegar Samples
2.3. Accelerated Aging by Ultrasound and Microwave Techniques
2.4. Analysis of Phenolics by Liquid Chromatography–Mass Spectrometry (LC–MS/MS)
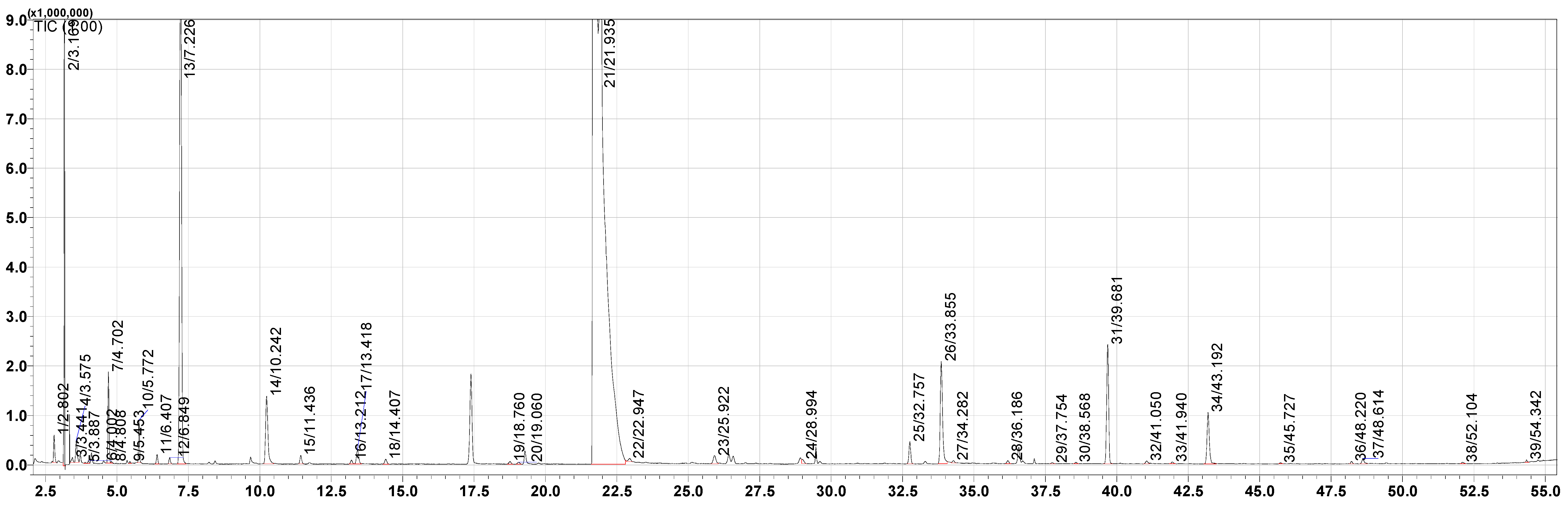
2.5. Analysis of Volatile Compounds by Gas Chromatography–Mass Spectrometry (GC–MS)
2.6. Analyses of Colorimetric Characteristics by UV–Vis Spectrophotometer
2.7. Descriptive Sensory Analysis
- Odor: overall odor, vinegar ID, winy character, raisin, ethyl acetate (chemical), alcohol/liquor, woody, fruity, spicy, vanilla, clove, toasted, nuts, and leather/old.
- Flavor: overall flavor, vinegar ID, winy character, raisin, ethyl acetate (chemical), alcohol/liquor, woody, fruity, vanilla, clove, toasted, nuts, and leather/old.
- Basic tastes: sweetness, sourness, and bitterness.
- Chemical sensations: astringency and pungency.
- Global attributes: aftertaste.
- Appearance: color and untuoso (texture).
- Defects: dirty (sucio), bacterial, cheese-like, and sawdust (wood shavings).
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Components by LC–MS/MS
3.2. Volatile Compounds by GC–MS/MS
