Abstract
Listeria monocytogenes is a significant foodborne pathogen associated with high rates of hospitalization and death, especially among vulnerable populations. Despite established regulatory standards and available antimicrobial intervention strategies, L. monocytogenes remains as a pathogen of concern in ready-to-eat (RTE) foods. This ultimately can lead to food recalls or listeriosis outbreak, highlighting its ongoing risks to food safety and public health. This review consolidates publicly accessible surveillance case counts and recall data of L. monocytogenes contamination from Australia, Europe, Canada, and the United States to assess the contamination trends in the RTE food supply chain. It also evaluates the effectiveness of antimicrobial intervention strategies, including both those currently implemented in industry and those that have been studied as potential interventions but are not yet widely adopted. Key factors affecting the efficiency of those strategies are identified, including food matrix composition, water activity (aw), fat content, and strain variability. Emerging multi-hurdle technology that integrates physical, chemical, and biological antimicrobial interventions are highlighted as promising approaches for maintaining both food safety and product quality. It also outlines the role of quantitative microbial risk assessment (QMRA) as a decision-support tool to select appropriate control strategies, predict recall risk and guide evidence-based risk management. Future research directions are proposed to expand the application of QMRA in managing recall risks throughout the RTE food supply chain due to L. monocytogenes.
1. Introduction
Listeria monocytogenes is a serious foodborne pathogen of public health concern, associated with listeriosis. Though listeriosis is rare, it is a serious illness with high hospitalization and mortality rates, particularly in vulnerable populations, such as the elderly, pregnant women, neonates, and immune-compromised individuals [1,2,3]. Globally, the World Health Organization (WHO) estimated an incidence of 0.2 cases per 100,000 population in 2010 [4,5]. In the United States (US), the rate of listeriosis is 0.24 cases per 100,000 population [6,7]. In Australia, the most recent data on the number of listeriosis cases per annum is from 2023, which was around 0.33 cases per 100,000 population per year [8,9]. In 30 countries of the European Union and European Economic Area (EU/EEA), the rate was 0.67 cases per 100,000 population in 2023 [10].
L. monocytogenes is ubiquitous and present in the environment, water, and raw foods of both plant and animal origin. It can persist in harsh conditions and thrive at refrigeration temperatures [11,12,13]. The bacterium’s ability to develop biofilms on food contact surfaces exacerbates sanitation challenges and heightens the danger of cross-contamination in food manufacturing and processing [13,14]. Ready-to-eat (RTE) foods such as pre-prepared salads, cold RTE chicken, deli meats, pasteurized milk, soft cheese, and smoked salmon are consumed without further cooking or pathogen inactivation steps [15,16]. This makes RTE foods a significant vehicle for listeriosis transmission, and they are considered high risk in terms of food safety [15,17]. The prevalence of L. monocytogenes in the food supply chain has led to numerous food recalls and listeriosis cases globally. Indeed, L. monocytogenes contamination was a leading cause of microbiological food recalls in Australia, Europe, the US, and Canada between 2012 and 2022 [18,19,20,21]. The recall data from the US and compliance data in Australia for both locally produced foods and imported foods also indicate that RTE foods are mostly recalled because of L. monocytogenes contamination [18,22,23,24,25,26]. These trends emphasize the need for robust, farm-to-fork control strategies to proactively manage L. monocytogenes contamination risks across the RTE food supply chain.
In food processing environments, the control of L. monocytogenes relies on stringent sanitation practices, environmental monitoring programs, and the implementation of robust preventive controls. Facilities adopt Good Manufacturing Practices (GMPs) and Hazard Analysis and Critical Control Point (HACCP) systems tailored to minimize contamination risks, particularly in high-risk areas, such as slicing, packaging, and post-lethality zones [27]. Preventive strategies include the design of hygienic equipment, strict zoning between raw and processing areas, air handling systems to prevent cross-contamination, and regular cleaning using validated sanitizers [28,29]. Environmental sampling plans focus on food contact and non-contact surfaces, allowing for early detection and response to Listeria species [29]. Additionally, biofilm control strategies, staff hygiene training, and raw material control are key components of ongoing risk mitigation [27]. Despite these practices, persistent contamination and the survival of L. monocytogenes in harborage sites continue to challenge food processors, reinforcing the importance of continuous verification and improvement of control measures.
Currently, the risk of L. monocytogenes in RTE foods is primarily managed through strict regulatory standards. These include microbiological limits and monitoring requirements of products and processing environments, along with disease surveillance measures, such as mandatory notification of listeriosis cases by general practitioners and laboratories to public health authorities [30,31]. Alongside regulatory compliance, food producers implement a range of antimicrobial intervention strategies such as thermal or non-thermal treatments and the use of biological agents, such as nisin or chemical preservatives, to control L. monocytogenes. However, each intervention strategy comes with specific advantages and limitations. Their effectiveness can vary depending on the food matrix, application method, and processing conditions. In some cases, interventions may negatively impact product quality or consumer acceptability. Strategies that maintain sensory quality and consumer appeal often show limited effectiveness when used individually and are generally more effective when applied in combination. As a result, there is a need to explore novel or integrated intervention strategies. Rather than relying solely on time-consuming and resource intensive experimental trials, quantitative microbial risk assessment (QMRA) frameworks offer a promising solution, enabling science-based evaluation of different interventions in reducing contamination risk. For example, the FAO/WHO QMRA for L. monocytogenes in RTE foods modeled how varying levels of contamination and intervention scenarios affected the probability of illness, providing a science based foundation for establishing microbiological criteria [32].
While numerous reviews have addressed L. monocytogenes prevalence, virulence, or individual control measures, there is limited synthesis that explicitly links recall, outbreak, and surveillance data with the effectiveness and limitations of antimicrobial interventions used in RTE foods. This review bridges that gap by integrating publicly available food safety data, including compliance testing, prevalence surveys, and recall notifications with a critical evaluation of intervention strategies applied across various RTE food categories. In addition, while QMRA has traditionally been used for predicting illness outcomes, this review highlights emerging applications of QMRA in estimating shelf-life and recall risk, which are particularly underexplored. This integrative approach not only strengthens the understanding of L. monocytogenes risk in RTE foods but also emphasizes the need for data-driven, industry-relevant decision support tools that align with evolving regulatory frameworks and consumer safety demands. By addressing these gaps, the review offers a novel perspective on improving food safety management from both a regulatory and operational standpoint.
This review aims to synthesize publicly available outbreak, surveillance, and recall data alongside widely used and emerging intervention strategies to provide a more comprehensive understanding of L. monocytogenes risk in RTE food. Hence, the objectives of this review are to (i) analyze the prevalence and risk of L. monocytogenes in RTE foods in Australia, with comparisons to trends in Europe, Canada, and the US; (ii) examine global antimicrobial intervention strategies for controlling L. monocytogenes in RTE foods and identify its limitations; and (iii) highlight the emerging opportunities for future research in the field of QMRA to address the risk of L. monocytogenes in RTE foods.
2. Listeria monocytogenes
L. monocytogenes is a Gram-positive, non-spore forming, facultatively anaerobic rod [33], and it is ubiquitous in nature [34]. It is present in soil, water, decomposing plants, animal excrement, and food processing environments [34]. The organism exhibits notable environmental adaptability. It can grow at temperatures from 4 to 45 °C, survives a wide pH range (4.7–9.2), and requires moderate water activity (aw > 0.92) [34]. This allows it to persist in food processing environments, survive various control strategies, and proliferate in food products [35,36]. Another significant factor in the persistence of L. monocytogenes in food environments is its ability to form biofilms on equipment and food contact surfaces [13,14,37]. These biofilms provide protection to the bacterial cells against environmental stressors, such as desiccation, nutrient limitation, and exposure to antimicrobial agents [38,39,40]. Moreover, they present a major challenge for sanitation efforts, as cells embedded within biofilms can survive standard cleaning and disinfection procedures [41,42]. This resilience makes L. monocytogenes particularly difficult to eliminate once established in processing environments. In fact, research has shown that certain L. monocytogenes strains are capable of long-term persistence in food facilities, surviving for several months or even years [13,43]. A study by Leong et al. [44] also reported that four out of seven Irish food processing facilities (isolated ≥ 6 months apart) had persistent L. monocytogenes strains (belonging to serotype 1/2a, 1/2b, 1/2c, 4b) in both environmental and food samples.
Potential sources of L. monocytogenes to food processing plants can be introduced from raw materials, food handlers, water, or equipment. However, the primary sources of contamination are often food handlers or equipment [13,40,43,44,45,46]. Additionally, Samelis and Metaxopoulos [47] concluded that post-processing contamination, particularly in areas such as cooked meat slicing, was a major issue. Their research indicated that sliced vacuum-packed cooked meats and country style sausages were more contaminated (6.7–10%) in comparison to non-sliced cooked meats in which L. monocytogenes was not detected [47]. Similarly, Zhang et al. [48] investigated L. monocytogenes contamination in two RTE food plants in Shanghai, China, and reported that 12.1% of 239 samples were positive, including 21 samples from plant A. In plant A, L. monocytogenes was primarily isolated from pickling, cooling, and packaging areas [48]. In another study, Muhterem-Uyar et al. [49] analyzed 2242 food processing environment samples collected from twelve facilities across six European countries (Austria, Ireland, Spain, Slovakia, Greece, and Romania) over one year. They outlined three contamination scenarios: (i) occasional contamination at the junction between raw material reception and hygienic areas; (ii) localized contamination hotspots in hygienic processing zones; and (iii) extensive contamination across the entire processing environment. Similarly, Ho et al. [50] conducted a three-year longitudinal study in a farmstead dairy processing facility and found that L. monocytogenes contamination was largely confined to drains and floors, with persistent PFGE subtypes (DUP-1052A in the processing plant; DUP-1039A in the milking parlor), and a subtype detected only once in each area (DUP-1030A), consistent with sporadic contamination. These findings emphasize site specific persistence and cross-contamination pathway, indicating the need to control post-lethality exposure (especially slicing lines), prioritize hygienic design and sanitation of wet niches (such as drains/floors), and maintain targeted environmental monitoring capable of detecting both persistent and sporadic events.
2.1. Importance of L. monocytogenes Biofilms
The ability of L. monocytogenes to form biofilms is a key factor in its long-term survival and persistence in food processing environments. Microbial biofilms are composed of microbial cells attached to a surface and embedded in an extracellular matrix. Development of biofilms typically proceeds through (1) attachment, (2) cell proliferation and production of extracellular polymeric substances (EPS), (3) early development of biofilm architecture, (4) maturation, and (5) dispersion [51,52,53]. The early stages (step 1–3) are facilitated by motility and adhesion factors; however, during maturation, cells lose motility. Matrix build-up and high cell density provide protection from antimicrobials and disinfectants, increasing tolerance and enabling persistence [39,40].
L. monocytogenes forms biofilms on a variety of food and non-contact food surfaces commonly found in RTE facilities (e.g., stainless steel, rubber, plastic, and even conveyor belts and drain covers) [37,43]. Persistent contamination has been reported on stainless steel, with adhesion enhanced at low temperatures [40,46]. When L. monocytogenes cells are embedded in biofilms, a subpopulation can enter a reversible dormant state (viable but non-culturable, VBNC), complicating detection because culture methods fail to recover these cells [53,54,55,56].
L. monocytogenes detection in processing environments should draw on learnings from in-product testing that has demonstrated that molecular-based methods are more efficient in detection contamination than culture-based methods. For instance, Panera-Martínez et al. [57] detected L. monocytogenes in 50.0% by culture (isolation on OCLA and confirmation by conventional PCR) and 66.7% by q-PCR (concentrations ranged 2.40–5.22 log10 CFU/g (total) and <2.15–3.93 log10 CFU/g viable L. monocytogenes) on raw poultry samples. v-PCR showed 100% sensitivity, 66.7% specificity, and 83.3% efficiency vs. the culture gold standard (kappa coefficient = 0.67), supporting v-PCR for detecting viable cells that culture may miss [57]. Extending this approach to chicken meat, Panera-Martínez et al. [58] reported L. monocytogenes in 75.0% of samples; v-PCR detected viable cells in 30.8% of samples versus 19.2% by plating. Mean counts (log10 CFU/g) were 4.01 (total), 3.21 (viable), 1.00 (viable-culturable), and 3.20 (VBNC), with VBNC cells comprising 15.66% of total and 99.38% of viable cells [58]. This evidence suggests that culture-dependent testing fails to capture the full extent of L. monocytogenes contamination and risk.
2.2. Food Safety Risk of L. monocytogenes
L. monocytogenes presents significant challenges to food safety and public health authorities worldwide due to its ability to cause listeriosis [1]. Human infection typically occurs through the consumption of contaminated food [59], and the incubation period can range from a few weeks to three months [32]. Additionally, L. monocytogenes can transiently reside in the human intestinal tract, with 2–10% of the population being asymptomatic carriers [60].
Listeriosis presents in two clinical forms: non-invasive and invasive form [2]. Non-invasive listeriosis only affects healthy individual and causes mild gastrointestinal disorder, which resolves without any treatment [2]. On the other hand, invasive listeriosis is severe and affects high-risk population groups, notably the elderly, neonates, pregnant women, and immune-compromised individuals [33,61,62]. The symptoms include septicemia, meningitis, and even miscarriage and death [1]. These vulnerable groups have reduced immune defenses, making them more susceptible to invasive infections [61]. In healthcare settings, the risk is amplified because of the presence of a large number of highly vulnerable individuals. For instance, during the 1999 outbreak in Finland linked to butter, 15 of the 25 affected patients were severely immunocompromised and had prolonged hospital stays, with 60% of cases occurring in a single tertiary hospital [63].
The severity of listeriosis is further emphasized by the low infectious dose (ID50) required to cause illness in susceptible individuals. The ID50, defined as the dose needed to infect 50% of a population, varies depending on host susceptibility and the characteristics of the food matrix [62,64]. In animal models such as mice, the oral ID50 for L. monocytogenes has been reported to be around 6.4 log10 CFU [65]. However, for high-risk human populations, a considerably lower dose may be sufficient to cause illness [32,63]. This was evident during an outbreak in Finland, where butter samples contained 0.7–1.8 log10 CFU/g of L. monocytogenes (one sample reaching 4.0 log10 CFU/g), yet still caused illness in highly vulnerable patients [32,63]. Certain food matrices, particularly high-fat products like butter or cheese, can enhance bacterial survival in the gastrointestinal tract, thus reducing the effective infectious dose [66]. Additionally, host-related factors, such as gastric acid suppression caused by medications like antacids or proton pump inhibitors, can impair natural barriers to infection and further increase susceptibility [67].
Beyond differences in host susceptibility and infectious dose, L. monocytogenes exhibits genetic diversity that influences its virulence, ecological niches, and persistence in food processing environments. L. monocytogenes is classified into 4 genetic lineages and 13 serotypes, out of which 1/2a, 1/2b, 1/2c, and 4b are mostly associated with listeriosis in humans [33,68,69,70,71]. Lineage I strains (serotype 1/2b, 3b, 3c, and 4b), particularly serotype 4b, are associated with major outbreaks and severe invasive disease, while lineage II strains (serotype 1/2a, 1/2c, and 3c), especially serotype 1/2a, are more prevalent in environmental and food isolates and are often associated with sporadic cases [33,69,71,72]. Lineage III (serotype 4a, 4b, and 4c) and IV strains (serotype 4a, 4b, and 4c) are rarely isolated from clinical cases but are predominately isolated from animal sources [72,73]. This distribution highlights that certain lineages and serotypes may have an enhanced capacity to persist in processing environments and cause listeriosis, reinforcing the importance of molecular surveillance and targeted intervention strategies in the food industry.
2.3. Global Trends and Impact of Listeriosis
In 2020, the WHO Foodborne Disease Burden Epidemiology Reference Group (WHO/FERG) estimated that foodborne listeriosis caused around 14,169 illnesses, 3175 deaths, and 118,340 DALYs worldwide based on the data compiled from 45 member states to generate global estimates [5,74]. Among these cases, 79.3% were non-invasive, while 20.7% were invasive, with fatality rates estimated at 14.9% and 25.9%, respectively [5,74]. Given the potential severity of illness and the high-risk nature of certain population groups, ongoing surveillance of listeriosis incidence and L. monocytogenes prevalence in the food supply chain is critical for effective food safety management. However, comprehensive surveillance data is only available for Australia and Europe, making these regions the primary focus for current trend analysis.
