Members of Velvet Complex FpVeA and FpVelB Regulate Asexual Development, Fumonisin Biosynthesis and Virulence in Fusarium proliferatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media and Culture Conditions
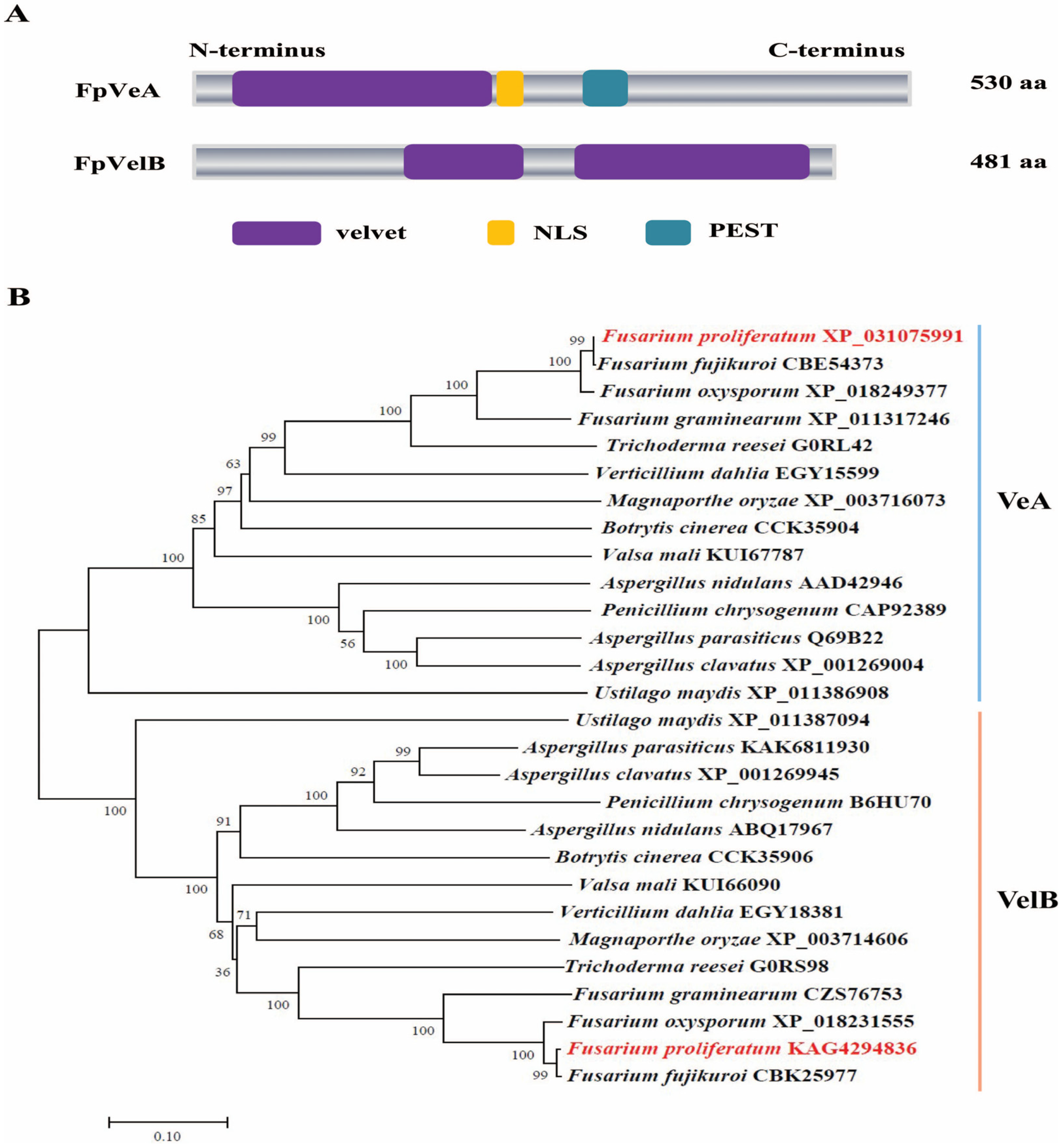
2.2. Characterization and Phylogenetic Analysis of Proteins
2.3. Gene Deletion and Complementation
2.4. Trehalose Assay
2.5. Measurement of Carotenoid Content
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Stress Susceptibility Test
2.8. Determination of FB1 Production
2.9. Pathogenicity and Penetration Assay
2.10. Statistical Analysis
3. Results
3.1. Identification of FpveA and FpvelB in F. proliferatum
3.2. FpVeA and FpVelB Are Involved in Vegetative Growth
3.3. FpVeA and FpVelB Negatively Regulate Asexual Sporulation
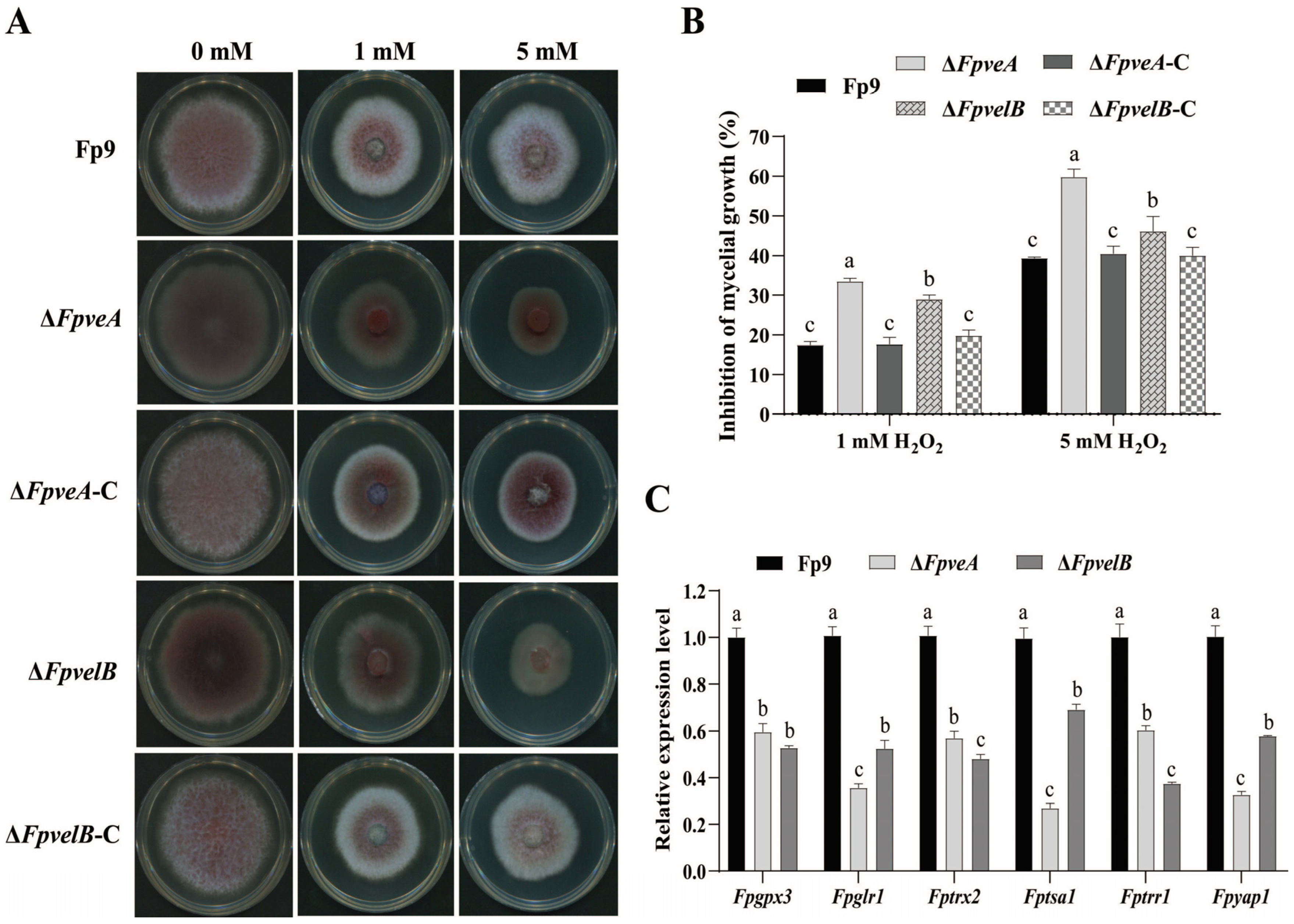
3.4. FpVeA and FpVelB Are Responsible for Tolerance to Oxidative Stress
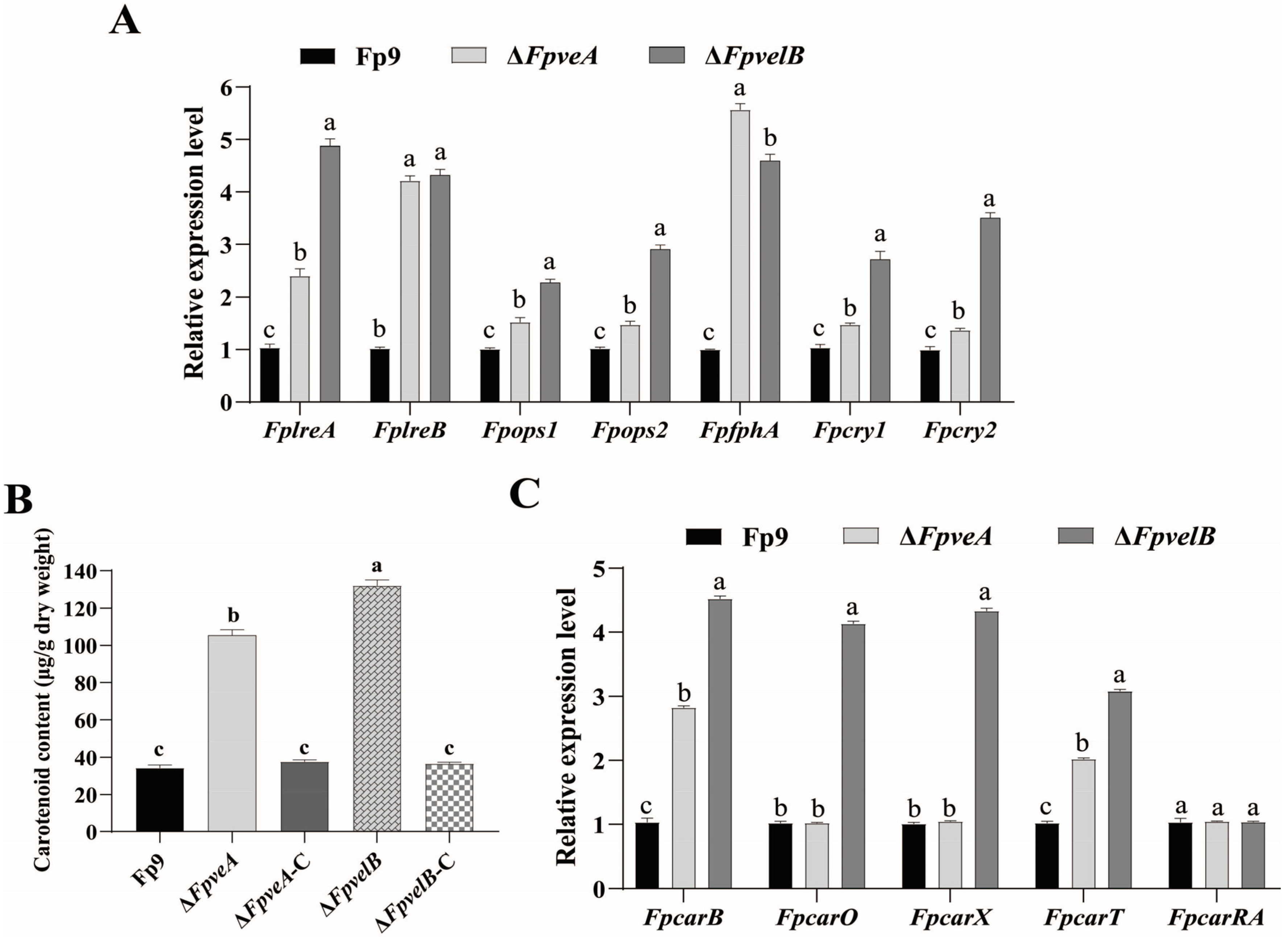
3.5. FpVeA and FpVelB Affect Light Perception and Carotenoid Accumulation
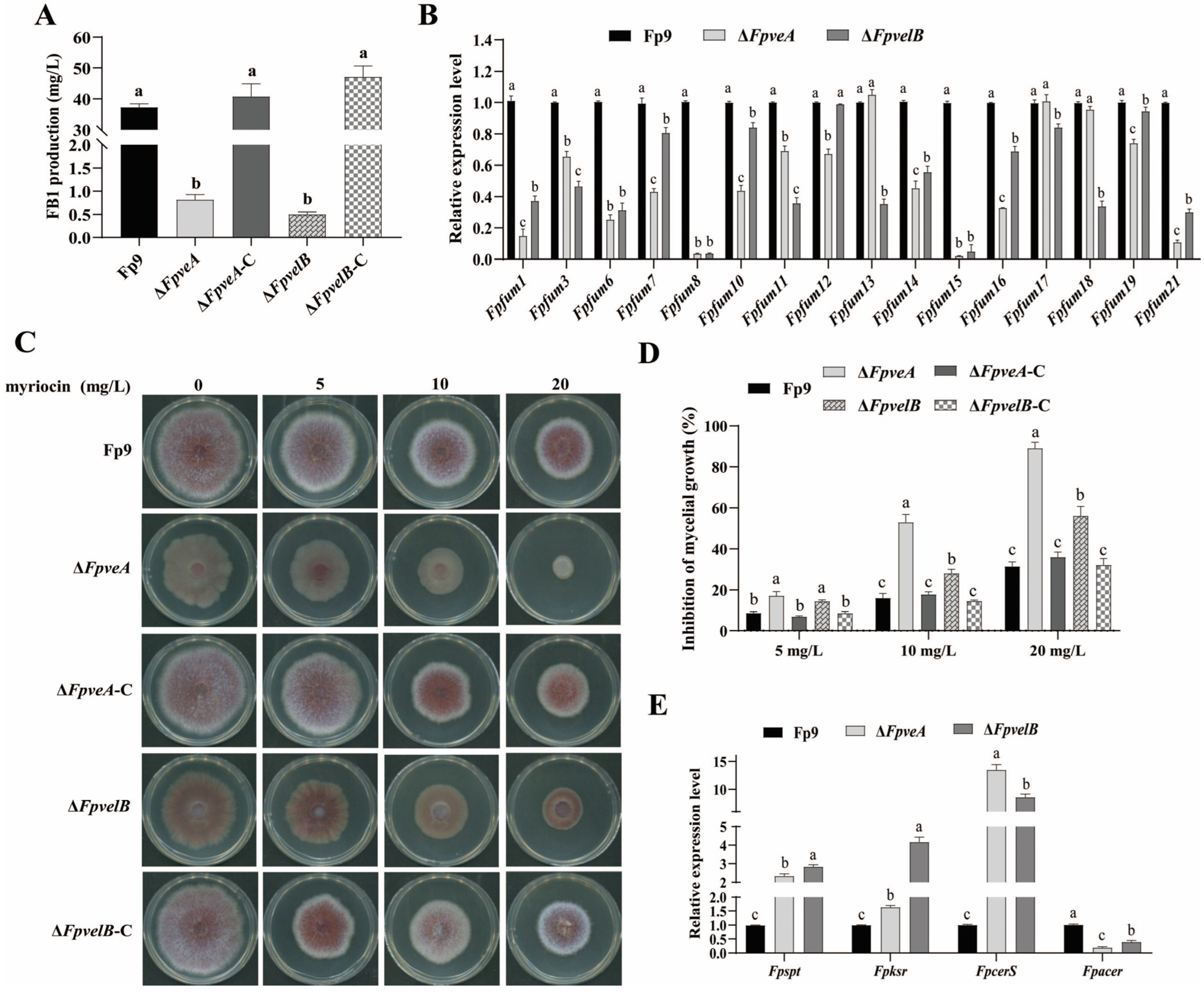
3.6. FpVeA and FpVelB Contribute to Fumonisin Biosynthesis
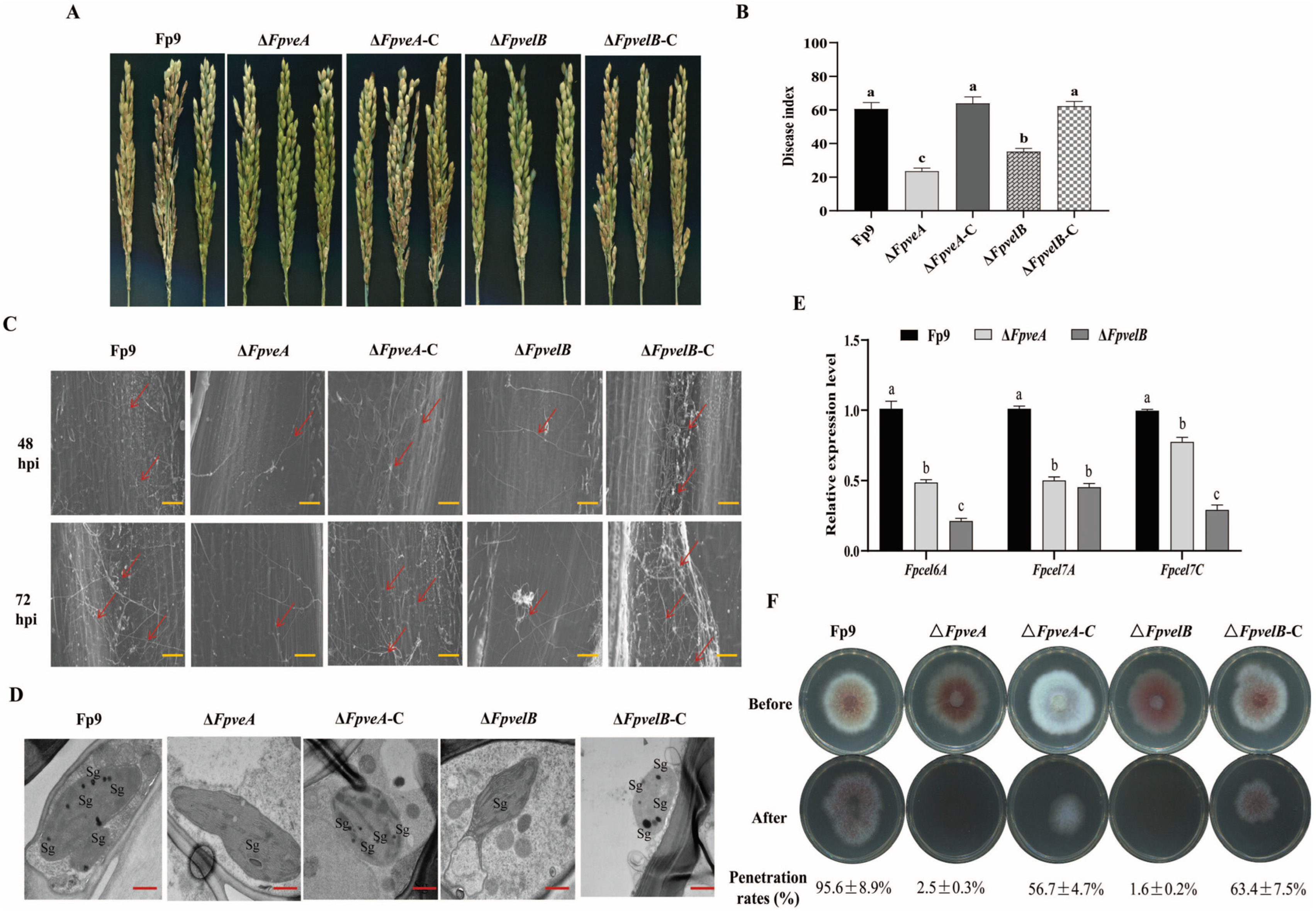
3.7. FpVeA and FpVelB Are Required for Full Virulence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.H.; Lee, S.; Nah, J.Y.; Kim, H.K.; Paek, J.S.; Lee, S.; Ham, H.; Hong, S.K.; Yun, S.H.; Lee, T. Species composition of and fumonisin production by the Fusarium fujikuroi species complex isolated from Korean cereals. Int. J. Food Microbiol. 2018, 267, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Anumudu, C.K.; Ekwueme, C.T.; Uhegwu, C.C.; Ejileugha, C.; Augustine, J.; Okolo, C.A.; Onyeaka, H. A review of the mycotoxin family of fumonisins, their biosynthesis, metabolism, methods of detection and effects on humans and animals. Int. J. Mol. Sci. 2024, 26, 184. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Chen, C.; Riley, R.T.; Wu, F. Dietary fumonisin and growth impairment in children and animals: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1448–1464. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. IARC Monog. Eval. Carc. 2010, 95, 9–38. [Google Scholar]
- Zentai, A.; Szeitzné-Szabó, M.; Mihucz, G.; Szeli, N.; Szabó, A.; Kovács, M. Occurrence and risk assessment of fumonisin B1 and B2 mycotoxins in maize-based food products in Hungary. Toxins 2019, 11, 709. [Google Scholar] [CrossRef]
- Chen, W.; Son, Y.E.; Cho, H.J.; Choi, D.; Park, H.S.; Yu, J.H. Phylogenomics analysis of velvet regulators in the fungal kingdom. Microbiol. Spectr. 2024, 12, e03717-23. [Google Scholar] [CrossRef]
- Bayram, Ö.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.; Keller, N.P.; Yu, J.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef]