3.3. Colorimetric Characteristics
3.4. Sensory Properties
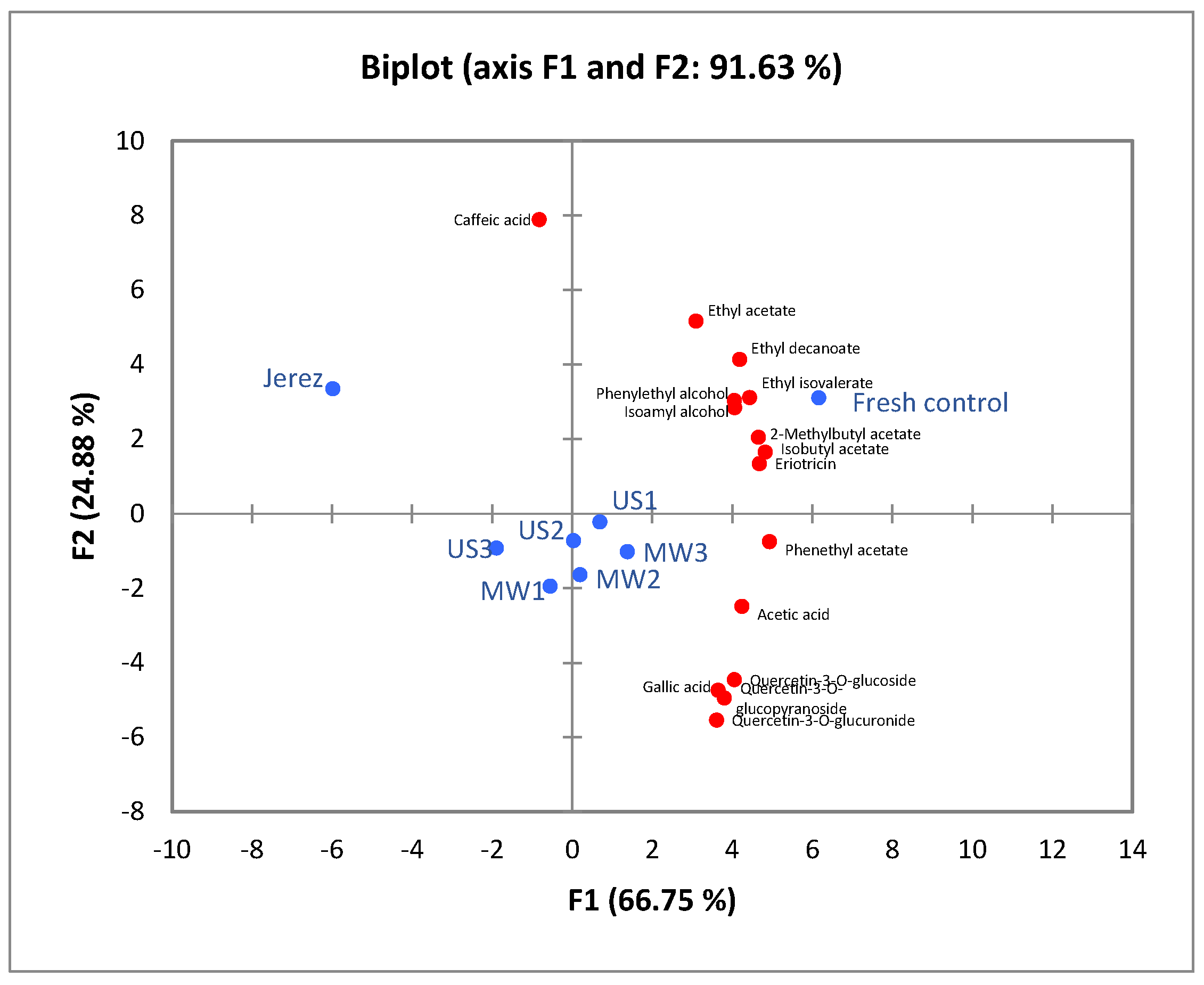
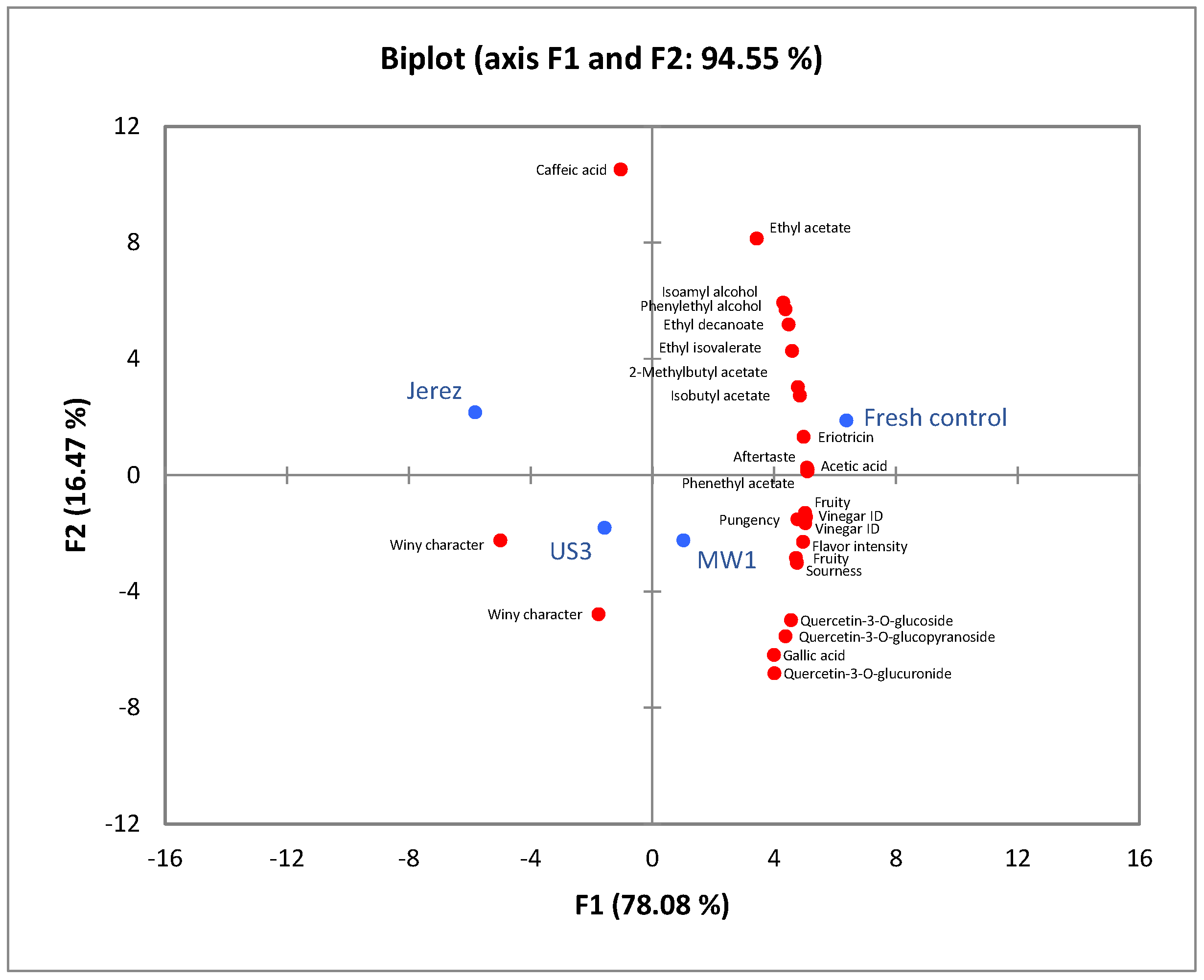
3.5. PCA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palacios, V.; Valcárcel, M.; Caro, I.; Pérez, L. Chemical and biochemical transformations during the industrial process of sherry vinegar aging. J. Agric. Food Chem. 2002, 50, 4221–4225. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.; García-Parrilla, M.; Troncoso, A. Sensory evaluation of Sherry wine vinegar. J. Sens. Stud. 2002, 17, 133–144. [Google Scholar] [CrossRef]
- Council of the European Union. Council Regulation (EC) No 510/2006 of 20 March 2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 2006, 93, 12–25. [Google Scholar]
- Consejería de Agricultura, Pesca, Agua y Desarrollo Rural de la Junta de Andalucía. Guía de Certificación de la Producción Ecológica; Junta de Andalucía: Sevilla, Spain, 2006; Available online: https://www.juntadeandalucia.es/export/drupaljda/Manual_Certificacion_Alta_2015.pdf (accessed on 21 October 2025).