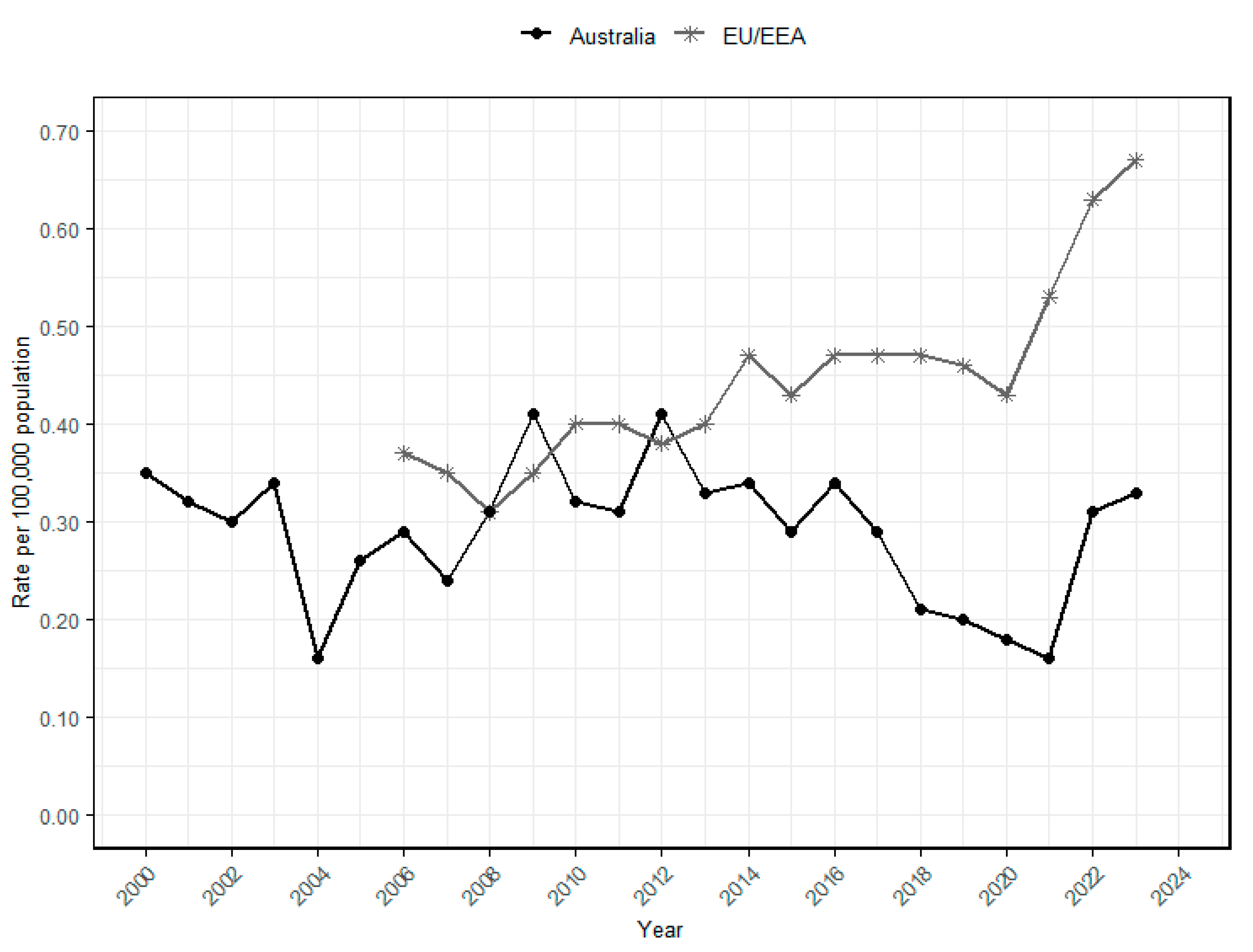
In Australia, the National Notifiable Diseases Surveillance System (NNDSS) records the incidence of communicable diseases, including foodborne infections like listeriosis. Surveillance data spanning 23 years (2000–2023) indicate a fluctuating trend in the incidence rate of listeriosis (Figure 1). Between 2000 and 2003, the rate remained relatively high (0.30–0.35 cases per 100,000 population) before declining in 2004 (0.16 cases per 100,000 population). Subsequently, the incidence rate generally increased and remained relatively stable through the 2010s (around 0.25–0.30 cases per 100,000 population). The rate then decreased during 2020–2021 (0.16–0.18 cases per 100,000 population), likely due to reduced surveillance and reporting during the COVID-19 pandemic, in addition to possible changes in exposure patterns. Factors such as reduced consumption of RTE foods because of limited dining out during lockdowns and improved hygiene practices (e.g., mandatory handwashing) may have contributed to the decline [75,76]. The rate rose again in 2022 and 2023 (0.31 and 0.33 cases per 100,000 population, respectively), indicating the continuing public health importance of L. monocytogenes in Australia.
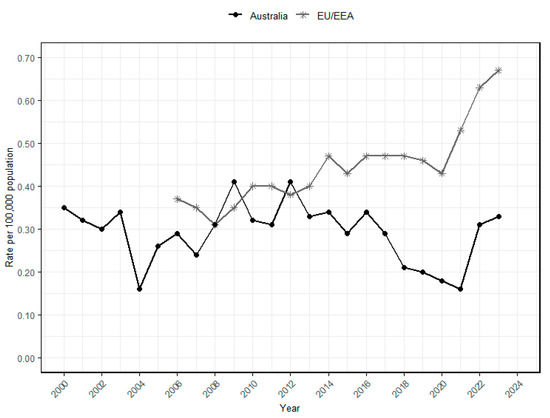
Figure 1.
Annual listeriosis incidence rates per 100,000 population in the European Union and European Economic Area (EU/EEA) and Australia. The surveillance data of Australia (2000–2023) was obtained from the National Notifiable Diseases Surveillance System (NNDSS) database [8,77,78], and annual population estimates were extracted from Australian Bureau of Statistic [79]. The listeriosis rates for the EU/EEA were obtained from the European Centre for Disease Prevention and Control’s (ECDC) Annual Epidemiological Reports. Starting in 2020, the rate from the United Kingdom was excluded from this report [10,80,81,82,83,84,85,86,87,88,89,90,91,92,93].
In contrast, the European Union and European Economic Area (EU/EEA) exhibited a generally increasing trend in incidence rates over 18 years (2006–2023), rising from around 0.35 cases per 100,000 population in 2006 to 0.46 cases per 100,000 population until 2019. A minor dip occurred in 2020 (0.43 cases per 100,000), coinciding with the COVID-19 pandemic and the withdrawal of the United Kingdom from EU surveillance. However, it gradually increased to about 0.67 cases per 100,000 population in 2023. This long-term upward trajectory highlights that L. monocytogenes remains a persistent food safety and public health concern in Europe.
Listeriosis has emerged as a major global public health issue, imposing substantial economic costs and social impacts. In Australia, the total annual cost of listeriosis was estimated to be around AUD 78.4 million annually in terms of medical expenses, lost productivity, premature mortality, and hospitalization [94]. This is similar to the US, where the cost is estimated to be around USD 2.8 billion in 2013, equivalent to AUD 4.3 billion [95]. Surveillance data from Australia and Europe, along with the economic burden in both Australia and the US, demonstrate that L. monocytogenes represents a significant public health concern not only in Australia but also in major countries like the US and Europe.
3. Ready-to-Eat (RTE) Foods
RTE foods are products that do not undergo any listericidal treatment and are consumed in the same state as they are sold [15]. Nuts in the shell, raw fruits, and vegetables intended for washing or peeling by consumers are not considered RTE foods [15]. Similarly in the EU, RTEs are foods that can be described as those consumed without the need for additional processing to meet safety and microbiological criteria [96]. They belong to a large heterogenous category of foods and are convenient for today’s lifestyle because they do not require cooking or further preparation [27].
RTE foods are broadly classified as high-risk or low-risk based on their ability to support the growth of L. monocytogenes. This classification is adopted by major food safety authorities, including Food Standards Australia New Zealand (FSANZ), the Codex Alimentarius Commission, the European Commission, and Health Canada. For example, FSANZ classifies RTE foods into two categories depending on whether they support L. monocytogenes growth, applying stricter limits (absence in 25 g) for higher-risk foods. Likewise, Canada uses a similar approach, classifying RTE foods into Category 1 (able to support the growth) and Category 2 (not supporting the growth) of L. monocytogenes, with stricter compliance limits for Category 1 products [97]. This classification is based on the intrinsic properties of RTE foods, mainly pH and water activity (aw). Table 1 provides a comparison of international criteria and examples of high-risk and low-risk RTE food categories. High-risk RTE foods are particularly susceptible to contamination during post-processing handling [98].

Table 1.
Regulatory classification of RTE foods by L. monocytogenes growth potential and risk level across regions.
Among RTE food categories, deli meats, smoked seafood (e.g., cold-smoked salmon), and soft cheeses (e.g., Brie, Camembert, Queso Fresco) are consistently considered high-risk for L. monocytogenes contamination. These products share several risk-enhancing characteristics that relate to their food matrix properties, post-processing contamination, and intervention strategies. In relation to food matrix properties, they typically have intrinsic properties, such as high water activity (aw > 0.92) and neutral pH (≥5.0), supporting the survival and growth of L. monocytogenes [99,100]. Intervention-wise, many of these products do not receive a listericidal treatment after packaging, making them particularly susceptible to post-processing contamination during slicing, handling, or repackaging. Physical interventions are prohibitive because they change the nature and properties of the product, while chemical intervention are restricted to very few approved additives or processing aids such as sodium lactate and sodium diacetate that prevents microbial growth [101,102,103]. Biological interventions (e.g., phages, bacteriocins) are likewise constrained to a limited set of approved uses [100,104,105,106,107,108]. Finally, these product groups are prone to post-processing contamination due to being handled and packaged into formats convenient to the consumer, such as slices or pieces, thus exposing their surface to contamination through knives and slicers. For example, cold-smoked salmon is not heat-treated after smoking, and RTE meats like ham or turkey slices are frequently re-contaminated at various stages of production and distribution [13,108,109,110,111]. L. monocytogenes can grow by 1.3 log10 CFU/g at 4 °C over 28 days in smoked salmon [112]. In a related real-world example, Stessl et al. [113] investigated a sporadic listeriosis cluster in Austria during 2015–2017 across three Austrian provinces and, in a subsequent five-year environmental monitoring program, identified a schnitzel-sorting machine as the likely contamination source.
4. Significance of L. monocytogenes in RTE Supply Chain
The susceptibility of RTE foods to L. monocytogenes contamination is well established due to their minimal processing prior to consumption and extended refrigerated shelf life [114]. Analysis of food recall and compliance data, with particular attention to RTE foods, reinforces this vulnerability by providing concrete evidence of contamination trends across product types and regions. When food products exceed regulatory thresholds for L. monocytogenes (further outlined in Section 5.1), regulatory actions, such as mandatory reporting and product recalls, are initiated to protect public health. In Australia, one such measure is the mandatory reporting of L. monocytogenes detection during routine or quality testing. Commercial laboratories and food businesses are required to notify the food safety authority within 24 h of detection, and reporting requirements differ from state to state [115]. Other key measures are the recall and withdrawal of non-compliant food products. A food recall is the process of removing food products from sale, distribution, and consumption to address safety risks to consumers [116]. Product withdrawal is an action taken to remove food products from sale as a result of quality defect or as a precautionary measure when further investigation is needed to analyze the potential health risks to consumers [116]. To identify the types of RTE foods most commonly associated with L. monocytogenes contamination, this section looks into the publicly available food recall data, primarily from the US and Australia due to data unavailability in other countries. Detection during production and resulting withdrawals typically trigger corrective actions, such as sanitation interventions, process modifications, or reformulation [16,97,117], while recalls from the market are often followed by root cause investigations, increased monitoring, and regulatory scrutiny to prevent recurrence [118,119].
4.1. National and International Food Recall Data
National and international food recall data provide valuable insights into the continued risk posed by L. monocytogenes in the food supply, particularly in RTE foods. Table 2 summarizes publicly available food recall data from Australia, Canada, and the US; for the EU, the iRASFF notification count is included. The recall data from the EU could not be obtained, as the EU does not publish consolidated EU wide recalls figures. The EU’s Alert and Cooperation Notification (ACN), shared through the iRASFF platform, records events by notification type rather than recall counts. The ACN covers the following aspects: (1) non-compliances with possible health risk; (2) non-compliances without health risk; (3) suspicions of fraud; (4) non-compliant consignments of plants and plant products, as well as issues related to contingency plans for plant pests and other plant health concerns; and (5) non-compliances and fraud suspicions related to companion animals and animal welfare. Notifications are classified as alert, information for attention, information for follow-up, border rejection, or news. Measures for outbreaks/food poisoning are recorded in in the follow-up section of the ACN annual reports [120].

Table 2.
Summary of food recall incidents due to L. monocytogenes and other microbial contaminants.
Across countries, recalls due to microbiological hazards account for 19.4–38.5% of total recalls (Australia, Canada, and the US), and recalls due to L. monocytogenes represent 31.1–43.2% of microbiological recalls (Australia, the US). For the EU, notifications due to microbiological hazards constitute 55.7% of all notifications, and L. monocytogenes account for 10.0% of microbiological notifications. While some data reflect all food types, RTE foods, particularly deli meats, pre-packaged salads, dairy products, and seafood, consistently emerge as high-risk categories within these recalls [18].
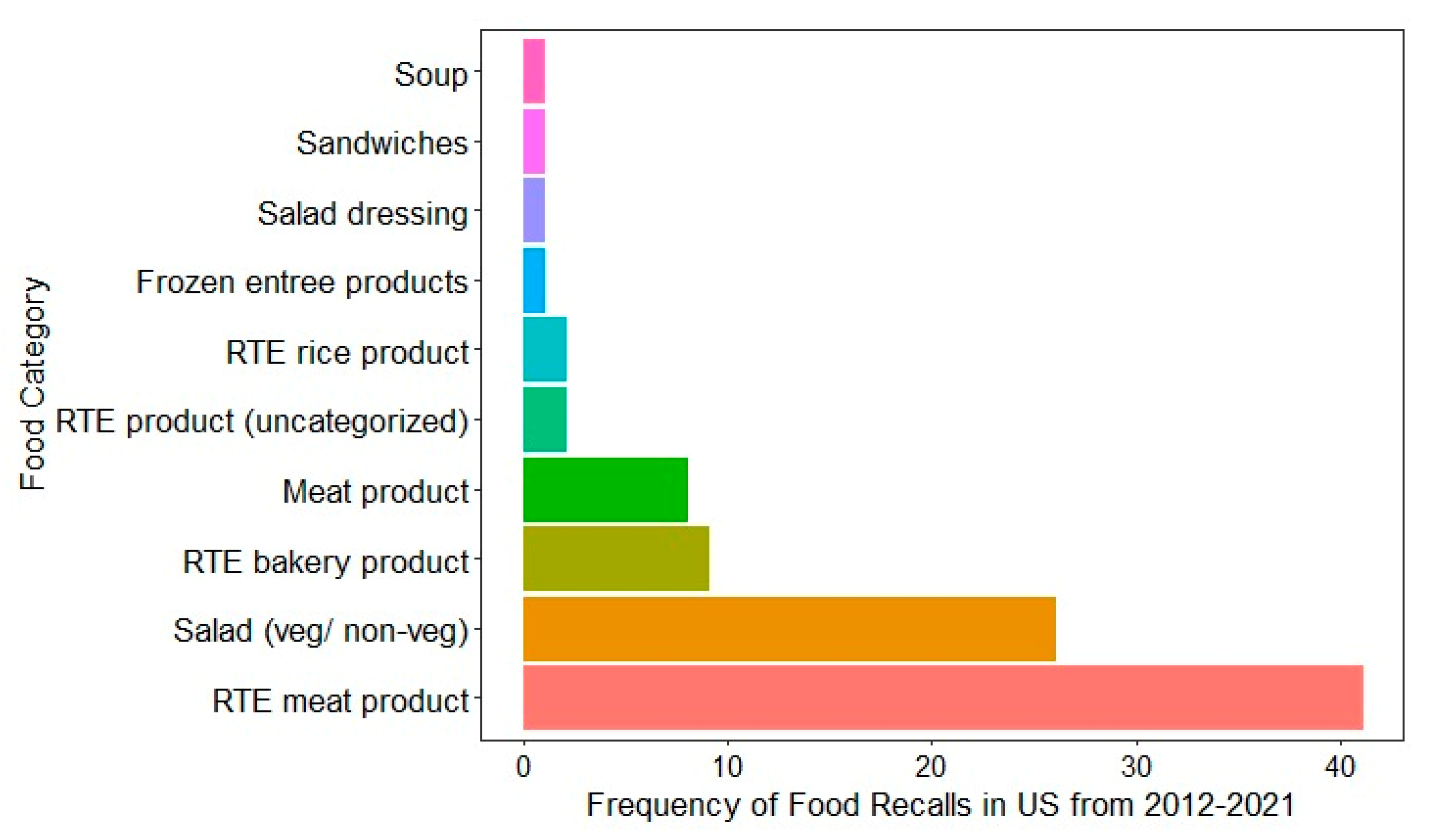
Recent food recall data from the US (2012–2021) highlights that the types of food products most commonly recalled due to L. monocytogenes contamination are RTE meat products, as shown in Figure 2 [18]. These products, such as deli meats, hot dogs, and sausages, are susceptible to contamination during processing, packaging, and handling, especially in facilities with inconsistent hygiene protocols. For instance, a notice of suspension was issued by the USDA FSIS to Boar’s Head Provisions, Co., Inc., to revoke the federal marks of inspection and suspend the operations of RTE products at Boar’s Head Provisions, Co., Inc., Virginia, due to unsanitary conditions of the processing unit [122]. The second most recalled food was pre-packaged salads, both vegetarian and non-vegetarian [18]. Comparable product-specific recall data were not publicly available for Australia, Canada, or the EU, where only aggregated recall figures or broad food categories are reported without specifying the pathogen responsible. This data gap limits cross-country comparisons and emphasizes the importance of improved transparency and harmonization in food recall reporting. The trends observed in the US emphasize the vulnerability of RTE products to L. monocytogenes and highlight the need for stringent monitoring and control measures in the production and handling of high-risk foods, such as RTE meats, dairy, fish, and fresh produce. This persistent association with contamination emphasizes the ongoing challenges in managing L. monocytogenes in RTE foods.
