Abstract
Stelechocarpus burahol (kepel) is valued for its aromatic fruits and medicinal leaves, yet its genomic and phytochemical features remain poorly characterized. This study estimated the nuclear DNA content of kepel leaves at 3.96 pg per haploid genome (genome size: 3873 Mbp) and comprehensively profiled their bioactive metabolites. Leaf extracts prepared with water and 70% ethanol, with or without pulsed electric field (PEF) treatment, were analyzed using HPLC-MS, UHPLC-QTOF-MS, HPLC-DAD, and GC-MS. Leaf extracts showed the highest phenolic and flavonoid contents, with PEF markedly improving ethanolic extraction efficiency. A total of 72 phenolics, 2 tocopherols, 3 tocotrienols, and several novel vitamin E derivatives were detected, alongside abundant catechins, tannic acid, and gallic acid. PEF significantly enhanced catechin recovery: catechin (C) increased from 153.7 to 846.8 mg/g and epicatechin (EC) from 338.2 to 921.4 mg/g in water extracts, while ethanolic extracts rose from 335.3 to 905.1 mg/g (C) and 245.0 to 616.9 mg/g (EC). Epigallocatechin 3-gallate (EGCG), absent in untreated leaves, reached 799.9 mg/g in water and 231.9 mg/g in ethanol extracts after PEF. In fruits, PEF reduced phenolic recovery in water extracts (C: 236.7 → 136.8 mg/g; EC: 135.4 → 118.2 mg/g; EGCG: 2892.2 mg/g → undetectable), but slightly improved ethanolic extracts (C: 237.8 → 289.4 mg/g). GC-MS identified 19 volatile compounds contributing to the fruit’s aroma. This work provides the first integrated report of kepel genome size and phytochemical composition, highlighting PEF as a promising strategy to enhance leaf catechin extraction and supporting kepel’s potential as a functional food and medicinal resource.
1. Introduction
Stelechocarpus burahol (Blume) Hook.f. & Thomson, known as Burahol and kepel, belongs to the Annonaceae family (https://www.ipni.org/n/urn:lsid:ipni.org:names:2139-1 accessed on 5 May 2025). It was first taxonomized as Uvaria burahol and had been published in the Flora van Nederlandsch Indie, Collation i:94 (1855). Heusden previously published descriptions of the general features of the kepel plant, including trees up to 25 m high; glabrous and lenticel twigs; coriaceous, membranous, shiny elliptic to elliptic oblong (8–31 cm long, 2.5–9.5 cm wide) brown to green leaves; obovoid, subglobos, ellipsoid (30–70 mm long, 15–45 mm in diameter) glabrous or pubescent fruits with minute hairs, scurfy to verruculose; and horizontal, ellipsoid to broadly ellipsoid, flatly shaped seeds [1]. Due to traditional restrictions and policies pertaining to Indonesian Royal trees, kepel has been previously categorized as a rare plant in Indonesia [2]. Nowadays, kepel trees are cultivated and distributed in areas across South and Southeast Asia including Sumatra, Borneo, Java, Bali, Malaysia, Thailand, and Vietnam.
In addition to its cultural and botanical significance, kepel fruit is consumed both fresh and processed, valued for its sweet, aromatic flavor. Traditionally, Javanese royal families regarded it not only as a delicacy but also as a natural deodorant, as regular consumption was believed to impart a pleasant fragrance to the body [3]. Nutritionally, kepel fruit contains a variety of bioactive compounds such as flavonoids, phenolic acids, and essential fatty acid esters, many of which contribute to its antioxidant and anti-inflammatory properties [4]. The fruit also provides dietary fiber, vitamins, and minerals that support digestive health and overall well-being. These nutritional benefits, together with its ethnopharmacological applications, make kepel a promising candidate for functional food development and natural health products.
Importantly, species identification based on morphological characters can be utilized to precisely and scientifically recognize and establish the inter-species or intra-species diversity of S. Burahol [5]. Genome size and deoxyribonucleic acid (DNA) content represent key components of the unreplicated, basic, gametic chromosome set, providing a unifying measure that relates to important biological and ecological traits. This could ultimately lead to a better understanding of plant genome evolution [6,7]. The amount of DNA (C) and the number of complete sets of chromosomes (N) within a cell can change during the following events: a haploid gamete (1C, 1N), fertilization (2C, 2N), DNA replication (4C, 2N), and mitosis (2C, 2N). By using a molecular technique, plant genetics and genomics can be employed to utilize DNA barcoding that would lead to better accuracy and faster taxonomic identification [5]. Therein, inter sample sequence repeat (ISSR) markers and start codon targeted (SCoT) polymorphism have allowed researchers to investigate the genetic diversity and relationships among different cultivars [8]. Furthermore, polymerase chain reaction (PCR) has been utilized, along with specific primers, to amplify DNA fragments. Recently, Probojati and colleagues have identified a S. burahol DNA fragment length of 500–600 basepair (bp) and an abundance value of 63.9% with AT bases [5].
At present, kepel mesocarp can be cultured in Murashige and Skoog medium supplemented with picloram, wherein flavonoid compounds are produced during the 15-day log phase of growth [3]. Interestingly, ethanolic extract derived from Stelechocarpus cauliflorus or ngam ngo in Thai name (Family Annonaceae) leaves contained two dihydroflavonol glycosides, namely engeletin and astilbin, and these two compounds exert antioxidant, anti-inflammatory, and anti-glycation effects [9,10]. Conventional extraction methods (e.g., solid-liquid, maceration, enzyme/chemical hydrolysis, and Soxhlet extraction), as well as certain green techniques (e.g., pressurized fluid, supercritical fluid, freeze-thawing, ultrasonication, microwave, and pulsed electric field (PEF) extraction) have been used to achieve high-value ingredients, while valorization can maximize the extraction yield and enhance any nutritional, biological, and pharmacological properties [11,12]. The colorimetric Folin–Ciocalteu method was used for the determination of total phenolic content (TPC), while aluminum chloride-based assay was used for determination of total flavonoid content (TFC) [13,14]. In addition, headspace-solid phase microextraction (HS-SPME), followed by gas chromatography (GC)/mass spectrometry (MS), was used to effectively characterize the volatile organic substances of the entire fruits [15]. Likewise, polar phytochemicals can be quantified using a powerful form of high-performance liquid chromatography (HPLC) coupled with an ultraviolet-visible (UV-Vis), photodiode array (PDA), MS, or tandem MS/MS detector(s). This was accomplished through automated matching of the results of the UV spectra to the authentic standards of the in-house library, thereby authenticating the identity [16]. An ultrahigh performance liquid chromatography-electrospray ionization-quadrupole time-of-flight-mass spectrometry (UHPLC-ESI-QTOF-MS) machine can provide comprehensive profiling of many phytochemicals of plant extracts [17]. Moreover, headspace solid-phase microextraction gas chromatography-mass spectrometry (HS-SPME-GC-MS) is a nondestructive, solvent-free method that uses a thermal-regulated HS above a sample to extract volatile compounds onto a stationary adsorbent coated-fiber combined with GC for separation and MS for identification [18]. Furthermore, PEF pretreatment can improve the conventional 50% ethanol extraction method to achieve 91.6% increased TPC values, which correlated with the antioxidant activity recorded from olive pomace [19]. Importantly, the PEF technique has been successfully applied for the enhancement of polyphenol extraction from fresh food products and by-products [20].
A recent study has revealed that the methanolic extract of kepel fruits contains dodecanoic acid-propanetriyl ester; its methanol peel extract contains hexadecanoic acid methyl ester, hexamethyl tetracosahexaene and dodecanoic acid-propanetriyl ester; its ethyl acetate peel extract contains dodecanoic acid-propanetriyl ester; and its ethyl acetate fruit extract contains pelargonidin-malonylrhamnoside, 8-epiiridodial glucoside tetraacetate, epigallocatechin 3-gallate (EGCG), 5-octadecenal, 1,5-dicaffeoylquinic acid, 1,6-di-O-galloylglucose, and luteolin 7-O-glucoside. Notably, all the extracts exerted strong antioxidant activity [4]. Not only kepel fruits but also kepel leaves exert important nutritional and ethnopharmacological effects, including antioxidant, anti-hyperuricemic, inhibitory xanthine oxidase and cyclooxygenase, and anti-microbial activities [3]. Thus, this study aimed to investigate DNA content and size, and to identify the chemical compositions of the water and ethanolic extracts obtained from kepel leaves and fruits with and without with the use of the PEF.
2. Materials and Methods
2.1. Chemicals and Reagents
PCR master mix reagent was purchased from iNtRON Biotechnology Company, Yeonggi-do, Republic of Korea. NucleoSpin filters, spin column, and Plant II reagent kit including lysis buffers PL1 and PL2, precipitation buffer PL3, PCR buffer, washing buffers PW1 and PW2, elution buffer PE, and ribonuclease A (RNase A) were obtained from Macherey-Nagel GmbH & Co. KG, Duren, Germany. Nucleic acid staining dye “SafeViewTM FireRed dye” was purchased from Applied Biological Materials Inc., Richmond, BC, Canada. Agarose powder (Product number A9539, a low electroendosmosis value of 0.09–0.13), 50–5000 bp DNA marker (Catalogue number P9577), Folin–Ciocalteu reagent, aluminum chloride, sodium carbonate, potassium chloride, sodium acetate, and Tris-borate-ethylenediamine tetraacetate (TBE) were purchased from Sigma-Aldrich Chemicals Company Limited, Saint Louis, MO, USA. DNA dye (6X Loading ViSafe Gel Green dye, Catalogue Number: SD0101) was obtained from Vivantis Technologies, Selangor, Malaysia. Authentic standards, including EGCG, catechin (C), epicatechin (EC), gallic acid (GA) and quercetin (Q) were also purchased from Sigma-Aldrich Chemicals Company. Solvents, including acetonitrile, ethanol, ethyl acetate, methanol, and formic acid, were of HPLC or the highest-pure grade.
