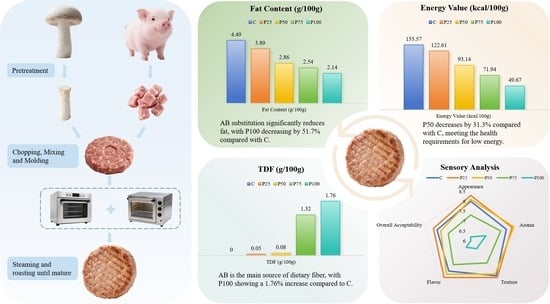
Physicochemical and Sensory Properties of Pork Patties with Partial Replacement of Lean Pork by Stalks of Agaricus bisporus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
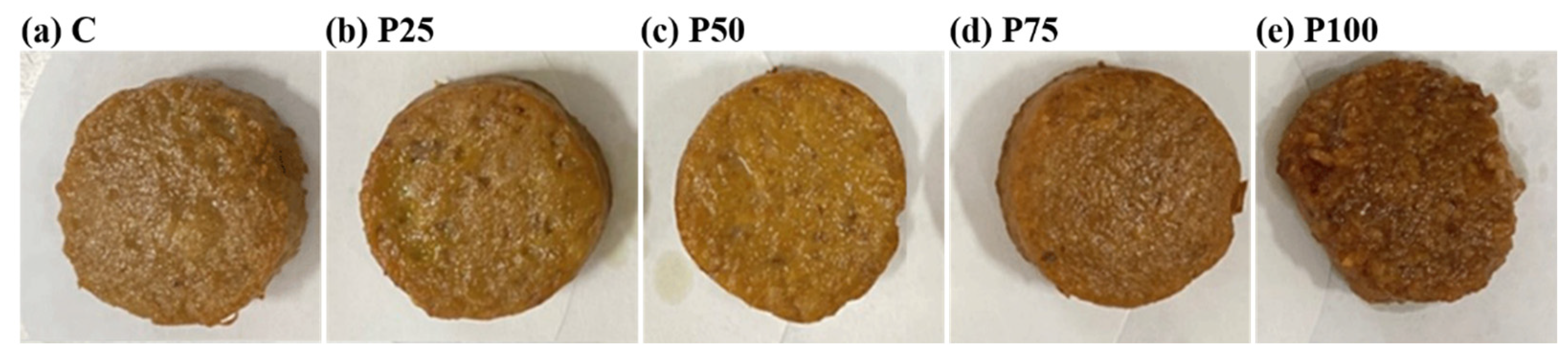
2.2. Preparation of Pork Patties
2.3. Analysis of Proximate Composition and Energy Value
2.4. Determination of Water Activity and pH
2.5. Determination of Cooking Loss and WHC
2.6. Color Measurement
2.7. Texture Profile Analysis (TPA)
2.8. Determination of Amino Acid Content
2.9. Electronic-Nose (E-Nose)
2.10. Volatile Compound
2.11. Sensory Characteristics
2.12. Determination of Microbiological Quality
2.13. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition and Energy Value Analysis
3.2. Water Activity and pH
3.3. Cooking Loss and WHC
3.4. TPA
3.5. Color Characteristics
3.6. Amino Acid Profile
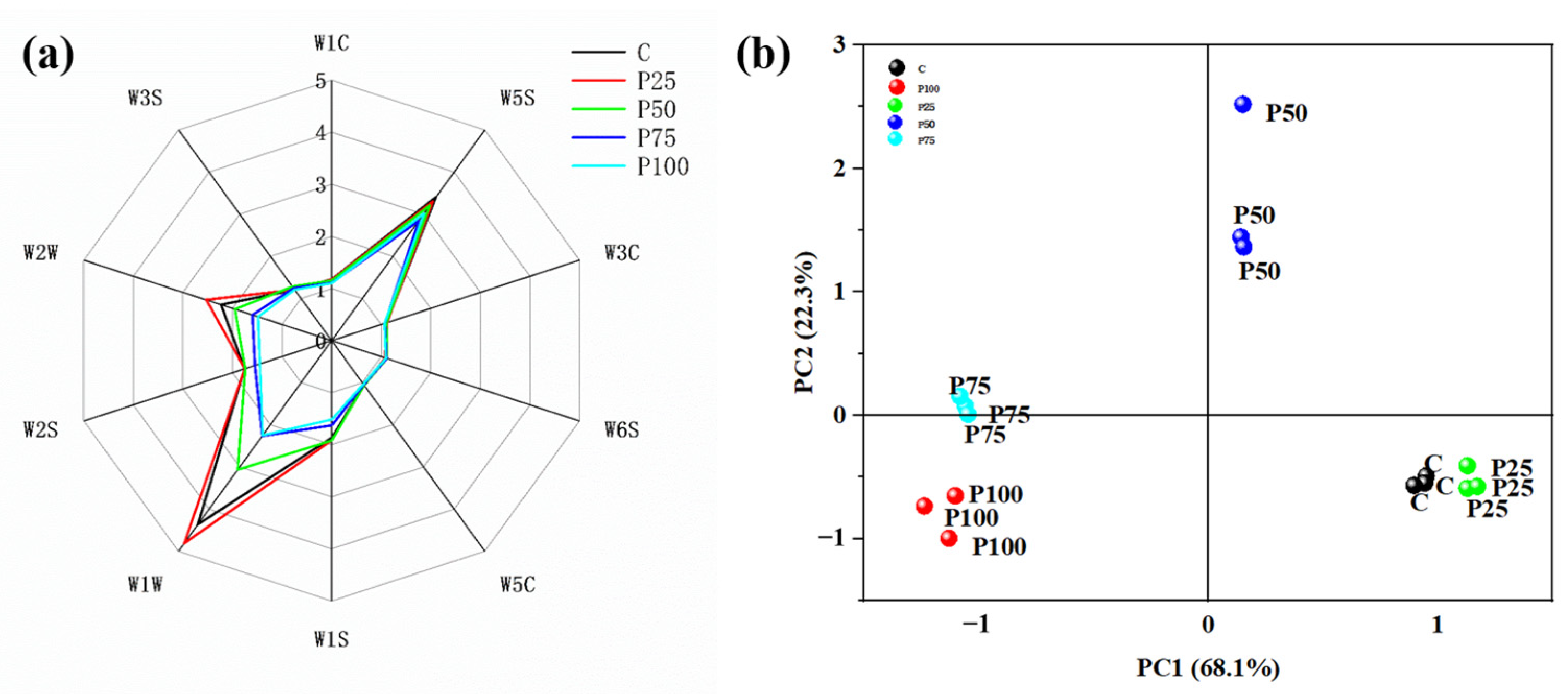
3.7. E-Nose Analysis
3.8. Volatile Compound Analysis
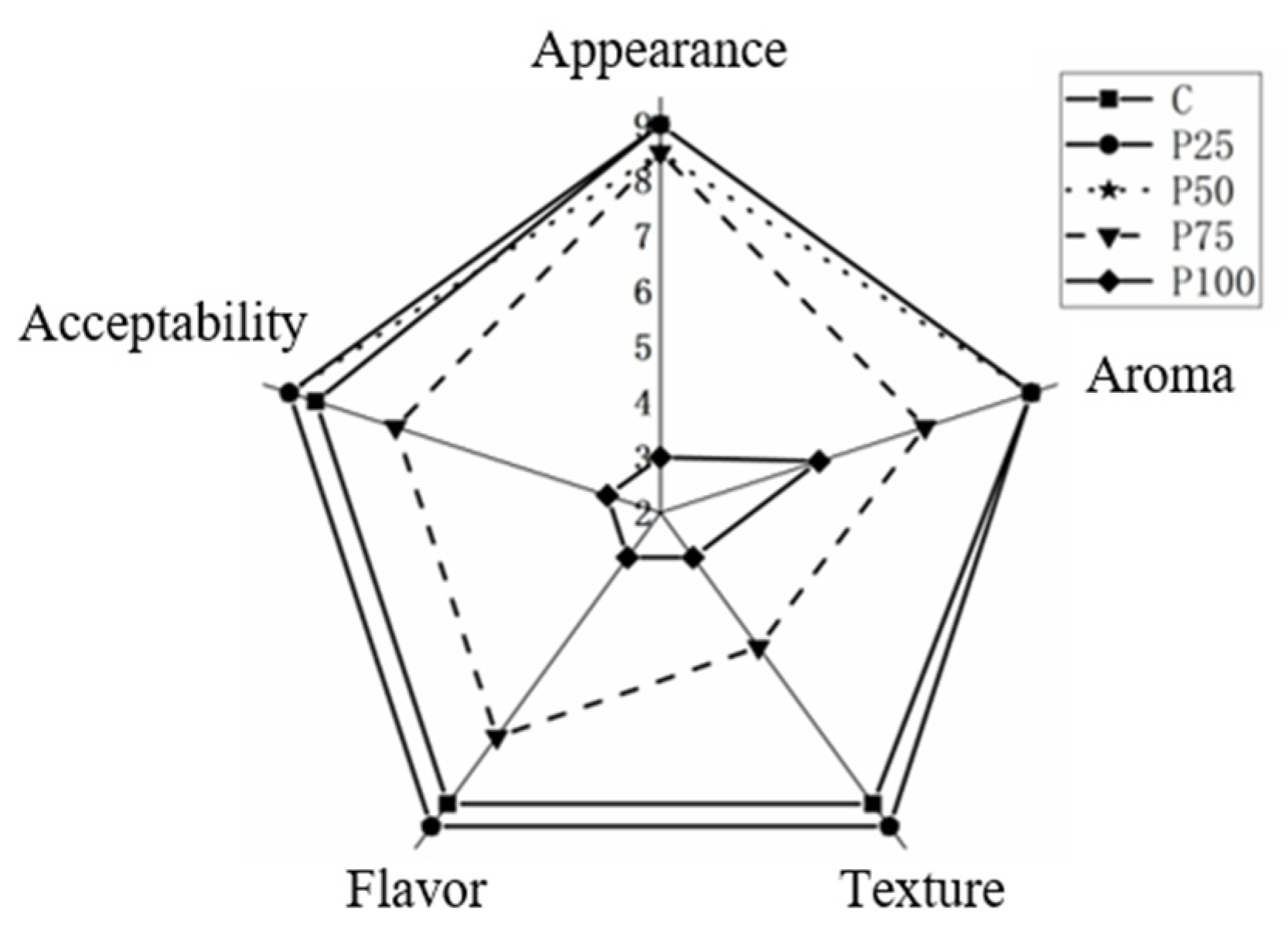
3.9. Sensory Analysis
3.10. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Li, X.; Sun, S.; Wang, Y.; Li, Z.; Kang, H.; Peng, X. Effects of carboxymethyl chitosan on the oxidation stability and gel properties of myofibrillar protein from frozen pork patties. Int. J. Biol. Macromol. 2023, 234, 123710. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Kovačević, D.B.; Putnik, P.; Mrkonjić, Ž.; Đurović, S.; Jokanović, M.; Ivić, M.; Škaljac, S.; et al. Supercritical extracts of wild thyme (Thymus serpyllum L.) by-product as natural antioxidants in ground pork patties. LWT 2020, 130, 109661. [Google Scholar] [CrossRef]
- Rosli, W.W.; Solihah, M.A. Nutritional composition and sensory properties of oyster mushroom-based patties packed with biodegradable packaging. Sains Malays 2014, 43, 65–71. [Google Scholar]
- Grummon, A.H.; Musicus, A.A.; Salvia, M.G.; Thorndike, A.N.; Rimm, E.B. Impact of Health, Environmental, and Animal Welfare Messages Discouraging Red Meat Consumption: An Online Randomized Experiment. J. Acad. Nutr. Diet. 2023, 123, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, M.; Sivakumar, N. Fungi: A Potential Future Meat Substitute. In Fungi in Sustainable Food Production; Springer International Publishing: Cham, Switzerland, 2021; pp. 181–195. [Google Scholar] [CrossRef]
- Naska, A.; Ververis, E.; Niforou, A.; Pires, S.M.; Poulsen, M.; Jakobsen, L.S.; Becker, N.; Lohmann, M.; Tessom, V.; Federighi, M.; et al. Novel foods as red meat replacers–an insight using Risk Benefit Assessment methods (the NovRBA project). EFSA Support. Publ. 2022, 19, 7316E. [Google Scholar] [CrossRef]