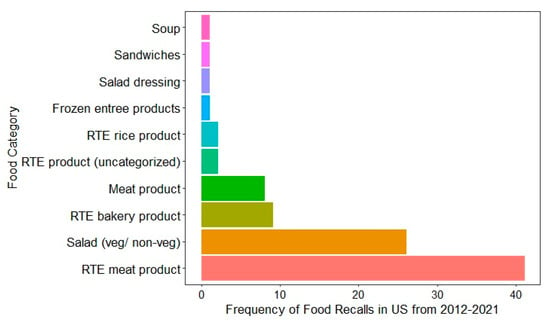
Figure 2.
Food categories associated with food recall due to L. monocytogenes contamination in the US from 2012 to 2021 [123].
4.2. National Compliance and Prevalence of L. monocytogenes in RTE Foods
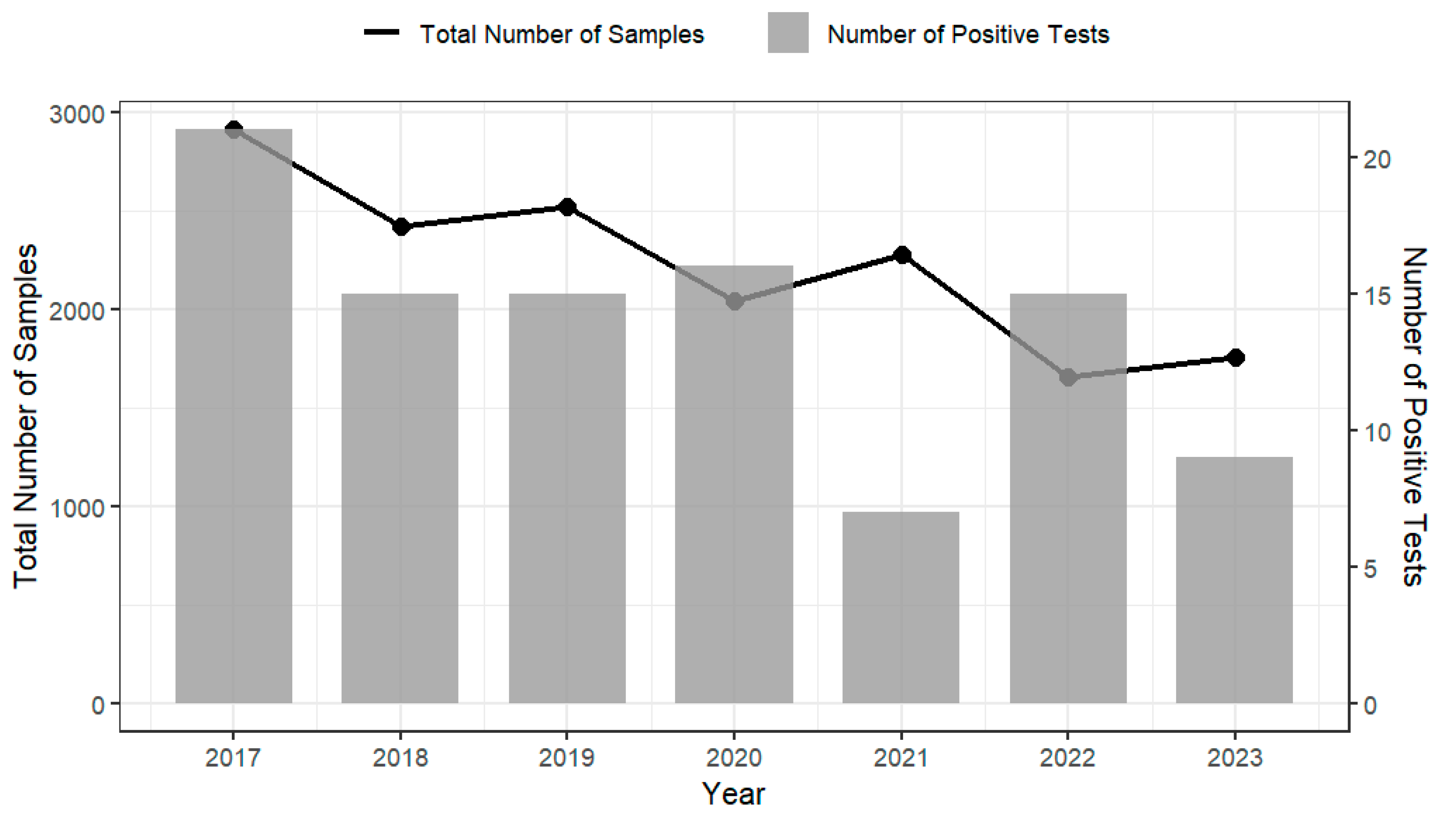
Analysis of testing data on imported products from the Australian Department of Agriculture, Fisheries and Forestry (2017–2023) indicates that products such as cheese, RTE seafood, and processed meats demonstrated consistently high compliance with regulatory requirements for L. monocytogenes, with annual compliance rates generally exceeding 99% [22]. However, despite these high compliance levels, non-compliant foods were recorded each year (see Figure 3), indicating that L. monocytogenes remains a manageable yet persistent risk. This trend is mirrored in the prevalence surveys of domestically produced RTE foods conducted by FSANZ and relevant health departments of ACT and NSW on various domestically produced RTE foods [23,24,25,26]. These surveys report L. monocytogenes prevalence rates ranging from 0.4% to 3.9% across different food types and regions (Table 3). While the data suggest a possible decline in contamination levels over time, the continued detection of L. monocytogenes demonstrate the importance of maintaining robust food safety controls and active surveillance throughout the RTE food supply chain. The compliance testing graph (Figure 3) reports regulatory non-compliance at the border (i.e., results assessed against microbiological criteria), while the prevalence survey (Table 3) reports positivity from targeted surveys of specific products and settings. These sources differ in sampling frame (imports/consignments vs. retail/food-service), design (rate-based regulatory screening vs. study-specific surveys), product scope (mixed imported RTE foods vs. single categories, like cooked prawns, sliced RTE meats, sandwiches), period (2017–2023 vs. 2003–2016), definitions (non-compliant vs. positive), methods and detection limits, and sample size (with some survey denominators marked NA). Due to these differences, the lower non-compliance rates (0.3–0.9%) from border compliance testing and the higher survey positivity (0.4–3.9%) are not directly comparable; cross-stream inferences are indicative only and should be interpreted with caution.
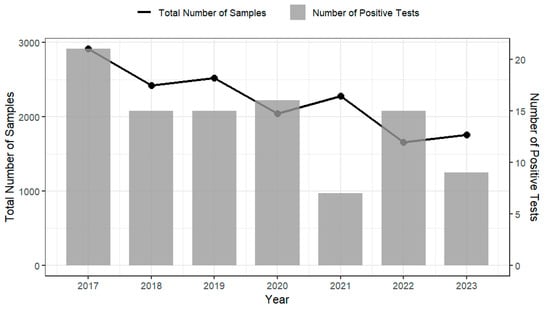
Figure 3.
Annual testing results of imported RTE foods for L. monocytogenes by the Australian Department of Agriculture, Fisheries and Forestry between 2017 and 2023 [22]. The left y-axis and black line represent the total number of samples tested each year; the right y-axis and grey bars represent the number of samples positive for L. monocytogenes.

Table 3.
Prevalence data for L. monocytogenes in RTE foods in Australia.
5. Current Intervention Strategies
5.1. Risk-Based Regulatory Framework
In recent years, food safety management has increasingly adopted a risk-based approach, grounded in the principles of risk analysis [124]. The risk analysis framework comprises three key components: risk assessment, risk management, and risk communication [125]. This framework enables evidence-based decision making by evaluating hazards and identifying appropriate control measures. Within this framework, risk assessment plays a central role in evaluating potential hazards in the food supply [124,125]. For microbiological hazards such as L. monocytogenes, quantitative microbial risk assessment (QMRA) is widely used to integrate data on contamination levels, consumption patterns, and dose–response relationships to estimate the probability of illness [126,127]. This scientific approach enables regulators to establish food safety limits based on both the likelihood of contamination and the severity of health outcomes.
A QMRA conducted by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) evaluated the risk of L. monocytogenes in various RTE food categories [128]. This international risk assessment has been widely referenced and used as a foundational document for guiding food safety policies and microbiological criteria for the food product. The outcomes of these assessments support the development of regulatory requirements tailored to specific regions, as shown in Table 4. To comply with such standards, food businesses may be required to demonstrate whether their products support the growth of L. monocytogenes [16,27,96,97,99]. This is often carried out through challenge testing, which assesses the pathogen’s behavior under specific storage and handling conditions. The results of such studies help determine whether additional control measures are necessary to ensure product safety throughout shelf life and to meet regulatory compliance for L. monocytogenes.

Table 4.
Microbiological criteria for L. monocytogenes in RTE foods.
5.2. Antimicrobial Intervention Strategies to Meet Requirements
To mitigate L. monocytogenes in RTE foods, food businesses utilize various intervention strategies that can be broadly classified into physical, chemical, and biological methods. These strategies aim to either eliminate the pathogen or suppress its proliferation to guarantee food safety for the entire shelf life [130]. Table 5 summarizes the different antimicrobial intervention strategies, including their type, brief descriptions, product types applied, efficacy and limitations. The studies included in the table were selected based on their relevance in demonstrating the efficacy of intervention strategies against L. monocytogenes in RTE foods. It is important to note that the efficacy of interventions varies with both product and process conditions (see Table 5). For RTE deli meats, a 3 log10 reduction may require 266 min at 55 °C, whereas high-pressure processing of cooked ham at 504 MPa for 3 min can achieve a 4 log10 reduction. The influence of product formulation is also seen; even at the same temperature, the time to a 7 log10 reduction differs (at 68.9 °C, it is 8.1 min for lean sausage and 8.4 min for fat sausage).
Beyond product and process effects, efficacy also varies with environmental and food-related stresses (acid, salt concentration, temperature) and strain/serotype variability to such stresses [131,132,133,134,135,136]. For instance, Zhou et al. [133] observed shorter lag times for clinical strains under 0.50% NaCl/0.04% bile salt, and Myintzaw et al. [135] reported better low-temperature growth among clinical isolates; serotype 4b (lineage I) has been reported as more osmotolerant than other serotypes. Thus, one-size-fits-all controls are inadequate, and this strain/serotype variability is not reflected in Table 5 (full discussion in Section 5.2.1 Challenges and Considerations in Implementing Intervention Strategies for details).
Physical interventions include both thermal (e.g., conventional pasteurization, infrared heating) and non-thermal treatments (e.g., high-pressure processing, irradiation, pulsed light, ultrasound). Thermal treatments are highly effective in achieving substantial log reductions (between 1 and 7 log10 reduction) of L. monocytogenes (see Table 5: Physical intervention strategies: Thermal); however, they may not be suitable for heat-sensitive products, such as cheese or vacuum-packed meats, due to potential changes in texture, color, flavor, or packaging integrity. For such products, non-thermal alternatives are increasingly preferred, as they offer gentler effects on food quality while still achieving notable 1–2 log10 reductions and sometimes complete elimination of L. monocytogenes (see Table 5; Physical intervention strategies: Non-thermal). Despite their benefits, some technologies remain costly, and certain methods, like irradiation, face barriers due to limited consumer acceptance.
Chemical interventions involve the use of approved additives, such as sodium lactate or sodium diacetate, and the use of organic acid. These substances help lower pH or create antimicrobial environments that inhibit L. monocytogenes proliferation. Under sufficient concentrations, this can also achieve non-thermal inactivation, but additives are not a substitute for a validated kill step [108,137]. Research has shown that while these interventions are effective in inhibiting growth, overuse may affect the sensory properties, such as increasing saltiness, or may lead to heat resistance in L. monocytogenes when used in combination with salt (see Table 5: Chemical intervention strategies). The use and approval of chemical preservatives also varies between countries. For example, sodium lactate (E325) is an approved food additive in many regions, but its use is not permitted in foods intended for infants and toddlers [102,103].
Biological interventions leverage natural antimicrobials, such as bacteriophages, bacteriocins, lactic acid bacteria, and essential oils, offering sustainable alternatives to physical or chemical interventions. These antimicrobials can be highly specific to combat L. monocytogenes, making them ideal for applications where clean-label or minimal processing is desired [138,139]. However, their effectiveness is often lower than that of physical or chemical methods, with reported reductions in L. monocytogenes ranging between 0.4 and 4.0 log10 or inhibition of growth for a short period of time (3–5 days) (see Table 5; Biological intervention strategies). Their efficacy can also depend on the food matrix. For example, bacteriophages are generally more effective in liquid products due to better distribution and contact with the bacterial cells [104,140]. Additionally, regulatory approval varies by country. For instance, nisin is a bacteriocin produced by certain strains of Lactococcus lactis. In Australia, the maximum limit of 12.5 mg/kg of nisin is permissible in processed meat products [105]. In the US, the limit is 5.5 mg/kg for RTE meats and poultry products [106]. However, in Europe and Canada, the nisin limit in RTE meat products is 25 mg/kg [100,107].

Table 5.
Efficacy of the intervention strategies used to control L. monocytogenes in RTE foods.
Table 5.
