Functional Foods Based on Postbiotics as a Food Allergy Treatment
Abstract
1. Introduction
2. Definition of Postbiotics
3. Functional Foods Based on Postbiotics
4. Postbiotics in FA Treatment
4.1. Bacteriocins
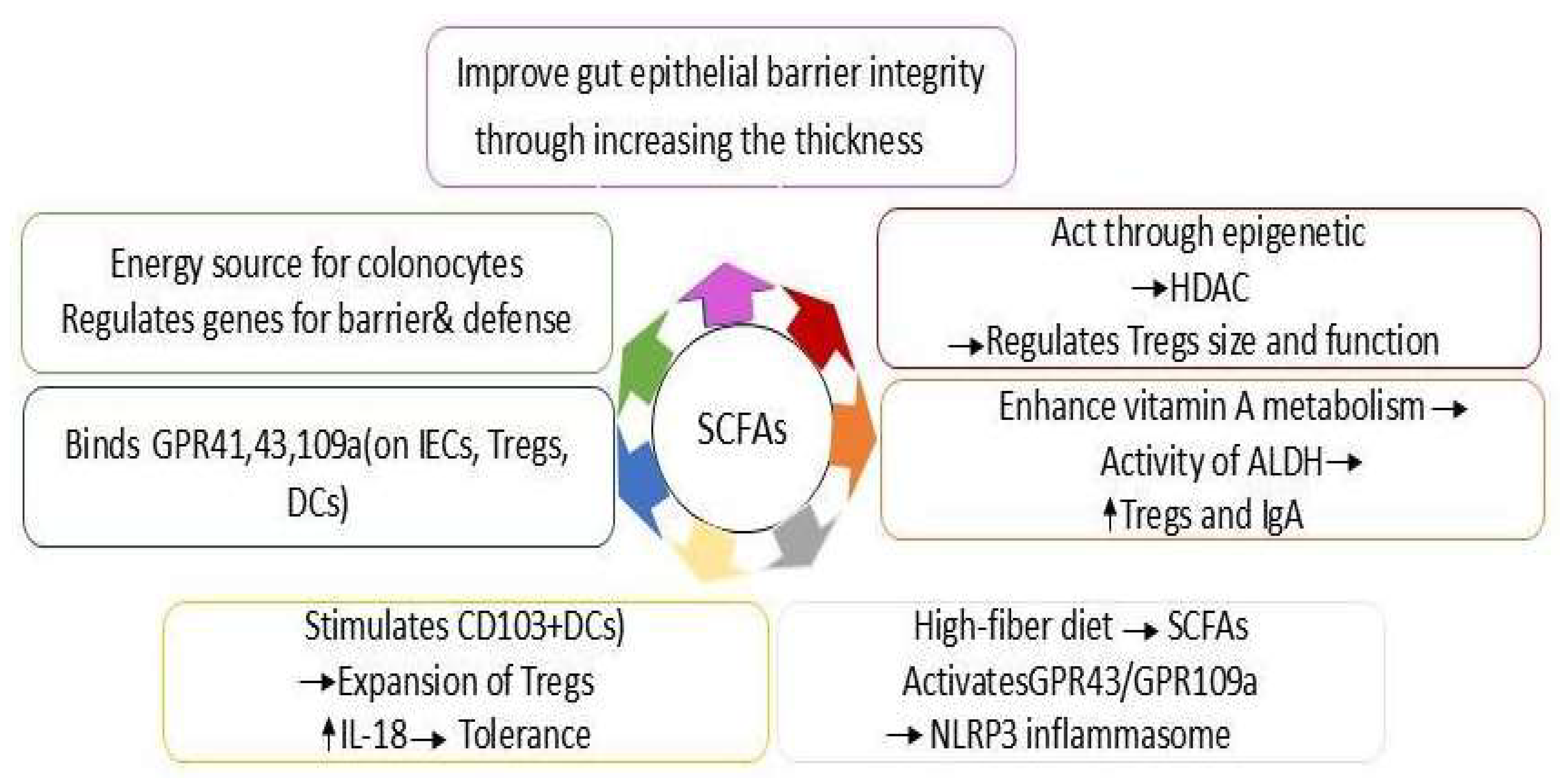
4.2. SCFAs
4.3. Lipoteichoic Acid (LTA)
5. The Dose–Response Effects of Postbiotics in FA
6. Functional Foods-Based on Postbiotics in FA Treatment
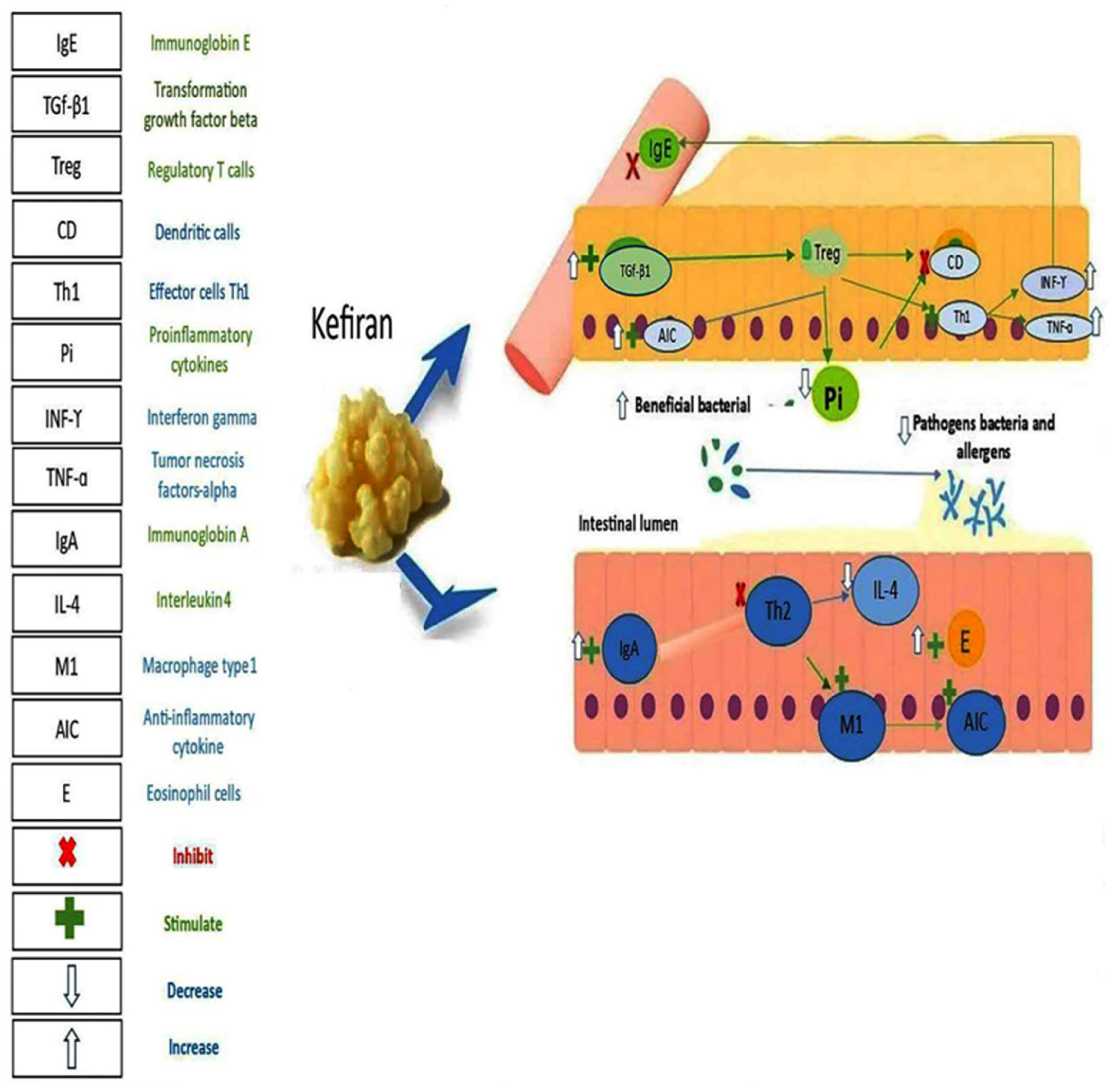
6.1. Kefir
6.2. Yogurt
6.3. Fermented Milk
6.4. Non-Dairy Sources
7. The Synergistic Effects of Postbiotics with Prebiotics
8. Future Prospects for Functional Food-Based on Postbiotics
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartha, I.; Almulhem, N.; Santos, A.F. Feast for thought: A comprehensive review of food allergy 2021–2023. J. Allergy Clin. Immunol. 2023, 153, 576–594. [Google Scholar] [CrossRef]
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food allergy across the globe. J. Allergy Clin. Immunol. 2021, 148, 1347–1364. [Google Scholar] [CrossRef]
- Khani, N.; Ashkezary, M.R.; Hosseinzadeh, N.; Aghapour, B.; Hosseini, A.; Soleimani, R.A.; Houshmandi, S.; Shokouhian, S.M.J.; Homayouni-Rad, A. The role of functional foods based on probiotics in improving fertility: A review. J. Funct. Foods 2025, 129, 106871. [Google Scholar] [CrossRef]
- Liu, C.; Ma, N.; Feng, Y.; Zhou, M.; Li, H.; Zhang, X.; Ma, X. From probiotics to postbiotics: Concepts and applications. Anim. Res. One Health 2023, 1, 92–114. [Google Scholar] [CrossRef]
- Rad, A.H.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Scarpellini, E.; Rinninella, E.; Basilico, M.; Colomier, E.; Rasetti, C.; Larussa, T.; Santori, P.; Abenavoli, L. From pre-and probiotics to post-biotics: A narrative review. Int. J. Environ. Res. Public Health 2021, 19, 37. [Google Scholar]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The concept of postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: Current evidence, controversies, and perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef]
- Pelton, R. Postbiotic metabolites: How probiotics regulate health. Integr. Med. Clin. J. 2020, 19, 25. [Google Scholar]
- Ali, M.S.; Lee, E.-B.; Hsu, W.H.; Suk, K.; Sayem, S.A.J.; Ullah, H.A.; Lee, S.-J.; Park, S.-C. Probiotics and postbiotics as an alternative to antibiotics: An emphasis on pigs. Pathogens 2023, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.; Dwivedi, M.K. The concept of probiotics, prebiotics, postbiotics, synbiotics, nutribiotics, and pharmabiotics. In Probiotics in the Prevention and Management of Human Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–11. [Google Scholar]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, W.; Feng, C.; Kwok, L.-Y.; He, Q.; Sun, Z. Stronger gut microbiome modulatory effects by postbiotics than probiotics in a mouse colitis model. NPJ Sci. Food 2022, 6, 53. [Google Scholar] [CrossRef]