- Bayram, Ö.; Braus, G.H. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya-Bayram, Ö.; Palmer, J.M.; Keller, N.; Braus, G.H.; Bayram, Ö. One Juliet and four Romeos: VeA and its methyltransferases. Front. Microbiol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Röhrig, J.; Yu, Z.; Chae, K.S.; Kim, J.H.; Han, K.H.; Fischer, R. The Aspergillus nidulans Velvet-interacting protein, VipA, is involved in light-stimulated heme biosynthesis. Mol. Microbiol. 2017, 105, 825–838. [Google Scholar] [CrossRef]
- Eom, T.J.; Moon, H.; Yu, J.H.; Park, H.S. Characterization of the velvet regulators in Aspergillus flavus. J. Microbiol. 2018, 56, 893–901. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Wang, Y.; Liu, F.; Li, E.; Ma, J.; Yang, B.; Zhang, C.; Li, L.; Liu, Y. Requirement of LaeA, VeA, and VelB on asexual development, ochratoxin A biosynthesis, and fungal virulence in Aspergillus ochraceus. Front. Microbiol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Brown, D.W.; Kleigrewe, K.; Bok, J.W.; Keller, N.P.; Humpf, H.U.; Tudzynski, B. FfVel1 and FfLae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol. Microbiol. 2010, 77, 972–994. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Hera, C.; Sulyok, M.; Schäfer, K.; Capilla, J.; Guarro, J.; Di Pietro, A. The velvet complex governs mycotoxin production and virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Microbiol. 2013, 87, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Myong, K.; Kim, J.E.; Kim, H.K.; Yun, S.H.; Lee, Y.W. FgVelB globally regulates sexual reproduction, mycotoxin production and pathogenicity in the cereal pathogen Fusarium graminearum. Microbiology 2012, 158, 1723–1733. [Google Scholar] [CrossRef]
- Merhej, J.; Urban, M.; Dufresne, M.; Hammond-Kosack, K.E.; Richard-Forget, F.; Barreau, C. The velvet gene, FgVe1, affects fungal development and positively regulates trichothecene biosynthesis and pathogenicity in Fusarium graminearum. Mol. Plant Pathol. 2012, 13, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F. Key role of LaeA and velvet complex proteins on expression of β-lactam and PR-toxin genes in Penicillium chrysogenum: Cross-talk regulation of secondary metabolite pathways. J. Ind. Microbiol. Biotechnol. 2017, 44, 525–535. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, L.; Yin, Z.; Dai, Q.; Gao, X.; Feng, H.; Voegele, R.T.; Huang, L. Two members of the velvet family, VmVeA and VmVelB, affect conidiation, virulence and pectinase expression in Valsa mali. Mol. Plant Pathol. 2018, 19, 1639–1651. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Ge, S.L.; Liang, W.H.; Liao, W.Y.; Li, W.; Jiao, G.; Wei, X.J.; Shao, G.N.; Xie, L.H.; et al. Genomic footprints related with adaptation and fumonisins production in Fusarium proliferatum. Front. Microbiol. 2022, 13, 1004454. [Google Scholar] [CrossRef]