2.2. Kepel Plants
Kepel (S. burahol) trees were planted in the Demonstration Fields at Ban Pang Khum, Tambol Yangmin, Amphur Samerng, Chiang Mai Province, Thailand, at an altitude of 14,000 m. The crop, including stalks, leaves, flowers, and fruits, aged 8 years and 1 month, was harvested and sent for botanical identification, genome analysis, and chemical composition analysis, as will be described below.
2.3. Botanical Identification and Description
The plant was then described and identified according to its taxonomic aspects using standard procedures at the Institute of Herbal Medicine, Department of Medical Science, Ministry of Public Health, Nonthaburi, Thailand. Additionally, specimens were registered in the Herbarium, Faculty of Pharmacy, Chiang Mai University. Chiang Mai, Thailand.
2.4. Molecular Fingerprinting Using Inter-Simple Sequence Repeats
The ISSR-PCR is used to produce multilocus genetic markers based on length variations between microsatellites and valuable for investigating taxonomy, genetic diversity and phylogenetics in plants and fungi. ISSRs are DNA segments with different sizes (100–3000 bp) that can be amplified using PCR with microsatellite core sequences as primers as following.
2.4.1. DNA Isolation
Firstly, 100 mg of fresh leaves of kepel, and Drawf Ylang-Ylang (Cananga fruticose) as a reference, were placed in a mortar and liquid nitrogen was poured over the samples. They were then crushed into a fine powder using a grinder. The pulverized samples were stored in 1.5 mL microcentrifuge tubes and DNA was extracted with a NucleoSpin Plant II reagent kit according to the manufacturer’s instructions. Firstly, 400 μL of a mixture of both lysis buffers PL1 and PL2 (1:1 by volume), and 10 μL of RNase A, were added to the powdered leaf samples. The mixture was then incubated in a 65 °C hot water bath for 30 min. Secondly, 1.5 mL of the cell lysate was transferred to a collection tube containing a NucleoSpin Filter and centrifuged at 11,000× g for 2 min. Thirdly, the filtrate was mixed with 450 μL of PCR PL3 buffer to precipitate DNA. Subsequently, the DNA was dissolved in 700 μL of buffer PW1, loaded onto a collection tube inserted with a NucleoSpin column (Silica membrane, dimension of 235 mm × 161 mm × 77 mm, binding capacity of 50 μg DNA with 50–50,000 bp size), and centrifuged at 11,000× g for 1 min. Fourthly, the DNA adsorbed onto the column was washed twice with 700 μL of buffer PW2 and eluted with 50 μL of buffer PE that had been prewarmed to 65 °C and subjected it to centrifugation at 11,000× g for 5 min twice. Finally, the pooled eluents were employed to determine the DNA amounts using a NanoDrop microvolume spectrophotometer (BioDrop Touch Duo, Product Number: 80-3006-61, Serial Number: BD1361, Cambridge, UK).
2.4.2. PCR Method
The obtained genomic DNA extract was amplified using ten ISSR-labeled primers (Table 1) according to the PCR protocol established by Sultana et al. [21]. A working PCR master mix reagent was prepared according to the prescribed procedure (Table 2) and placed in the PCR machine (LifePro Thermal Cycler, Model: TC-96/G/H(b)A, Serial Number: BYQ6063E-245, Hangzhou BIOER TECHNOLOGY Company Limited, Hangzhou, China) in order to initiate the reaction for 35 cycles (Table 3).

Table 1.
ISSR-labeled primers used in this assay.

Table 2.
Compositions of the PCR master mix reagent.

Table 3.
PCR steps.
2.4.3. Agarose Gel Electrophoresis
For preparation of 1.5% agarose gel, 0.75 mg of agarose was melted in a microwave for 2 min, mixed with 50 mL of 1× TBE buffer pH 8.3, and allowed to solidify at room temperature for 15 min. In the assay, 5 μL of PCR products obtained from KP and KS leaves, along with 50–5000 bp DNA marker (M) were gently mixed with 3 μL of non-toxic SafeViewTM FireRed dye (sensitivity limit: 0.1–0.3 ng of DNA per band) at room temperature for 30 min. The mixtures were then dropped into the agar wells and run at a potential difference of 100 volts with constant current for 40 min. Finally, the gel was exposed to a UV-Transilluminator (Model: BECXF-20.M V1, Serial Number: 10 102612, VILBER LOURMAT Technologies, Marne-la-Vallée Cedex 3, Rue de Lamirault, Collégien, France), viewed for red fluorescence intensity (excitation wavelength of 540 nm and emission wavelength of 630 nm) of DNA bands, and the images were photographed for data analysis. The FI of DNA fragments from PCR product were compared against those of DNA standards or ladder and calculated their DNA contents per sample. In calculation, genome size was estimated using the conversion factor 1 pg of DNA ≈ 978 Mbp. DNA mass was measured by spectrophotometry, and genome size (Mbp) was calculated as DNA content (pg) × 978.
2.5. Preparation of Kepel Extracts
Firstly, 100 kg of fresh weight (FW) Kepel leaves were dried in a hot-air oven at 50–55 °C overnight, and the dry leaves (10 kg) were then ground using an electric blender (SharpThai Company, Limited, Bangkok, Thailand). Additionally, whole kepel fruits (10 kg) were chopped into small pieces, lyophilized with a freeze dryer (Labfreez Instruments Company, Limited, Changsha, Hunan, China), and ground using an electric blender. Afterward, 1 g of dry kepel leaves and fruits were extracted one time with 100 mL of DI water at 80 °C for 10 min or 100 mL of 70% (v/v) ethanol at room temperature overnight using the maceration method. After extraction, the samples were filtered through gauze sheets and Whatman No. 1 cellulose paper using a vacuum pump. The kepel leaf water extract (KLWE) and fruit water extract (KFWE) were dried using a freeze dryer. The filtrates of kepel leaf ethanolic extract (KLEE) and fruit ethanolic extract (KFEE) were then subjected to drying using a rotary evaporator at 40 rpm, 47.7–50.0 °C, and by applying the freeze-drying method, respectively [22].
2.6. PEF-Assisted Extractions
In assistance with PEF instrument (Heidolph Hei-VAP, Becthai Equipment & Chemical Company, Limited, Bangkok, Thailand), the generator of electric fields was powered by 220 VAC/50 Hz, and 500 watts. The chamber received a high voltage electric field (0–10 kV/cm and 1 μs) from the rotating gap switches. The coaxial-cylinder PEF chamber has a volume of 500 mL. The pulse repetition frequency (10 Hz), duration (1 μs), electric field strength (3 kV/cm), number of pulses (1200 pulses) were set, and the specific energy was accordingly calculated to be 2.61 kJ/kg of kepel powder [23,24]. In PEF treatment, dried leaf and fruit powder specimens (1 g) were mixed with DI water of 70% ethanol (100 mL) and extracted with PEF (^) at a field strength of 3 kV/cm, 5 min/cycle for 30 min at room temperature (25 ± 1 °C). The extraction chamber had a rectangular shape of 4.2 × 13.2 cm with electrodes spaced 2.0 cm apart. This was performed at a frequency of 50 Hz and the number of pulses recorded at 1200. The extraction chamber had a rectangular shape of 4.2 × 13.2 cm with electrodes spaced 2.0 cm apart. After extraction, the samples were filtered as described above. The kepel leaf water extract (KLWE^), leaf ethanolic extract (KLEE^), fruit water extracts (KFWE^) and fruit ethanolic extract (KFEE^) were dried using the methods as described above.
2.7. Colorimetric Determination of Chemical Compositions
2.7.1. TPC
Kepel extract solution (100 µL) was mixed with 10% (v/v) Folin–Ciocalteu reagent (200 µL) and 10% (w/v) sodium carbonate (800 µL). The mixture was incubated at 25 °C for 30 min, and optical density (OD) was measured at a wavelength of 700 nm against a reagent blank using a double-beam UV-VIS spectrophotometer (Model UV-1800, Shimadzu Corporation, Kyoto, Japan). TPC was determined from a standard curve of GA and reported in terms of mg gallic acid equivalent (GAE)/g [13].
2.7.2. TFC
Flavonoid content was determined using the colorimetric method based on the formation of a stable complex between aluminum ion and flavonoids [25]. Kepel extract solution (250 µL) was mixed with a chromogenic reagent containing 10% (w/v) aluminum chloride (50 µL), 1 M potassium acetate (50 µL), and DI (2.15 mL). The mixture was then incubated in the dark at 25 °C for 30 min, and OD was measured at a wavelength of 415 nm against a reagent blank using a UV-Vis spectrophotometer [14]. TFC was determined from a standard curve of Q and reported as mg quercetin equivalent/g (QE/g).