- Khani, N.; Shakeri, A.H.; Bonyadi, M.; Khorrami, R.; Homayouni-Rad, A. Microbial and Chemical Safety Aspects of Postbiotics: As their Tools in Improving Food Safety. Curr. Nutr. Food Sci. 2025, 21, 593–607. [Google Scholar] [CrossRef]
- Khani, N.; Shakeri, A.H.; Houshmandi, S.; Ziavand, M.; Abedi-Soleimani, R.; Hosseinzadeh, N.; Homayouni-Rad, A. The Promising Biological Role of Postbiotics in Treating Human Infertility. Probiotics Antimicrob. Proteins 2025, 2025, 1–13. [Google Scholar] [CrossRef]
- Khani, N.; Shakeri, A.H.; Moosavy, M.-H.; Fard, M.S.; Soleimani, R.A.; Khorrami, R.; Hosseinzadeh, N.; Homayouni-Rad, A. Potential Application of Postbiotics as a Natural Preservative in Cheese. Probiotics Antimicrob. Proteins 2025, 2025, 1–15. [Google Scholar] [CrossRef]
- Khani, N.; Soleimani, R.A.; Homayouni-Rad, A. Potential of Postbiotics for the Biodegradation of Xenobiotics: A Review. Curr. Nutr. Food Sci. 2025, 21, 653–670. [Google Scholar] [CrossRef]
- Khani, N.; Soleimani, R.A.; Rad, A.H. Characterization and Antimicrobial Activity of Postbiotic from Lactobacillus Acidophilus LA5 on Staphylococcus Aureus in Food Model and In vitro. Curr. Nutr. Food Sci. 2025, 21, 379–387. [Google Scholar] [CrossRef]
- Khani, N.; Bonyadi, M.; Soleimani, R.A.; Raziabad, R.H.; Ahmadi, M.; Homayouni-Rad, A. Postbiotics: As a Promising Tools in the Treatment of Celiac Disease. Probiotics Antimicrob. Proteins 2024, 17, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Chen, C.; Razali, U.H.M.; Saikim, F.H.; Mahyudin, A.; Noor, N.Q.I.M. Morus alba L. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- Matos, J.; Afonso, C.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M. Yogurt Enriched with Isochrysis galbana: An Innovative Functional Food. Foods 2021, 10, 1458. [Google Scholar] [CrossRef]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef]
- Palanivelu, J.; Thanigaivel, S.; Vickram, S.; Dey, N.; Mihaylova, D.; Desseva, I. Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability. Appl. Sci. 2022, 12, 455. [Google Scholar] [CrossRef]
- Küçükgöz, K.; Trząskowska, M. Nondairy Probiotic Products: Functional Foods That Require More Attention. Nutrients 2022, 14, 753. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Guimarães, J.F.; Coutinho, N.M.; Pimentel, T.C.; Ranadheera, C.S.; Santillo, A.; Albenzio, M.; Cruz, A.G.; Sant’Ana, A.S. The future of functional food: Emerging technologies application on prebiotics, probiotics and postbiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2560–2586. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, B.; Bai, J.; Zhang, Y.; Liu, C.; Suo, H.; Wang, C. Postbiotics are a candidate for new functional foods. Food Chem. X 2024, 23, 101650. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.É.d.L.; Rocha, C.d.S.; de Moura, M.S.B.; Barcelos, M.P.; Silva, C.H.T.d.P.d.; Hage-Melim, L.I.d.S. Potential beneficial effects of kefir and its postbiotic, kefiran, on child food allergy. Food Funct. 2021, 12, 3770–3786. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Cheng, L.; Wang, J.; Raghavan, V. Gut microbiome modulation by probiotics, prebiotics, synbiotics and postbiotics: A novel strategy in food allergy prevention and treatment. Crit. Rev. Food Sci. Nutr. 2022, 64, 5984–6000. [Google Scholar] [CrossRef]