Efficacy of the intervention strategies used to control L. monocytogenes in RTE foods.
| Types of Intervention Strategies | Description | Product Type | Example of Efficacy | Reference | Limitation 1 |
|---|---|---|---|---|---|
| Physical intervention strategies: Thermal | |||||
| Pasteurization using conventional heating | Use of thermal heat above 72 °C to destroy L. monocytogenes. | RTE deli turkey | 1.95–3 log10 reduction at 93.3 °C for 2–5 min | [141] | High temperatures might affect the texture, flavor or nutritional quality. Possible issue with the packaging, as it will need to withstand high temperatures. |
| RTE deli meats | 3 log10 reduction within 266 min at 55 °C or 44 min at 60 °C or 23 min at 62.5 °C or 5 min at 65 °C | [142] | |||
| RTE chicken drumette | 7 log10 reduction at 54 min and 28 min at 60 °C and 70 °C, respectively, or at 18 min and 19 min at 80 °C and 90 °C, respectively | [143] | |||
| Lean sausage | 7 log10 reduction within 8.1 min at 68.9 °C | [130] | |||
| Fat sausage | 7 log10 reduction within 8.4 min at 68.9 °C | [130] | |||
| Lean ham | 7 log10 reduction within 6.3 min at 67.9 °C | [130] | |||
| Fat ham | 7 log10 reduction within 5.8 min at 68.9 °C | [130] | |||
| Pasteurization via infrared heating | Use of infrared radiation to kill L. monocytogenes. | Deli ham | 0.75–1.85 log10 reductions when processed for 45–75 s | [144] | Limited penetration capability for complete destruction of the bacteria [145]. |
| Roast beef | 3.8 log10 reductions when processed for 60–90 s | [144] | |||
| Turkey frankfurters | 3.5, 4.3, and 4.5 log10 reductions at temperatures of 70 °C, 75 °C, and 80 °C for 82 s, 92 s, and 103 s, respectively | [146] | |||
| Sliced ham | 4.16 log10 reduction after 50 s | [147] | |||
| Cooked chicken | 3.7 log10 reduction 62 °C within 9.3 min; 4.9 log10 reduction at 68 °C within 5.2 min; 6.0 log10 reduction at 75 °C within 3.6 min | [145] | |||
| Physical intervention strategies: Non-thermal | |||||
| High-pressure processing (HPP) | Use of high pressure (400–600 MPa) for microbial inactivation [148,149]. | Fresh Hispánico-type cheese | 3.8 log10 reduction at high pressure of 400 MPa for 3 min | [150] | High equipment cost and possible issue with the packaging, as it will need to withstand high pressure [151]. |
| Ripe Mahón cheese | 1 log10 reduction at 400 MPa for 18 min | [150] | |||
| RTE cooked chicken | 3.3 log10 reduction at pressure level of 600 MPa for 2 min | [152] | |||
| RTE sliced cooked ham | 4 log10 reduction at 504 MPa for 3 min | [153] | |||
| Sliced mortadella | 4 log10 reduction at 526 MPa for 3 min | [153] | |||
| Sliced dry cured ham (white pig) | 3.9 log10 reduction at 600 MPa for 5 min | [154] | |||
| Sliced dry cured ham (Iberian pig) | 1.9 log10 reduction at 600 MPa for 5 min | [154] | |||
| RTE cooked chicken | 1.4 log10 reduction at 600 MPa for 2 min | [155] | |||
| Dry cured ham | 0.92–3.92 log10 reduction at 450 MPa for 5 min | [156] | |||
| Dry cured ham | 6.28–6.82 log10 reduction at 600 MPa for 5 min | [156] | |||
| Dry cured ham | 5.26–7.96 log10 reduction at 750 MPa for 5 min | [156] | |||
| Mozzarella | 3–4 log10 reduction at 400 MPa for 10 min | [157] | |||
| Mozzarella | Not detected at 500 MPa for 10 min | [157] | |||
| Smoked salmon | 1.5 log10 reduction at 500 MPa for 10 min | [157] | |||
| Salchichon (dry cured sausage) | 3.42 log10 reduction at 600 MPa for 8 min | [158] | |||
| Dry cured loin | 3.08 log10 reduction at 600 MPa for 8 min | [158] | |||
| Bacon | 1.68 log10 reduction at 593 MPa for 5 min at 4.0 °C | [159] | |||
| Smoked rainbow trout | 1.0–1.82 log10 reduction at 500 MPa for 5 min at 4.4 °C | [160] | |||
| Smoked rainbow trout | 1.2–2.73 log10 reduction at 500 MPa for 5 min at 4.4 °C | [160] | |||
| Irradiation | Use of ionizing radiation to eliminate L. monocytogenes by destroying the DNA of L. monocytogenes, thereby leading to its inactivation. | Vacuum packed cooked ham | Achievement of FSC (102 CFU) and zero tolerance at doses of 1 kGy and 2.5 kGy of e-beam radiation | [161] | Possible issue with consumer acceptance, and expensive [162]. |
| Chicken breast Adobo | Complete elimination at a minimum dose of 25 kGy of gamma irradiation with total irradiation time of 4.6 h | [163] | |||
| Pulse light (PL) | Use of short flashes with wavelengths of 200–1100 nm, including UV light known as pulse light, which disrupts DNA transcription and replication in L. monocytogenes [164]. | Cooked ham | 2 log10 CFU/cm2 reduction at pulse light of 8.4 J/cm2 | [101] | Limited penetration capability [101]. Higher fluences may lead to change in sensory quality [101]. |
| Cooked bologna | 1 log10 CFU/cm2 reduction at pulse light of 8.4 J/cm2 | [101] | |||
| RTE cured meat products | 1.5–1.8 log10 CFU/cm2 reduction at pulse light of 11.9 J/cm2 | [165] | |||
| Ultrasound | Washing technique applied by using bath-type ultrasound between frequencies of 20–100 kHz to destroy and detach the microorganisms from the food surface [166,167,168,169]. | Lettuce | 0.42 log10 CFU/cm2 at 10 min treatment time | [170] | May increase lipid oxidation. Ultrasound parameters should be optimized for each product type [168]. |
| Lettuce | 0.39 log10 CFU/cm2 at 30 min treatment time | [170] | |||
| Lettuce | 0.40 log10 CFU/cm2 at 60 min treatment time | [170] | |||
| Chemical intervention strategies | |||||
| Chemical additives | The application of chemical substances, referred to as preservatives (e.g., sodium diacetate, sodium lactate) to reduce pH, therefore establishing an environment unfavorable to pathogens. | Vacuum-packaged frankfurters | Inhibited the growth and increased the storage time to 120 days when sodium lactate and sodium acetate used together (all at 0.25%) | [109] | Affects the sensory properties of the product [171,172]. May lead to heat resistance of L. monocytogenes when used in combination with salt [173]. |
| Weiners | Prevented the growth during storage at 4.5 °C for 60 days when ≥3% sodium acetate and combination of ≥1% lactate plus ≥ 0.1% diacetate was used | [137]. | |||
| Use of organic acids | Use of GRAS (generally recognized as safe) organic acid, such as such as acetic acid, lactic acid, citric acid, malic acid, and peracetic acid, to disrupt cell function. | Lettuce | 1.18 log10 CFU/cm2 when 2% acetic acid used for 10 min | [170] | May affect the organoleptic quality of the product due to residue organic acid left on the product and also due to organic acid-specific odor and taste |
| Lettuce | 1.87 log10 CFU/cm2 when 2% acetic acid used for 30 min | [170] | |||
| Lettuce | 2.68 log10 CFU/cm2 when 2% acetic acid used for 60 min | [170] | |||
| Lettuce | 1.21 log10 CFU/cm2 when 2% lactic acid used for 10 min | [170] | |||
| Lettuce | 2.12 log10 CFU/cm2 when 2% lactic acid used for 10 min | [170] | |||
| Lettuce | 2.99 log10 CFU/cm2 when 2% lactic acid used for 10 min | [170] | |||
| Lettuce | 6.01 log10 CFU/g when 1% acetic used for 0.5 min | [174] | |||
| Lettuce | 5.33 log10 CFU/g when 1% acetic used for 0.5 min | [174] | |||
| Lettuce | 5.04 log10 CFU/g when 1% acetic used for 1 min | [174] | |||
| Lettuce | 4.34 log10 CFU/g when 1% acetic used for 1 min | [174] | |||
| Biological intervention strategies | |||||
| Use of lactic acid bacteria (LAB) starter cultures | LAB inhibit L. monocytogenes by (i) acidification (pH reduction), (ii) competitive exclusion for nutrients and niches, and (iii) producing antimicrobials (organic acids, hydrogen peroxide, bacteriocins, such as nisin) [175,176]. | Cheese | 1.48–4.16 log10 for lactococci 1.96–4.21 for lactobacilli | [177] | Should be used as a part of hurdle technology, as this singly will not ensure complete safety [178]. Strains used should be GRAS-approved or assigned Qualified Presumption of Safety (QPS) [178,179]. Food matrix might influence the survival or replication of L. monocytogenes and LAB [179]. |
| Gorgonzola cheese | Not detected in 25 g after addition of two strains of lactic acid bacteria, Lactococcus lactis subsp. Cremoris FT27. | [180] | |||
| Soft cheese | 0.5–1 log10 CFU/g reduction when a mixture comprising three lactic acid bacteria was added | [181] | |||
| Dry-cured fermented sausage, “salchichon” | 1.6–2.2 log10 CFU/g reduction after addition of Lactobacillus sakei 205 | [182] | |||
| Bacteriophages | Use of bacteriophages, which are viruses that infect and replicate within bacteria, thus killing the host [183]. E.g., phages such as A511 and P100 were effective in controlling L. monocytogenes in RTE foods [184]. | Fresh-cut red delicious apples | 0.4 log10 reduction after application of nisin and phage mixture | [185] | Efficiency is dependent on intrinsic and extrinsic parameters, such as phage concentration, food matrix, and storage conditions. More efficient in liquid products. |
| Honeydew melons stored at 10 °C | 2.0–4.6 log10 reduction after application of nisin and phage mixture | [185] | |||
| Cooked turkey | 2.1 log10 CFU/cm2 reduction when PhageGuard ListexTM P100 was used at 4 °C | [186] | |||
| Cooked turkey | 1.5 log10 CFU/cm2 reduction when PhageGuard ListexTM P100 was used at 10 °C | [186] | |||
| Roast beef | 1.7 log10 CFU/cm2 reduction when PhageGuard ListexTM P100 was used at 4 °C | [186] | |||
| Roast beef | 1.7 log10 CFU/cm2 reduction when PhageGuard ListexTM P100 was used at 10 °C | [186] | |||
| Cheese | 0.7 log10 reduction by using the commercially available phage, ListShield | [187] | |||
| Smoked salmon | 1 log10 reduction by using the commercially available phage, ListShield | [187] | |||
| Smoked salmon | 0.85, 2.4, 2.75, 2.34, and 1.58 log10 reduction at 1, 5, 10, 15, and 30 days after application of bacteriophage P100 | [188] | |||
| Hot dog | >2 log10 concentration reduction after addition of lactic acid bacteria | [189]. | |||
| Bacteriocins | Use of antimicrobial peptides (e.g., nisin) produced by certain bacteria, such as lactic acid bacteria, which attacks the cell membrane of the pathogen, resulting in damage to the membrane structure [190,191,192]. | RTE sliced pork ham | 2.83 log10 reduction by bacteriophage P100 (LISTEXTM P100) | [193] | Higher concentrations of bacteriocin are required to inhibit growth [139]. Intrinsic properties of food will affect the stability of bacteriocin, which is unstable to pH changes [139]. Country-specific restriction in the use of nisin in food [100,104,105,106,107]. Should be used as a part of hurdle technology. |
| Cooked ham | 1.85 log10 reduction by 200 AU/cm2 nisin A (N200) | [194] | |||
| Cooked ham | 1.80 log10 reduction by 200 AU/g nisin plus 1.8% potassium lactate | [194] | |||
| Cooked ham | 4 log10 reduction by interleavers (containing mixture of enterocins, sakacin, nisin, potassium lactate) | [194] | |||
| Cooked ham | 4 log10 reduction by interleavers (containing mixture of enterocins, sakacin, nisin, potassium lactate) | [194] | |||
| RTE Russian type salad | Not detected in 25 g until day 5 when 40 µg/g of enterocin AS-48 was used at 10 °C | [195] | |||
| RTE turkey ham | 4 log10 CFU/g reduction at 0.4% and 0.5% nisin treatment after 7 and 14 days of storage, respectively. | [196] | |||
| Sliced dry cured ham (white pig) | 0.80 log10 reduction when nisin was applied directly (200 AU/mL), and 3 log10 reduction after 60 days of storage | [154] | |||
| Sliced dry cured ham (Iberian pig) | 1.24 log10 reduction when nisin was applied directly (200 AU/mL), and 3 log10 reduction after 60 days of storage | [154] | |||
| Smoked salmon | 2, 3.4, 4.5, 4.25, and 4.25 log10 reductions at 1, 5, 10, 15, and 30 days after spraying with enterocin AS-48 | [188] | |||
| Gorgonzola cheese | Not detected after addition of two strains of lactic acid bacteria, Lactococcus lactis subsp. Cremoris FT27 | [180] | |||
| Soft cheese | 0.5–1 log10 CFU/g reduction when a mixture comprising three lactic acid bacteria was added. | [181] | |||
| Dry-cured fermented sausage, “salchichon” | 1.6–2.2 log10 CFU/g reductions after addition of Lactobacillus sakei 205 | [182] | |||
| Sardinian dairy products | 3–4 log10 CFU/g reductions when a mixture of lactic acid bacteria was added | [177] | |||
| Jben (a Moroccan fresh cheese) | 1.46 log10 CFU/g reduction) in comparison to control after addition of enterocin OS1 (200 AU/g) after 15 days of storage at 8 °C | [197] | |||
| Spices, herbs, and essential oils | Use of spices, herbs, and essential oil derived from plants (e.g., thyme, rosemary), to provide bioactive components, such as phenolic acids, terpenes, aldehydes, and flavonoids, which have antimicrobial properties that help damage the cellular structure of the pathogen [198]. | Ham slices | 2.5, 2.6, and 3.0 log10 reductions with addition of clove, rosemary, cassia bark, and licorice, and 2.9, 3.0, and 3.2 log10 reductions in MAP | [199] | May affect taste and aroma of products. |
| Cheese (curd) | No growth observed at concentration of 2000 ppm (day 1–5) | [200] | |||
| Smoked Salmon | No growth observed at concentration of 4000 ppm (day 1–3) | [200] | |||
| Italian mortadella | Growth was 2.29–2.79 log10 CFU/g less in samples treated with thyme and rosemary compared to the control samples after 30 days of storage | [201] | |||
Note: 1 The limitations presented here are only overall limitations of the particular intervention strategies and are not specific to any of the studies mentioned.
5.2.1. Challenges and Considerations in Implementing Intervention Strategies
Implementing effective safety interventions for L. monocytogenes in RTE foods present multiple challenges. The efficacy of these interventions depends largely on product specific factors, such as the type of RTE food, the product formulation, the properties of the food matrix, and the characteristics of the contaminating strains. By tailoring interventions to specific product types and processing environments, the food industry can enhance food safety while addressing the aforementioned challenges. This section focuses primarily on these factors influencing intervention effectiveness. Other considerations, such as consumer preference, clean label demand, and economic feasibility, are also important but are beyond the primary scope of this review.
- (a)
- Influence of different types of RTE foods: Intervention strategies must be adapted depending on the characteristics of RTE foods, such as deli meats, cheeses, and salads. For example, thermal treatments above 72 °C are effective in destroying L. monocytogenes in deli meats without significantly affecting sensory quality [142]. However, for heat-sensitive products like RTE salads, such treatments can negatively impact texture and flavor. In these cases, non-thermal methods like high-pressure processing (HPP), which keeps the product temperatures below 45 °C, are more suitable [149,150]. Koutsoumanis et al. [127] reported that RTE salad can be processed at 400–600 MPa for 1.5–3.0 min at 4–8 °C to meet the microbiological criteria. Similarly, irradiation at 2.5 kGy may eliminate L. monocytogenes in RTE cooked meats but can cause undesirable odors, leading to consumer rejection [161]. This illustrates the importance of balancing microbial safety with sensory qualities, such as taste, texture, and appearance, which are critical for consumer acceptance [202].
- (b)
- Influence of food matrix properties: The intrinsic properties of RTE food, such as pH, aw, fat content, and nutrient composition play a crucial role in determining the effectiveness of intervention strategies. For example, Verheyen et al. [203] showed that fat enhanced L. monocytogenes inactivation in emulsions but offered protection in gelled emulsions (mimicking processed fish products), likely due to differences in heat transfer and shielding effects. Similarly, studies using various meat products show that fat content and aw can modulate microbial lethality, sometimes enhancing or limiting inactivation depending on the processing method [153,156,204]. In ground beef, fat reduced heat resistance at lower temperature (51.7 °C) but increased it when subjected at higher temperatures (57.2 °C and 62.8 °C) [204]. Furthermore, Bover-Cid et al. [156] compared two types of dry-cured ham during HPP treatment: one with a higher aw (0.92) and lower fat content (14.25%), and the other one with a lower aw (0.88) and higher fat content (33.26%). The former showed greater inactivation (5.26 log10 reduction at 750 MPa), while the latter was more resistant (0.92 log10 reduction at 450 MPa). Conversely, in cooked ham and mortadella, higher fat content was linked to lower HPP effectiveness [153]. Salt concentration, in combination with temperature, can also significantly influence L. monocytogenes survival and growth dynamics. In meat emulsions with 20% fat, different NaCl concentrations (2.5%, 5.0%, and 7.5%) produced markedly different growth outcomes depending on storage temperature (10, 20, and 30 °C) [205]. At higher temperatures (20–30 °C), lower salt levels (2.5% and 5.0%) allowed for faster growth, whereas 7.5% NaCl had an inhibitory effect [205]. However, at 10 °C, L. monocytogenes was able to grow even at 7.5% NaCl, reaching 6.8 log10 CFU/g after 12 days, indicating its ability to survive under cold, high-salt conditions [205]. Similarly, in a challenge test involving twelve RTE salad products, L. monocytogenes was able to grow in two types: tomato–cucumber without salt and lemon juice, and tahini salad at 4, 10, and 24 °C [206]. This was attributed to higher pH (>4.6) and release of nutrients from the tomato and cucumber [206]. In another study about Pinata (RTE Italian sausage), it has also been reported that the concentration of LAB and low aw (0.91) and pH (5.8–5.9) acted as the key hurdles to the growth of L. monocytogenes [207]. These examples illustrate how fat content interacts with other matrix factors, such as aw, pH, and nutrient composition, to influence microbial survival, emphasizing the need for tailored interventions that consider the specific properties of each RTE product.