2.8. HPLC-ESI-MS Identification of Phenolic Compounds
Kepel extracts were analyzed for anthocyanins using the HPLC-MS method established by Cuyckens and colleagues with slight modifications [13,26]. The HPLC system (Agilent Technologies 1100 Series, Deutschland GmbH, Waldbronn, Germany) consisted of a quaternary pump (G1311A), an online vacuum degasser (G1322A), an autosampler (G1313A), a thermostated column compartment (G1316A), and a PDA detector (G1315A). The outlet of the PDA was coupled directly to the atmospheric pressure ESI interface of the MS detector (Agilent Technologies 1100 LC/MSD SL, Palo Alto, CA, USA) through a flow splitter (1:1). In terms of the analysis, kepel extracts were constituted in 1.0 mL of a mixture comprising solvent A (acetonitrile) and solvent B (10 mM formate buffer pH 4.0) (1:1, v/v), and then filtered through a syringe polytetrafluoroethylene (PTFE) membrane filter (25 mm diameter, 0.45-μm pore size, Corning Incorporation, Corning, NY, USA) before being analyzed. In the analysis, the sample (20 μL) was injected into the HPLC system, and the separation was carried out on a column (LiChroCART RP-18e, 150 mm × 4.6 mm, 5 µm particle size; Purospher STAR, Merck, Darmstadt, Germany) operated at 40 °C. Mobile phases A and B were run at a flow rate of 1.0 mL/min under the gradient program of 100% B (0% A) for an initial period of 5 min, 0–20% A from 5 to 10 min, 20% A from 10 to 20 min, 20–40% A from 20 to 60 min, 40% A for 3 min, and followed by an initial step of 100% B for 5 min. PDA detection was set at 270 nm. MS analysis was performed in positive ESI mode, and spectra were acquired within the mass-to-charge ratio (m/z) range from 100 to 700. For the single quadrupole MS system, the ESI energy was set at 70 eV, while the temperatures of the ion source and the interface were set at 150 °C and 230 °C, respectively. Nitrogen was used as the nebulizing, drying, and collision gas. The capillary temperature was set to 320 °C, the nebulizer pressure was set to 60 pounds•inch2, and the drying gas flow rate was set to 13 L/min. Capillary voltages were set to 3500 V (positive) and 150 V (negative). The oven temperature was programmed as follows: 80 °C (held for 3 min), ramped to 110 °C at 10 °C/min (held for 5 min), increased to 190 °C (held for 3 min), ramped to 220 °C at 10 °C/min (held for 4 min), and increased to 280 °C at 15 °C/min (held for 13 min). Accurate mass measurements were performed by employing the auto mass calibration method using an external mass calibration solution (ESI-L Low Concentration Tuning Mix; Agilent calibration solution B). Herein, the limit of detection (LOD), limit of quantitation (LOQ), and recovery value were found to be 0.5 mg/kg, 1.20 mg/kg, and 70–110%, respectively. The chromatographic and mass spectrometric analyses, and a prediction of the chemical formula, including the exact mass calculation, were performed by Mass Hunter software version B.04.00 built to 4.0.479.0 (Agilent Technologies, Deutschland GmbH, Waldbronn, Germany). In addition, MS data were searched for in published literature repositories.
2.9. UHPLC-ESI-QTOF-MS Analysis of Phenolic Compounds
Phenolic compositions of kepel extracts were analyzed using the comprehensive UHPLC-ESI-QTOF-MS method previously described by Hodgson and colleagues [27] with slight modifications [28]. The UHPLC-ESI-QTOF-MS system was composed of an UHPLC machine (Agilent 1260 Infinity II LC, Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with an ESI-QTOF-MS. In the MS system, nitrogen gas nebulization was set at 45 pounds per inch2 with a flow rate of 5.0 L/min at 300 °C, the sheath gas was set at 11.0 L/min at 250 °C, and the capillary and nozzle voltage values were set at 3.5 kV and 500 V, respectively. A complete mass scan was conducted with m/z values ranging from 200 to 3200. All the operations, acquisitions, and analyses of the data were monitored using Agilent UHPLC-ESI-QTOF-MS MassHunter Acquisition Software Version B.04.00 “Find by Be” algorithm to generate a list of precise mass matches compounds. Peak identification was performed in positive modes using the library database, and the identification scores were further selected for characterization and m/z verification.
Lyophilized kepel extracts were reconstituted in a mixture of absolute methanol (100 µL) and Milli Q water (100 µL), ultrasonicated on an ice bath for 10 min, and centrifuged at 17,000× g at room temperature for 10 min. Afterward, the supernatant was passed through a syringe filter (cellulose ester type, 0.45 µm pore size, Merck Amicon filter, Sigma-Aldrich Chemical Company, Limited, Saint Louis, MO, USA). In the analysis, the filtrate (5 μL) was injected into the UHPLC system using an autosampler and fractionated on a column (InfinityLab Poroshell 120 EC octadecyl silane type, 2.1 mm × 100 mm, 2.7 µm particle size, Agilent Technologies Company, Santa Clara, CA, USA) that had been thermally regulated at 40 °C. It was then eluted in the linear gradient mode using mobile phase A (0.1% formic acid in DI) and mobile phase B (0.1% formic acid in acetonitrile) at a flow rate of 0.35 mL/min for 60 min. The timing program employed for gradient elution was as follows: 0 → 15 min; %A/B (100/0 → 90/10), 15 → 30 min; %A/B (90/10 → 40/60); 30 → 45 min; %A/B (40/60 → 10/90); and 45 → 60 min; %A/B (10/90 → 0/100). Peak identification was carried out in a positive mode using the library database, and the identification scores were sorted out for the purposes of characterization and m/z verification. For interpretation of MS/MS fragments, a stable molecular ion and characteristic analyte fragment ions were used to confirm structural features, differentiate between isomers, and assign Match Confidence Levels (MCL) beyond a single MS spectrum, following approach established by Schymanski et al. [29]. This system defines five confidential levels: Level 1, confirmed structure, supported by a reference standard with matching TR and MS/MS spectrum; Level 2a, probable structure, based on a library spectrum; Level 2b, probable structure, supported by diagnostic evidence but lacking a reference spectrum); Level 3, tentative candidate, where multiple possible structures remain but are not fully confirmed; Level 4, unequivocal molecular formula assignment only; and Level 5, exact mass of interest only.
2.10. HPLC-DAD Quantification of Catechins
Briefly, the kepel extracts (10 mg) were reconstituted in 1 mL of DI water and passed through a syringe PTFE membrane filter. Then, 20 µL of the extract solution (1.0 g/100 mL) was injected into the HPLC-DAD system (Model 1290 Infinity II, Agilent Technologies, Inc., Santa Clara, CA, USA) and fractionated on a column (ODS type, 150 mm × 4.6 mm, 5 µm particle size, Agilent Technologies, Inc., Santa Clara, CA, USA) capped with a guard column (10 mm × 4.7 mm, 5 µm particle size, Agilent Technologies, Inc., Santa Clara, CA, USA). Individual catechins were eluted isocratically with mobile-phase solvent containing 0.05% H2SO4: acetonitrile: ethyl acetate (86:12:2, v/v/v) at a flow rate of 1.0 mL/min, and OD was detected at 280 nm with DAD. Standard C and EGCG at concentrations of 0–1 mg/mL were used to position the eluted EGCG peak, thereby generating a standard curve [30,31]. Accordingly, C, EC and EGCG concentrations of the kepel extracts were determined from the curves.
2.11. HS-SPME-GC-MS Analysis of Volatile Organic Compounds
The analysis was performed using the Agilent Instrument system (Agilent Technologies Company, Santa Clara, CA, USA). In principle, volatile organic compounds with small molecules by preparing the sample in the HS autosampler (Model Agilent PN 7697A), which involved the use of an oven heated to 70 °C. This would allow the analyte to evaporate into gas, move through and be captured by SPME resin before being eluted with a helium gas vehicle, fractionated on a stationary-phase column of GC machine (Model Agilent 7890B), and detected by an MS detector (Model Agilent 5977B) based on the m/z value. In the analysis, 1 μL of the sample obtained from HS-SPME was loaded onto a GC system equipped with a silica capillary column (30 m × 0.25 mm ID, 0.25 μm film thickness; Agilent 19091S-433) with carrier helium gas at a flow rate of 1.0 mL/min (injection temperature set from 60 °C for 2 min and then increased to 250 °C at a rate of 4 °C/min for 20 min), passed through an MS instrument set to scan at 70 and 230 electron volts over the m/z range of 29 to 350 amu, and the detected volatile compounds were characterized by comparison with the spectra of standard compounds [32]. For interpretation of MS/MS fragments, a stable molecular ion and characteristic analyte fragment ions were used to confirm structural features, differentiate between isomers, and assign MCL beyond a single MS spectrum, following approach established by Schymanski et al. [29]. This system defines five confidential levels: Level 1, confirmed structure, supported by a reference standard with matching TR and MS/MS spectrum; Level 2a, probable structure, based on a library spectrum; Level 2b, probable structure, supported by diagnostic evidence but lacking a reference spectrum); Level 3, tentative candidate, where multiple possible structures remain but are not fully confirmed; Level 4, unequivocal molecular formula assignment only; and Level 5, exact mass of interest only.
2.12. Statistical Analysis
All quantitative experiments were performed in biological triplicates (n = 3) unless otherwise specified. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) Statistics for Windows version 22 Program IBM Corporation, Armonk, NY, USA) and expressed as values of mean ± standard deviation (SD). For comparisons involving more than two groups, Significance was investigated using the one-way analysis of variance (ANOVA) test, followed by Tukey’s HSD post hoc test to correct for multiple comparisons. For pairwise comparisons, a two-tailed Student’s t-test was used. Where multiple hypotheses were tested simultaneously, false discovery rate correction was applied to minimize type I error. A threshold of p < 0.05 was considered statistically significant. When data did not meet the assumption of normal distribution, nonparametric tests were applied to determine significance.