- Haripriyaa, M.; Suthindhiran, K.; Jayasri, M. Prevention of Food Allergies Using Postbiotics. In Postbiotics; Elsevier: Amsterdam, The Netherlands, 2025; pp. 437–458. [Google Scholar]
- Feng, L.; Guo, Z.; Zhao, J.; Yao, W.; Li, X.; Wu, L.; Mu, G.; Zhu, X. Screening of anti-allergic Lactiplantibacillus species by splenocyte sensitization model and evaluate their probiotic and postbiotic characteristics. Food Biosci. 2023, 57, 103440. [Google Scholar] [CrossRef]
- Sathvik, G.; Ravi, L. Anti-Allergy Activity of Postbiotics. In Postbiotics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 233–241. [Google Scholar]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The microbiome-shaping roles of bacteriocins. Nat. Rev. Microbiol. 2021, 19, 726–739. [Google Scholar] [CrossRef]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024, 22, 556–571. [Google Scholar] [CrossRef]
- Poto, R.; Fusco, W.; Rinninella, E.; Cintoni, M.; Kaitsas, F.; Raoul, P.; Caruso, C.; Mele, M.C.; Varricchi, G.; Gasbarrini, A.; et al. The Role of Gut Microbiota and Leaky Gut in the Pathogenesis of Food Allergy. Nutrients 2023, 16, 92. [Google Scholar] [CrossRef]
- Yang, H.; Qu, Y.; Gao, Y.; Sun, S.; Wu, R.; Wu, J. Research Progress on the Correlation between the Intestinal Microbiota and Food Allergy. Foods 2022, 11, 2913. [Google Scholar] [CrossRef]
- Wang, S.; Wei, Y.; Liu, L.; Li, Z. Association Between Breastmilk Microbiota and Food Allergy in Infants. Front. Cell. Infect. Microbiol. 2022, 11, 770913. [Google Scholar] [CrossRef]
- Jensen, C.; Antonsen, M.F.; Lied, G.A. Gut Microbiota and Fecal Microbiota Transplantation in Patients with Food Allergies: A Systematic Review. Microorganisms 2022, 10, 1904. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, L.; Wang, J.; Hao, M.; Che, H. Antibiotic-Induced Gut Microbiota Dysbiosis Damages the Intestinal Barrier, Increasing Food Allergy in Adult Mice. Nutrients 2021, 13, 3315. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C. Dysbiosis in food allergy and implications for microbial therapeutics. J. Clin. Investig. 2021, 131, e144994. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yan, J.; Xiang, Q.; Dai, H.; Wang, Y.; Fang, L.; Huang, K.; Zhang, W. Early-life gut microbiota in food allergic children and its impact on the development of allergic disease. Ital. J. Pediatr. 2023, 49, 148. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Sigmatulin, I.A.; Gigani, O.O.; Lipkina, S.A. Mechanisms of Regulation Allergic and Autoimmune Reactions by Bacterial Origin Bioregulators. Rudn. J. Med. 2023, 27, 470–482. [Google Scholar] [CrossRef]