- Vilela, A. Microbial dynamics in sour–sweet wine vinegar: Impacts on chemical and sensory composition. Appl. Sci. 2023, 13, 7366. [Google Scholar] [CrossRef]
- Uysal, R.S. Effects of Aging in Wood Casks on Anthocyanins Compositions, Volatile Compounds, Colorimetric Properties, and Sensory Profile of Jerez Vinegars. Fermentation 2024, 10, 469. [Google Scholar] [CrossRef]
- Uysal, R.S.; Issa-Issa, H.; Sendra, E.; Carbonell-Barrachina, Á.A. Changes in anthocyanin pigments, trans-resveratrol, and colorimetric characteristics of Fondillón wine and other “Monastrell” wines during the aging period. Eur. Food Res. Technol. 2023, 249, 1821–1831. [Google Scholar] [CrossRef]
- Tesfaye, W.; Morales, M.L.; García-Parrilla, M.C.; Troncoso, A.M. Jerez vinegar. In Vinegars of the World; Springer: Milan, Italy, 2009; pp. 179–195. [Google Scholar]
- Parrilla, M.G.a.; Heredia, F.J.; Troncoso, A.M. Sherry wine vinegars: Phenolic composition changes during aging. Food Res. Int. 1999, 32, 433–440. [Google Scholar] [CrossRef]
- Toulaki, A.K.; Bozinou, E.; Athanasiadis, V.; Chatzimitakos, T.; Mantanis, G.I.; Dourtoglou, V.G.; Lalas, S.I. Accelerating xinomavro red wine flavor aging using a pulsed electric field and various wood chips. Appl. Sci. 2023, 13, 12844. [Google Scholar] [CrossRef]
- Munoz-Garcia, R.; Díaz-Maroto, M.C.; Villena, M.A.; Perez-Coello, M.S.; Alanon, M.E. Ultrasound and microwave techniques as physical methods to accelerate oak wood aged aroma in red wines. LWT 2023, 179, 114597. [Google Scholar] [CrossRef]
- García, R.M.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Marchante, L.; Díaz-Maroto, M.C.; Porras, P.P.; Ortín, A.B.; Gómez-Plaza, E.; Pérez-Coello, M.S. Ultrasound and microwave techniques for assisting ageing on lees of red wines. Food Chem. 2023, 426, 136660. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research progress and application of ultrasonic-and microwave-assisted food processing technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Okonkwo, C.E.; Inyinbor, A.A.; Yagoub, A.E.A.; Olaniran, A.F. Ultrasound, infrared and its assisted technology, a promising tool in physical food processing: A review of recent developments. Crit. Rev. Food Sci. Nutr. 2023, 63, 1587–1611. [Google Scholar] [CrossRef]
- Pérez-Porras, P.; Bautista-Ortín, A.B.; Jurado, R.; Gómez-Plaza, E. Using high-power ultrasounds in red winemaking: Effect of operating conditions on wine physico-chemical and chromatic characteristics. LWT 2021, 138, 110645. [Google Scholar] [CrossRef]
- Gao, X.; Feng, T.; Liu, E.; Shan, P.; Zhang, Z.; Liao, L.; Ma, H. Ougan juice debittering using ultrasound-aided enzymatic hydrolysis: Impacts on aroma and taste. Food Chem. 2021, 345, 128767. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Chu, R.; Ratchaneesiripap, P. An ultrasound-assisted extraction system to accelerate production of Mhiskey, a rice spirit-based product, inside oak barrel: Total phenolics, color, and energy consumption. J. Food Process Eng. 2022, 45, e13861. [Google Scholar] [CrossRef]
- Delgado-González, M.J.; García-Moreno, M.d.V.; Guillén-Sánchez, D.A. A theoretical approximation of the accelerating effects of ultrasound about the extraction of phenolic compounds from wood by wine spirits. Foods 2022, 11, 517. [Google Scholar] [CrossRef]
- Ferraretto, P.; Cacciola, V.; Batllò, I.F.; Celotti, E. Ultrasounds application in winemaking: Grape maceration and yeast lysis. Ital. J. Food Sci./Riv. Ital. Sci. Degli Aliment. 2013, 25, 160. [Google Scholar]
- Celotti, E.; Ferraretto, P. Recent Applications of Ultrasound in Winemaking: From the Maceration to the Wine Aging. In Proceedings of the II International Conference on Ultrasonic-Based Applications: From analysis to Synthesis, Caparica, Portugal, 6–8 June 2016; pp. 89–90. [Google Scholar]
- Andreou, V.; Giannoglou, M.; Xanthou, M.; Metafa, M.; Katsaros, G. Aging acceleration of balsamic vinegar applying micro-oxygenation technique. Food Chem. 2023, 419, 136077. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, X.; Wang, X.; Zhang, H.; Ji, Y.; Ren, D.; Lu, J. An efficient method using ultrasound to accelerate aging in crabapple (Malus asiatica) vinegar produced from fresh fruit and its influencing mechanism investigation. Ultrason. Sonochem. 2021, 72, 105464. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Liu, F.; Zhang, C.; Ma, H.; Wang, L.; Zhao, S. Effects of ultrasonic treatment on the maturation of Zhenjiang vinegar. Ultrason. Sonochem. 2017, 39, 272–280. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Liu, Y.; Xu, M.; Zhang, T.H.; Ren, H.; Liu, W.; Li, M.Y. Accelerated aging of grape pomace vinegar by using additives combined with physical methods. J. Food Process Eng. 2020, 43, e13398. [Google Scholar] [CrossRef]