- Boehm, E.; Borzekowski, D.; Ververis, E.; Lohmann, M.; Böl, G.F. Communicating food risk-benefit assessments: Edible insects as red meat replacers. Front. Nutr. 2021, 8, 749696. [Google Scholar] [CrossRef]
- Pintado, T.; Delgado-Pando, G. Towards more sustainable meat products: Extenders as a way of reducing meat content. Foods 2020, 9, 1044. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Liu, X.; Jiang, G.; Li, C.; Li, X.; Li, Y. Roles of Lentinula edodes as the pork lean meat replacer in production of the sausage. Meat Sci. 2019, 156, 44–51. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary nutrients in edible mushroom, Agaricus bisporus and their radical scavenging, antibacterial, and antifungal effects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food proteins from plants and fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Caroline, A.; Jiménez-Quero, A.; Martínez-Abad, A.; Vilaplana, F.; et al. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible Mushrooms as a Natural Source of Food Ingredient/Additive Replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef]
- Singh, U.; Tiwari, P.; Kelkar, S.; Kaul, D.; Tiwari, A.; Kapri, M.; Sharma, S. Edible mushrooms: A sustainable novel ingredient for meat analogs. EFood 2023, 4, e122. [Google Scholar] [CrossRef]
- Vargas-Sanchez, R.D.; Torres-Martinez, B.D.M.; Huerta-Leidenz, N.; Ibarra-Arias, F.J.; Fernandez-Lopez, J.; Torrescano-Urrutia, G.R.; Perez-Alvarez, J.A.; Sanchez-Escalante, A. Antioxidant and Antibacterial Effect of Agaricus brasiliensis Extract on Raw and Cooked Pork Patties during Storage. Agriculture 2023, 13, 346. [Google Scholar] [CrossRef]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar] [PubMed]
- Reis, G.C.; Guidi, L.R.; Fernandes, C.; Godoy, H.T.; Gloria, M.B.A. UPLC-UV method for the quantification of free amino acids, bioactive amines, and ammonia in fresh, cooked, and canned mushrooms. Food Anal. Method 2020, 13, 1613–1626. [Google Scholar] [CrossRef]
- Yin, X.; Wen, R.; Sun, F.; Wang, Y.; Kong, B.; Chen, Q. Collaborative analysis on differences in volatile compounds of Harbin red sausages smoked with different types of woodchips based on gas chromatography–mass spectrometry combined with electronic nose. LWT 2021, 143, 111144. [Google Scholar] [CrossRef]