3. Results
3.1. Taxonomy and Molecular Fingerprints of Kepel
The male flower samples of the kepel tree can be identified by plant taxonomy with the scientific name S. burahol (Blume) Hook.f. & Thomson Family: Annonaceae with a voucher number 0023315. According to the AGE method, ISSR markers and 10 tested primers were employed. DNA bands obtained from kepel leaves appeared at 300, 490, 600, 690, 900, and 1200 bp when using the UBC-807 primer, at 800 and 980 bp when using the UBC-808 primer, at 300, 490, 500, and 810 bp when using the UBC-809 primer, at 400, 500, 1400, 1500, 1600, and 2000 bp when using the primer UBC-811, at 330, 420, 500, 980, and 1300 bp when using the primer UBC-812, at 200, 500, 700, 800, 1000, 1400, and 1600 bp when using the primer UBC-834, at 400, 600, 890, 1100, 900, and 1200 bp when using the primer UBC-840, at 250, 280, 310, 400, 600, 820, 1000, 1200, and 1500 bp when using the primer UBC-841, and at 200, 300, 400, 590, 690, 700, 880, 900, 1000, 1300, 1500, 2000, and 2800 bp when using the primer UBC-842; nonetheless, no DNA bands were seen when the primer UBC-810 was used (Figure 1).
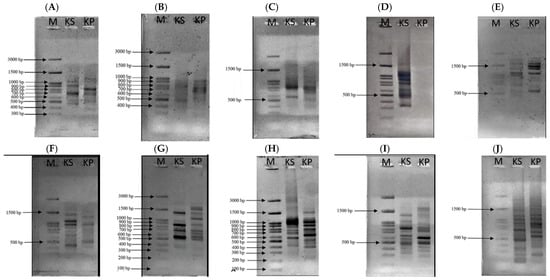
Figure 1.
AGE analysis of DNA using primers UBC-807 (A), UBC-808 (B), UBC-809 (C), UBC-810 (D), UBC-811 (E), UBC-812 (F), UBC-834 (G), UBC-840 (H), UBC-841 (I), and UBC-842 (J). From the left, lane 1 = DNA marker, lane 2 = kepel leaves and lane 3 = Dwarf Ylang Ylang leaves.
Genomic analysis of kepel leaves revealed that the nuclear DNA content was estimated to be 3.96 pg per haploid genome, which corresponds to a genome size of approximately 3873 Mbp (3.9 Gbp) [33].
3.2. Kepel Leaf and Fruit Extracts
As is shown in Table 4, the water and 70% ethanolic extracts of kepel leaves and fruits without or with PEF revealed different appearances, of which the ethanolic extracts had a more greas, intense brownness than the water extracts. Stoichiometrically, amounts of KLWE, KLEE, KFWE, KFEE, KLWE^, KLEE^, KFWE^, and KFEE^ were recorded at 11.8 ± 2.6, 8.8 ± 1.5, 16.8 ± 1.9, 10.4 ± 3.2, 11.8 ± 2.6, 8.8 ± 1.5, 16.8 ± 1.9, and 10.4 ± 3.2 g/100 g FW, respectively.

Table 4.
Appearance and yields of water and ethanolic extracts obtained from kepel leaves and fruits with and without PEF(^). Amount of fresh, dry, and lyophilized kepel samples are reported in absolute values of g/100 g FW.
3.3. Total Phenolic and Flavonoid Contents in Kepel Extracts
As can be seen in Figure 2A, TPC values were 39.92 ± 0.26, 26.53 ± 1.30, 29.54 ± 0.29, 24.97 ± 0.33 mg GAE/g) (n = 3) for KLWE, KLEE, KFWE and KFEE, respectively, using conventional extractions. Using PEF-assisted extractions, TPC values were 26.26 ± 0.07, 22.38 ± 1.25, 36.38 ± 0.33 and 43.08 ± 0.40 mg GAE/g (n = 3) for KLWE^, KLEE^, KFWE^ and KFEE^, respectively. In comparison, the TPC values in KLWE were significantly higher than KLEE (p < 0.001, Tukey’s HSD, n = 3), KFWE higher than KFEE (p < 0.01) The results suggest that the leaves contained more phenolic contents than the fruits. Notably, the water extraction method revealed greater amounts of phenolic contents than the ethanolic extraction method, while PEF enhanced the release of phenolic compounds. In comparison, TFC values obtained from three separate experiments were significantly higher in KLWE (3.28 ± 0.02 mg QE/g) than in KLEE (1.70 ± 0.02 mg QE/g) (p < 0.01), in KLEE^ (5.54 ± 0.09 mg QE/g) than in KLWE^ (2.03 ± 0.05 mg QE/g), and in KFEE^ (1.68 ± 0.09 mg QE/g) than in KFWE^ (0.30 ± 0.12 mg QE/g) (Figure 2B). Taken together, the results suggest that kepel leaves are richer in phenolics and flavonoids than kepel fruits. Water is better for phenolics whereas ethanol with PEF is better for flavonoids. PEF treatment significantly improves extraction efficiency.
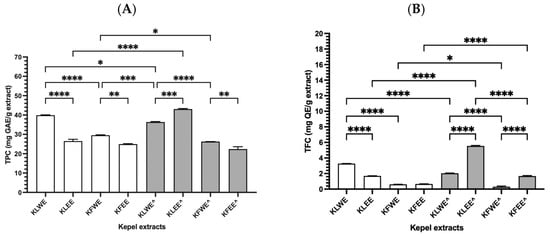
Figure 2.
TPC (A) and TFC (B) values in the water and ethanolic extracts of kepel leaves and fruits with and without PEF (^) assistance. Data obtained from triplicate analysis are presented as mean ± SD values, for which * p < 0.05, ** p < 0.01, *** p < 0.005, and **** p < 0.001.
3.4. HPLC-ESI-MS Profiles and Amounts of Phenolics in Kepel Extracts
The results of HPLC-ESI-MS analysis demonstrated that the water and ethanolic extracts obtained from kepel leaves and fruits contained gallic acid, catechins, and tannic acid; however, they were more abundant in KLEE and KFEE than in KLWE and KFWE. In addition, eriodyctoyl was found in 105.88 mg/kg of KLWE and 359.75 mg/kg of KLEE; isoquercetin in 257.26 mg/kg of KLEE and 1.88 mg/kg of KFWE; and quercetin in 29.13 mg/kg of KLEE. Nonetheless, hydroquinone, apigenin, and kaempferol were not detected in all the kepel extracts (Figure 3 and Table 5).
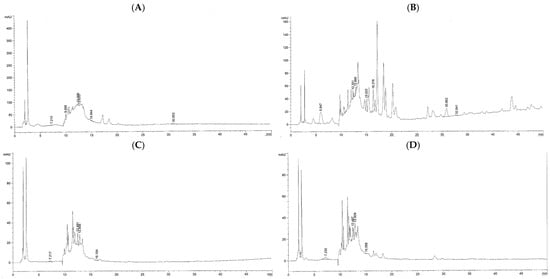
Figure 3.
HPLC-ESI-MS profile of phenolic compounds obtained from KLWE (A), KLEE (B), KFWE (C), and KFEE (D).

Table 5.
Absolute values of phenolic content obtained from KLWE, KLEE, KFWE, and KFEE samples assayed by the HPLC-ESI-MS method.
3.5. UHPLC-ESI-QTOF-MS Identification of the Phytochemicals of the Kepel Extracts
3.5.1. Phenolic Compounds
Compound identifications were based on accurate mass measurements and database/library searches using UHPLC-ESI-QTOF-MS. As authentic standards were not available for most compounds and MS/MS fragmentation data were not acquired, the results should be considered tentative or putative identifications. The findings (Figure 4) revealed a diverse profile of phenolic compounds in KLWE (A), KLEE (B), KFWE (C), and KFEE (D) samples. Correspondingly, Table 6 provides details of the tentatively identified compounds, including their retention time (minutes), observed and reference molecular weights (g/mol), mass error (ppm), and molecular formulas. The identified compounds can be categorized into five major groups as follows. Phenolic acids such as cinnamic acid, coumaric acid, ferulic acid, caffeic acid, chlorogenic acid, and sinapic acid were detected across all extracts. These were particularly abundant in ethanol-based extracts (Figure 4B,D), consistent with ethanol’s ability to solubilize medium-polarity phenolics. Flavonoids and their derivatives, including quercetin glycosides (e.g., quercetin 3-(2-glucosylrhamnoside), quercetin-3′-glucuronide), luteolin glycosides (e.g., luteolin 7-rhamnosylgalactoside, luteolin 5-malonylglucoside), and malvidin glycosides, were mainly present in the ethanol extracts. Their solubility pattern reflects the affinity of flavonoid aglycones and glycosides for alcoholic solvents. curcuminoids such as curcumin I, curcumin III, demethoxycurcumin, bisdemethoxycurcumin, hexahydrocurcumin, 5′-methoxycurcumin, and cyclocurcumin were concentrated in the ethanol extracts (KLEE and KFEE). This highlights the efficiency of ethanol in extracting hydrophobic phenolic compounds, which are poorly soluble in water. Gallic acid derivatives (e.g., gallic acid 3-O-galloylglucoside, glucogallic acid, ascorbyl-epigallocatechin gallate) were more frequently detected in water extracts (KLWE and KFWE; Figure 4A,C). Tannins are highly polar polyphenols, explaining their preferential extraction in aqueous media. A range of other bioactive compounds, including hydroxytyrosol glucoside, N-feruloyltyramine, urolithin A-glucuronide, carnosic acid, carnosol, and rosmanol derivatives were detected predominantly in ethanol extracts. These molecules are generally lipophilic, aligning with ethanol’s stronger extraction capacity for such compounds.