- Wang, L.; Ge, S.; Liang, W.; Liao, W.; Li, W.; Jiao, G.; Wei, X.; Shao, G.; Xie, L.; Sheng, Z.; et al. Genome-wide characterization reveals variation potentially involved in pathogenicity and mycotoxins biosynthesis of Fusarium proliferatum causing spikelet rot disease in rice. Toxins 2022, 14, 568. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular evolutionary genetic analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Ge, S.; Sheng, Z.; Hu, S.; Jiao, G.; Shao, G.; Xie, L.; Tang, S.; Hu, P. The role of FpfetC from Fusarium proliferatum in iron acquisition, fumonisin B1 production, and virulence. Int. J. Mol. Sci. 2025, 26, 2883. [Google Scholar] [CrossRef]
- Yu, J.H.; Hamari, Z.; Han, K.H.; Seo, J.A.; Reyes-Domínguez, Y.; Scazzocchio, C. Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 2004, 41, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Yu, J.H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS ONE 2007, 2, e970. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cendoya, E.; Nichea, M.J.; Monge, M.P.; Sulyok, M.; Chiacchiera, S.M.; Ramirez, M.L. Fumonisin occurrence in wheat-based products from Argentina. Food Addit. Contam. Part B Surveill. 2019, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.W.; Wang, L.; Liu, L.M.; Tang, S.Q.; Zhu, D.F.; Savary, S. Rice spikelet rot disease in China–2. Pathogenicity tests, assessment of the importance of the disease, and preliminary evaluation of control options. Crop Prot. 2011, 30, 10–17. [Google Scholar] [CrossRef]
- Ruger-Herreros, M.; Nordzieke, S.; Vega-Álvarez, C.; Avalos, J.; Limón, M.C. Relation between CarS expression and activation of carotenogenesis by stress in Fusarium fujikuroi. Front. Bioeng. Biotechnol. 2022, 10, 1000129. [Google Scholar] [CrossRef]
- Hou, X.; Liu, L.; Xu, D.; Lai, D.; Zhou, L. Involvement of LaeA and Velvet proteins in regulating the production of mycotoxins and other fungal secondary metabolites. J. Fungi 2024, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.E.; Yu, J.H.; Park, H.S. Regulators of the asexual life cycle of Aspergillus nidulans. Cells 2023, 12, 1544. [Google Scholar] [CrossRef]
- Dhingra, S.; Andes, D.; Calvo, A.M. VeA regulates conidiation, gliotoxin production, and protease activity in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1531–1543. [Google Scholar] [CrossRef]