- Martín, J.F.G.; Sun, D.-W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state-of-the-art research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Hu, Q.; He, Y.; Wang, F.; Wu, J.; Ci, Z.; Chen, L.; Xu, R.; Yang, M.; Lin, J.; Han, L. Microwave technology: A novel approach to the transformation of natural metabolites. Chin. Med. 2021, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Loira, I.; Morata, A.; Suárez-Lepe, J.; González, M.; Rauhut, D. Shortening the ageing on lees process in wines by using ultrasound and microwave treatments both combined with stirring and abrasion techniques. Eur. Food Res. Technol. 2016, 242, 559–569. [Google Scholar] [CrossRef]
- Sánchez-Córdoba, C.; Durán-Guerrero, E.; Castro, R. Olfactometric and sensory evaluation of red wines subjected to ultrasound or microwaves during their maceration or ageing stages. LWT 2021, 144, 111228. [Google Scholar] [CrossRef]
- OIV—International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis; Edition 2022; OIV: Paris, France, 2022; Volume 1‒2. [Google Scholar]
- Glories, Y. The colour of red wines. Conn. Vigne Vin 1984, 18, 195–217. [Google Scholar]
- OECCA. Ficha de Cata de Vinagres; OECCA: Jerez de la Frontera, Spain, 2022. [Google Scholar]
- Ferreira, I.M.; Pérez-Palacios, M.T. Anthocyanic Compounds and Antioxidant Capacity in Fortified Wines. In Processing and Impact on Antioxidants in Beverages; Elsevier: Oxford, UK, 2014; pp. 3–14. [Google Scholar]
- Cerezo, A.B.; Cuevas, E.; Winterhalter, P.; Carmen Garcia-Parrilla, M.; Troncoso, A.M. Anthocyanin composition in Cabernet Sauvignon red wine vinegar obtained by submerged acetification. Food Res. Int. 2010, 43, 1577–1584. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Dangles, O.; Brouillard, R. Coupling reactions between flavylium ions and catechin. Phytochemistry 1996, 41, 1583–1592. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.; Durán-Guerrero, E.; Rodríguez-Dodero, M.C.; Barroso, C.G.; Castro, R. Use of ultrasound at a pilot scale to accelerate the ageing of sherry vinegar. Ultrason. Sonochem. 2020, 69, 105244. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.-D.; de Lerma, N.L.; Cotea, V.V.; Zamfir, C.-I.; Peinado, R.A. Effect of aging time, dosage and toasting level of oak chips on the color parameters, phenolic compounds and antioxidant activity of red wines (var. Fetească neagră). Eur. Food Res. Technol. 2016, 242, 2171–2180. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, X.; Yao, Y.; Qu, A.; Ding, K.; Zhao, G.; Liu, S.Q. Insights into the microbiota and driving forces to control the quality of vinegar. LWT 2022, 157, 113085. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, X.; Zhang, C.; Zheng, Y.; Zheng, B.; Duan, X.; Tian, Y. Microbial dynamics and flavor formation during the traditional brewing of Monascus vinegar. Food Res. Int. 2019, 125, 108531. [Google Scholar] [CrossRef]
- Durán Guerrero, E.; Chinnici, F.; Natali, N.; Marín, R.N.; Riponi, C. Solid-phase extraction method for determination of volatile compounds in traditional balsamic vinegar. J. Sep. Sci. 2008, 31, 3030–3036. [Google Scholar] [CrossRef]
- Kahn, J.; Nickol, G.; Conner, H. Analysis of vinegar by gas-liquid chromatography. J. Agric. Food Chem. 1966, 14, 460–465. [Google Scholar] [CrossRef]
- Chen, T.; Gui, Q.; Shi, J.J.; Zhang, X.Y.; Chen, F.S. Analysis of variation of main components during aging process of Shanxi Aged Vinegar. Acetic Acid Bact. 2013, 2, 31–38. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Changes in major antioxidant compounds during aging of traditional balsamic vinegar. J. Food Biochem. 2010, 34, 152–171. [Google Scholar] [CrossRef]
- Larios, A.; García, H.S.; Oliart, R.M.; Valerio-Alfaro, G. Synthesis of flavor and fragrance esters using Candida antarctica lipase. Appl. Microbiol. Biotechnol. 2004, 65, 373–376. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, J.; Wang, L.; Li, Z. Development of a SPME-GC-MS method for the determination of volatile compounds in Shanxi aged vinegar and its analytical characterization by aroma wheel. J. Food Sci. Technol. 2016, 53, 171–183. [Google Scholar] [CrossRef]