- (c)
- Strain variability and stress resistance: Different L. monocytogenes strains exhibit varying degrees of tolerance to common stresses encountered in food processing and within the host, such as acid, osmotic, and thermal stress, as well as biofilm production [131,132,133,208,209]. The glutamate decarboxylase (GAD) system, a key acid-resistance mechanism, also varies between strains, with higher GAD activity correlating with improved survival in gastric juice [208,209]. Mechanistically, the L. monocytogenes GAD system comprises intracellular GAD (GADi) and an antiport arm (GADe), which are activated at different pH levels and differ by strain and medium, helping explain between-strain differences in acid tolerance. For example, when strains 10403S and LO28 were both exposed to pH 2.7, 10403S survived, whereas LO28 lost viability [210]. Notably, preliminary studies suggest that clinical L. monocytogenes isolates often possess significantly higher GAD activities compared to food isolates, indicating that strains more capable of causing human infection may be inherently more resistant to acidic conditions [208,211]. Additionally, clinical strains produced biofilm at higher incubation temperatures compared to strains isolated from food factories [209]. Consistent with this variability, Aryani et al. [212] reported D-values (time required at a specific temperature and condition to reduce the microbial population by one decimal) of twenty L. monocytogenes strains ranging from 9 to 30 min at 55 °C, 0.6 to 4.0 min at 60 °C, and 0.1 to 0.60 min at 65 °C, highlighting broad differences in thermal resistance. Similarly, Wang et al. [213] reported wide kinetic ranges across 33 L. monocytogenes strains, with maximum growth rates (µmax), lag times (λ), and (D60) time required for 1 log10 reduction at 60 °C ranging between 0.20 and 0.45 h−1, 0.24 and 3.36 h, and 0.52 and 3.93 min, respectively. They also showed that the mild acid adaptation (pH 5.5) increased heat resistance for most strains and produced survival curves with a shoulder during 60 °C inactivation, while leaving growth kinetics largely unchanged [213]. Heredia et al. [214] studied the inter-strain variability of twenty-six clinical and food L. monocytogenes isolates and concluded that strains from the meat category exhibited the lowest average pHmin (4.57), indicating potential acid adaptation. This inherent variability means that a universal approach to L. monocytogenes control may be inadequate, as highly resistant strains could persist despite interventions employed. Beyond strain-level variation, there are different serotypes of L. monocytogenes, out of which serotype 4b is associated with 50% of human outbreaks and serotypes 1/2a, 1/2b, and 1/2c are mostly isolated from food [68,71]. These differences in serotype distribution and virulence further underline that a universal approach to L. monocytogenes control may be inadequate, as certain serotypes and strains may persist or remain infectious despite interventions. A more nuanced understanding of both strain- and serotype-specific resistance is therefore critical to developing targeted and robust intervention strategies. A risk-based, virulence, and pathogenicity classification developed by the Joint FAO/WHO for L. monocytogenes should be considered similar to the approach used for Shiga toxin-producing E. coli (STEC) in food [215,216].
- (d)
- Other considerations: Beyond technical efficacy, factors such as consumer perception and cost can significantly influence the adoption of intervention strategies. For example, consumer concerns about irradiation and chemical preservatives have driven demand for clean-label, minimally processed products [217,218,219,220]. This shift has encouraged the food industry to explore novel biocontrol approaches for managing L. monocytogenes in RTE foods, such as bacteriophages, bacteriocins, spices, herbs, and essential oils [177,181,186,200,202]. Likewise, advanced technologies like HPP and pulsed electric fields (PEFs), while effective, may be prohibitively expensive for small and medium-sized producers [221]. Although these factors are outside the main scope of this section, they remain important in determining the real-world feasibility of intervention implementation.
Overall, the successful implementation of L. monocytogenes intervention strategies in RTE foods requires a tailored, evidence-based approach. Factors such as product-specific characteristics, food matrix composition, and strain variability must be considered. The demand for minimally processed, clean-label products call for the application of biological interventions, where feasible. Balancing microbial safety with product quality remains a critical challenge for food producers and regulators, highlighting the importance of continued research and innovation in food safety management.
5.2.2. Multi-Hurdle Approaches for Controlling L. monocytogenes
Controlling L. monocytogenes is particularly challenging due to its persistence in food processing environments and its ability to proliferate at refrigeration temperatures, even under modified atmospheric packaging. These challenges coupled with the limitations of single intervention have led to the development of multi-hurdle approaches that combine intervention strategies. Overall, studies consistently show that combining high-pressure processing (HPP) with natural antimicrobials, such as plant-derived compounds, bacteriocins, or bacteriophages, produces greater and more sustained L. monocytogenes reductions than when these treatments are applied individually [127,154,188,193,194,222,223]. While the magnitude of the benefit varies with product type, the general trend is that multi-hurdle treatments both accelerate pathogen inactivation and prolong the period before regrowth occurs. For instance, Bleoancă et al. [223] evaluated a combined treatment of thyme-derived natural antimicrobials with post-processing (HPP) in cheese. This combination resulted in a maximum reduction of 5.12 log10 CFU/g of L. monocytogenes, compared to only 1.68 log10 CFU/g when HPP was applied alone [223]. Similarly, Komora et al. [222] studied the impact of HPP (300 MPa) and bacteriophage P100 and pediocin PA-1 in fermented sausage, which resulted in 5 log10 reduction, completely eliminating L. monocytogenes immediately after processing. However, using these interventions individually did not eliminate the pathogen. Even the combination of HPP with P100 alone did not eliminate the pathogen even after 60 days of storage [222]. The combination of HPP and pediocin PA-1 did eliminate L. monocytogenes, but the effect was delayed by 72 h [222]. Figueiredo and Almeida [193] also found that combining nisin with bacteriophage P100 in RTE sliced pork ham resulted in a 3 log10 reduction of L. monocytogenes after 72 h of storage at 6–8 °C. These emphasize the synergistic effect between natural compounds and non-thermal inactivation methods.
Another study by Baños et al. [188] on smoked salmon fillets concluded that bacteriocin AS-48 alone reduced L. monocytogenes by 2-4.5 log10 CFU/cm2 during storage (below detection at day 10), while phage P100 alone achieved 0.85-2.75 log10 reductions. When combined, bacteriocin AS-48 and P100 maintained L. monocytogenes below detection (<10 CFU/cm2) from days 1-15, with only slight regrowth to 0.78 log10 CFU/cm2 by day 30 [188]. Similarly, Hereu et al. [154] concluded that the combined effect of HPP (600 MPa for 5 min) and nisin-activated polyvinyl film containing 200 AU/cm2 resulted in reductions of 5.9 log10 and 4.1 log10 in dry cured ham with aw of 0.92 and 0.88, respectively. This is in contrast to the single treatment of HPP, which only resulted in 3.9 and 1.9 log10 reductions, respectively [154]. In another study by Jofre et al. [194], the combination of interventions (HPP and bacteriocins) not only resulted in the elimination of L. monocytogenes but also extended shelf life. When cooked ham was pressurized at 400 MPa for 10 min and then treated with interleavers (thin sheets placed between slices) containing bacteriocins, i.e., 200 and 200 AU/cm2 of enterocins (E200 and E2000), 200 and 200 AU/cm2 of sakacin (S200 and S2000), 200 AU/cm2 of nisin A (N), 1.8% potassium lactate (L), a combination of 200 AU/g nisin plus 1.8% of potassium lactate (NL), 4.2-4.5 log10 reductions were achieved, with S2000 yielding absence of L. monocytogenes in 25 g samples [194]. During storage, S200, N, and NL kept L. monocytogenes below 3 MPN/g for 45 days, and S2000, E200, and E2000 maintained this for an additional 15 days [194]. These findings demonstrate that while single interventions can be effective, combining strategies often provides greater and more consistent reductions in L. monocytogenes, providing hurdles that ensure food safety, extend shelf life, and maintain the sensory and nutritional quality of RTE foods.
Among the various multi-hurdle strategies, some approaches show greater practicality and promise for industry adoption based on effectiveness, cost, regulatory acceptance, and compatibility with existing processing lines. In current practice, processors commonly employ organic acid salts (e.g., potassium lactate, sodium diacetate) and nisin [224]. High-pressure processing (HPP) is widely adopted by many RTE meat producers due to its ability to inactivate L. monocytogenes without significant impact on product quality and its regulatory acceptance as a non-thermal listericidal treatment [225]. When combined with GRAS (Generally Recognized as Safe) antimicrobials, such as nisin or organic acids (e.g., potassium lactate), this strategy becomes even more robust. Bacteriophage P100, approved for use in several jurisdictions (e.g., US, EU, Australia), also presents a promising addition, particularly because it can be applied post-packaging via spraying or dipping without requiring major processing changes. However, standalone phage application often shows limited efficacy and is more effective when used in combination with HPP or bacteriocins [194]. In summary, HPP combined with bacteriocins, or organic acid-based preservatives is currently the most feasible multi-hurdle approach for broad industrial application in RTE foods due to its proven efficacy, regulatory clearance, and scalability. Other promising methods, like lactic acid bacteria biocontrol, natural preservatives, and active packaging systems, are emerging but may require further optimization, regulatory clarity, and cost–benefit evaluation before widespread implementation.
5.3. Future Studies
Despite significant advancements in understanding the risk of listeriosis and implementation of relevant control measures for RTE foods, further research is needed to enhance food safety outcomes. The effectiveness of these interventions can be strongly influenced by product-specific intrinsic factors, such as pH, aw, and fat content. These intrinsic properties can affect how L. monocytogenes grows, survives, or become inactivated under different processing conditions. Strain variability plays a critical role in determining the pathogen’s resistance to environmental and technological stresses [208,209,212]. However, existing studies have primarily focused on a limited range of RTE products and have used a narrow selection of L. monocytogenes strains [153,156,203,204,226], which can result in under- or overestimation of intervention efficacy.
To account for this variability, reduce uncertainty, and improve food safety decision making, researchers have increasingly turned to QMRA models. Several well-established models (e.g., [227,228,229] integrate predictive microbiology (Baranyi for growth, Bigelow/Weibull for inactivation, etc.) to simulate L. monocytogenes behavior and the impact of interventions, such as thermal processing and HPP. These models represent a significant advancement in QMRA by linking growth or inactivation kinetics of L. monocytogenes, but they are often based on data generated under controlled laboratory settings and may not reflect the full complexity of commercial food processing environments. In particular, accurate determination of growth and inactivation parameters under real-world conditions, including variability in temperature, pH, aw, and product composition, are critical to improving the reliability of predictive models. For instance, the predictive model for L. monocytogenes in seafood and meat products developed by Mejlholm and Dalgaard [230] includes the effects of 12 environmental parameters, including temperature, pH, aw, salt, CO2, smoke components, nitrite, acetic acid, benzoic acid, citric acid, diacetate, lactic acid, and sorbic acid [230,231,232]. It also accounts for microbial interaction with lactic acid bacteria via the Jameson effect [233]. Inclusion of such multi-factor growth models within the QMRA will not only strengthen risk assessments but will also support more targeted and effective L. monocytogenes control strategies in RTE foods. Importantly, the application of QMRA could extend beyond illness risk estimation to predicting recall risks and determining microbiologically safe shelf life. This is where the approach could differ from existing models. For example, Chen et al. [234] applied QMRA to estimate the likelihood of regulatory non-compliance and recall risk for smoked salmon, while Koutsoumanis et al. [30] incorporated spoilage modeling to guide shelf-life setting. Building on these concepts, future work should develop industry-relevant, QMRA based decision-support tools that (i) integrate predictive growth accounting for strain variability and key environmental factors (temperature, pH, aw, nitrite, organic acids, CO2), including microbial interaction via the Jameson effect; (ii) include inactivation models; and (iii) evaluate single and multi-hurdle interventions (e.g., HPP with bacteriocins/protective cultures) to simulate their combined impact on L. monocytogenes control, shelf life, and recall risk. Such tools would enable food businesses to proactively manage contamination, optimize product shelf life, ensure regulatory compliance, and minimize the likelihood of costly recalls, ultimately contributing to safer RTE food systems.
6. Conclusions
L. monocytogenes remains one of the most significant foodborne pathogens affecting RTE foods, posing serious risks to public health and food industry operations. Its ability to persist in food processing environments, form protective biofilms, and grow at refrigeration temperatures makes it particularly difficult to control. Despite strict regulatory requirements set for L. monocytogenes in RTE foods, it continues to be associated with food recalls, especially high-risk RTE products. Comparisons between Australian surveillance and recall data, and available data from the US, Europe, and Canada confirm that RTE foods are common vehicles for L. monocytogenes contamination. Although compliance rates in Australia remain high, occasional non-compliant samples (detection of L. monocytogenes in food) and ongoing listeriosis cases still occur, indicating ongoing risk. The increasing demand for minimally processed, clean label foods also present new challenges for managing the risk of L. monocytogenes in RTE foods, particularly where conventional interventions like pasteurization may compromise product quality.
This review highlights the need for intervention strategies to be tailored to the intrinsic properties of specific RTE foods and the strain variabilities of L. monocytogenes while also balancing safety and sensory quality. Although QMRA tools have been primarily used for prediction of probability of illness, there is a growing interest in their use for predicting shelf life and recall risk. Future research should prioritize the development of a robust, industry-relevant, QMRA-based decision support tool that integrates predictive growth and inactivation models capturing strain variability, key environmental factors (temperature, pH, aw, nitrite, organic acids, CO2), and microbial interactions (e.g., the Jameson effect). Unlike the existing tool, it will output the optimum shelf life and recall risk of RTE food based on the multi-factor growth and inactivation models. Such a tool would align with evolving regulatory standards and help businesses proactively manage L. monocytogenes risks across the RTE supply chain, thereby reducing recall associated losses. By advancing these models and strengthening food safety practices across the RTE food supply chain, it will be possible to reduce public health risks, minimize product recalls and support safer food systems globally. Overall, controlling L. monocytogenes in RTE foods requires a comprehensive and adaptive approach that integrates scientific innovation, industry collaboration, and predictive modeling to achieve safer RTE food systems and protect public health.
Author Contributions
Conceptualization, J.Y., D.S. and C.K.; writing—original draft preparation, J.Y.; writing—review and editing, J.Y., D.S., L.R., R.V. and C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Dipon Sarkar is employed by the company Victual Pty Ltd., Sydney, Australia, and declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Victual Pty Ltd. had no role in the conception and design of the review, literature search or selection, synthesizing or interpretation, manuscript preparation, or the decision to publish. Dipon Sarkar contributed to the manuscript in his capacity as a Research Adjunct at the University of Tasmania.
References
- New South Wales Government. Listeriosis. Available online: https://www.health.nsw.gov.au/Infectious/factsheets/Pages/listeriosis.aspx (accessed on 14 May 2025).
- World Health Organization. Listeriosis. Available online: http://who.int/news-room/fact-sheets/detail/listeriosis (accessed on 1 February 2025).
- US Centers for Disease Control and Prevention. People at Increased Risk for Listeria Infection. Available online: https://www.cdc.gov/listeria/risk-factors/index.html#cdc_risk_factors_who-pregnant-people-and-newborns (accessed on 8 June 2025).
- World Health Organization. Foodborne Illnesses Per 100,000 (Median, 95% Uncertainty Interval). Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/foodborne-illnesses-per-100-000--2010-(median--95--uncertainty-interval) (accessed on 12 May 2025).