- Todorov, S.D.; Popov, I.; Weeks, R.; Chikindas, M.L. Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns. Foods 2022, 11, 3145. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The current and future perspectives of postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Singh, B.; Halestrap, A.P.; Paraskeva, C. Butyrate can act as a stimulator of growth or inducer of apoptosis in human colonic epithelial cell lines depending on the presence of alternative energy sources. Carcinogenesis 1997, 18, 1265–1270. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Zhang, K.; Xue, W. The role of gut microbiota and its metabolites short-chain fatty acids in food allergy. Food Sci. Hum. Wellness 2023, 12, 702–710. [Google Scholar] [CrossRef]
- Sasaki, M.; Suaini, N.H.A.; Afghani, J.; Heye, K.N.; O’MAhony, L.; Venter, C.; Lauener, R.; Frei, R.; Roduit, C. Systematic review of the association between short-chain fatty acids and allergic diseases. Allergy 2024, 79, 1789–1811. [Google Scholar] [CrossRef]
- Oliva, C.T.; Musa, I.; Kopulos, D.; Ardalani, F.; Maskey, A.; Wilson, A.; Yang, N.; Li, X.-M. The gut microbiome and cross-reactivity of food allergens: Current understanding, insights, and future directions. Front. Allergy 2025, 5, 1503380. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Li, T.; Liang, D.; Gao, J.; Liu, X.; Mao, X. Milk fat globule membrane supplementation protects against β-lactoglobulininduced food allergy in mice via upregulation of regulatory T cells and enhancement of intestinal barrier in a microbiota-derived short-chain fatty acids manner. Food Sci. Hum. Wellness 2024, 13, 124–136. [Google Scholar] [CrossRef]
- Takahashi, H.; Fujii, T.; Yamakawa, S.; Yamada, C.; Fujiki, K.; Kondo, N.; Funasaka, K.; Hirooka, Y.; Tochio, T. Combined oral intake of short and long fructans alters the gut microbiota in food allergy model mice and contributes to food allergy prevention. BMC Microbiol. 2023, 23, 266. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, M.M.; Shetty, R.; Bang-Berthelsen, C.H.; Mudnakudu-Nagaraju, K.K. Role of mesenchymal stem cells and short chain fatty acids in allergy: A prophylactic therapy for future. Immunol. Lett. 2023, 260, 1–10. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef]
- Di Costanzo, M.; De Paulis, N.; Biasucci, G. Butyrate: A link between early life nutrition and gut microbiome in the development of food allergy. Life 2021, 11, 384. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as a bioactive human milk protective component against food allergy. Allergy 2020, 76, 1398–1415. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Aitoro, R.; Simeoli, R.; Amoroso, A.; Paparo, L.; Nocerino, R.; Pirozzi, C.; di Costanzo, M.; Meli, R.; De Caro, C.; Picariello, G.; et al. Extensively hydrolyzed casein formula alone or with L. rhamnosus GG reduces β-lactoglobulin sensitization in mice. Pediatr. Allergy Immunol. 2017, 28, 230–237. [Google Scholar] [CrossRef]