- Liang, J.; Xie, J.; Hou, L.; Zhao, M.; Zhao, J.; Cheng, J.; Wang, S.; Sun, B.-G. Aroma constituents in Shanxi aged vinegar before and after aging. J. Agric. Food Chem. 2016, 64, 7597–7605. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Zhu, W.; Zhang, C.; Yin, L.; Li, L.; Liu, J. Effect of temperature on chemical compounds of Cupei (precursor of bran vinegar) during in-situ aging and revelation of functional microorganisms in the process. LWT 2023, 182, 114912. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Yang, X. Influence of different fumigation processes on aroma compounds of Shanxi aged vinegar. Food Sci. 2015, 36, 90–94. [Google Scholar]
- Corsini, L.; Castro, R.; Barroso, C.G.; Durán-Guerrero, E. Characterization by gas chromatography-olfactometry of the most odour-active compounds in Italian balsamic vinegars with geographical indication. Food Chem. 2019, 272, 702–708. [Google Scholar] [CrossRef]
- Li, H.; Ming, X.; Liu, Z.; Xu, L.; Xu, D.; Hu, L.; Mo, H.; Zhou, X. Accelerating vinegar aging by combination of ultrasonic and magnetic field assistance. Ultrason. Sonochem. 2021, 78, 105708. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Falcone, P.M.; Qiu, J.; Ren, C.-Z.; Li, Z.-G. Effect of ageing on rheological properties and quality of Shanxi aged vinegar. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changsha, China, 18–20 September 2020; p. 012096. [Google Scholar]
- Bozkurt, H.; Göğüş, F.; Eren, S. Nonenzymic browning reactions in boiled grape juice and its models during storage. Food Chem. 1999, 64, 89–93. [Google Scholar] [CrossRef]
- Suslick, K.S. The chemical effects of ultrasound. Sci. Am. 1989, 260, 80–87. [Google Scholar] [CrossRef]
- Denominación de Origen Vinagre de Jerez; Consejo Regulador de las Denominaciones de Origen Jerez-Xérès-Sherry, Manzanilla-Sanlúcar de Barrameda & Vinagre de Jerez. Dossier de Información Vinagre de Jerez; Spain. Available online: https://www.vinagredejerez.org/noticias/Dossier_de_prensa_Vinagre_de_Jerez.pdf (accessed on 21 October 2025).
- Issa-Issa, H.; Noguera-Artiaga, L.; Sendra, E.; Pérez-López, A.J.; Burló, F.; Carbonell-Barrachina, Á.A.; López-Lluch, D. Volatile composition, sensory profile, and consumers’ acceptance of Fondillón. J. Food Qual. 2019, 2019, 5981762. [Google Scholar] [CrossRef]
- Junge, J.Y.; Bertelsen, A.S.; Mielby, L.A.; Zeng, Y.; Sun, Y.-X.; Byrne, D.V.; Kidmose, U. Taste interactions between sweetness of sucrose and sourness of citric and tartaric acid among Chinese and Danish consumers. Foods 2020, 9, 1425. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Marchand, S.; de Revel, G.; Barbe, J.-C. How do esters and dimethyl sulphide concentrations affect fruity aroma perception of red wine? Demonstration by dynamic sensory profile evaluation. Food Chem. 2016, 194, 196–200. [Google Scholar] [CrossRef]
- Chang, A.C.; Chen, F.C. The application of 20 kHz ultrasonic waves to accelerate the aging of different wines. Food Chem. 2002, 79, 501–506. [Google Scholar] [CrossRef]
- Lucena, G.N.; dos Santos, C.C.; Pinto, G.C.; Piazza, R.D.; Guedes, W.N.; Júnior, M.J.; de Paula, A.V.; Marques, R.F.C. Synthesis and characterization of magnetic cross-linked enzyme aggregate and its evaluation of the alternating magnetic field (AMF) effects in the catalytic activity. J. Magn. Magn. Mater. 2020, 516, 167326. [Google Scholar] [CrossRef]
| Chemical Family | Code | Compound | RT (min) | [M–H] (m/z) | MS/MS (m/z) | Fresh Control | Jerez | US1 | US2 | US3 | MW1 | MW2 | MW3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stilbenes | 1 | (E)-resveratrol | 8.16 | 229 | 135.10/107.10 †/91.10 | ND ‡ d | 25.9 ± 0.4 a | 27.7 ± 0.3 a | ND d | 21.4 ± 0.3 b | 15.3 ± 0.2 c | ND d | 18.5 ± 0.6 b |
| Flavones | 2 | Luteolin-7-O-glucoside | 6.68 | 448.90 | 287.10/153.10/135.15 | 8.38 ± 0.4 b | 5.26 ± 0.3 c | 10.0 ± 0.2 a | 8.35 ± 0.3 b | 7.95 ± 0.3 b | 8.3 ± 0.2 b | 5.58 ± 0.1 c | 8.93 ± 0.3 b |
| Flavanones | 3 | Eriotricin | 5.95 | 595.20 | 287.05/150.09/135.05 | 65.8 ± 0.6 a | ND c | 24.9 ± 0.2 b | 24.8 ± 0.2 b | 24.8 ± 0.3 b | 25.2 ± 0.4 b | 25.0 ± 0.3 b | 25.0 ± 0.2 b |
| 4 | Hesperidin | 7.02 | 609.20 | 301/163.90/150.95 | ¶ tr b | ND a | ND a | ND a | ND a | ND a | ND a | ND a | |
| Flavonols | 5 | Myricetin-3-O-glucoside | 5.69 | 481.10 | 319.10/273.10/153 | 32.3 ± 0.3 a | 28.6 ± 0.2 a | 31.6 ± 0.2 a | 32.5 ± 0.5 a | 30.7 ± 0.3 a | 32.1 ± 0.4 a | ND b | 32.7 ± 0.3 a |
| 6 | Quercetin-3-O-glucoside | 5.95 | 463.25 | 300.15/271.15/255.20 | 232 ± 6.1 a | 60.2 ± 0.7 c | 234 ± 5.2 a | 210 ± 6.4 a | 175 ± 4.6 b | 224 ± 6.5 a | 217 ± 6.2 a | 230 ± 5.5 a | |