- Park, H.S.; Bayram, Ö.; Braus, G.H.; Kim, S.C.; Yu, J.H. Characterization of the velvet regulators in Aspergillus fumigatus. Mol. Microbiol. 2012, 86, 937–953. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Y.; Ma, Z. Involvement of BcVeA and BcVelB in regulating conidiation, pigmentation and virulence in Botrytis cinerea. Fungal Genet. Biol. 2013, 50, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Leng, Y.; Shrestha, S.; Zhong, S. Coordinated and independent functions of velvet-complex genes in fungal development and virulence of the fungal cereal pathogen Cochliobolus sativus. Fungal Biol. 2016, 120, 948–960. [Google Scholar] [CrossRef]
- Cea-Sánchez, S.; Martín-Villanueva, S.; Gutiérrez, G.; Cánovas, D.; Corrochano, L.M. VE-1 regulation of MAPK signaling controls sexual development in Neurospora crassa. mBio 2024, 15, e0226424. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K.; Scharfenstein, L.L.; Li, P.; Ehrlich, K.C. Aspergillus flavus VelB acts distinctly from VeA in conidiation and may coordinate with FluG to modulate sclerotial production. Fungal Genet. Biol. 2013, 58–59, 71–79. [Google Scholar] [CrossRef]
- Calvo, A.M.; Bok, J.; Brooks, W.; Keller, N.P. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 2004, 70, 4733–4739. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, J.H.; Kim, K.S.; Lee, Y.H. Comparative functional analysis of the velvet gene family reveals unique roles in fungal development and pathogenicity in Magnaporthe oryzae. Fungal Genet. Biol. 2014, 66, 33–43. [Google Scholar] [CrossRef]
- Liu, K.; Dong, Y.; Wang, F.; Jiang, B.; Wang, M.; Fang, X. Regulation of cellulase expression, sporulation, and morphogenesis by velvet family proteins in Trichoderma reesei. Appl. Microbiol. Biotechnol. 2016, 100, 769–779. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Yin, Y.; Ma, Z. Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum. PLoS ONE 2011, 6, e28291. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yun, Y.; Liu, Y.; Ma, Z. FgVELB is associated with vegetative differentiation, secondary metabolism and virulence in Fusarium graminearum. Fungal Genet. Biol. 2012, 49, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Brooks, W.; Calvo, A.M. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell 2003, 2, 1178–1186. [Google Scholar] [CrossRef]
- Park, H.S.; Yu, J.H. Velvet regulators in Aspergillus spp. Microbiol. Biotechnol. Lett. 2017, 44, 409–419. [Google Scholar]
- Thammahong, A.; Puttikamonkul, S.; Perfect, J.R.; Brennan, R.G.; Cramer, R.A. Central role of the trehalose biosynthesis pathway in the pathogenesis of human fungal infections: Opportunities and challenges for therapeutic development. Microbiol. Mol. Biol. Rev. 2017, 81, e00053-16. [Google Scholar] [CrossRef]
- Park, H.S.; Ni, M.; Jeong, K.C.; Kim, Y.H.; Yu, J.H. The role, interaction and regulation of the velvet regulator VelB in Aspergillus nidulans. PLoS ONE 2012, 7, e45935. [Google Scholar] [CrossRef]