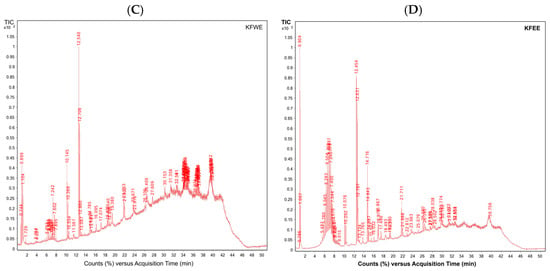
Figure 4.
UHPLC-ESI-QTOF-MS profiles of phenolics, tocopherols, tocotrienols and their derivatives obtained from KLWE (A), KLEE (B), KFWE (C), and KFEE (D).

Table 6.
Tentative existence of phenolic compounds in KLWE (A), KLEE (B), KFWE (C), and KFEE (D) assayed using UHPLC-ESI-QTOF-MS.
3.5.2. Tocopherols and Tocotrienols in Kepel Leaf and Fruit Extracts
Interestingly, UHPLC-ESI-QTOF-MS analysis tentatively showed the presence of several tocopherols, tocotrienols, and related derivatives in kepel leaf extracts (Figure 4 and Table 7). α-Tocopherol (m/z 430.38) and δ-tocotrienol (m/z 396.30) were tentatively detected in the KFEE, while α-tocotrienol (m/z 424.33) was present in both KFWE and KFEE. In contrast, γ-tocotrienol (m/z 410.31) and tocopherol quinone (m/z 446.37) were tentatively identified only in the KLWE and KLEE. Tocopherol (m/z 416.36) was consistently observed across all extracts, indicating it as the predominant and most stable vitamin E derivative in kepel leaves. Tocopherol nicotinate (m/z 535.40) appeared solely in the KLEE. These findings suggest that fermentation and extraction solvents influence the selective enrichment or degradation of specific tocopherol and tocotrienol isomers in kepel leaf preparations.

Table 7.
Existence of tocopherols and tocotrienols in KLWE (A), KLEE (B), KFWE (C), and KFEE (D) assayed using UHPLC-ESI-QTOF-MS.
3.5.3. Amounts of C, EC and EGCG
Using HPLC-DAD with authentic standards, C, EC, and EGCG were confirmed and quantified in kepel extracts. The chromatograms (Figure 5A–H) and quantitative data (Table 8) clearly demonstrate that C, EC, and EGCG contents in S. burahol extracts are strongly influenced by solvent type and PEF treatment.
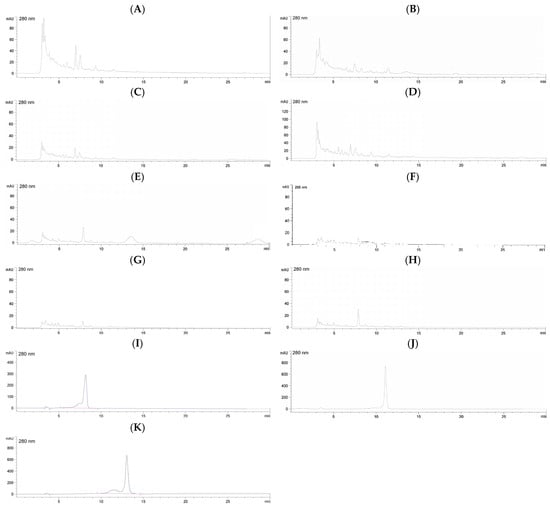
Figure 5.
HPLC-DAD profiles of catechins in KLWE (A), KLWE^ (B), KLEE (C), KLEE^ (D), KFWE (E), KFWE^ (F), KFEE (G), KFEE^ (H) (5 mg/mL each), C (I), EC (J) and EGCG (K) (1 mg/mL each). Symbol: ^ = PEF.

Table 8.
Absolute values of C, EC and EGCG contents in the water and ethanolic extracts from kepel leaves and fruits, without and with PEF treatment.
For leaf extracts, both KLWE and KLEE showed substantial increases in catechin and epicatechin levels after PEF. Catechin rose more than fivefold in water extracts (from 153.7 to 846.8 mg/g) and nearly tripled in ethanol extracts (from 335.3 to 905.1 mg/g). Similarly, epicatechin increased almost threefold in both solvents (KLWE: from 338.2 to 921.4 mg/g; KLEE: from 245.0 to 616.9 mg/g). Strikingly, EGCG, which was undetectable in untreated leaves, became abundant with PEF (799.9 mg/g in water extract and 231.9 mg/g in ethanolic extract). These results indicate that PEF disrupts leaf cell matrices, enhancing the release of flavan-3-ols and enabling detection of EGCG. In contrast, fruit extracts responded differently. Water extracts (KFWE) showed reductions in both C (from 236.7 to 136.8 mg/g) and EC (from 135.4 to 118.2 mg/g) after PEF, with EGCG completely lost (from 2892.2 mg/g to undetectable). Ethanolic extract (KFEE), however, showed modest improvements in C (from 237.8 to 289.4 mg/g) and EC (from 147.6 to 224.2 mg/g), but EGCG remained undetectable.
This suggests that PEF may degrade or destabilize certain catechins in fruit tissues, particularly EGCG, while improving recovery of other phenolics in ethanolic systems. Overall, the chromatographic profiles highlight a differential PEF effect: strongly positive in leaves, but solvent-dependent in fruits. These findings underline the importance of optimizing extraction conditions depending on plant tissue type and targeted phytochemicals.
3.5.4. Volatile Organic Compounds
GC-MS analysis of kepel fruits (Figure 6A,B) identified a diverse range of volatile organic compounds (Table 9). Regarding extraction principle and physicochemical properties of the volatiles HS-SPME is more sensitive to light, volatile monoterpenes while ethanolic extraction is more effective for heavier, less volatile, and more polar sesquiterpenes. Monoterpenes such as α-pinene, β-pinene, β-myrcene, limonene, and eucalyptol dominated the early eluting fractions, while sesquiterpenes including α-humulene, trans-β-caryophyllene, α-farnesene, and α-bisabolene were more prominent at higher retention times. Compounds were assigned MCL ranging from Level 2a (probable structure, library spectrum match) to Level 4 (unequivocal molecular formula only). The majority of identifications fell into Level 2a, supported by strong spectral matches (>90%), while a subset of sesquiterpenes and oxygenated derivatives such as nerolidol B, α-eudesmol, and bulnesol were assigned to Level 4 due to limited reference data. These findings indicate that both HS-SPME and ethanolic extraction capture complementary profiles of volatile constituents, with HS-SPME favoring more volatile monoterpenes and ethanolic extraction yielding higher molecular weight sesquiterpenes and alcohols. Both extraction approaches provided complementary qualitative profiles of the fruit’s volatile composition, highlighting the complexity of terpenoid constituents. Notably. Notably, some compounds appeared exclusively in either HS-SPME or ethanolic extracts. These differences are reported descriptively only, without inference of quantitative or statistical significance.
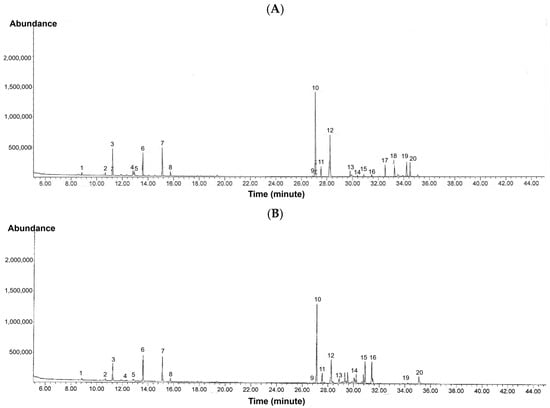
Figure 6.
GC-MS profiles of volatile organic compounds in kepel fruits obtained using (A) HS-SPME and (B) ethanolic extraction.

Table 9.
Volatile organic compounds in kepel fruits processed by HS-SPME and ethanolic extraction and identified using GC-MS.
In addition, abundances of the volatiles per HS-SPME method versus ethanolic extraction) are expressed as relative % peak area of the total ion count (TIC). As the results, kepel fruit volatiles showed the presence of twenty compounds with varying relative abundances. The dominant constituent was trans-caryophyllene (30.82% of TIC), followed by α-humulene (10.30%), terpinolene (9.75%), and myrcene (8.70%). Other notable analytes included ocimene (7.63%), δ-cadinene (4.26%), γ-gurjunene (4.22%), β-eudesmol (6.05%), and bulnesol (5.17%). Compounds present in moderate to low proportions were α-pinene, β-pinene, limonene, eucalyptol, tricyclene, bergamotene isomers, farnesene, sesquiphellandrene, bisabolene, and nerolidol B, each representing less than 2% of the total ion current.
Moreover, the principal component analysis (PCA) and heatmap plots were created based on the simulated profiles of GC-MS and illustrated for comparing the HS-SPME versus ethanolic extraction. The PCA score plot (Figure 7A) shows a clear separation of compounds along PC1, with monoterpenes clustering toward HS-SPME (more volatile) and sesquiterpenes/oxygenated sesquiterpenes clustering toward ethanolic extraction (less volatile), suggesting the complementary selectivity of the two methods have been added. The heatmap plot (Figure 7B) highlights compound-specific enrichment patterns, with HS-SPME favoring light monoterpenes (such as α-pinene, myrcene, limonene and ocimene) and ethanol favoring heavier sesquiterpenes and oxygenated derivatives (such as trans-caryophyllene, α-humulene, eudesmol and bulnesol). Color intensity corresponds to relative abundance (% of TIC).