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. About Listeria Infection. Available online: https://www.cdc.gov/listeria/about/ (accessed on 10 August 2025).
- US Centers for Disease Control and Prevention. Clinical Overview of Listeriosis. Available online: https://www.cdc.gov/listeria/hcp/clinical-overview/index.html (accessed on 12 August 2025).
- Department of Health Disability and Ageing. National Notifiable Diseases Surveillance System (NNDSS) Fortnightly Reports. Available online: https://www.health.gov.au/resources/collections/nndss-fortnightly-reports#2025 (accessed on 12 August 2025).
- Australian Bureau of Statistics. National, State and Territory Population. 2023. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/dec-2023#data-downloads (accessed on 31 August 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2023; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2025; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2023 (accessed on 15 September 2025).
- Possas, A.; Hernández, M.; Esteban-Carbonero, Ó.; Valero, A.; Rodríguez-Lázaro, D. Listeria monocytogenes survives better at lower storage temperatures in regular and low-salt soft and cured cheeses. Food Microbiol. 2022, 104, 103979. [Google Scholar] [CrossRef]
- US Department of Agriculture. Listeria monocytogenes, Listeriosis and You. Available online: https://www.usda.gov/about-usda/news/blog/listeria-monocytogenes-listeriosis-and-you (accessed on 14 April 2025).
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar]
- Di Ciccio, P.; Conter, M.; Zanardi, E.; Ghidini, S.; Vergara, A.; Paludi, D.; Festino, A.; Ianieri, A. Listeria monocytogenes: Biofilms in food processing. Ital. J. Food Sci. 2012, 24, 203–213. Available online: https://scholar.google.com/scholar_lookup?title=Listeria+monocytogenes:+Biofilms+in+food+processing&author=Di+Ciccio,+P.&author=Conter,+M.&author=Zanardi,+E.&author=Ghidini,+S.&author=Vergara,+A.&author=Paludi,+D.&author=Festino,+A.R.&author=Ianieri,+A.&publication_year=2012&journal=Ital.+J.+Food+Sci.&volume=24&pages=203%E2%80%93213 (accessed on 1 August 2025).
- Food Standards Australia New Zealand. Australia New Zealand Food Standards Code—Standard 3.2.2—Food Safety Practices and General Requirements (Australia Only); Food Standards Australia New Zealand: Canberra, Australia, 2000. [Google Scholar]
- Center for Food Safety and Applied Nutrition. Control of Listeria Monocytogenes in Ready-to-Eat Foods: Guidance for Industry (Draft Guidance); US Food and Drug Authority (FDA): Rockville, MD, USA, 2017. [Google Scholar]
- Buchanan, R.L.; Gorris, L.G.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- USDA Food Safety and Inspection Service. Annual Recall Summaries. Available online: https://www.fsis.usda.gov/food-safety/recalls-public-health-alerts/annual-recall-summaries (accessed on 8 March 2025).
- Food Standards Australia New Zealand. Australian Food Recall Statistics. Available online: https://www.foodstandards.gov.au/food-recalls/recallstats (accessed on 21 January 2025).
- European Commission. RASFF Window_ Notification 2024.1499 (Listeria monocytogenes in Cooked Ham). Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/667121 (accessed on 8 March 2025).
- Government of Canada. Statistics: Food Recall Incidents and Food Recalls. Available online: https://inspection.canada.ca/food-safety-for-consumers/canada-s-food-safety-system/food-recall-incidents-and-food-recalls/eng/1348756225655/1348756345745 (accessed on 8 March 2025).
- Department of Agriculture, Fisheries and Forestry. Imported Food Data Reports and Surveys from 2017–2023. Available online: https://www.agriculture.gov.au/biosecurity-trade/import/goods/food/inspection-testing/surveys-data (accessed on 1 June 2025).
- Food Standards Australia New Zealand. Listeria Monocytogenes in Cooked Prawns; Food Standards Australia New Zealand: Canberra, Australia, 2004. [Google Scholar]
- New South Wales Food Authority. Microbiological Quality of Packaged Sliced Ready-to-Eat Meat Products; New South Wales (NSW) Food Authority: Silverwater, Australia, 2009. [Google Scholar]
- Krsteski, R.; Rockliff, S. Microbiological Quality of Ready-to-Eat Foods: July 2014–June 2015; ACT Health Protection Service: Canberra, Australia, 2015. [Google Scholar]
- New South Wales Food Authority. Survey of Listeria monocytogenes in Sliced Pre-Packaged RTE Meats; NSW Food Authority: Silverwater, Australia, 2013. [Google Scholar]
- Codex Alimentarius Commission. Guidelines on the Application of General Principles of Food Hygiene to the Control of Listeria monocytogenes in Foods—CAC/GL 61—2007; Codex Alimentarius Commission: Rome, Italy, 2007. [Google Scholar]
- Aalto-Araneda, M.; Lunden, J.; Markkula, A.; Hakola, S.; Korkeala, H. Processing plant and machinery sanitation and hygiene practices associate with listeria monocytogenes occurrence in ready-to-eat fish products. Food Microbiol. 2019, 82, 455–464. [Google Scholar] [CrossRef]
- Gupta, P.; Adhikari, A. Novel Approaches to Environmental Monitoring and Control of Listeria monocytogenes in Food Production Facilities. Foods 2022, 11, 1760. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Tsaloumi, S.; Aspridou, Z.; Tassou, C.; Gougouli, M. Application of quantitative microbiological risk assessment (QMRA) to food spoilage: Principles and methodology. Trends Food Sci. Technol. 2021, 114, 189–197. [Google Scholar] [CrossRef]
- Victoria State Government. Listeriosis. Available online: https://www.health.vic.gov.au/infectious-diseases/listeriosis (accessed on 10 August 2025).
- FAO/WHO. Risk Assessment of Listeria monocytogenes in Ready-to-Eat Foods; Annali Della Facoltà di Medicina Veterinaria di: Pisa, Italy, 2004. [Google Scholar]
- Farber, J.M.; Peterkin, P. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef] [PubMed]
- Petran, R.; Zottola, E. A study of factors affecting growth and recovery of Listeria monocytogenes Scott A. J. Food Sci. 1989, 54, 458–460. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Holch, A.; Webb, K.; Lukjancenko, O.; Ussery, D.; Rosenthal, B.M.; Gram, L. Genome sequencing identifies two nearly unchanged strains of persistent Listeria monocytogenes isolated at two different fish processing plants sampled 6 years apart. Appl. Environ. Microbiol. 2013, 79, 2944–2951. [Google Scholar] [CrossRef]
- Bonsaglia, E.; Silva, N.; Júnior, A.F.; Júnior, J.A.; Tsunemi, M.; Rall, V. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control 2014, 35, 386–391. [Google Scholar] [CrossRef]
- Blaschek, H.P.; Wang, H.H.; Agle, M.E. Biofilms in the Food Environment; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes–how this pathogen survives in food-production environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.; Wang, Q.; Yang, J.; Zhong, Q. The mixed biofilm formed by Listeria monocytogenes and other bacteria: Formation, interaction and control strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 8570–8586. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Breidt, F., Jr.; Kathariou, S. Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment. Appl. Environ. Microbiol. 2006, 72, 7711–7717. [Google Scholar] [CrossRef]
- Zhang, H.; Que, F.; Xu, B.; Sun, L.; Zhu, Y.; Chen, W.; Ye, Y.; Dong, Q.; Liu, H.; Zhang, X. Identification of Listeria monocytogenes contamination in a ready-to-eat meat processing plant in China. Front. Microbiol. 2021, 12, 628204. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, B.; Cerf, O. Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Leong, D.; Alvarez-Ordóñez, A.; Jordan, K. Monitoring occurrence and persistence of Listeria monocytogenes in foods and food processing environments in the Republic of Ireland. Front. Microbiol. 2014, 5, 436. [Google Scholar] [CrossRef]
- Tompkin, R.B. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef]
- da Silva, D.A.L.; de Melo Tavares, R.; Camargo, A.C.; Yamatogi, R.S.; De Martinis, E.C.P.; Nero, L.A. Biofilm growth by Listeria monocytogenes on stainless steel and expression of biofilm-related genes under stressing conditions. World J. Microbiol. Biotechnol. 2021, 37, 119. [Google Scholar] [CrossRef]
- Samelis, J.; Metaxopoulos, J. Incidence and principal sources of Listeria Spp. And Listeria monocytogenes contamination in processed meats and a meat processing plant. Food Microbiol. 1999, 16, 465–477. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Chang, Z.; Liu, X.; Chen, W.; Yu, Y.; Wang, X.; Dong, Q.; Ye, Y.; Zhang, X. Listeria monocytogenes contamination characteristics in two ready-to-eat meat plants from 2019 to 2020 in Shanghai. Front. Microbiol. 2021, 12, 729114. [Google Scholar] [CrossRef]
- Muhterem-Uyar, M.; Dalmasso, M.; Bolocan, A.S.; Hernandez, M.; Kapetanakou, A.E.; Kuchta, T.; Manios, S.G.; Melero, B.; Minarovičová, J.; Nicolau, A.I. Environmental sampling for Listeria monocytogenes control in food processing facilities reveals three contamination scenarios. Food Control 2015, 51, 94–107. [Google Scholar] [CrossRef]
- Ho, A.; Lappi, V.; Wiedmann, M. Longitudinal monitoring of Listeria monocytogenes contamination patterns in a farmstead dairy processing facility. J. Dairy Sci. 2007, 90, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.P.; De Martinis, E.C.P. Current knowledge and perspectives on biofilm formation: The case of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2013, 97, 957–968. [Google Scholar] [CrossRef]
- Lasa, I. Towards the identification of the common features of bacterial biofilm development. Int. Microbiol. 2006, 9, 21–28. [Google Scholar] [PubMed]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. The One Health approach in food safety: Challenges and opportunities. Food Front. 2024, 5, 1837–1865. [Google Scholar] [CrossRef]
- Lotoux, A.; Milohanic, E.; Bierne, H. The viable but non-culturable state of Listeria monocytogenes in the one-health continuum. Front. Cell. Infect. Microbiol. 2022, 12, 849915. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, A.R. Assessment of bacterial viability: A comprehensive review on recent advances and challenges. Microbiology 2019, 165, 593–610. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.-W.; Ding, T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Panera-Martínez, S.; Capita, R.; García-Fernández, C.; Alonso-Calleja, C. Viability and Virulence of Listeria monocytogenes in Poultry. Microorganisms 2023, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Panera-Martinez, S.; Rodriguez-Melcon, C.; Del Campo, C.; Alonso-Calleja, C.; Capita, R. Prevalence and levels of cells of Salmonella spp. and Listeria monocytogenes in various physiological states naturally present in chicken meat. Food Control 2025, 167, 110770. [Google Scholar] [CrossRef]
- European Food Safety Authority. Request for updating the former SCVPH opinion on Listeria monocytogenes risk related to ready-to-eat foods and scientific advice on different levels of Listeria monocytogenes in ready-to-eat foods and the related risk for human illness-scientific opinion of the panel on biological hazards. EFSA J. 2008, 6, 599. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Expert Consultation on Risk Assessment of Microbiological Hazards in Foods; World Health Organization and Food and Agriculture Organization of the United Nations: Rome, Italy, 2000. [Google Scholar]
- Swaminathan, B.; Gerner-Smidt, P. The Epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.L.; Killinger, A. Listeria monocytogenes and listeric infections. Bacteriol. Rev. 1966, 30, 309–382. [Google Scholar] [CrossRef] [PubMed]
- Lyytikäinen, O.; Autio, T.; Maijala, R.; Ruutu, P.; Honkanen-Buzalski, T.; Miettinen, M.; Hatakka, M.; Mikkola, J.; Anttila, V.-J.; Johansson, T. An outbreak of Listeria monocytogenes serotype 3a infections from butter in Finland. J. Infect. Dis. 2000, 181, 1838–1841. [Google Scholar] [CrossRef]
- Yezli, S.; Otter, J.A. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ. Virol. 2011, 3, 1–30. [Google Scholar] [CrossRef]
- Notermans, S.; Dufrenne, J.; Teunis, P.; Chackraborty, T. Studies on the risk assessment of Listeria monocytogenes. J. Food Prot. 1998, 61, 244–248. [Google Scholar] [CrossRef]
- Sprong, R.C.; Hulstein, M.F.; Van der Meer, R. High intake of milk fat inhibits intestinal colonization of Listeria but not of Salmonella in rats. J. Nutr. 1999, 129, 1382–1389. [Google Scholar] [CrossRef]
- Heras, V.; Clooney, A.G.; Ryan, F.J.; Cabrera-Rubio, R.; Casey, P.G.; Hueston, C.M.; Pinheiro, J.; Rudkin, J.K.; Melgar, S.; Cotter, P. Short-term consumption of a high-fat diet increases host susceptibility to Listeria monocytogenes infection. Microbiol. Soc. 2020, 7, 7. [Google Scholar] [CrossRef]
- Burall, L.S.; Grim, C.J.; Datta, A.R. A clade of Listeria monocytogenes serotype 4b variant strains linked to recent listeriosis outbreaks associated with produce from a defined geographic region in the US. PLoS ONE 2017, 12, e0176912. [Google Scholar] [CrossRef] [PubMed]
- Kramarenko, T.; Roasto, M.; Meremäe, K.; Kuningas, M.; Põltsama, P.; Elias, T. Listeria monocytogenes prevalence and serotype diversity in various foods. Food Control 2013, 30, 24–29. [Google Scholar] [CrossRef]
- Pontello, M.; Guaita, A.; Sala, G.; Cipolla, M.; Gattuso, A.; Sonnessa, M.; Gianfranceschi, M.V. Listeria monocytogenes serotypes in human infections (Italy, 2000–2010). Ann. Dell’ist. Super. Sanità 2012, 48, 146–150. [Google Scholar] [CrossRef]
- Maury, M.M.; Tsai, Y.-H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A. uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef]
- Roberts, A.; Nightingale, K.; Jeffers, G.; Fortes, E.; Kongo, J.M.; Wiedmann, M. Genetic and phenotypic characterization of listeria monocytogenes lineage III. Microbiology 2006, 152, 685–693. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Listeria Monocytogenes in Ready-to-Eat (RTE) Food: Attribution, Characterization and Monitoring: Meeting Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- European Food Safety Authority. The European Union One Health 2021 zoonoses report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Nepomuceno, F.V.; Akutsu, R.d.C.C.d.A.; Draeger, C.L.; da Silva, I.C.R. Foodborne diseases: A study before and during the covid-19 pandemic in Brazil. Nutrients 2023, 16, 60. [Google Scholar] [CrossRef]
- Department of Health Disability and Ageing. Australia’s Notifiable Disease Status, 2015—Annual Report of the National Notifiable Diseases Surveillance System; Department of Health Disability and Ageing: Canberra, ACT, Australia, 2019. [Google Scholar]
- Amy, B.; NNDSS Annual Report Working Group; O’Dwyer, M.R.; Trungove, M. Australia’s Notifiable Disease Status, 2016: Annual Report of the National Notifiable Diseases Surveillance System. Commun. Dis. Intell. 2021, 45. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. National, State and Territory Population. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population (accessed on 8 October 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2009 [2007 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2009; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2009-2007-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2010 [2008 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2010; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2010-2008-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2011 [2009 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2011; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2011-2009-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2012 [2010 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2013; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2012-2010-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2013 [2011 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2013; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2013-2011-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report 2016 [2014 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2017; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2016-2014-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2015; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2015 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2016; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2016 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2017; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2017 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2018; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2018 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2019 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2020; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2020 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2021; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2021 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2022; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2022 (accessed on 15 September 2025).