- Wu, M.Y.; Mead, M.E.; Lee, M.K.; Neuhaus, G.F.; Adpressa, D.A.; Martien, J.I.; Son, Y.E.; Moon, H.; Amador-Noguez, D.; Han, K.H.; et al. Transcriptomic, protein-DNA interaction, and metabolomic studies of VosA, VelB, and WetA in Aspergillus nidulans asexual spores. mBio 2021, 12, e03128-20. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Rodrigues-Pousada, C.; Devaux, F.; Caetano, S.M.; Pimentel, C.; da Silva, S.; Cordeiro, A.C.; Amaral, C. Yeast AP-1 like transcription factors (Yap) and stress response: A current overview. Microb. Cell 2019, 6, 267–285. [Google Scholar] [CrossRef]
- Wu, D.; Oide, S.; Zhang, N.; Choi, M.Y.; Turgeon, B.G. ChLae1 and ChVel1 regulate T-toxin production, virulence, oxidative stress response, and development of the maize pathogen Cochliobolus heterostrophus. PLoS Pathog. 2012, 8, e1002542. [Google Scholar] [CrossRef]
- Gao, J.X.; Yu, C.J.; Wang, M.; Sun, J.N.; Li, Y.Q.; Chen, J. Involvement of a velvet protein ClVelB in the regulation of vegetative differentiation, oxidative stress response, secondary metabolism, and virulence in Curvularia lunata. Sci. Rep. 2017, 7, 46054. [Google Scholar] [CrossRef]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, L.M. Light in the fungal world: From photoreception to gene transcription and beyond. Ann. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Purschwitz, J.; Müller, S.; Fischer, R. Mapping the interaction sites of Aspergillus nidulans phytochrome FphA with the global regulator VeA and the White Collar protein LreB. Mol. Genet. Genom. 2009, 281, 35–42. [Google Scholar] [CrossRef]
- Igbalajobi, O.; Yu, Z.; Fischer, R. Red- and blue-light sensing in the plant pathogen Alternaria alternata depends on phytochrome and the white-collar protein LreA. mBio 2019, 10, e00371-19. [Google Scholar] [CrossRef]
- Castrillo, M.; Avalos, J. The flavoproteins CryD and VvdA cooperate with the white collar protein WcoA in the control of photocarotenogenesis in Fusarium fujikuroi. PLoS ONE 2015, 10, e0119785. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodríguez-Ortiz, R.; Hornero-Méndez, D.; Limón, M.C. Carotenoid Biosynthesis in Fusarium. J. Fungi 2017, 3, 39. [Google Scholar] [CrossRef]
- Bayram, Ö.S.; Dettmann, A.; Karahoda, B.; Moloney, N.M.; Ormsby, T.; McGowan, J.; Cea-Sánchez, S.; Miralles-Durán, A.; Brancini, G.T.P.; Luque, E.M.; et al. Control of development, secondary metabolism and light-dependent carotenoid biosynthesis by the Velvet complex of Neurospora crassa. Genetics 2019, 212, 691–710. [Google Scholar] [CrossRef]