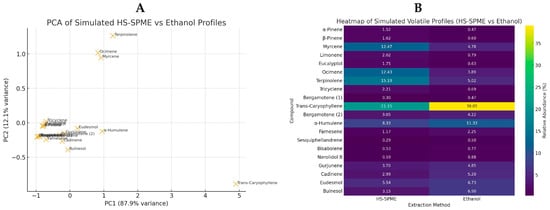
Figure 7.
PCA (A) and heatmap (B) plots of volatile compounds detected in kepel fruits using simulated HS-SPME and ethanolic extraction profiles.
Overall, the volatile profile was characterized by a predominance of sesquiterpenes, especially trans-caryophyllene and α-humulene, supported by notable levels of monoterpenes such as myrcene, terpinolene, and ocimene. The presence of oxygenated sesquiterpenes, including β-eudesmol and bulnesol, further contributed to the complexity of the aroma, suggesting a sesquiterpene-abundant composition associated with woody, spicy, and herbal notes.
4. Discussion
Plant genome size refers to the total amount of DNA in a plant cell’s nucleus. It is usually measured in pg or bp. It can vary dramatically across plant species, with some having genomes more than 2000 times larger than others. In this case, it is important to recognize that approximately 90% of the nucleotide sequence can be repetitive. A unique DNA sequence is found only once per genome (or more than once, but in small numbers). In general, micro-satellite repeats consist of short repeats of one to six (and possibly as many as 10) bases and are approximately 16–25 nucleotides in length. In addition, the genomes of each organism contain varying numbers of tandem repeats distributed throughout the genome, resulting in a high degree of diversity, which make repeated nucleotide sequences useful as molecular markers in the study of plants and animals. Interestingly, ISSR markers use only one primer per reaction to amplify the DNA fragments located between two adjacent micro-satellite regions throughout the genomes. In contrast, random amplification of polymorphic DNA markers utilizes primers containing simple sequence repeats. Thus, ISSR markers are used to study the relationships of closely related organisms in populations and detect alleles specific to any organism. Regarding the advantages of employing ISSR markers, the method is not complicated, the results can be tested quickly, and the cost is relatively low [34]. Remarkably, ISSR markers have been chosen to analyze the apple genome and to possibly reveal polymorphisms in other plants [1,35,36,37].
Previous studies have identified universal DNA barcodes from chloroplast genomes such as ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit, maturase K, transfer RNA-leucine (trnL), and transfer RNA-phenylalanine, which were used to optimize identification for the Family Annonaceae [38,39]. Recently, Probojati and colleagues used trnL intron sequences as a non-coding DNA barcode for the taxonomic identification of S. burahol and sister species complexes of the Annonaceae Family [5]. In addition, the genome sizes of other plants have been reported to be around 866.53 Mbp or 781.82 Mbp for Orophea yunnanensis and 44.7 Mbp for Carica papaya [40]. In this study, ISSR markers were employed. These markers are dominant, non-coding, non-quantitative, and are widely applied in genetic fingerprinting and diversity assessments. However, they do not provide comprehensive genome characterization since they yield only fragmentary information on genomic variation rather than quantitative or coding sequence data. As the results, the ISSR markers were successfully amplified and sequenced in S. burahol with a high-quality value. In this finding, the average DNA content was 3.96 pg per haploid genome and the genome size was 3873 Mbp. This study result has proved and confirmed the power of ISSR as a DNA barcode. It should, thus, be recommended for use in the identification of S. burahol and other species within the Annonaceae family.
By HPLC-MS analysis, ethyl acetate extract obtained from kepel fruit flesh was comprised of pelargonidin-malonylrhamnoside, 8-epiiridodial glucoside tetraacetate, EGCG, and 5-octadecenal, while ethyl acetate extract obtained from kepel fruit peel contained 1,5-dicaffeoylquinic acid, 1,6-di-O-galloylglucose, luteolin 7-O-glucoside, and 2-hydroxy-3-ethylidene-5-(methoxycarbonyl)-3,4-dihydro-2H-pyran-4-acetic acid 2-(3,4-dihydroxyphenyl)ethyl ester [4]. Moreover, antioxidant compounds, such as tocopherols, β-carotene, L-ascorbic acid, alkaloids, and saponins, as well as certain phenolics, namely flavonoids, cinnamic acid derivates, tannins, and coumarin, were reported [4]. Accordingly, these phytochemicals were found to exert antioxidant and antibacterial activities.
The present study has revealed that water and ethanolic extracts obtained from S. burahol leaves and fruits were abundant with phenolic and flavonoid contents such as catechins (HPLC-DAD analysis). Herein, the PEF operation significantly enhanced the ethanolic extraction of the phenolics and the flavonoids obtained from kepel leaves and fruits, but it did not influence the water extraction process. Notably, flavonoids are produced from S. burahol cultures in the log phase, for which the maximum production was reported on the 15th day [41]. Specifically, we highlight that while genera such as Annona, Polyalthia, and Uvaria are rich in flavonoids, phenolic acids, and acetogenins, S. burahol appears to be particularly abundant in polyphenols and flavonoids. This distinctive profile may account for its strong antioxidant activity and suggests complementary health benefits compared to its relatives
In our study, a comprehensive UHPLC-ESI-QTOF-MS technique could tentatively identify at least 72 phenolics and glycosides; α- and β-tocopherols; α- and δ-tocotrienols; γ-tocotrienol; α-tocopherolquinone; β-tocopherol; and α-tocopherol nicotinate in kepel leaves and fruits. In the findings, a broad spectrum of phenolic compounds across the four tested extracts (KLWE, KLEE, KFWE, KFEE). The tentative identifications encompassed phenolic acids, flavonoids, curcuminoids, tannins, and other bioactive phenolics, each showing distinct distribution patterns depending on the solvent used for extraction. The extraction solvent played a decisive role in shaping the phenolic composition. Water extracts (KLWE and KFWE) were particularly enriched in polar phenolic acids (e.g., cinnamic acid, caffeic acid, chlorogenic acid) and tannins/ellagitannins (e.g., gallic acid derivatives), consistently with previous reports highlighting the high polarity and water solubility of hydroxybenzoic and hydroxycinnamic acids as well as condensed tannins [42]. Conversely, ethanol extracts (KLEE and KFEE) exhibited a more complex profile dominated by curcuminoids, flavonoid glycosides, and diterpenoid phenolics, reflecting ethanol’s ability to dissolve medium- to low-polarity compounds and suggesting that the polarity index of phenolic compounds strongly dictates their extractability. Additionally, curcumin and its analogs have repeatedly been reported as predominant in ethanolic extracts of Curcuma spp. [43], while chlorogenic acid and gallic acid derivatives are often abundant in aqueous plant extracts [44].
The HPLC-DAD results reveal distinct solvent- and tissue-dependent effects of PEF on catechin recovery from S. burahol. In leaves, PEF markedly enhanced extraction of C, EC, and enabled the detection of EGCG, which was absent in untreated controls. This strong positive effect is likely due to electroporation-induced disruption of leaf cell walls and vacuoles, facilitating solvent penetration and metabolite release. Similar improvements in flavonoid recovery have been reported in other plant matrices subjected to PEF, underscoring its utility as a non-thermal intensification technique. Conversely, in fruits, PEF treatment did not enhance C recovery in water extracts and instead caused sharp reductions, particularly the complete loss of EGCG. Possibly, fruit tissues, richer in sugars, organic acids, and oxidative enzymes, may foster catechin degradation under PEF-induced stress. EGCG is known to be highly unstable and prone to oxidation, which could account for its disappearance post-treatment. The modest improvements observed in ethanolic fruit extracts suggest that ethanol may provide partial stabilization of phenolics against degradation, though not sufficient to preserve EGCG. The contrasting outcomes highlight the complexity of PEF-assisted extraction. In leaves, PEF acts as an effective enhancer of phenolic recovery, whereas in fruits its impact is solvent-dependent and may even be detrimental to certain labile compounds. This dual response underscores the need for tailored optimization of PEF parameters and solvent systems for different plant tissues. Importantly, the discovery of PEF-released EGCG in leaves expands the known phytochemical profile of kepel and supports its potential as a functional food resource.
The HS-SPME-GC-MS platform is a powerful analytical method developed for extracting and identifying volatile organic compounds. It offers the advantages of easy solvent-free sample preparation, simple manipulation, high purity of volatile analytes in the extracts, potential automation, and enhanced sensitivity. HS-SPME-GC-MS analysis demonstrated that pulp methanolic extracts contained dodecanoic acid-propanetriyl ester (lauric acid ester); the peel methanolic extract contained hexadecanoic acid methyl ester (or methyl palmitate), hexamethyl-tetracosahexaene (or squalene) and dodecanoic acid-propanetriyl ester; and the peel ethyl acetate extract contained dodecanoic acid-propanetriyl ester [4]. Wang and colleagues have reported a total of 348 volatile compounds. These included 60 esters, 55 alkenes, 45 aldehydes, 45 ketones, 37 alcohols, 20 aromatic hydrocarbons, and 66 other compounds that were identified in citrus blend black tea by using HS-SPME-GC-MS [45]. In contrast, our findings have revealed at least 20 volatile compounds from ripe kepel fruits. Regarding extraction principle and physicochemical properties of the volatiles HS-SPME is more sensitive to light, volatile monoterpenes while ethanolic extraction is more effective for heavier, less volatile, and more polar sesquiterpenes. In this study, the findings revealed that both HS-SPME and ethanolic extraction capture complementary profiles of volatile constituents, with HS-SPME favoring more volatile monoterpenes and ethanolic extraction yielding higher molecular weight sesquiterpenes and alcohols. Regarding PCA and heatmap plots, HS-SPME emphasizes the fresh, herbal aroma from monoterpenes while ethanolic extraction highlights the woody, spicy notes from sesquiterpenes, providing complementary volatile profiles of kepel fruits.