| 7 | Quercetin-3-O-glucuronide | 5.97 | 478.95 | 303.05/229/153/112.8 | 1806 ± 10 a | 1029 ± 7 c | 1768 ± 7 b | 1772 ± 9 b | 1738 ± 6 b | 1863 ± 15 a | 1852 ± 11 a | 1877 ± 15 a | |
| 8 | Quercetin-3-O-galactoside | 5.98 | 465 | 303.10/229.1/164/153 | 240 ± 2.1 a | 61.9 ± 0.8 c | 232 ± 1 b | 231 ± 3 b | 228 ± 2 b | 241 ± 2 a | 242 ± 2 a | 248 ± 3 a | |
| 9 | Quercetin-3-O-rutinoside | 6.20 | 608.90 | 301/299.95/270.95 | 54.5 ± 0.5 a | ND b | 56.4 ± 0.4 a | 56.0 ± 0.5 a | 51.5 ± 0.3 b | 51.4 ± 0.4 b | 51.9 ± 0.3 b | ND b | |
| 10 | Quercetin-3-O-glucopyranoside | 6.41 | 463.10 | 300.95/300/270.95 | 202 ± 4 a | 79.7 ± 0.7 d | 175 ± 1 b | 184 ± 2 b | 161 ± 2 c | 211 ± 4 a | 206 ± 2 a | 215 ± 3 a | |
| Phenolic acids and derivatives | 11 | Gallic acid | 1.18 | 169.10 | 124.95/124.30/78.95 | 1191 ± 8 a | 642 ± 4 c | 1120 ± 7 b | 1180 ± 7 a | 1178 ± 7 a | 1133 ± 6 b | 1147 ± 6 b | 1109 ± 8 b |
| 12 | Chlorogenic acid | 3.22 | 353.30 | 191.05/92.95/85.05 | 23.4 ± 0.3 b | 30.6 ± 0.3 a | 15.1 ± 0.2 c | 15.0 ± 0.2 c | ND d | 14.8 ± 0.1 c | 15.2 ± 0.3 c | 15.0 ± 0.2 c | |
| 13 | Caffeic acid | 4.12 | 179.10 | 135/134/106.95 | 307 ± 7 a | 336 ± 8 a | 239 ± 2 b | 238 ± 2 b | 251 ± 7 b | 198 ± 2 d | 214 ± 3 d | 225 ± 2 c | |
| Anthocyanins | 14 | Peonidin-3,5-di-O-glucoside | 4.89 | 625.15 | 462.95/300.95/285.95 | ND b | ND b | ND b | ND b | ND b | ND b | 11.9 ± 0.1 a | ND b |
| 15 | Cyanidin-3-O-rutinoside | 4.94 | 595.10 | 449/287.10 | tr a | ND a | ND a | ND a | ND a | ND a | ND a | ND a | |
| 16 | Pelargonidin-3-O-glucoside | 5.04 | 433 | 271.10/121.10 | tr b | 15.3 ± 0.2 a | ND b | ND b | tr b | tr b | tr b | ND b | |
| 17 | Peonidin-3-O-glucoside | 5.14 | 463 | 301.10/286/201.05 | ND a | ND a | ND a | tr a | ND a | ND a | ND a | ND a | |
| 18 | Malvidin-3-O-glucoside | 5.16 | 493.10 | 331.10/315.10/287.10 | tr a | ND a | ND a | ND a | ND a | ND a | ND a | ND a | |
| 19 | Malvidin-3-O-galactoside | 5.21 | 493.20 | 331.10/315.10/287.10 | tr a | ND a | ND a | ND a | ND a | ND a | ND a | ND a | |
| Total of polyphenolic compounds | 4230 a | 2515 d | 3943 c | 3951 c | 3967 c | 4016 b | 3988 c | 4034 b |
| Chemical Family | Compound | Code | RT (min) ‡ | RI † | Fresh Control | Jerez | US1 | US2 | US3 | MW1 | MW2 | MW3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esters | Methyl acetate | V1 | 2.80 | 857 | 3.76 ± 0.2 a | 1.36 ± 0.2 d | 2.51 ± 0.2 c | 2.51 ± 0.05 c | 2.30 ± 0.2 c | 2.37 ± 0.2 c | 2.54 ± 0.3 c | 3.24 ± 0.4 b |
| Ethyl acetate | V2 | 3.17 | 888 | 43.9 ± 2.1 a | 32.5 ± 1.5 c | 38.6 ± 1.7 b | 32.4 ± 0.9 c | 25.9 ± 1.8 d | 29.5 ± 1.2 c | 29.8 ± 1.1 c | 30.2 ± 1.5 c | |
| Ethyl propanoate | V5 | 3.89 | 947 | 0.08 ± 0.01 b | 0.17 ± 0.02 a | 0.06 ± 0.02 b | 0.07 ± 0.01 b | 0.06 ± 0.01 b | 0.05 ± 0.01 b | 0.05 ± 0.01 b | 0.06 ± 0.01 b | |
| Ethyl isobutyrate | V6 | 4.01 | 957 | 0.33 ± 0.03 a | 0.15 ± 0.01 b | 0.20 ± 0.02 b | 0.19 ± 0.01 b | 0.15 ± 0.02 b | 0.16 ± 0.01 b | 0.17 ± 0.02 b | 0.19 ± 0.01 b | |
| Isobutyl acetate | V7 | 4.71 | 1007 | 12.3 ± 0.2 a | 3.99 ± 0.4 c | 8.01 ± 0.7 b | 7.60 ± 0.2 b | 6.36 ± 0.4 b | 6.32 ± 0.5 b | 6.82 ± 0.8 b | 7.98 ± 0.2 b | |
| Methyl isovalerate | V8 | 4.82 | 1012 | 0.24 ± 0.06 a | 0.09 ± 0.01 c | 0.14 ± 0.01 b | 0.14 ± 0.04 b | 0.14 ± 0.04 b | 0.11 ± 0.02 b | 0.12 ± 0.04 b | 0.13 ± 0.01 b | |
| Ethyl 2-methylbutanoate | V9 | 5.46 | 1041 | 0.19 ± 0.01 a | 0.08 ± 0.01 c | 0.14 ± 0.01 b | 0.11 ± 0.01 b | 0.09 ± 0.02 c | 0.09 ± 0.01 c | 0.09 ± 0.01 c | 0.12 ± 0.02 b | |
| Ethyl isovalerate | V10 | 5.78 | 1056 | 5.33 ± 0.7 a | 1.78 ± 0.1 b | 3.59 ± 0.4 b | 2.85 ± 0.1 b | 2.46 ± 0.4 b | 2.28 ± 0.05 b | 2.42 ± 0.4 b | 2.89 ± 0.3 b | |
| 2-Methylbutyl acetate | V13 | 7.22 | 1113 | 125 ± 1 a | 36.6 ± 3.9 d | 85.7 ± 6.1 b | 74.7 ± 2.4 b | 61.6 ± 6.4 c | 58.2 ± 4.6 c | 62.2 ± 6.1 c | 71.5 ± 4.3 b | |
| Ethyl hexanoate | V15 | 11.45 | 1225 | 1.84 ± 0.1 a | 0.32 ± 0.02 e | 1.06 ± 0.2 b | 0.95 ± 0.03 b | 0.79 ± 0.1 d | 0.71 ± 0.06 d | 0.76 ± 0.1 d | 0.86 ± 0.08 c | |
| Hexyl acetate | V16 | 13.22 | 1262 | 0.81 ± 0.1 c | 0.94 ± 0.1 c | 0.46 ± 0.2 d | 0.92 ± 0.2 c | 1.48 ± 0.2 b | 1.65 ± 0.1 b | 2.31 ± 0.1 a | 2.61 ± 0.3 a | |
| 2-Methylbutyl isovalerate | V18 | 14.42 | 1288 | 1.34 ± 0.1 a | 0.19 ± 0.06 d | 0.57 ± 0.1 b | 0.50 ± 0.06 b | 0.37 ± 0.1 c | 0.35 ± 0.04 c | 0.25 ± 0.04 d | 0.42 ± 0.02 c | |
| Acetoin acetate | V19 | 18.79 | 1370 | 0.52 ± 0.06 a | 0.28 ± 0.06 d | 0.32 ± 0.1 c | 0.38 ± 0.04 c | 0.33 ± 0.02 c | 0.38 ± 0.08 c | 0.44 ± 0.05 b | 0.48 ± 0.08 a | |