- Jin, S.-K.; Choi, J.S.; Yang, H.-S.; Park, T.-S.; Yim, D.-G. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Noipha, S. Active film from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Baune, M.C.; Broucke, K.; Ebert, S.; Gibis, M.; Weiss, J.; Enneking, U.; Profeta, A.; Terjung, N.; Heinz, V. Meat hybrids–An assessment of sensorial aspects, consumer acceptance, and nutritional properties. Front. Nutr. 2023, 10, 1101479. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Panpipat, W.; Chaijan, M.; Shetty, K.; Rawdkuen, S. Role of plant protein on the quality and structure of meat analogs: A new perspective for vegetarian foods. Future Foods 2023, 8, 100280. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, W.; Zhou, G. Effects of glutinous rice flour on the physiochemical and sensory qualities of ground pork patties. LWT-Food Sci. Technol. 2014, 58, 135–141. [Google Scholar] [CrossRef]
- Bach, F.; Haminiuk, C.W.I.; Helm, C.V. Avaliação do Potencial Nutricional, Antioxidante e Antibacteriano de Cogumelos Comestíveis. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2017. [Google Scholar]
- Selani, M.M.; Shirado, G.A.; Margiotta, G.B.; Saldaña, E.; Spada, F.P.; Piedade, S.M.S.; Contreras-Castillo, C.J.; Canniatti-Brazaca, S.G. Effects of pineapple byproduct and canola oil as fat replacers on physicochemical and sensory qualities of low-fat beef burger. Meat Sci. 2016, 112, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Choi, J.H.; Han, D.J.; Kim, H.Y.; Lee, M.A.; Kim, H.W.; Jeong, J.Y.; Kim, C.J. Characteristics of low-fat meat emulsion systems with pork fat replaced by vegetable oils and rice bran fiber. Meat Sci. 2009, 82, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.T.; Pires, M.A.; Baldin, J.C.; Munekata, P.E.S.; de Carvalho, F.A.L.; Rodrigues, I.; Polizer, Y.J.; de Mello, J.L.M.; Lapa-Guimarães, J.; Trindade, M.A. Partial replacement of meat and fat with hydrated wheat fiber in beef burgers decreases caloric value without reducing the feeling of satiety after consumption. Meat Sci. 2019, 147, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D. The eating quality of meat: IV—Water holding capacity and juiciness. In Lawrie’s Meat Science; Woodhead Publishing: Cambridge, UK, 2023; pp. 457–508. [Google Scholar]
- Kurt, A.; Gençcelep, H. Enrichment of meat emulsion with mushroom (Agaricus bisporus) powder: Impact on rheological and structural characteristics. J. Food Eng. 2018, 237, 128–136. [Google Scholar] [CrossRef]