Beneficially, the sophisticated analytical instruments used in this study disclose new polar phenolic constituents in kepel leaves. The kepel leaves were found to possess herbal properties and contain volatile organic constituents, thereby potentially offering aromatic and nutraceutical benefits. Another key aspect of our findings is the role of PEF in enhancing phytochemical extraction. PEF is a nonthermal, environmentally friendly technology that permeabilizes plant cell membranes, thereby facilitating the release of phenolics, flavonoids, and other intracellular compounds. This approach preserves thermolabile bioactivities, maintains antioxidant activity, and increases overall extraction yields compared with conventional methods. In terms of the relevant limitations, there are not many kepel trees in Thailand. Moreover, kepel fruits are very expensive, as they are considered a seasonal fruit. Therefore, kepel seedlings and kepel tissue cultures need to be propagated within the country, so that the biological and pharmacological activities of kepel plants can be urgently investigated.
Collectively, we elaborated on the role of different primers (ISSR and SCoT), highlighting how their amplification of distinct genomic regions provides complementary insights into the genetic diversity of S. burahol. We also discussed the importance of integrating additional marker systems (e.g., SSRs, SNPs) in future work for higher resolution and validation. We strengthened our interpretation by emphasizing the biological relevance of the identified compound classes (flavonoids, phenolic acids, anthocyanins, fatty acid esters) and their contribution to the observed antioxidant activity. Notably, water and ethanol extractions yield complementary sets of phenolics. Water preferentially extracts highly polar compounds, while ethanol favors less polar, lipophilic bioactive phytochemicals such as curcuminoids and diterpenoids. However, the combined use of aqueous and ethanolic extractions may maximize recovery of bioactive compounds, enhancing both nutraceutical value and functional applications of the extracts. We also noted the potential synergistic effects of these compounds within the extracts. We acknowledged that some compounds were identified based on mass spectral libraries only. We have added a statement recommending validation with authentic analytical standards (e.g., EGCG, luteolin-7-O-glucoside, caffeoylquinic acid derivatives) in future studies to enhance confidence in compound identification and quantification.
5. Conclusions
Overall, the chemical compositions of the water and ethanol extracts can differ due to the differing polarity of the solvents, which can then affect the contents and antioxidant activity. Pulse electric field operation can enhance the efficiency of the solvent extractions of the flavonoids, but not the phenolics, that were obtained from kepel leaves and fruits. Among the phenolic constituents of kepel extracts, catechin, epicatechin, and epigallocatechin 3-gallate were confirmed and quantified using authentic standards, while the remaining compounds were tentatively identified by UHPLC-QTOF-MS. These findings highlight the rich phenolic composition of kepel, although further work using additional standards and MS/MS validation is needed to confirm the full phytochemical profile. Future studies should assess the extracts’ toxicities and anti-hepatic lipid deposition in cell cultures and in vivo models to provide confirmation.
Author Contributions
Conceptualization, S.S., P.K., S.J., A.Y. and A.W.; methodology, O.K., P.K., N.P., P.W. and A.Y.; formal analysis, O.K., N.P. and N.C.; investigation, O.K., N.P. and P.W.; resources, A.W.; writing—original draft preparation, O.K. and S.S.; writing—review and editing, S.S.; supervision, S.S., P.K. and N.P.; project administration, S.S. and P.K.; funding acquisition, S.S., P.K., N.P., S.J., N.C. and A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agriculture Research Development Agency (Public) (Grant number: PRP6605030970/2023) and the Faculty of Medicine Fund, Chiang Mai University (Grant number: 129/2568), Thailand. The Article Processing Charge was funded by Chiang Mai University, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge the Distinguished Research, National Research Council of Thailand (Grant number: N42A670732) for providing authentic standard catechins and myricetin for HPLC analysis. We thank Akarawat Wutttidetchotipokin for supplying the kepel fruits and leaves used in this study. We also express our sincere thanks to Sakwichai Ontong, Department of Medical Science, Ministry of Public Health, Thailand for confirming the botanical identification and description of the kepel plant.
Conflicts of Interest
Amorntip Wongmuangsinghanat, was employed by the Golden–T Siam Company Limited, Pai District, Maehongsorn Province, Thailand. Her contribution is described above. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations/Symbols
The following abbreviations and symbols are used in this manuscript:
| ANOVA | Analysis of Variance |
| bp | Base pair |
| C | Catechin |
| C | Content |
| DAD | Diode array detector |
| DI | Deionized water |
| DNA | Deoxyribonucleic acid |
| dNTPs | Deoxyribonucleotide triphosphates |
| EC | Epicatechin |
| EGCG | Epigallocatechin 3-gallate |
| ESI | Electrospray ionization |
| GA | Gallic acid |
| GAE | Gallic acid equivalent |
| GC | Gas chromatography |
| HPLC | High-performance liquid chromatography |
| HS | Headspace |
| ISSR | Inter sample sequence repeat |
| KFEE | Ethanolic extract of kepel fruits |
| KLEE | Ethanolic extract of kepel leaves |
| KFWE | Water extract of kepel fruits |
| KLWE | Water extract of kepel leaves |
| LOD | Limit of detection |
| LOQ | Limit of quantitation |
| M | Marker |
| MCL | Match Confidence Levels |
| MS | Mass spectrometry |
| m/z | Mass-to-charge ratio |
| N | Number |
| OD | Optical density |
| PCR | Polymerase chain reaction |
| PDA | Photodiode array |
| PEF, ^ | Pulsed electric field |
| PTFE | Polytetrafluoroethylene |
| Q | Quercetin |
| QE | Quercetin equivalent |
| QTOF | Quadrupole time-of-flight |
| RNase A | Ribonuclease A |
| rpm | Revolution per minute |
| S. burahol | Stelechocarpus burahol |
| SCoT | Start codon targeted |
| SPME | Solid phase microextraction |
| TBE | Tris-borate-ethylenediamine tetraacetate |
| TFC | Total flavonoid content |
| TIC | Total ion count |
| Tm | Melting temperature |
| TPC | Total phenolic content |
| trnL | Transfer RNA-leucine |
| UHPLC | Ultrahigh performance liquid chromatography |
| UV-Vis | Ultraviolet-visible |
| w/v | Weight by volume |
| °C | Degree Celsius |
References
- Joshi, S.P.; Gupta, V.S.; Aggarwal, R.K.; Ranjekar, P.K.; Brar, D.S. Genetic diversity and phylogenetic relationship as revealed by inter-simpl sequence repeat (ISSR) polymorphism in the genus Oryza. Theor. Appl. Genet. 2000, 100, 1311–1320. [Google Scholar] [CrossRef]
- Angio, M.H.; Firdiana, E.R. Kepel (Stelechocarpus burahol (Blume) Hook & Thompson), Buah Langka Khas Keraton Yogyakarta: Sebuah Koleksi Kebun Raya Purwodadi. War. Kebun Raya 2021, 19, 7–13. [Google Scholar]
- Radji, M. Bioactivity and the prospect of Stelechocarpus burahol as oral deodorant. EC Pharmacol. Toxicol. 2022, 10, 46–53. [Google Scholar]
- Sundary, D.; Handayani, D.; Suryanti, V. Chemical compositions, antioxidant and antibacterial activities of kepel (Stelechocarpus burahol) fruit flesh and peel extracts. Biodiversitas J. Biol. Divers. 2023, 24, 4668–4675. [Google Scholar] [CrossRef]
- Probojati, R.; Hadiyanti, N.; Hapsari, L. Assessment for Identification of Stelechocarpus burahol and sister species complex of Annonaceae Family using trnL intron sequences. J. Biol. Trop. 2023, 23, 643–649. [Google Scholar] [CrossRef]
- Soltis, D.E.; Soltis, P.S.; Bennett, M.D.; Leitch, I.J. Evolution of genome size in the angiosperms. Am. J. Bot. 2003, 90, 1596–1603. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Moles, A.T.; Leitch, I.J.; Bennett, M.D.; Dickie, J.B.; Knight, C.A. Correlated evolution of genome size and seed mass. New Phytol. 2007, 173, 422–437. [Google Scholar] [CrossRef]
- Smolik, M.; Krzysztoszek, O. Evaluation of genetic variability in choosen apple (Malus × domestica Borkh.) cultivars by ISSR-PCR analysis. Genetika 2010, 46, 923–931. [Google Scholar] [CrossRef]