| Ethyl decanoate | V25 | 32.78 | 1630 | 5.08 ± 0.5 a | 0.29 ± 0.1 d | 1.22 ± 0.07 b | 1.00 ± 0.1 b | 0.53 ± 0.08 c | 0.78 ± 0.1 c | 0.78 ± 0.05 c | 1.14 ± 0.09 b | |
| Diethyl succinate | V27 | 34.31 | 1664 | 0.40 ± 0.09 b | 0.79 ± 0.03 a | 0.24 ± 0.08 d | 0.23 ± 0.04 d | 0.24 ± 0.03 d | 0.20 ± 0.03 d | 0.22 ± 0.05 d | 0.31 ± 0.08 c | |
| Benzyl acetate | V28 | 36.21 | 1706 | 0.67 ± 0.1 a | 0.36 ± 0.01 d | 0.34 ± 0.08 d | 0.42 ± 0.08 c | 0.30 ± 0.04 d | 0.41 ± 0.02 c | 0.32 ± 0.02 d | 0.51 ± 0.08 b | |
| Methyl salicylate | V29 | 37.76 | 1745 | 0.20 ± 0.01 a | 0.25 ± 0.04 a | 0.15 ± 0.05 b | 0.14 ± 0.02 b | 0.12 ± 0.01 b | 0.17 ± 0.01 b | 0.17 ± 0.04 b | 0.20 ± 0.03 a | |
| Ethyl benzeneacetate | V30 | 38.60 | 1765 | 0.27 ± 0.05 a | 0.13 ± 0.02 c | 0.13 ± 0.02 c | 0.13 ± 0.01 c | 0.13 ± 0.01 c | 0.12 ± 0.02 c | 0.15 ± 0.01 c | 0.16 ± 0.03 b | |
| Phenethyl acetate | V31 | 39.70 | 1792 | 27.5 ± 0.8 a | 7.87 ± 0.4 d | 19.3 ± 0.4 b | 18.1 ± 0.3 b | 15.3 ± 0.8 c | 18.1 ± 0.7 b | 18.8 ± 0.9 b | 21.5 ± 1.1 b | |
| Isopropyl myristate | V36 | 48.24 | 2010 | 0.41 ± 0.07 c | 0.22 ± 0.03 d | 0.66 ± 0.06 b | 0.97 ± 0.1 a | 0.30 ± 0.1 d | 0.23 ± 0.09 d | 0.28 ± 0.07 d | 0.12 ± 0.03 d | |
| Acids | Acetic acid | V21 | 21.9 | 1427 | 716 ± 20 a | 314 ± 6.6 d | 659 ± 15 a | 597 ± 18 b | 451 ± 16 c | 533 ± 1.8 b | 715 ± 25 a | 716 ± 28 a |
| Isobutyric acid | V24 | 29.0 | 1554 | 0.95 ± 0.1 b | 0.92 ± 0.03 b | 1.18 ± 0.6 b | 1.03 ± 0.04 b | 1.06 ± 0.1 b | 1.13 ± 0.1 b | 1.23 ± 0.08 a | 1.32 ± 0.2 a | |
| Isovaleric acid | V26 | 33.87 | 1654 | 26.8 ± 1.2 a | 18.2 ± 1.1 b | 21.6 ± 1.5 b | 25.4 ± 1.8 a | 23.1 ± 1.5 b | 28.0 ± 2.0 a | 30.5 ± 3.1 a | 32.8 ± 2.5 a | |
| Hexanoic acid | V32 | 41.07 | 1828 | 0.72 ± 0.02 a | 0.33 ± 0.03 d | 0.34 ± 0.06 d | 0.46 ± 0.1 c | 0.50 ± 0.09 c | 0.55 ± 0.08 c | 0.68 ± 0.09 b | 0.75 ± 0.01 a | |
| Octanoic acid | V37 | 48.64 | 2013 | 1.10 ± 0.1 a | 0.41 ± 0.01 d | 0.62 ± 0.1 c | 0.80 ± 0.1 b | 0.85 ± 0.1 b | 1.12 ± 0.1 a | 1.12 ± 0.2 a | 1.21 ± 0.2 a | |
| Decanoic acid | V39 | 54.35 | 1386 | 0.28 ± 0.06 a | 0.07 ± 0.01 c | 0.17 ± 0.01 b | 0.20 ± 0.01 b | 0.19 ± 0.02 b | 0.26 ± 0.02 a | 0.25 ± 0.01 a | 0.28 ± 0.02 a | |
| Alcohols | Ethanol | V4 | 3.58 | 922 | 4.17 ± 0.5 a | 2.91 ± 0.1 b | 3.04 ± 0.03 b | 2.87 ± 0.1 c | 2.53 ± 0.3 c | 2.91 ± 0.05 b | 3.19 ± 0.4 b | 4.32 ± 0.7 a |
| Isobutyl alcohol | V11 | 6.44 | 1085 | 1.49 ± 0.1 a | 0.71 ± 0.04 d | 0.88 ± 0.1 c | 0.91 ± 0.03 c | 0.81 ± 0.07 c | 0.93 ± 0.1 b | 0.98 ± 0.08 b | 1.13 ± 0.02 b | |
| Isoamyl alcohol | V14 | 10.26 | 1200 | 15.5 ± 1.3 a | 10.2 ± 1.2 b | 10.4 ± 1.3 b | 11.2 ± 1.3 b | 9.36 ± 0.4 c | 10.7 ± 1.0 b | 11.4 ± 1.0 b | 13.5 ± 0.8 a | |
| Benzyl alcohol | V33 | 41.97 | 1852 | 0.42 ± 0.03 a | 0.38 ± 0.05 b | 0.26 ± 0.03 c | 0.21 ± 0.05 c | 0.20 ± 0.01 c | 0.26 ± 0.03 c | 0.24 ± 0.07 c | 0.30 ± 0.04 b | |
| Phenylethyl alcohol | V34 | 43.22 | 1885 | 12.7 ± 0.8 a | 7.96 ± 0.1 c | 7.93 ± 0.5 c | 8.32 ± 0.6 c | 7.67 ± 0.6 c | 8.36 ± 0.6 c | 9.32 ± 0.7 b | 10.4 ± 0.8 b | |
| Dodecyl alcohol | V35 | 45.74 | 1957 | 0.17 ± 0.02 a | 0.19 ± 0.03 a | 0.12 ± 0.03 b | 0.15 ± 0.05 b | 0.14 ± 0.03 b | 0.23 ± 0.06 a | 0.23 ± 0.05 a | 0.22 ± 0.03 a | |
| Aldehydes | Isovaleraldehyde | V3 | 3.45 | 911 | 0.87 ± 0.02 a | 0.04 ± 0.01 d | 0.25 ± 0.07 c | 0.18 ± 0.01 c | 0.19 ± 0.02 c | 0.19 ± 0.03 c | 0.20 ± 0.01 c | 0.42 ± 0.04 b |
| Furfural | V22 | 22.97 | 1445 | 0.56 ± 0.07 a | 0.36 ± 0.01 b | 0.83 ± 0.03 c | 0.89 ± 0.08 c | 0.85 ± 0.03 c | 0.78 ± 0.08 c | 0.90 ± 0.08 c | 1.00 ± 0.07 c | |
| Benzaldehyde | V23 | 25.95 | 1496 | 2.52 ± 0.3 a | 0.23 ± 0.06 d | 1.48 ± 0.2 c | 1.46 ± 0.2 c | 1.18 ± 0.04 c | 1.48 ± 0.2 c | 1.41 ± 0.2 c | 1.70 ± 0.06 b | |
| Ketones | Acetoin | V17 | 13.46 | 1268 | 3.54 ± 0.5 a | 2.56 ± 0.1 c | 2.19 ± 0.4 c | 2.31 ± 0.07 c | 2.01 ± 0.1 c | 2.37 ± 0.07 c | 2.52 ± 0.5 c | 3.01 ± 0.3 b |
| 2-Nonanone | V20 | 19.08 | 1375 | 0.47 ± 0.03 a | 0.11 ± 0.01 c | 0.19 ± 0.03 b | 0.19 ± 0.05 b | 0.16 ± 0.05 b | 0.14 ± 0.03 c | 0.15 ± 0.02 b | 0.16 ± 0.02 b | |
| Phenolic compounds | 2-Ethylphenol | V38 | 52.13 | 2047 | 0.37 ± 0.01 a | 0.27 ± 0.01 b | 0.16 ± 0.01 c | 0.16 ± 0.01 c | 0.13 ± 0.01 d | 0.16 ± 0.01 c | 0.19 ± 0.01 c | 0.25 ± 0.01 b |
| Others | Linalool 3,7-oxide | V12 | 6.83 | 1102 | 0.90 ± 0.01 a | 0.17 ± 0.01 d | 0.56 ± 0.01 b | 0.44 ± 0.01 b | 0.37 ± 0.01 c | 0.31 ± 0.01 c | 0.32 ± 0.01 c | 0.47 ± 0.01 b |
| Total | N = 39 | 1019 a | 449 e | 876 c | 799 c | 623 d | 716 d | 910 b | 950 a |
| Vinegar Type | A420 ¶ | A520 ¶ | A620 ¶ | Color Intensity | Tonality | Color Density | Y ¶ (%) | R ¶ (%) | B ¶ (%) |