- Shao, H.; Min, F.; Huang, M.; Wang, Z.; Bai, T.; Lin, M.; Li, X.; Chen, H. Novel perspective on the regulation of food allergy by probiotic: The potential of its structural components. Crit. Rev. Food Sci. Nutr. 2022, 64, 172–186. [Google Scholar] [CrossRef]
- Mizuno, H.; Arce, L.; Tomotsune, K.; Albarracin, L.; Funabashi, R.; Vera, D.; Islam, A.; Vizoso-Pinto, M.G.; Takahashi, H.; Sasaki, Y.; et al. Lipoteichoic Acid Is Involved in the Ability of the Immunobiotic Strain Lactobacillus plantarum CRL1506 to Modulate the Intestinal Antiviral Innate Immunity Triggered by TLR3 Activation. Front. Immunol. 2020, 11, 571. [Google Scholar] [CrossRef]
- Lan, C.C.; Hsieh, C.C.; Chen, Y.S.; Syu, M.R.; Hsu, C.H.; Leu, S.F.; Hsu, C.L.; Huang, C.C.; Tsai, C.C. Functional Evaluation of Lactobacillus plantarum and Pediococcus acidilacticito Anti-Allergy and Anti-Inflammatory In Vitro and In Vivo. Int. J. Probiotics Prebiotics 2016, 11, e144994. [Google Scholar]
- El Far, M.S.; Zakaria, A.S.; Kassem, M.A.; Wedn, A.; Guimei, M.; Edward, E.A. Promising biotherapeutic prospects of different probiotics and their derived postbiotic metabolites: In-vitro and histopathological investigation. BMC Microbiol. 2023, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Qaisar, S.A.; Janeh, T.; Karimpour, H.; Darbandi, M.; Moludi, J. Clinical trial of the effects of postbiotic supplementation on inflammation, oxidative stress, and clinical outcomes in patients with CVA. Sci. Rep. 2024, 14, 24021. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Palomares, F.; Cañas, J.A.; Pérez-Sánchez, N.; Núñez, R.; Torres, M.J.; Gómez, F. New Insights in Therapy for Food Allergy. Foods 2021, 10, 1037. [Google Scholar] [CrossRef]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; Berg, M.P.M.v.D.; de Bruijn, M.J.W.; van Ijcken, W.F.J.; Junt, T.; Tam, S.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2018, 74, 799–809. [Google Scholar] [CrossRef]
- López-Enríquez, S.; Múnera-Rodríguez, A.M.; Leiva-Castro, C.; Sobrino, F.; Palomares, F. Modulation of the Immune Response to Allergies Using Alternative Functional Foods. Int. J. Mol. Sci. 2023, 25, 467. [Google Scholar] [CrossRef]
- Sousa, M.A.D.; Rama, G.R.; Volken de Souza, C.F.; Granada, C.E. Acid lactic lactobacilli as a biotechnological toll to improve food quality and human health. Biotechnol. Prog. 2020, 36, e2937. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Microbial interactions in kefir: A natural probiotic drink. Biotechnol. Lact. Acid Bact. Nov. Appl. 2010, 2010, 327. [Google Scholar]
- Kothari, D.; Patel, S.; Kim, S.-K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Z.; Kalanpour, N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Shimizu, H.; Shioya, S. Enhanced kefiran production by mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. J. Biotechnol. 2003, 100, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.G. Food Allergy Prevention and Treatment by Targeted Nutrition. Ann. Nutr. Metab. 2018, 72, 33–45. [Google Scholar] [CrossRef]