- Wirasathien, L.; Pengsuparp, T.; Suttisri, R.; Ueda, H.; Moriyasu, M.; Kawanishi, K. Inhibitors of aldose reductase and advanced glycation end-products formation from the leaves of Stelechocarpus cauliflorus R.E. Fr. Phytomedicine 2007, 14, 546–550. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, G.; Jiang, K.; Li, C.; Qiu, C.; Deng, G. Engeletin alleviates lipopolysaccharide-induced endometritis in mice by inhibiting TLR4-mediated NF-kappaB activation. J. Agric. Food Chem. 2016, 64, 6171–6178. [Google Scholar] [CrossRef]
- Bermudez, G.; Terenzi, C.; Medri, F.; Andrisano, V.; Montanari, S. Extraction and analytical methods for the characterization of polyphenols in marine microalgae: A Review. Mar Drugs 2024, 22, 538. [Google Scholar] [CrossRef]
- Anusha Siddiqui, S.; Redha, A.A.; Esmaeili, Y.; Mehdizadeh, M. Novel insights on extraction and encapsulation techniques of elderberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 5937–5952. [Google Scholar] [CrossRef] [PubMed]
- Hutachok, N.; Angkasith, P.; Chumpun, C.; Fucharoen, S.; Mackie, I.J.; Porter, J.B.; Srichairatanakool, S. Anti-platelet aggregation and anti-cyclooxygenase activities for a range of coffee extracts (Coffea arabica). Molecules 2020, 26, 10. [Google Scholar] [CrossRef] [PubMed]
- Petry, R.D.; Ortega, G.G.; Silva, W.B. Flavonoid content assay: Influence of the reagent concentration and reaction time on the spectrophotometric behavior of the aluminium chloride—Flavonoid complex. Pharmazie 2001, 56, 465–470. [Google Scholar] [PubMed]
- Ferreira, L.; Perestrelo, R.; Caldeira, M.; Camara, J.S. Characterization of volatile substances in apples from Rosaceae family by headspace solid-phase microextraction followed by GC-qMS. J. Sep. Sci. 2009, 32, 1875–1888. [Google Scholar] [CrossRef]
- Saleem, A.; Harris, C.S.; Asim, M.; Cuerrier, A.; Martineau, L.; Haddad, P.S.; Arnason, J.T. A RP-HPLC-DAD-APCI/MSD method for the characterisation of medicinal Ericaceae used by the Eeyou Istchee Cree First Nations. Phytochem. Anal. 2010, 21, 328–339. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Algieri, F.; Rodriguez-Nogales, A.; Galvez, J.; Segura-Carretero, A. Phytochemical profiling of anti-inflammatory Lavandula extracts via RP-HPLC-DAD-QTOF-MS and -MS/MS: Assessment of their qualitative and quantitative differences. Electrophoresis 2018, 39, 1284–1293. [Google Scholar] [CrossRef]
- Ngamdokmai, N.; Ingkaninan, K.; Chaichamnong, N.; Chootip, K.; Neungchamnong, N.; Waranuch, N. Development, characterization, and stability evaluation of the anti-cellulite emgel containing herbal extracts and essential oils. Pharmaceuticals 2021, 14, 842. [Google Scholar] [CrossRef]
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of pulsed electric fields and high pressure on improved recovery of high-added-value compounds from olive pomace. J. Food Sci. 2020, 85, 1500–1512. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Polyphenol extraction from food (by) products by pulsed electric field: A Review. Int. J. Mol. Sci. 2023, 24, 15914. [Google Scholar] [CrossRef]
- Sultana, K.W.; Das, S.; Chandra, I.; Roy, A. Efficient micropropagation of Thunbergia coccinea Wall. and genetic homogeneity assessment through RAPD and ISSR markers. Sci. Rep. 2022, 12, 1683. [Google Scholar] [CrossRef]
- Paradee, N.; Howes, M.R.; Utama-Ang, N.; Chaikitwattna, A.; Hider, R.C.; Srichairatanakool, S. A chemically characterized ethanolic extract of Thai Perilla frutescens (L.) Britton fruits (nutlets) reduces oxidative stress and lipid peroxidation in human hepatoma (HuH7) cells. Phytother. Res. 2019, 33, 2064–2074. [Google Scholar] [CrossRef]
- Thikham, S.; Tongdonyod, S.; Kantala, C.; Therdtatha, P.; Klangpetch, W. Enhancing enzymatic production efficiency of crude pectic oligosaccharides by pulsed electric field and study of prebiotic potential. J. Food Sci. Technol. 2024, 61, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Salee, N.; Chaiyana, W.; Yawootti, A.; Naruenartwongsakul, S.; Klangpetch, W.; Walter, P.; Utama-Ang, N. Optimization of the pulse electric field assisted extraction of black rice grain for antioxidant and sirtuin1 enzyme stimulation activities. Sci. Rep. 2022, 12, 6459. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Claeys, M. Optimization of a liquid chromatography method based on simultaneous electrospray ionization mass spectrometric and ultraviolet photodiode array detection for analysis of flavonoid glycosides. Rapid Commun. Mass Spectrom. 2002, 16, 2341–2348. [Google Scholar] [CrossRef]
- Hodgson, A.B.; Randell, R.K.; Mahabir-Jagessar, T.K.; Lotito, S.; Mulder, T.; Mela, D.J.; Jeukendrup, A.E.; Jacobs, D.M. Acute effects of green tea extract intake on exogenous and endogenous metabolites in human plasma. J. Agric. Food Chem. 2014, 62, 1198–1208. [Google Scholar] [CrossRef]
- Prommaban, A.; Utama-Ang, N.; Chaikitwattana, A.; Uthaipibull, C.; Porter, J.B.; Srichairatanakool, S. Phytosterol, lipid and phenolic composition, and biological activities of guava seed oil. Molecules 2020, 25, 2474. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Srichairatanakool, S.; Ounjaijean, S.; Thephinlap, C.; Khansuwan, U.; Phisalpong, C.; Fucharoen, S. Iron-chelating and free-radical scavenging activities of microwave-processed green tea in iron overload. Hemoglobin 2006, 30, 311–327. [Google Scholar] [CrossRef]
- Thephinlap, C.; Ounjaijean, S.; Khansuwan, U.; Fucharoen, S.; Porter, J.B.; Srichairatanakool, S. Epigallocatechin-3-gallate and epicatechin-3-gallate from green tea decrease plasma non-transferrin bound iron and erythrocyte oxidative stress. Med. Chem. 2007, 3, 289–296. [Google Scholar] [CrossRef]
- Ma, X.W.; Su, M.Q.; Wu, H.X.; Zhou, Y.G.; Wang, S.B. Analysis of the volatile profile of core Chinese mango germplasm by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Molecules 2018, 23, 1480. [Google Scholar] [CrossRef]
- Dolezel, J.; Bartos, J.; Voglmayr, H.; Greilhuber, J. Nuclear DNA content and genome size of trout and human. Cytom. A 2003, 51, 127–128. [Google Scholar] [CrossRef]
- Powell, W.; Morgante, M.; Doyle, J.J.; McNicol, J.W.; Tingey, S.V.; Rafalski, A.J. Genepool variation in genus Glycine subgenus Soja revealed by polymorphic nuclear and chloroplast microsatellites. Genetics 1996, 144, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Prevost, A.; Wilkinson, M.J. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor. Appl. Genet. 1999, 98, 107–112. [Google Scholar] [CrossRef]
- Xue-Jun, G.; Yan, Y.; Nan-Xiao, Z.; Hai-Shan, C.H.; Wen-Qing, Q. Genetic variation in the endangered Inner Magnolia endemic shrub Tetraena mongolica Maxim. (Zygophyllaceae). Biol. Conserv. 2003, 111, 427–434. [Google Scholar]
- Smolik, M.; Rzepka-Plevneš, D.; Stankiewicz, I.; Chełpiński, P.; Kowalczys, K. Analysis of genetic similarity of apple tree cultivars. Folia Hort. 2004, 16, 87–94. [Google Scholar]
- Chaowasku, T.; Damthongdee, A.; Jongsook, H.; Ngo, D.T.; Le, H.T.; Tran, D.M.; Suddee, S. Enlarging the monotypic Monocarpieae (Annonaceae, Malmeoideae): Recognition of a second genus from Vietnam informed by morphology and molecular phylogenetics. Candollea 2018, 73, 261–275. [Google Scholar] [CrossRef]
- Guo, X.; Thomas, D.C.; Saunders, R.M.K. Gene tree discordance and coalescent methods support ancient intergeneric hybridisation between Dasymaschalon and Friesodielsia (Annonaceae). Mol. Phylogenet. Evol. 2018, 127, 14–29. [Google Scholar] [CrossRef]
- Xiaolong, Y.; Yuexian, Y.; Meihua, M.; Chuanguang, Z.; Yi, W. Prediction and analysis of genome size of Orophea yunnanensis. J. Southwest For. Univ. 2025, 45, 202–207. [Google Scholar] [CrossRef]
- Aini Habibah, N.; Moeljopawiro, S.; Dewi, K.; Indrianto, A. Flavonoid production, growth and differentiation of Stelechocarpus burahol (Bl.) Hook. F. and Th. Cell suspension culture. Pak. J. Biol. Sci. 2017, 20, 197–203. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Merlin, N.; Karling, M.; Carpes, S.T.; Alencar, S.M.; Morales, R.G.F.; Silva, E.A.D.; Pilau, E.J. Bioguided extraction of phenolic compounds and UHPLC-ESI-Q-TOF-MS/MS characterization of extracts of Moringa oleifera leaves collected in Brazil. Food Res. Int. 2019, 125, 108647. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Shi, J.; Yan, H.; Wang, M.; Ma, W.; Zhang, Y.; Peng, Q.; Chen, Y.; Lin, Z. Discrimination and identification of aroma profiles and characterized odorants in citrus blend black tea with different citrus species. Molecules 2020, 25, 4208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).