|---|---|---|---|---|---|---|---|---|---|
| Fresh control | 1.031 | 0.305 | 0.091 | 1.43 ± 0.05 c | 3.39 ± 0.02 a | 1.34 ± 0.02 c | 72.3 ± 0.1 a | 21.4 ± 0.1 b | 6.3 ± 0.1 c |
| Jerez | 2.049 | 0.784 | 0.294 | 3.13 ± 0.07 a | 2.62 ± 0.07 b | 2.83 ± 0.05 a | 65.5 ± 0.1 b | 25.1 ± 0.1 a | 9.4 ± 0.1 a |
| US1 | 1.085 | 0.334 | 0.108 | 1.53 ± 0.02 c | 3.25 ± 0.08 a | 1.42 ± 0.03 c | 71.1 ± 0.1 a | 21.9 ± 0.1 b | 7.0 ± 0.1 b |
| US2 | 1.124 | 0.346 | 0.124 | 1.58 ± 0.03 c | 3.22 ± 0.08 a | 1.46 ± 0.08 b | 70.3 ± 0.1 a | 21.9 ± 0.1 b | 7.8 ± 0.1 b |
| US3 | 1.253 | 0.382 | 0.160 | 1.82 ± 0.05 b | 3.31 ± 0.09 a | 1.65 ± 0.10 b | 69.7 ± 0.1 a | 21.1 ± 0.1 b | 9.2 ± 0.1 a |
| MW1 | 1.047 | 0.313 | 0.092 | 1.45 ± 0.02 c | 3.35 ± 0.11 a | 1.36 ± 0.07 c | 72.2 ± 0.1 a | 21.5 ± 0.1 b | 6.3 ± 0.1 c |
| MW2 | 1.078 | 0.319 | 0.098 | 1.50 ± 0.03 c | 3.38 ± 0.10 a | 1.40 ± 0.06 c | 72.1 ± 0.1 a | 21.3 ± 0.1 b | 6.6 ± 0.1 c |
| MW3 | 1.055 | 0.314 | 0.094 | 1.46 ± 0.02 c | 3.37 ± 0.15 a | 1.37 ± 0.05 c | 72.2 ± 0.1 a | 21.4 ± 0.1 b | 6.4 ± 0.1 c |
| Attribute | ANOVA | Sample | |||
|---|---|---|---|---|---|
| Fresh Control | Jerez | US3 | MW1 | ||
| Appearance | |||||
| Color | *** | 4.4 c | 9.1 a | 5.5 b | 4.1 c |
| Untuoso (texture) | ǂ NS | 4.4 a | 4.9 a | 4.4 a | 4.4 a |
| Odor | |||||
| Odor intensity | *** | 2.9 a | 1.8 bc | 2.3 ab | 2.6 b |
| Vinegar ID | *** | 8.0 a | 5.1 d | 6.5 c | 6.9 b |
| Winy character | *** | 1.4 b | 2.7 a | 2.4 a | 2.2 a |
| Raisin | *** | 0.0 c | 0.6 a | 0.6 a | 0.3 b |
| Ethyl acetate | *** | 0.0 a | 0.1 a | 0.2 a | 0.1 a |
| Alcohol/liquor | *** | 0.0 b | 0.3 a | 0.1 b | 0.0 b |
| Woody | *** | 0.0 c | 0.9 a | 0.9 a | 0.4 b |
| Fruity | *** | 6.3 a | 3.1 c | 5.3 b | 5.1 b |
| Spicy | ** | 0.0 b | 0.2 b | 0.7 a | 0.1 b |
| Vanilla | NS | 0.0 a | 0.1 a | 0.0 a | 0.1 a |
| Clove | ** | 0.0 b | 0.1 b | 0.4 a | 0.0 b |
| Toasted | *** | 0.0 b | 0.4 a | 0.3 a | 0.1 b |
| Nuts | ** | 0.0 b | 0.1 b | 0.4 a | 0.0 b |
| Leather/old | ** | 0.0 b | 0.4 a | 0.2 a | 0.1 b |
| Defects | NS | 0.0 b | 0.3 a | 0.0 b | 0.0 b |
| Flavor | |||||
| Flavor intensity | *** | 8.0 a | 5.6 c | 6.6 b | 7.5 ab |
| Vinegar ID | *** | 8.1 a | 5.6 c | 6.6 b | 7.4 a |
| Winy character | *** | 2.7 b | 2.9 b | 3.9 a | 2.7 b |
| Raisin | NS | 0.0 a | 0.2 a | 0.1 a | 0.0 a |
| Ethyl acetate | NS | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Alcohol/liquor | NS | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Woody | ** | 0.0 b | 0.4 a | 0.1 b | 0.0 b |
| Fruity | *** | 5.1 a | 2.8 c | 3.6 bc | 4.46 b |
| Spicy | NS | 0.0 a | 0.1 a | 0.1 a | 0.0 a |
| Vanilla | NS | 0.0 a | 0.0 a | 0.0 a | 0.1 a |
| Clove | NS | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Toasted | NS | 0.0 a | 0.1 a | 0.1 a | 0.0 a |
| Nuts | ** | 0.5 a | 0.3 b | 0.3 b | 0.4 a |
| Leather/old | NS | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Sweetness | *** | 1.1 b | 1.6 a | 1.6 a | 1.1 b |
| Sourness | *** | 8.1 a | 5.6 c | 6.6 b | 7.9 a |
| Bitterness | NS | 0.4 a | 0.4 a | 0.4 a | 0.4 a |
| Astringency | NS | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Pungency | *** | 4.1 a | 2.0 b | 2.5 b | 3.8 a |
| Defects | NS | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Global | |||||
| Aftertaste | *** | 6.5 a | 4.3 c | 4.9 c | 5.6 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uysal, R.S.; Issa-Issa, H.; Carbonell-Barrachina, Á.A.; Sendra, E. Exploring Ultrasound and Microwave-Assisted Accelerated Aging of Jerez Vinegar: Impacts on Phenolic, Volatile, Colorimetric, and Sensory Properties. Foods 2025, 14, 3665. https://doi.org/10.3390/foods14213665
Uysal RS, Issa-Issa H, Carbonell-Barrachina ÁA, Sendra E. Exploring Ultrasound and Microwave-Assisted Accelerated Aging of Jerez Vinegar: Impacts on Phenolic, Volatile, Colorimetric, and Sensory Properties. Foods. 2025; 14(21):3665. https://doi.org/10.3390/foods14213665
Chicago/Turabian StyleUysal, Reyhan Selin, Hanán Issa-Issa, Ángel A. Carbonell-Barrachina, and Esther Sendra. 2025. "Exploring Ultrasound and Microwave-Assisted Accelerated Aging of Jerez Vinegar: Impacts on Phenolic, Volatile, Colorimetric, and Sensory Properties" Foods 14, no. 21: 3665. https://doi.org/10.3390/foods14213665
APA StyleUysal, R. S., Issa-Issa, H., Carbonell-Barrachina, Á. A., & Sendra, E. (2025). Exploring Ultrasound and Microwave-Assisted Accelerated Aging of Jerez Vinegar: Impacts on Phenolic, Volatile, Colorimetric, and Sensory Properties. Foods, 14(21), 3665. https://doi.org/10.3390/foods14213665