- Niehaus, E.M.; Münsterkötter, M.; Proctor, R.H.; Brown, D.W.; Sharon, A.; Idan, Y.; Oren-Young, L.; Sieber, C.M.; Novák, O.; Pěnčík, A.; et al. Comparative “Omics” of the Fusarium fujikuroi species complex highlights differences in genetic potential and metabolite synthesis. Genome Biol. Evol. 2016, 8, 3574–3599. [Google Scholar] [CrossRef]
- Jian, Q.; Li, T.; Wang, Y.; Zhang, Y.; Zhao, Z.; Zhang, X.; Gong, L.; Jiang, Y. New insights into fumonisin production and virulence of Fusarium proliferatum underlying different carbon sources. Food Res. Int. 2019, 116, 397–407. [Google Scholar] [CrossRef]
- Kohut, G.; Adám, A.L.; Fazekas, B.; Hornok, L. N-starvation stress induced FUM gene expression and fumonisin production is mediated via the HOG-type MAPK pathway in Fusarium proliferatum. Int. J. Food Microbiol. 2009, 130, 65–69. [Google Scholar] [CrossRef]
- Jiménez, M.; Mateo, J.J.; Hinojo, M.J.; Mateo, R. Sugars and amino acids as factors affecting the synthesis of fumonisins in liquid cultures by isolates of the Gibberella fujikuroi complex. Int. J. Food Microbiol. 2003, 89, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Spadaro, D.; Prelle, A.; Gullino, M.L.; Garibaldi, A. Light affects fumonisin production in strains of Fusarium fujikuroi, Fusarium proliferatum, and Fusarium verticillioides isolated from rice. Int. J. Food Microbiol. 2013, 166, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gong, L.; Wang, Y.; Chen, F.; Gupta, V.K.; Jian, Q.; Duan, X.; Jiang, Y. Proteomics analysis of Fusarium proliferatum under various initial pH during fumonisin production. J. Proteom. 2017, 164, 59–72. [Google Scholar] [CrossRef]
- Xie, L.; Yang, Q.; Wu, Y.; Xiao, J.; Qu, H.; Jiang, Y.; Li, T. Fumonisin B1 biosynthesis is associated with oxidative stress and plays an important role in Fusarium proliferatum infection on Banana fruit. J. Agric. Food Chem. 2023, 71, 5372–5381. [Google Scholar] [CrossRef]
- Cendoya, E.; Monge, M.D.P.; Chiacchiera, S.M.; Farnochi, M.C.; Ramirez, M.L. Influence of water activity and temperature on growth and fumonisin production by Fusarium proliferatum strains on irradiated wheat grains. Int. J. Food Microbiol. 2018, 266, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.A.; Susca, A.; Haidukowski, M.; Stea, G.; Cendoya, E.; Ramírez, M.L.; Chulze, S.N.; Farnochi, M.C.; Moretti, A.; Torres, A.M. Genetic variability and fumonisin production by Fusarium proliferatum isolated from durum wheat grains in Argentina. Int. J. Food Microbiol. 2015, 201, 35–41. [Google Scholar] [CrossRef]
- Singh, H.; Kaur, H.; Hunjan, M.S.; Sharma, S. Unveiling toxigenic Fusarium species causing maize ear rot: Insights into fumonisin production potential. Front. Plant Sci. 2025, 16, 1516644. [Google Scholar] [CrossRef]