- Solomon, M.B.; Eastridge, J.S.; Paroczay, E.W.; Bowker, B.C. Measuring meat texture. In Handbook of Muscle Foods Analysis; CRC Press: Boca Raton, FL, USA, 2008; pp. 499–522. [Google Scholar]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the key aroma and phenolic compounds of champignon (Agaricus bisporus) and oyster (Pleurotus ostreatus) mushrooms after two cooking treatments as elucidated by GC–MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021, 354, 129576. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Xiao, J.; Liu, J.; Tang, N.; Zhou, A. Variations of volatile flavour compounds in finger citron (Citrusmedica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Li, X.; Liu, Y.; Yan, W. Effects of partial replacement of pork back fat by a camellia oil gel on certain quality characteristics of a cooked style Harbin sausage. Meat Sci. 2018, 146, 154–159. [Google Scholar] [CrossRef]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef]
- Petričević, S.; Radovčić, N.M.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Dinnella, C.; Napolitano, F.; Spinelli, S.; Monteleone, E.; Pacelli, C.; Braghieri, A. Factors affecting stated liking for meat products: Focus on demographics, oral responsiveness, personality, and psycho-attitudinal traits. Meat Sci. 2023, 195, 109004. [Google Scholar] [CrossRef]
| Ingredients (%) | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|
| Pork lean meat | 76 | 57 | 38 | 19 | 0 |
| Stalk of Agaricus bisporus | 0 | 19 | 38 | 57 | 76 |
| Additives | |||||
| Salt | 1.15 | 1.15 | 1.15 | 1.15 | 1.15 |
| Sugar | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| Monosodium glutamate | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| Sodium pyrophosphate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Sodium tripolyphosphate | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamin C | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| White pepper | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Carrageenan | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Isolated soy protein | 3 | 3 | 3 | 3 | 3 |
| Dry starch | 5 | 5 | 5 | 5 | 5 |
| Sodium pyrophosphate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Soy sauce | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| Ice | 11.8 | 11.8 | 11.8 | 11.8 | 11.8 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Parameters | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|
| Fat (%) | 4.49 ± 0.26 a | 3.89 ± 0.29 b | 2.86 ± 0.13 c | 2.54 ± 0.71 c | 2.17 ± 0.25 d |
| Protein (%) | 23.79 ± 0.30 a | 21.88 ± 0.13 b | 16.81 ± 0.26 c | 11.63 ± 0.13 d | 6.70 ± 0.22 e |
| Moisture (%) | 62.81 ± 0.64 e | 64.71 ± 0.45 d | 70.55 ± 0.46 c | 74.38 ± 0.30 b | 77.85 ± 0.07 a |
| TDF (%) | ND | 0.05 ± 0.01 a | 0.08 ± 0.01 b | 1.32 ± 0.04 c | 1.76 ± 0.05 d |
| Energy value (%) | 135.57 a | 122.61 b | 93.14 c | 71.94 d | 49.67 e |