- Rachid, R.; Chatila, T.A. The role of the gut microbiota in food allergy. Curr. Opin. Pediatr. 2016, 28, 748–753. [Google Scholar] [CrossRef]
- Monaco, S.; Russo, G.; Romano, A.; Liotti, L.; Verga, M.; Sopo, S.M. Yogurt is tolerated by the majority of children with IgE-mediated cow’s milk allergy. Allergol. Immunopathol. 2019, 47, 322–327. [Google Scholar] [CrossRef]
- Lee, N.-K.; Kim, S.-Y.; Han, K.J.; Eom, S.J.; Paik, H.-D. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT-Food Sci. Technol. 2014, 58, 130–134. [Google Scholar] [CrossRef]
- Krzych-Fałta, E.; Furmańczyk, K.; Tomaszewska, A.; Olejniczak, D.; Samoliński, B.; Samolińska-Zawisza, U. Probiotics: Myths or facts about their role in allergy prevention. Adv. Clin. Exp. Med. 2018, 27, 119–124. [Google Scholar] [CrossRef]
- Laiho, K.; Ouwehand, A.; Salminen, S.; Isolauri, E. Inventing probiotic functional foods for patients with allergic disease. Ann. Allergy Asthma Immunol. 2002, 89, 75–82. [Google Scholar] [CrossRef]
- Abdi-Moghadam, Z.; Darroudi, M.; Mahmoudzadeh, M.; Mohtashami, M.; Jamal, A.M.; Shamloo, E.; Rezaei, Z. Functional yogurt, enriched and probiotic: A focus on human health. Clin. Nutr. ESPEN 2023, 57, 575–586. [Google Scholar] [CrossRef]
- Szydłowska, A.; Sionek, B. Probiotics and Postbiotics as the Functional Food Components Affecting the Immune Response. Microorganisms 2022, 11, 104. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, F.; Xie, Q.; Zhang, Y.; Evivie, S.E.; Li, X.; Liang, S.; Chen, Q.; Xin, B.; Li, B.; et al. Co-fermented cow milk protein by Lactobacillus helveticus KLDS 1.8701 and Lactobacillus plantarum KLDS 1.0386 attenuates its allergic immune response in Balb/c mice. J. Dairy Sci. 2022, 105, 7190–7202. [Google Scholar] [CrossRef]
- Morisset, M.; Aubert-Jacquin, C.; Soulaines, P.; Moneret-Vautrin, D.-A.; Dupont, C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 2010, 65, 175–183. [Google Scholar] [CrossRef]
- Yamakawa, M.; Wada, K.; Hayashi, M.; Ezaki, T.; Nakashima, Y.; Nagata, C.; Sumoto, Y. Milk and dairy product intakes, intestinal bacteria, and respiratory infections in children of elementary school age and older in Japan. Nutrition 2023, 115, 112145. [Google Scholar] [CrossRef]
- Tomasik, P.; Tomasik, P. Probiotics, non-dairy prebiotics and postbiotics in nutrition. Appl. Sci. 2020, 10, 1470. [Google Scholar] [CrossRef]
- Mishra, A.; Chakravarty, I.; Mandavgane, S. Current trends in non-dairy based synbiotics. Crit. Rev. Biotechnol. 2021, 41, 935–952. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Tariq, M.; Saris, P.E.J.; Zaidi, A. Evaluation of the probiotic and postbiotic potential of lactic acid bacteria from artisanal dairy products against pathogens. J. Infect. Dev. Ctries 2021, 15, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Ozcan, T. Pro-pre and Postbiotic Fermentation of the Dietetic Dairy Matrix with Prebiotic Sugar Replacers. Probiotics Antimicrob. Proteins 2023, 16, 726–736. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. Overview of Dairy-based Products with Probiotics: Fermented or Non-fermented Milk Drink. Korean J. Food Sci. Anim. Resour. 2024, 44, 255–268. [Google Scholar] [CrossRef]