- Australian National University; Food Standards Australia New Zealand. The Annual Cost of Foodborne Illness in Australia; Food Standards Australia New Zealand: Canberra, Australia, 2022. [Google Scholar]
- Hoffman, S.; Maculloch, B.; Batz, M. Economic Burden of Major Foodborne Illnesses Acquired in the United States; U.S. Department of Agriculture, Economic Research Service: Washingtn, DC, USA, 2015. [Google Scholar]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission: Brussels, Belgium, 2005. [Google Scholar]
- Health Canada. Policy on Listeria Monocytogenes in Ready-to-Eat Foods; Health Canada: Ottawa, ON, Canada, 2023. [Google Scholar]
- Piet, J.; Kieran, J.; Dara, L. Listeria monocytogenes in food: Control by monitoring the food processing environment. Afr. J. Microbiol. Res. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Australia New Zealand Food Standards Code—Standard 1.6.1—Microbiological Limits in Food; Food Standards Australia New Zealand: Canberra, ACT, Australia, 2015. [Google Scholar]
- Health Canada. Health Canada’s Proposal to Enable the Use of a New Food Additive, Nisin, as an Antimicrobial Preservative in or on Various Foods; Health Canada: Ottawa, ON, Canada, 2017. [Google Scholar]
- Hierro, E.; Barroso, E.; De la Hoz, L.; Ordóñez, J.A.; Manzano, S.; Fernández, M. Efficacy of pulsed light for shelf-life extension and inactivation of Listeria monocytogenes on ready-to-eat cooked meat products. Innov. Food Sci. Emerg. Technol. 2011, 12, 275–281. [Google Scholar] [CrossRef]
- Watson. E325 Sodium Lactate. Available online: https://food-detektiv.de/en/additives/?enummer=Sodium%20lactate (accessed on 12 August 2025).
- Food-Info. E325: Sodium Lactate. Available online: https://www.food-info.net/uk/e/e325.htm (accessed on 12 August 2015).
- Hudson, J.A.; McIntyre, L.; Billington, C. Application of bacteriophages to control pathogenic and spoilage bacteria in food processing and distribution. In Bacteriophages in the Control of Food and Waterborne Pathogens; Wiley: Hoboken, NJ, USA, 2010; pp. 119–135. [Google Scholar]
- Food Standards Australia New Zealand. Final Assessment Report- Use of Nisin in Processed Meat Products; Food Standards Australia New Zealand: Canberra, Australia, 2007. [Google Scholar]
- Rhodia Inc. Gras Notice No. Grn 000065: Nisin—Use in Frankfurter Casings and Cooked RTE Meat and Poultry Products; Rhodia Inc.: La Défense, France, 2001. [Google Scholar]
- European Food Safety Authority. Safety of Nisin (E 234) as a Food Additive in the Light of New Toxicological Data and the Proposed Extension of Use; European Food Safety Authority: Parma, Italy, 2017. [Google Scholar]
- Samelis, J.; Bedie, G.K.; Sofos, J.N.; Belk, K.E.; Scanga, J.A.; Smith, G.C. Control of Listeria monocytogenes with combined antimicrobials after postprocess contamination and extended storage of frankfurters at 4 ℃ in vacuum packages. J. Food Prot. 2002, 65, 299–307. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- South Australia Health. Guideline for the Control of Listeria in Food Service to Vulnerable Persons; South Australia Health: Adelaide, Australia, 2019. [Google Scholar]
- USDA Food Safety and Inspection Service. FSIS Compliance Guideline: Controlling Listeria monocytogenes in Post-Lethality Exposed Ready-to-Eat Meat and Poultry Products; USDA Food Safety and Inspection Service: Washington, DC, USA, 2014. [Google Scholar]
- Uyttendaele, M.; Busschaert, P.; Valero, A.; Geeraerd, A.; Vermeulen, A.; Jacxsens, L.; Goh, K.; De Loy, A.; Van Impe, J.; Devlieghere, F. Prevalence and challenge tests of Listeria monocytogenes in Belgian Produced and retailed mayonnaise-based deli-salads, cooked meat products and smoked fish between 2005 and 2007. Int. J. Food Microbiol. 2009, 133, 94–104. [Google Scholar] [CrossRef]
- Stessl, B.; Ruppitsch, W.; Wagner, M. Listeria monocytogenes post-outbreak management-when could a food production be considered under control again? Int. J. Food Microbiol. 2022, 379, 109844. [Google Scholar] [CrossRef]
- Chilled Food Association Ltd. Shelf Life of Ready to Eat Food in Relation to L. monocytogenes—Guidance for Food Business Operators; Chilled Food Association Ltd.: Kettering, UK, 2010. [Google Scholar]
- Victoria State Government. Notification Procedures for Infectious Diseases. Available online: https://www.health.vic.gov.au/infectious-diseases/notification-procedures-for-infectious-diseases (accessed on 14 May 2025).
- Food Standards Australia New Zealand. Food Recalls- What Is Food Recalls? Available online: https://www.foodstandards.gov.au/industry/foodrecalls/recalls/pages/whatisafoodrecall.aspx (accessed on 21 August 2025).
- Government of Canada. Toolkit for Food Businesses New to the Safe Food for Canadians Regulations. Available online: https://inspection.canada.ca/en/food-safety-industry/toolkit-food-businesses (accessed on 7 July 2025).
- Food Standards Australia New Zealand. How to Recall Food. Available online: https://www.foodstandards.gov.au/food-recalls/how-to-recall-food (accessed on 7 July 2025).
- US Food & Drug Administration. Post-Outbreak Response and Prevention Strategies to Enhance Food Safety (Updated 17 January 2025). Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/post-outbreak-response-and-prevention-strategies-enhance-food-safety-updated-january-17-2025 (accessed on 7 July 2025).
- European Commission. Regulation (EC) No 178/2002 General food law regulation. In Article 50 (Rapid Alert System); European Commission: Brussels, Belgium, 2002. [Google Scholar]
- European Commission, and Directorate-General SANTE. Alert and Cooperation Network (ACN) Annual Reports and Publications. Available online: https://food.ec.europa.eu/safety/acn/reports-and-publications_en (accessed on 20 September 2025).
- USDA Food Safety and Inspection Service. Frequently Requested Records; US Department of Agriculture (USDA): Annapolis, MD, USA, 2025. Available online: https://www.fsis.usda.gov/about-fsis/freedom-information-act-foia/frequently-requested-records (accessed on 15 June 2025).
- USDA Food Safety and Inspection Service. Understanding FSIS Food Recalls. Available online: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/understanding-fsis-food-recalls (accessed on 8 March 2025).
- Koutsoumanis, K.P.; Aspridou, Z. Moving towards a risk-based food safety management. Curr. Opin. Food Sci. 2016, 12, 36–41. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Principles and Guidelines for the Conduct of Microbiological Risk Assessment CAC/GL 30-1999; Codex Alimentarius Commission: Rome, Italy, 1999. [Google Scholar]
- Teunis, P.; Schijven, J.F. Generic Guidance to Quantitative Microbial Risk Assessment for Food and Water; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2019. [Google Scholar]
- Koutsoumanis, K.; Pavlis, A.; Nychas, G.-J.E.; Xanthiakos, K. Probabilistic model for Listeria monocytogenes growth during distribution, retail storage, and domestic storage of pasteurized milk. Appl. Environ. Microbiol. 2010, 76, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Risk assessment of Listeria monocytogenes in Ready-to-Eat Foods: Technical Report: Food & Agriculture Org; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- US Food & Drug Administration. CPG Sec 555.320 Listeria monocytogenes; US Food & Drug Administration: Silver Spring, MD, USA, 2008. [Google Scholar]
- Ahn, J.; Lee, H.-Y.; Knipe, L.; Balasubramaniam, V. Effect of a post-packaging pasteurization process on inactivation of a Listeria innocua surrogate in meat products. Food Sci. Biotechnol. 2014, 23, 1477–1481. [Google Scholar] [CrossRef]
- Sibanda, T.; Buys, E.M. Listeria monocytogenes pathogenesis: The role of stress adaptation. Microorganisms 2022, 10, 1522. [Google Scholar] [CrossRef]
- Lakicevic, B.Z.; Den Besten, H.M.; De Biase, D. Landscape of stress response and virulence genes among Listeria monocytogenes strains. Front. Microbiol. 2022, 12, 738470. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Pan, Y.; Zhao, Y.; Liu, H. Phenotypic heterogeneity and pathogenicity of Listeria monocytogenes under complex salinities of bile salts and sodium salts stress. Arch. Microbiol. 2025, 207, 101. [Google Scholar] [CrossRef]
- Aalto-Araneda, M.; Pöntinen, A.; Pesonen, M.; Corander, J.; Markkula, A.; Tasara, T.; Stephan, R.; Korkeala, H. Strain variability of Listeria monocytogenes under NaCl stress elucidated by a high-throughput microbial growth data assembly and analysis protocol. Appl. Environ. Microbiol. 2020, 86, e02378-19. [Google Scholar] [CrossRef]
- Myintzaw, P.; Pennone, V.; McAuliffe, O.; Begley, M.; Callanan, M. Variability in cold tolerance of food and clinical Listeria monocytogenes isolates. Microorganisms 2022, 11, 65. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Glass, K.A.; Granberg, D.A.; Smith, A.L.; Mcnamara, A.M.; Hardin, M.; Mattias, J.; Ladwig, K.; Johnson, E.A. Inhibition of Listeria monocytogenes by sodium diacetate and sodium lactate on wieners and cooked bratwurst. J. Food Prot. 2002, 65, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.; Silva, S.P.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Muriana, P.; Gande, N.; Robertson, W.; Jordan, B.; Mitra, S. Effect of prepackage and postpackage pasteurization on postprocess elimination of Listeria monocytogenes on deli turkey products. J. Food Prot. 2004, 67, 2472–2479. [Google Scholar] [CrossRef]
- Selby, T.; Berzins, A.; Gerrard, D.; Corvalan, C.; Grant, A.; Linton, R. Microbial heat resistance of Listeria monocytogenes and the impact on ready-to-eat meat quality after post-package pasteurization. Meat Sci. 2006, 74, 425–434. [Google Scholar] [CrossRef]
- Li, M.; Pradhan, A.; Cooney, L.; Mauromoustakos, A.; Crandall, P.; Slavik, M.; Li, Y. A predictive model for the inactivation of Listeria innocua in cooked poultry products during postpackage pasteurization. J. Food Prot. 2011, 74, 1261. [Google Scholar] [CrossRef]
- Gande, N.; Muriana, P. prepackage surface pasteurization of ready-to-eat meats with a radiant heat oven for reduction of Listeria monocytogenes. J. Food Prot. 2003, 66, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sites, J. Elimination of Listeria monocytogenes on cooked chicken breast meat surfaces by near-infrared surface pasteurization prior to final packaging. J. Food Process Eng. 2012, 35, 1–15. [Google Scholar] [CrossRef]
- Huang, L. Infrared surface pasteurization of turkey frankfurters. Innov. Food Sci. Emerg. Technol. 2004, 5, 345–351. [Google Scholar] [CrossRef]
- Ha, J.-W.; Ryu, S.-R.; Kang, D.-H. Evaluation of near-infrared pasteurization in controlling Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes in ready-to-eat sliced ham. Appl. Environ. Microbiol. 2012, 78, 6458–6465. [Google Scholar] [CrossRef]
- European Food Safety Authority. High-Pressure Processing: Food Safety Without Compromising Quality. Available online: https://www.efsa.europa.eu/en/news/high-pressure-processing-food-safety-without-compromising-quality (accessed on 15 June 2025).
- Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. The efficacy and safety of high-pressure processing of food. EFSA J. 2022, 20, e07128. [Google Scholar] [CrossRef]
- Morales, P.; Calzada, J.; Rodríguez, B.; De Paz, M.; Gaya, P.; Nuñez, M. Effect of cheese water activity and carbohydrate content on the barotolerance of Listeria monocytogenes Scott A. J. Food Prot. 2006, 69, 1328–1333. [Google Scholar] [CrossRef]
- Rastogi, N.; Raghavarao, K.; Balasubramaniam, V.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef]
- Patterson, M.; Mackle, A.; Linton, M. Effect of high pressure, in combination with antilisterial agents, on the growth of Listeria monocytogenes during extended storage of cooked chicken. Food Microbiol. 2011, 28, 1505–1508. [Google Scholar] [CrossRef]
- Hereu, A.; Dalgaard, P.; Garriga, M.; Aymerich, T.; Bover-Cid, S. Modeling the high pressure inactivation kinetics of Listeria monocytogenes on RTE cooked meat products. Innov. Food Sci. Emerg. Technol. 2012, 16, 305–315. [Google Scholar] [CrossRef]
- Hereu, A.; Bover-Cid, S.; Garriga, M.; Aymerich, T. High hydrostatic pressure and biopreservation of dry-cured ham to meet the Food Safety Objectives for Listeria monocytogenes. Int. J. Food Microbiol. 2011, 154, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Stratakos, A.C.; Delgado-Pando, G.; Linton, M.; Patterson, M.F.; Koidis, A. Synergism between high-pressure processing and active packaging against Listeria monocytogenes in ready-to-eat chicken breast. Innov. Food Sci. Emerg. Technol. 2015, 27, 41–47. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Belletti, N.; Aymerich, T.; Garriga, M. Modeling the Protective effect of aw and fat content on the high pressure resistance of Listeria monocytogenes in dry-cured ham. Food Res. Int. 2015, 75, 194–199. [Google Scholar] [CrossRef]
- Misiou, O.; van Nassau, T.J.; Lenz, C.A.; Vogel, R.F. The preservation of Listeria-critical foods by a combination of endolysin and high hydrostatic pressure. Int. J. Food Microbiol. 2018, 266, 355–362. [Google Scholar] [CrossRef]
- Cava, R.; Higuero, N.; Ladero, L. High-pressure processing and storage temperature on Listeria monocytogenes, microbial counts and oxidative changes of two traditional dry-cured meat products. Meat Sci. 2021, 171, 108273. [Google Scholar] [CrossRef] [PubMed]
- Cetin-Karaca, H.; Cruzen, S.M.; Ebbing, D. Survival of Listeria monocytogenes on cooked and high pressure treated bacon. Appl. Food Res. 2023, 3, 100259. [Google Scholar] [CrossRef]
- Kafle, R.; Fouladkhah, A.C. Effects of thermally-assisted and high-pressure processing on background microbiota and the Listeria monocytogenes load of a minimally processed commodity. Microorganisms 2024, 12, 1858. [Google Scholar] [CrossRef]
- Cabeza, M.C.; Cambero, I.; de la Hoz, L.; Ordóñez, J.A. Optimization of e-beam irradiation treatment to eliminate Listeria monocytogenes from ready-to-eat (RTE) cooked ham. Innov. Food Sci. Emerg. Technol. 2007, 8, 299–305. [Google Scholar] [CrossRef]
- Tauxe, R.V. Food safety and irradiation: Protecting the public from foodborne infections. Emerg. Infect. Dis. 2001, 7, 516. [Google Scholar] [CrossRef]
- Feliciano, C.P.; De Guzman, Z.M.; Tolentino, L.M.M.; Cobar, M.L.C.; Abrera, G.B. Radiation-treated ready-to-eat (RTE) chicken breast adobo for immuno-compromised patients. Food Chem. 2014, 163, 142–146. [Google Scholar] [CrossRef]
- Wang, T.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Pulsed ultra-violet inactivation spectrum of Escherichia coli. Water Res. 2005, 39, 2921–2925. [Google Scholar] [CrossRef]
- Ganan, M.; Hierro, E.; Hospital, X.F.; Barroso, E.; Fernández, M. Use of pulsed light to increase the safety of ready-to-eat cured meat products. Food Control 2013, 32, 512–517. [Google Scholar] [CrossRef]
- Sagong, H.-G.; Lee, S.-Y.; Chang, P.-S.; Heu, S.; Ryu, S.; Choi, Y.-J.; Kang, D.-H. Combined effect of ultrasound and organic acids to reduce Escherichia Coli O157: H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 2011, 145, 287–292. [Google Scholar] [CrossRef]
- Scouten, A.; Beuchat, L. Combined effects of chemical, heat and ultrasound treatments to kill Salmonella and Escherichia Coli O157: H7 on alfalfa seeds. J. Appl. Microbiol. 2002, 92, 668–674. [Google Scholar] [PubMed]
- Gómez-Salazar, J.A.; Galván-Navarro, A.; Lorenzo, J.M.; Sosa-Morales, M.E. Ultrasound effect on salt reduction in meat products: A review. Curr. Opin. Food Sci. 2021, 38, 71–78. [Google Scholar] [CrossRef]
- Paul, I.D.; Jayachandran, L.E.; Kumar, P. Ultrasound and high-pressure processing of RTE: Recent trends. In Recent Advances in Ready-to-Eat Food Technology; CRC Press: Boca Raton, FL, USA, 2024; pp. 52–64. [Google Scholar]