| Ash (%) | 3.27 ± 0.02 d | 3.33 ± 0.03 d | 3.46 ± 0.03 c | 3.52 ± 0.02 b | 3.73 ± 0.03 a |
| Water activity | 0.97 ± 0.00 a | 0.97 ± 0.00 a | 0.97 ± 0.01 a | 0.97 ± 0.01 a | 0.97 ± 0.01 a |
| pH | 6.31 ± 0.02 e | 6.36 ± 0.01 d | 6.38 ± 0.05 c | 6.53 ± 0.15 b | 6.79 ± 0.00 a |
| Parameters | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|
| Cooking loss (%) | 11.42 ± 0.15 d | 12.50 ± 0.16 c | 15.00 ± 0.11 b | 15.82 ± 0.93 b | 16.95 ± 0.15 a |
| Water holding capacity (%) | 89.51 ± 0.22 a | 87.48 ± 0.20 b | 83.26 ± 0.06 c | 77.56 ± 0.07 d | 69.67 ± 0.17 e |
| Hardness (N) | 104.97 ± 2.81 a | 76.80 ± 1.39 b | 53.0 ± 6.68 c | 18.40 ± 0.46 d | 3.30 ± 0.30 e |
| Cohesiveness | 0.50 ± 0.02 a | 0.47 ± 0.01 a | 0.34 ± 0.02 b | 0.32 ± 0.02 b | 0.22 ± 0.02 c |
| Gumminess (N) | 78.53 ± 0.70 a | 67.33 ± 0.80 b | 56.43 ± 0.25 c | 52.90 ± 0.20 d | 2.73 ± 0.05 e |
| Chewiness (mJ) | 201.85 ± 10.94 a | 100.95 ± 0.55 b | 32.43 ± 2.65 c | 11.58 ± 1.34 d | 0.57 ± 0.02 e |
| Springiness (mm) | 6.51 ± 0.11 a | 6.43 ± 0.01 a | 5.39 ± 0.22 b | 3.70 ± 0.02 b | 0.22 ± 0.02 c |
| Cooking loss (%) | 11.42 ± 0.15 d | 12.50 ± 0.16 c | 15.00 ± 0.11 b | 15.82 ± 0.93 b | 16.95 ± 0.15 a |
| C | P25 | P50 | P75 | P100 | |
|---|---|---|---|---|---|
| External color | |||||
| L* | 44.44 ± 0.08 a | 44.31 ± 0.28 a | 43.44 ± 0.08 b | 42.45 ± 0.46 c | 40.57 ± 0.20 d |
| a* | 12.58 ± 0.03 ab | 12.48 ± 0.06 bc | 12.41 ± 0.11 c | 12.69 ± 0.06 a | 12.54 ± 0.09 abc |
| b* | 24.40 ± 0.14 a | 22.50 ± 0.21 d | 22.60 ± 0.08 cd | 22.76 ± 0.02 bc | 22.92 ± 0.02 b |
| ∆E* | ND | 1.91 ± 0.08 d | 2.07 ± 0.13 c | 2.58 ± 0.05 b | 4.14 ± 0.16 a |
| Inter color | |||||
| L* | 56.04 ± 0.04 a | 53.51 ± 0.03 b | 52.60 ± 0.09 c | 49.45 ± 0.23 d | 48.32 ± 0.25 e |
| a* | 8.30 ± 0.01 d | 8.54 ± 0.04 c | 8.64 ± 0.06 c | 8.80 ± 0.06 b | 8.91 ± 0.05 a |
| b* | 23.55 ± 0.08 c | 23.50 ± 0.03 c | 23.28 ± 0.16 d | 24.45 ± 0.06 b | 24.95 ± 0.02 a |
| ∆E* | ND | 2.54 ± 0.05 d | 4.00 ± 0.08 c | 6.67 ± 0.14 b | 7.87 ± 0.12 a |
| Parameters | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|
| Essential amino acid (g/100 g) | |||||
| Valine | 17.50 ± 0.06 a | 10.45 ± 0.04 b | 11.07 ± 0.02 c | 7.65 ± 0.03 d | 2.60 ± 0.08 e |
| Methionine | 5.10 ± 0.04 a | 5.14 ± 0.06 b | 5.72 ± 0.03 c | 6.10 ± 0.07 d | 6.26 ± 0.02 e |
| Lysine | 17.83 ± 0.03 a | 10.02 ± 0.04 b | 10.19 ± 0.03 c | 6.95 ± 0.03 d | 1.88 ± 0.01 e |
| Isoleucine | 9.12 ± 0.14 a | 5.31 ± 0.05 b | 5.71 ± 0.08 c | 4.01 ± 0.07 d | 1.35 ± 0.13 e |
| Leucine | 17.49 ± 0.08 a | 10.45 ± 0.07 b | 11.07 ± 0.09 c | 7.65 ± 0.08 d | 2.60 ± 0.10 e |