- Gálvez, L.; Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł.; Palmero, D. Fusarium proliferatum—Causal agent of garlic bulb rot in Spain: Genetic variability and mycotoxin production. Food Microbiol. 2017, 67, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Zhang, H.; Hu, C.; Wang, W.; Calvo, A.M.; Harris, S.D.; Chen, S.; Li, S. Coordinated and distinct functions of velvet proteins in Fusarium verticillioides. Eukaryot. Cell 2014, 13, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Su, X.; Qu, H.; Duan, X.; Jiang, Y. Biosynthesis, regulation, and biological significance of fumonisins in fungi: Current status and prospects. Crit. Rev. Microbiol. 2022, 48, 450–462. [Google Scholar] [CrossRef]
- Riley, R.T.; Merrill, A.H. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Rashidfarrokhi, A.; Schmidt, F.I.; Freinkman, E.; Dougan, S.; Dougan, M.; Esteban, A.; Maruyama, T.; Strijbis, K.; Ploegh, H.L. Disruption of sphingolipid biosynthesis blocks phagocytosis of Candida albicans. PLoS Pathog. 2015, 11, e1005188. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Sun, L.; Chen, X.; Gao, J.; Zhao, Y.; Liu, L.M.; Hou, Y.X.; Wang, L.; Huang, S.W. Effects of disruption of five FUM genes on fumonisin biosynthesis and pathogenicity in Fusarium proliferatum. Toxins 2019, 11, 327. [Google Scholar] [CrossRef]
- Myung, K.; Zitomer, N.C.; Duvall, M.; Glenn, A.E.; Riley, R.T.; Calvo, A.M. The conserved global regulator VeA is necessary for symptom production and mycotoxin synthesis in maize seedlings by Fusarium verticillioides. Plant Pathol. 2012, 61, 152–160. [Google Scholar] [CrossRef]
- El Hajj Assaf, C.; Snini, S.P.; Tadrist, S.; Bailly, S.; Naylies, C.; Oswald, I.P.; Lorber, S.; Puel, O. Impact of veA on the development, aggressiveness, dissemination and secondary metabolism of Penicillium expansum. Mol. Plant Pathol. 2018, 19, 1971–1983. [Google Scholar] [CrossRef]
- Tahtah, N.; Zetina-Serrano, C.; Rocher, O.; Naylies, C.; Lippi, Y.; El Khoury, A.; Atoui, A.; Jamin, E.L.; Oswald, I.P.; Lorber, S.; et al. Implication of VelB in the development, pathogenicity, and secondary metabolism of Penicillium expansum. Postharvest Biol. Technol. 2023, 195, 112121. [Google Scholar] [CrossRef]
- Schumacher, J.; Pradier, J.M.; Simon, A.; Traeger, S.; Moraga, J.; Collado, I.G.; Viaud, M.; Tudzynski, B. Natural variation in the VELVET gene bcvel1 affects virulence and light-dependent differentiation in Botrytis cinerea. PLoS ONE 2012, 7, e47840. [Google Scholar] [CrossRef]