- Vilela, A.; Cosme, F.; Inês, A. Wine and Non-Dairy Fermented Beverages: A Novel Source of Pro- and Prebiotics. Fermentation 2020, 6, 113. [Google Scholar] [CrossRef]
- Vera-Santander, V.E.; Hernández-Figueroa, R.H.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Health Benefits of Consuming Foods with Bacterial Probiotics, Postbiotics, and Their Metabolites: A Review. Molecules 2023, 28, 1230. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, L.; Borisova, D.; Paunova-Krasteva, T.; Dimitrova, P.D.; Hubenov, V.; Atanasova, N.; Ivanov, I.; Danova, S. From traditional dairy product “katak” to beneficial lactiplantibacillus plantarum strains. Microorganisms 2023, 11, 2847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Y.; Wang, G.; Yong, J.; Li, N. Recent progress in postbiotics and their applications in dairy products. J. Dairy Sci. Technol. 2022, 45, 47. [Google Scholar]
- Bai, J.; Zhao, X.; Zhang, M.; Xia, X.; Yang, A.; Chen, H. Gut microbiota: A target for prebiotics and probiotics in the intervention and therapy of food allergy. Crit. Rev. Food Sci. Nutr. 2022, 64, 3623–3637. [Google Scholar] [CrossRef]
- Fiocchi, A.; Pecora, V.; Dahdah, L. Probiotics, prebiotics & food allergy prevention: Clinical data in children. J. Pediatr. Gastroenterol. Nutr. 2016, 63, S14–S17. [Google Scholar]
- Sabahi, S.; Rad, A.H.; Aghebati-Maleki, L.; Sangtarash, N.; Ozma, M.A.; Karimi, A.; Hosseini, H.; Abbasi, A. Postbiotics as the new frontier in food and pharmaceutical research. Crit. Rev. Food Sci. Nutr. 2022, 63, 8375–8402. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Berin, M.C. Food allergy and the microbiome: Current understandings and future directions. J. Allergy Clin. Immunol. 2019, 144, 1468–1477. [Google Scholar] [CrossRef]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the Molecular Mechanisms Underlying the Protective Effects of Microbial SCFAs on Intestinal Tolerance and Food Allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Di Costanzo, M.; Carucci, L.; Berni Canani, R.; Biasucci, G. Gut microbiome modulation for preventing and treating pediatric food allergies. Int. J. Mol. Sci. 2020, 21, 5275. [Google Scholar] [CrossRef]


| Aspect | Probiotics | Postbiotics | References |
|---|---|---|---|
| Definition | Live microorganisms that confer health benefits when consumed in adequate amounts. | Non-living microbial cells, components, or metabolites that provide health benefits. | [1] |
| Nature | Living, self-replicating organisms. | Non-living, inanimate compounds. | [2] |
| Examples | Lactobacillus, Bifidobacterium, Saccharomyces boulardii. | SCFAs, cell wall fragments, peptides, enzymes. | [3] |