| Phenylalanine | 9.53 ± 0.07 b | 9.73 ± 0.05 a | 9.12 ± 0.03 c | 8.49 ± 0.05 d | 8.93 ± 0.04 e |
| Threonine | 10.21 ± 0.10 a | 9.89 ± 0.03 b | 9.32 ± 0.08 c | 8.35 ± 0.10 d | 8.54 ± 0.06 e |
| Total EAA | 86.78 ± 0.52 a | 60.99 ± 0.34 b | 62.20 ± 0.36 c | 49.20 ± 0.43 d | 32.16 ± 0.44 e |
| Non-essential amino acid (g/100 g) | |||||
| Histidine | 7.71 ± 0.03 a | 5.72 ± 0.04 b | 4.52 ± 0.02 c | 2.88 ± 0.06 d | 0.72 ± 0.07 e |
| Serine | 9.26 ± 0.07 b | 9.52 ± 0.12 a | 8.94 ± 0.02 d | 9.06 ± 0.14 c | 8.87 ± 0.13 e |
| Arginine | 16.42 ± 0.08 a | 16.43 ± 0.18 a | 15.89 ± 0.05 c | 15.36 ± 0.09 b | 14.95 ± 0.08 d |
| Glycine | 8.44 ± 0.02 a | 4.99 ± 0.01 b | 5.53 ± 0.06 c | 3.65 ± 0.03 d | 1.31 ± 0.02 e |
| Aspartic | 20.85 ± 0.11 a | 11.52 ± 0.08 b | 12.10 ± 0.10 c | 9.09 ± 0.08 d | 3.58 ± 0.09 e |
| Glutamic | 36.63 ± 0.04 a | 37.54 ± 0.09 b | 36.69 ± 0.11 a | 37.18 ± 0.15 c | 37.56 ± 0.17 b |
| Tyrosine | 7.76 ± 0.14 a | 4.57 ± 0.06 b | 4.79 ± 0.05 c | 3.39 ± 0.04 d | 1.20 ± 0.03 e |
| Alanine | 12.19 ± 0.04 a | 7.58 ± 0.03 b | 7.69 ± 0.07 c | 5.07 ± 0.05 d | 1.83 ± 0.04 e |
| Proline | 7.85 ± 0.02 a | 3.05 ± 0.07 b | 3.33 ± 0.06 c | 3.50 ± 0.04 d | 1.05 ± 0.07 e |
| Cysteine | 0.35 ± 0.06 a | 0.32 ± 0.08 a | 0.26 ± 0.01 b | 0.15 ± 0.07 c | 0.08 ± 0.09 d |
| Total NEAA | 127.46 ± 0.61 a | 101.24 ± 0.76 b | 99.74 ± 0.55 c | 89.33 ± 0.75 d | 71.15 ± 0.79 e |
| Sensor Name | Responsive Substance | Performance Description |
|---|---|---|
| W3C W6S | Aromatic Hydrogen | Sensitive aroma, ammonia Mainly selective for hydrides |
| W5C W1S W1W W2S W2W W3S | Arom-aliph Broad-methance Sulfur-alcohol Broad-alcohol Sulph-chlor Methane-aliph | Short-chain alkance aromatic component Sensitive to methyl Sensitive to sulfides Sensitive to alcohols, aldehydes and ketones Aromatic ingredients, sensitive to organic Sulfides Sensitive to long-chain alkanes |
| W1C | Aromatic | Aromatic costituents, benzene |
| W5S | Broad range | High sensitivity and sensitive to nitrogen oxides |
| No | Compound | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|---|
| 1 | Octanal | 0.552 | 0.336 | 0.883 | 0.337 | ND |
| 2 | Nonanal | 2.188 | 3.328 | 4.158 | 2.423 | 2.135 |
| 3 | Decanal | 1.318 | ND | 2.349 | 0.263 | ND |
| 4 | Benzaldehyde | 2.359 | 3.829 | 2.38 | 1.291 | 3.045 |
| 5 | 2,4-Decadienal, (E,E)- | 1.225 | 1.959 | 3.155 | 6.599 | 16.614 |
| 6 | Butanal, 2-methyl | 0.586 | 0.395 | ND | 0.284 | ND |
| 7 | Pentanal, 3-methyl- | 6.215 | 8.995 | 8.731 | 8.554 | ND |
| 8 | 2-Octenal | ND | ND | ND | 0.78 | 1.147 |
| 9 | 2-UNDecenal | ND | ND | ND | 0.34 | 0.727 |
| 10 | 2-Heptene aldehyde | ND | ND | ND | ND | 1.067 |