- Chettri, P.; Calvo, A.M.; Cary, J.W.; Dhingra, S.; Guo, Y.; McDougal, R.L.; Bradshaw, R.E. The veA gene of the pine needle pathogen Dothistroma septosporum regulates sporulation and secondary metabolism. Fungal Genet. Biol. 2012, 49, 141–151. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Liu, L.M.; Hou, Y.X.; Xu, Y.Y.; Liang, M.Q.; Gao, J.; Li, Q.Q.; Huang, S.W. Infection and colonization of pathogenic fungus Fusarium proliferatum in rice spikelet rot disease. Rice Sci. 2019, 26, 60–68. [Google Scholar] [CrossRef]
- Karakkat, B.B.; Gold, S.E.; Covert, S.F. Two members of the Ustilago maydis velvet family influence teliospore development and virulence on maize seedlings. Fungal Genet. Biol. 2013, 61, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Leroch, M.; Schumacher, J.; Zimmer, D.; Könnel, A.; Klug, K.; Leisen, T.; Scheuring, D.; Sommer, F.; Mühlhaus, T.; et al. Investigations on VELVET regulatory mutants confirm the role of host tissue acidification and secretion of proteins in the pathogenesis of Botrytis cinerea. New Phytol. 2018, 219, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, V.; Vélëz, H.; Tzelepis, G. The role of glycoside hydrolases in phytopathogenic fungi and Oomycetes virulence. Int. J. Mol. Sci. 2021, 22, 9359. [Google Scholar] [CrossRef]



| LOD [μg/L] | LOQ [μg/L] | Linear Range [μg/L] | Regression Coefficient (r2) | Recovery, Low (μg/L) | Recovery, Medium (μg/L) | Recovery, High (μg/L) | Intra-Day Repeatability | Inter-Day Repeatability | SSE |
|---|---|---|---|---|---|---|---|---|---|
| 0.513 | 2.5 | 2.5–50 | 0.999 | 81.8 ± 2.9 (5) | 97.5 ± 3.2 (10) | 108.4 ± 7.1 (25) | 14.0 | 16.2 | 116.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Tang, S.; Liao, W.; Sheng, Z.; Hu, S.; Jiao, G.; Shao, G.; Xie, L.; Hu, P. Members of Velvet Complex FpVeA and FpVelB Regulate Asexual Development, Fumonisin Biosynthesis and Virulence in Fusarium proliferatum. Foods 2025, 14, 3666. https://doi.org/10.3390/foods14213666
Wang L, Tang S, Liao W, Sheng Z, Hu S, Jiao G, Shao G, Xie L, Hu P. Members of Velvet Complex FpVeA and FpVelB Regulate Asexual Development, Fumonisin Biosynthesis and Virulence in Fusarium proliferatum. Foods. 2025; 14(21):3666. https://doi.org/10.3390/foods14213666
Chicago/Turabian StyleWang, Ling, Shaoqing Tang, Weiyang Liao, Zhonghua Sheng, Shikai Hu, Gui’ai Jiao, Gaoneng Shao, Lihong Xie, and Peisong Hu. 2025. "Members of Velvet Complex FpVeA and FpVelB Regulate Asexual Development, Fumonisin Biosynthesis and Virulence in Fusarium proliferatum" Foods 14, no. 21: 3666. https://doi.org/10.3390/foods14213666
APA StyleWang, L., Tang, S., Liao, W., Sheng, Z., Hu, S., Jiao, G., Shao, G., Xie, L., & Hu, P. (2025). Members of Velvet Complex FpVeA and FpVelB Regulate Asexual Development, Fumonisin Biosynthesis and Virulence in Fusarium proliferatum. Foods, 14(21), 3666. https://doi.org/10.3390/foods14213666