| Mechanism of Action | Colonize the gut, compete with pathogens, produce beneficial metabolites, modulate immunity. | Act directly through bioactive compounds, modulating immunity, inflammation, and gut barrier. | [4] |
| Stability | Sensitive to heat, pH, oxygen, and storage conditions. | More stable; longer shelf life; unaffected by conditions that kill live microbes. | [5] |
| Safety | Risk of infection in immunocompromised individuals. | Safer; no risk of infection since non-living | [6] |
| Source | Fermented foods (yogurt, kefir, kimchi) and supplements. | Derived from probiotic fermentation byproducts or heat-killed probiotics. | [7] |
| Delivery Requirement | Must survive stomach acid and bile to reach the gut alive. | Do not need to survive digestion; already bioactive. | [8] |
| Clinical Evidence | Well-studied for digestive health, immunity, IBS, antibiotic-associated diarrhea. | Emerging evidence for immunity, anti-inflammatory effects, gut barrier support. | [9] |
| Individual Variability | Effects vary with strain, dose, and host microbiome. | Less dependent on host microbiome; effects are more consistent. | [10] |
| Regulatory Status | Regulated as supplements or functional foods; strain-specific claims. | Less regulated; definitions still evolving in many countries. | [11] |
| Use Cases | Restoring gut microbiota, supporting digestion, preventing infections. | Complementing probiotics, reducing inflammation, immune modulation. | [12] |
| Limitations | Require careful storage, strain-specific effects, possible side effects. | Less diverse effects than probiotics; research still growing. | [13] |
| Synergy | Can be combined with prebiotics (synbiotics) and postbiotics. | Can complement probiotics or replicate some of their effects. | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khani, N.; Shirkhan, F.; Ashkezary, M.R.; Sarabi Aghdam, V.; Soleimani, R.A.; Shokouhian, S.M.J.; Hosseinzadeh, N.; Homayouni-Rad, A. Functional Foods Based on Postbiotics as a Food Allergy Treatment. Foods 2025, 14, 3584. https://doi.org/10.3390/foods14203584
Khani N, Shirkhan F, Ashkezary MR, Sarabi Aghdam V, Soleimani RA, Shokouhian SMJ, Hosseinzadeh N, Homayouni-Rad A. Functional Foods Based on Postbiotics as a Food Allergy Treatment. Foods. 2025; 14(20):3584. https://doi.org/10.3390/foods14203584
Chicago/Turabian StyleKhani, Nader, Faezeh Shirkhan, Mansour Rabie Ashkezary, Vahideh Sarabi Aghdam, Roya Abedi Soleimani, Seyed Mohamad Javad Shokouhian, Negin Hosseinzadeh, and Aziz Homayouni-Rad. 2025. "Functional Foods Based on Postbiotics as a Food Allergy Treatment" Foods 14, no. 20: 3584. https://doi.org/10.3390/foods14203584
APA StyleKhani, N., Shirkhan, F., Ashkezary, M. R., Sarabi Aghdam, V., Soleimani, R. A., Shokouhian, S. M. J., Hosseinzadeh, N., & Homayouni-Rad, A. (2025). Functional Foods Based on Postbiotics as a Food Allergy Treatment. Foods, 14(20), 3584. https://doi.org/10.3390/foods14203584