| 11 | 2-Decenal, (E)- | ND | ND | ND | 0.341 | 1.097 |
| 12 | 4-Methylhexanal | ND | ND | ND | ND | 0.477 |
| 13 | 2,4-Decadienal, (E,E)- | ND | ND | ND | ND | 1.069 |
| Total | 14.443 | 18.842 | 21.656 | 21.212 | 27.918 | |
| No | Compound | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|---|
| 1 | Methanethiol | 0.227 | ND | ND | ND | ND |
| 2 | 1-Octen-3-ol | 0.915 | 0.665 | 0.855 | 0.962 | 0.859 |
| 3 | 1-Hexanol, 2-ethyl- | 2.558 | 1.713 | ND | 1.188 | ND |
| 4 | Benzyl alcohol | ND | 0.867 | 1.659 | 2.903 | 3.388 |
| 5 | 1-Hexanol | ND | ND | 1.016 | ND | ND |
| 6 | 1-Hexanol, 2-ethyl- | ND | ND | 1.815 | ND | ND |
| 7 | 2-Furanmethanol | ND | ND | ND | 0.211 | ND |
| Total | 3.7 | 3.245 | 5.345 | 5.264 | 4.247 | |
| No | Compound | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|---|
| 1 | Nonane, 5-methylene- | 0.435 | ND | ND | ND | ND |
| 2 | 1-Decene | 0.412 | 0.486 | 0.335 | 0.472 | 0.859 |
| 3 | Caryophyllene | ND | 0.257 | 0.417 | ND | ND |
| 4 | 5-Tetradecene, (E)- | 0.211 | ND | ND | ND | 3.388 |
| 5 | 2-Nonene, 3-methyl- | 0.758 | 0.921 | 0.579 | 0.892 | ND |
| 6 | 4-Nonene, 5-methyl- | ND | ND | 0.441 | 0.377 | ND |
| 7 | 3-Ethyl, 2-methyl- | ND | ND | 0.283 | 0.702 | ND |
| 8 | 2-Dodecene | ND | ND | ND | 0.467 | ND |
| 9 | 1-Undecene, 2-methyl- | ND | 0.62 | ND | ND | ND |
| Total | 1.816 | 2.284 | 2.055 | 2.91 | 4.247 | |
| No | Compound | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|---|
| 1 | Acetoin | 1.06 | 0.875 | ND | ND | ND |
| 2 | Acetophenone | 0.605 | ND | ND | ND | ND |
| 3 | 5-Chloro-5-methylnonane | ND | 0.39 | ND | ND | ND |
| 4 | 5-Hepten-2-one, 6-methyl- | ND | 0.304 | ND | ND | ND |
| 5 | 3-Octanone | ND | ND | 0.772 | ND | ND |
| 6 | 5,9-UNDecadien-2-one | ND | ND | 0.338 | ND | ND |
| 7 | 2-Heptanone | ND | ND | 1.468 | ND | ND |
| Total | 1.665 | 1.569 | 2.578 | ND | ND | |
| No | Compound | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|---|
| 1 | Acetic acid | 1.413 | 0.648 | 1.736 | 1.413 | ND |
| 2 | Butanoic acid | 0.986 | 1.013 | 1.142 | 0.601 | ND |
| 3 | Hexanoic acid | 0.832 | 1.292 | 1.467 | 0.99 | 0.488 |
| 4 | Octanoic acid | 0.325 | 0.372 | ND | ND | ND |
| 5 | Nonanoic acid | 0.575 | 0.346 | 0.375 | ND | ND |
| 6 | n-Decanoic acid | 1.043 | 0.69 | 0.39 | ND | ND |
| 7 | Tetradecanoic acid | 0.493 | 0.646 | 0.493 | 0.479 | 0.341 |
| 8 | Cis-7-Hexadecenoic acid (7Z) | 0.341 | ND | ND | ND | ND |
| 9 | Pentadecanoic acid | 0.257 | 0.255 | ND | 0.197 | ND |
| Total | 6.265 | 1.569 | 2.578 | ND | ND | |
| No | Compound | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|---|