- Turhan, E.U.; Polat, S.; Erginkaya, Z.; Konuray, G. Investigation of synergistic antibacterial effect of organic acids and ultrasound against pathogen biofilms on lettuce. Food Biosci. 2022, 47, 101643. [Google Scholar] [CrossRef]
- Ruusunen, M.; Vainionpää, J.; Puolanne, E.; Lyly, M.; Lähteenmäki, L.; Niemistö, M.; Ahvenainen, R. Effect of sodium citrate, carboxymethyl cellulose and carrageenan levels on quality characteristics of low-salt and low-fat bologna type sausages. Meat Sci. 2003, 64, 371–381. [Google Scholar] [CrossRef]
- Bloukas, J.; Paneras, E.; Fournitzis, G. Sodium lactate and protective culture effects on quality characteristics and shelf-life of low-fat frankfurters produced with olive oil. Meat Sci. 1997, 45, 223–238. [Google Scholar] [CrossRef]
- Juneja, V.; Mukhopadhyay, S.; Marks, H.; Mohr, T.B.; Warning, A.; Datta, A. Predictive thermal inactivation model for effects and interactions of temperature, NaCl, sodium pyrophosphate, and sodium lactate on Listeria monocytogenes in ground beef. Food Bioprocess Technol. 2014, 7, 437–446. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, M.R.; Park, J.W.; Park, K.H.; Chung, M.S.; Ryu, S.; Kang, D.H. Use of organic acids to inactivate Escherichia Coli O157: H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh apples and lettuce. J. Food Sci. 2011, 76, M293–M298. [Google Scholar] [CrossRef] [PubMed]
- Kasra-Kermanshahi, R.; Mobarak-Qamsari, E. Inhibition effect of lactic acid bacteria against foodborne pathogen, Listeria monocytogenes. Appl. Food Biotechnol. 2015, 2, 11–19. [Google Scholar] [CrossRef]
- Webb, L.; Ma, L.; Lu, X. Impact of lactic acid bacteria on the control of Listeria monocytogenes in ready-to-eat foods. Food Qual. Saf. 2022, 6, fyac045. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Viale, S.; Deplano, M.; Mereu, F.; Blažić, M.; Cosentino, S. Inhibitory effect of Lactiplantibacillus Plantarum and Lactococcus Lactis autochtonous strains against Listeria monocytogenes in a laboratory cheese model. Foods 2022, 11, 715. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Delgado, J.; Córdoba, J.J. Strategies for biocontrol of Listeria monocytogenes using lactic acid bacteria and their metabolites in ready-to-eat meat-and dairy-ripened products. Foods 2022, 11, 542. [Google Scholar] [CrossRef]
- Yap, P.-C.; MatRahim, N.-A.; AbuBakar, S.; Lee, H.Y. Antilisterial potential of lactic acid bacteria in eliminating Listeria monocytogenes in host and ready-to-eat food application. Microbiol. Res. 2021, 12, 234–257. [Google Scholar] [CrossRef]
- Morandi, S.; Silvetti, T.; Vezzini, V.; Morozzo, E.; Brasca, M. How we can improve the antimicrobial performances of lactic acid bacteria? a new strategy to control Listeria monocytogenes in gorgonzola cheese. Food Microbiol. 2020, 90, 103488. [Google Scholar] [CrossRef]
- Panebianco, F.; Giarratana, F.; Caridi, A.; Sidari, R.; De Bruno, A.; Giuffrida, A. Lactic acid bacteria isolated from traditional italian dairy products: Activity against Listeria monocytogenes and modelling of microbial competition in soft cheese. LWT-Food Sci. Technol. 2021, 137, 110446. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Sánchez-Montero, L.; Padilla, P.; Córdoba, J.J. Effect of the dry-cured fermented sausage “salchichón” processing with a selected lactobacillus sakei in Listeria monocytogenes and microbial population. Foods 2021, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of phage-based inhibition of Listeria monocytogenes in food products and food processing environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef]
- Leverentz, B.; Conway, W.S.; Camp, M.J.; Janisiewicz, W.J.; Abuladze, T.; Yang, M.; Saftner, R.; Sulakvelidze, A. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 2003, 69, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage Listex™ P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef]
- Perera, M.N.; Abuladze, T.; Li, M.; Woolston, J.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food Microbiol. 2015, 52, 42–48. [Google Scholar] [CrossRef]
- Baños, A.; García-López, J.D.; Núñez, C.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Biocontrol of Listeria monocytogenes in fish by enterocin as-48 and listeria lytic bacteriophage p100. LWT-Food Sci. Technol. 2016, 66, 672–677. [Google Scholar] [CrossRef]
- Vijayakumar, P.P.; Muriana, P.M. Inhibition of Listeria monocytogenes on ready-to-eat meats using bacteriocin mixtures based on mode-of-action. Foods 2017, 6, 22. [Google Scholar] [CrossRef]
- Muriana, P.M. Bacteriocins for control of Listeria spp. In Food. J. Food Prot. 1996, 59, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Abee, T.; Krockel, L.; Hill, C. Bacteriocins: Modes of action and potentials in food preservation and control of food poisoning. Int. J. Food Microbiol. 1995, 28, 169–185. [Google Scholar] [CrossRef]
- Furlaneto-Maia, L.; Ramalho, R.; Rocha, K.R.; Furlaneto, M.C. Antimicrobial activity of enterocins against Listeria sp. and other food spoilage bacteria. Biotechnol. Lett. 2020, 42, 797–806. [Google Scholar] [CrossRef]
- Figueiredo, A.C.L.; Almeida, R.C. Antibacterial efficacy of nisin, bacteriophage p100 and sodium lactate against Listeria monocytogenes in ready-to-eat sliced pork ham. Braz. J. Microbiol. 2017, 48, 724–729. [Google Scholar] [CrossRef]
- Jofre, A.; Garriga, M.; Aymerich, T. Inhibition of Listeria monocytogenes in cooked ham through active packaging with natural antimicrobials and high-pressure processing. J. Food Prot. 2007, 70, 2498–2502. [Google Scholar] [CrossRef]
- Molinos, A.C.; Abriouel, H.; López, R.L.; Omar, N.B.; Valdivia, E.; Gálvez, A. Enhanced bactericidal activity of enterocin as-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against Listeria monocytogenes in ready-to-eat salad. Food Chem. Toxicol. 2009, 47, 2216–2223. [Google Scholar] [CrossRef]
- Ruiz, A.; Williams, S.; Djeri, N.; Hinton, A., Jr.; Rodrick, G. Nisin affects the growth of Listeria monocytogenes on ready-to-eat turkey ham stored at four degrees celsius for sixty-three days. Poult. Sci. 2010, 89, 353–358. [Google Scholar] [CrossRef]
- Ananou, S.; Bouraqqadi, M.; Zouhri, N.; El Kinany, S.; Manni, L. Control of Listeria monocytogenes in a fresh cheese using aromatic and medicinal plants and enterocin: A comparative study. Lett. Appl. Microbiol. 2023, 76, ovad076. [Google Scholar] [CrossRef]
- Yousefi, M.; Khorshidian, N.; Hosseini, H. Potential application of essential oils for mitigation of Listeria monocytogenes in meat and poultry products. Front. Nutr. 2020, 7, 577287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, B.; Xiong, Y.L.; Sun, X. Antimicrobial activities of spice extracts against pathogenic and spoilage bacteria in modified atmosphere packaged fresh pork and vacuum packaged ham slices stored at 4 °C. Meat Sci. 2009, 81, 686–692. [Google Scholar] [CrossRef]
- Takahashi, H.; Kashimura, M.; Koiso, H.; Kuda, T.; Kimura, B. Use of ferulic acid as a novel candidate of growth inhibiting agent against Listeria monocytogenes in ready-to-eat food. Food Control 2013, 33, 244–248. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Ragonese, C.; Beninati, C.; Sciarrone, D.; Ziino, G.; Mondello, L.; Giuffrida, A.; Panebianco, A. Antimicrobial activity of combined thyme and rosemary essential oils against Listeria monocytogenes in Italian mortadella packaged in modified atmosphere: Thyme & rosemary EOs vs L monocytogenes. J. Essent. Oil Res. 2016, 28, 467–474. [Google Scholar] [CrossRef]
- Emma, M.-L.; Palou, E.; López-Malo, A. Biopreservatives as agents to prevent food spoilage. In Microbial Contamination and Food Degradation; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Verheyen, D.; Govaert, M.; Seow, T.K.; Ruvina, J.; Mukherjee, V.; Baka, M.; Skåra, T.; Van Impe, J.F. The complex effect of food matrix fat content on thermal inactivation of Listeria monocytogenes: Case study in emulsion and gelled emulsion model systems. Front. Microbiol. 2020, 10, 3149. [Google Scholar] [CrossRef]
- Fain, A.R., Jr.; Line, J.E.; Moran, A.B.; Martin, L.M.; Lechowich, R.V.; Carosella, J.M.; Brown, W.L. Lethality of heat to Listeria monocytogenes Scott A: D-value and Z-value determinations in ground beef and turkey. J. Food Prot. 1991, 54, 756–761. [Google Scholar] [CrossRef]
- Karina, P.; Julio, C.; Leda, G.; Noemi, Z. Behavior of Listeria monocytogenes type1 355/98 (85) in meat emulsions as affected by temperature, pH, water activity, fat and microbial preservatives. Food Control 2011, 22, 1573–1581. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Ghoush, M.A.; Al-Holy, M.; Hilal, H.A.; Al-Nabulsi, A.A.; Osaili, T.M.; Ayyash, M.; Holley, R.A. Survival and growth of Listeria monocytogenes and Staphylococcus aureus in ready-to-eat mediterranean vegetable salads: Impact of storage temperature and food matrix. Int. J. Food Microbiol. 2021, 346, 109149. [Google Scholar] [CrossRef] [PubMed]
- Polese, P.; Del Torre, M.; Venir, E.; Stecchini, M.L. A simplified modelling approach established to determine the Listeria monocytogenes behavior during processing and storage of a traditional (Italian) ready-to-eat food in accordance with the European commission regulation n 2073/2005. Food Control 2014, 36, 166–173. [Google Scholar] [CrossRef]
- Hill, C.; Cotter, P.D.; Sleator, R.D.; Gahan, C.G. Bacterial stress response in Listeria monocytogenes: Jumping the hurdles imposed by minimal processing. Int. Dairy J. 2002, 12, 273–283. [Google Scholar] [CrossRef]
- Nilsson, R.E.; Ross, T.; Bowman, J.P. Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int. J. Food Microbiol. 2011, 150, 14–24. [Google Scholar] [CrossRef]
- Karatzas, K.-A.G.; Suur, L.; O’Byrne, C.P. Characterization of the intracellular glutamate decarboxylase system: Analysis of its function, transcription, and role in the acid resistance of various strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2012, 78, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Olier, M.; Rousseaux, S.; Piveteau, P.; Lemaıtre, J.-P.; Rousset, A.; Guzzo, J. Screening of glutamate decarboxylase activity and bile salt resistance of human asymptomatic carriage, clinical, food, and environmental isolates of Listeria monocytogenes. Int. J. Food Microbiol. 2004, 93, 87–99. [Google Scholar] [CrossRef]
- Aryani, D.; Den Besten, H.; Hazeleger, W.; Zwietering, M. Quantifying strain variability in modeling growth of Listeria monocytogenes. Int. J. Food Microbiol. 2015, 208, 19–29. [Google Scholar] [CrossRef]
- Wang, X.; Tian, S.; Wu, Y.; Li, H.; Bai, L.; Liu, H.; Zhang, X.; Dong, Q. Strain variability in growth and thermal inactivation characteristics of Listeria monocytogenes strains after acid adaptation. J. Food Prot. 2021, 84, 2229–2236. [Google Scholar] [CrossRef]
- Heredia, S.M.S.; Le Marc, Y.; Martín, J.S.; Possas, A.; Jiménez, E.C.; Díaz, A.V. Inter-strain variability on the cardinal parameters (pH and aw) of clinical and food isolates of Listeria monocytogenes using turbidimetric measurements. Int. J. Food Microbiol. 2024, 411, 110521. [Google Scholar] [CrossRef]
- FAO/WHO. Hazard identification and characterization: Criteria for categorizing shiga toxin–producing Escherichia coli on a risk basis. J. Food Prot. 2019, 82, 7–21. [Google Scholar] [CrossRef]
- FAO/WHO. Shiga Toxin-Producing Escherichia coli (STEC) and food: Attribution, Characterization, and Monitoring; WHO: Rome, Italy, 2018. [Google Scholar]
- Eustice, R.F.; Bruhn, C.M. Consumer acceptance and marketing of irradiated foods. In Food Irradiation Research and Technology; Wiley: Hoboken, NJ, USA, 2012; pp. 173–195. [Google Scholar]
- Maherani, B.; Hossain, F.; Criado, P.; Ben-Fadhel, Y.; Salmieri, S.; Lacroix, M. World market development and consumer acceptance of irradiation technology. Foods 2016, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Bodie, A.R.; Wythe, L.A.; Dittoe, D.K.; Rothrock, M.J., Jr.; O’Bryan, C.A.; Ricke, S.C. Alternative additives for organic and natural ready-to-eat meats to control spoilage and maintain shelf life: Current perspectives in the United States. Foods 2024, 13, 464. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D. Cost analysis and environmental impact of pulsed electric fields and high pressure processing in comparison with thermal pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.; Castro, S.M.; Saraiva, J.A.; Teixeira, P. Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model-high pressure processing assisted by bacteriophage P100 and bacteriocinogenic pediococcus acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef] [PubMed]
- Bleoancă, I.; Saje, K.; Mihalcea, L.; Oniciuc, E.-A.; Smole-Mozina, S.; Nicolau, A.I.; Borda, D. Contribution of high pressure and thyme extract to control Listeria monocytogenes in fresh cheese-a hurdle approach. Innov. Food Sci. Emerg. Technol. 2016, 38, 7–14. [Google Scholar] [CrossRef]
- Australian Meat Industry Council. Guidelines for the Safe Manufacture of Smallgoods, 3rd ed.; Meat & Livestock Australia: North Sydney, Australia, 2025. [Google Scholar]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.S.; de Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-pressure processing of meat: Molecular impacts and industrial applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. [Google Scholar] [CrossRef]
- Chhabra, A.; Carter, W.; Linton, R.; Cousin, M. A Predictive model to determine the effects of ph, milkfat, and temperature on thermal inactivation of Listeria monocytogenes. J. Food Prot. 1999, 62, 1143–1149. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Carrasco, E.; Bover-Cid, S.; Jofré, A.; Valero, A. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready-to-eat (RTE) foods: Activity 2, a quantitative risk characterization on L. monocytogenes in RTE foods; starting from the retail stage. EFSA Support. Publ. 2017, 14, 1252E. [Google Scholar] [CrossRef]
- Foerster, C.; Figueroa, G.; Evers, E. Risk assessment of Listeria monocytogenes in poultry and beef. Br. Food J. 2015, 117, 779–792. [Google Scholar] [CrossRef]
- Possas, A.; Valdramidis, V.; García-Gimeno, R.M.; Pérez-Rodríguez, F. High hydrostatic pressure processing of sliced fermented sausages: A quantitative exposure assessment for Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2019, 52, 406–419. [Google Scholar] [CrossRef]
- Mejlholm, O.; Dalgaard, P. Development and validation of an extensive growth and growth boundary model for Listeria monocytogenes in lightly preserved and ready-to-eat shrimp. J. Food Prot. 2009, 72, 2132–2143. [Google Scholar] [CrossRef]
- Mejlholm, O.; Bøknæs, N.; Dalgaard, P. Development and validation of a stochastic model for potential growth of Listeria monocytogenes in naturally contaminated lightly preserved seafood. Food Microbiol. 2015, 45, 276–289. [Google Scholar] [CrossRef]
- Mejlholm, O.; Gunvig, A.; Borggaard, C.; Blom-Hanssen, J.; Mellefont, L.; Ross, T.; Leroi, F.; Else, T.; Visser, D.; Dalgaard, P. predicting growth rates and growth boundary of Listeria monocytogenes—an international validation study with focus on processed and ready-to-eat meat and seafood. Int. J. Food Microbiol. 2010, 141, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Mejlholm, O.; Dalgaard, P. Modelling and predicting the simultaneous growth of Listeria monocytogenes and psychrotolerant lactic acid bacteria in processed seafood and mayonnaise-based seafood salads. Food Microbiol. 2015, 46, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Orsi, R.H.; Guariglia-Oropeza, V.; Wiedmann, M. Development of a modeling tool to assess and reduce regulatory and recall risks for cold-smoked salmon due to Listeria monocytogenes contamination. J. Food Prot. 2022, 85, 1335–1354. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).