| 1 | Ethyl Acetate | 1.633 | 1.074 | 3.503 | 0.689 | ND |
| 2 | Acetic acid, butyl ester | 1.86 | 2.283 | 1.523 | ND | ND |
| 3 | Propyl acetate | ND | ND | ND | 0.254 | ND |
| 4 | Acetic acid, hexyl ester | 0.224 | ND | ND | ND | ND |
| Total | 3.717 | 3.357 | 5.026 | 0.943 | ND | |
| No | Compound | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|---|
| 1 | Octane, 4-ethyl- | 0.35 | 0.248 | 0.522 | ND | 0.56 |
| 2 | 2,7-dimethyluNDecanone | 0.227 | 0.323 | ND | ND | ND |
| 3 | Nonane, 3-methyl-5-propyl- | 1.008 | 0.779 | ND | 1.058 | 0.886 |
| 4 | Undecane, 3-methyl- | 1.935 | 2.911 | ND | 1.631 | 3.647 |
| 5 | Dodecane | 2.952 | 3.765 | 3.816 | ND | ND |
| 6 | Tetradecane | 0.56 | 0.498 | ND | ND | ND |
| 7 | Decane, 4-ethyl- | ND | 0.278 | 0.495 | 0.285 | ND |
| 8 | Cyclooctasiloxane, hexadecamethyl- | ND | 0.333 | 0.729 | ND | 0.503 |
| 9 | Nonane, 2-methyl-5-propyl- | ND | ND | 1.259 | ND | ND |
| 10 | UNDecane, 3-methylene- | ND | ND | ND | 0.333 | ND |
| Total | 7.032 | 9.135 | 6.281 | 3.307 | 5.596 | |
| No | Compound | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|---|
| 1 | Maltol | 0.376 | 0.366 | ND | ND | ND |
| 2 | Furan, 2-ethyl- | ND | 0.182 | 0.29 | 0.383 | ND |
| Microorganisms (log CFU/g) | C | P25 | P50 | P75 | P100 |
|---|---|---|---|---|---|
| Total viable counts | 1.35 ± 0.30 a | 1.65 ± 0.35 a | 1.15 ± 0.15 a | 0.85 ± 0.15 a | 1.55 ± 0.25 a |
| Coliform | ND | ND | ND | ND | ND |
| Staphylococcus aureus | ND | ND | ND | ND | ND |
| Mold | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, S.; Tu, H.; Yan, X.; Hu, Z.; Sun, H. Physicochemical and Sensory Properties of Pork Patties with Partial Replacement of Lean Pork by Stalks of Agaricus bisporus. Foods 2025, 14, 3655. https://doi.org/10.3390/foods14213655
Wang L, Li S, Tu H, Yan X, Hu Z, Sun H. Physicochemical and Sensory Properties of Pork Patties with Partial Replacement of Lean Pork by Stalks of Agaricus bisporus. Foods. 2025; 14(21):3655. https://doi.org/10.3390/foods14213655
Chicago/Turabian StyleWang, Liyan, Shuo Li, Huajie Tu, Xiaoxia Yan, Zhiqing Hu, and Hongrui Sun. 2025. "Physicochemical and Sensory Properties of Pork Patties with Partial Replacement of Lean Pork by Stalks of Agaricus bisporus" Foods 14, no. 21: 3655. https://doi.org/10.3390/foods14213655
APA StyleWang, L., Li, S., Tu, H., Yan, X., Hu, Z., & Sun, H. (2025). Physicochemical and Sensory Properties of Pork Patties with Partial Replacement of Lean Pork by Stalks of Agaricus bisporus. Foods, 14(21), 3655. https://doi.org/10.3390/foods14213655