Development of Fermented Cricket Paste and Its Characteristic Comparison with Traditional Fermented Shrimp Paste (terasi)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cricket Paste
2.2. Microbial Load Analysis
2.3. Proximate Analysis
2.4. Physiochemical Analysis
2.4.1. Physiochemical Properties
2.4.2. In Vitro Digestibility
2.4.3. Trichloroacetic Acid (TCA)-Soluble Peptide, Total Volatile Base Nitrogen (TVB-N), Thiobarbituric Acid Reactive Substances (TBARS), Reducing Sugar, Histamine, and Acrylamide Assays
2.5. Preparation of Cricket Chili Paste
2.6. Sensory Evaluation
2.6.1. Discriminative Test (Triangle Test)
2.6.2. Descriptive Intensity Rating
2.6.3. Hedonic Rating
2.7. Statistical Analysis
3. Results
3.1. Physiochemical Characteristics of Cricket Paste
3.2. Nutritional Characteristics of Cricket Paste
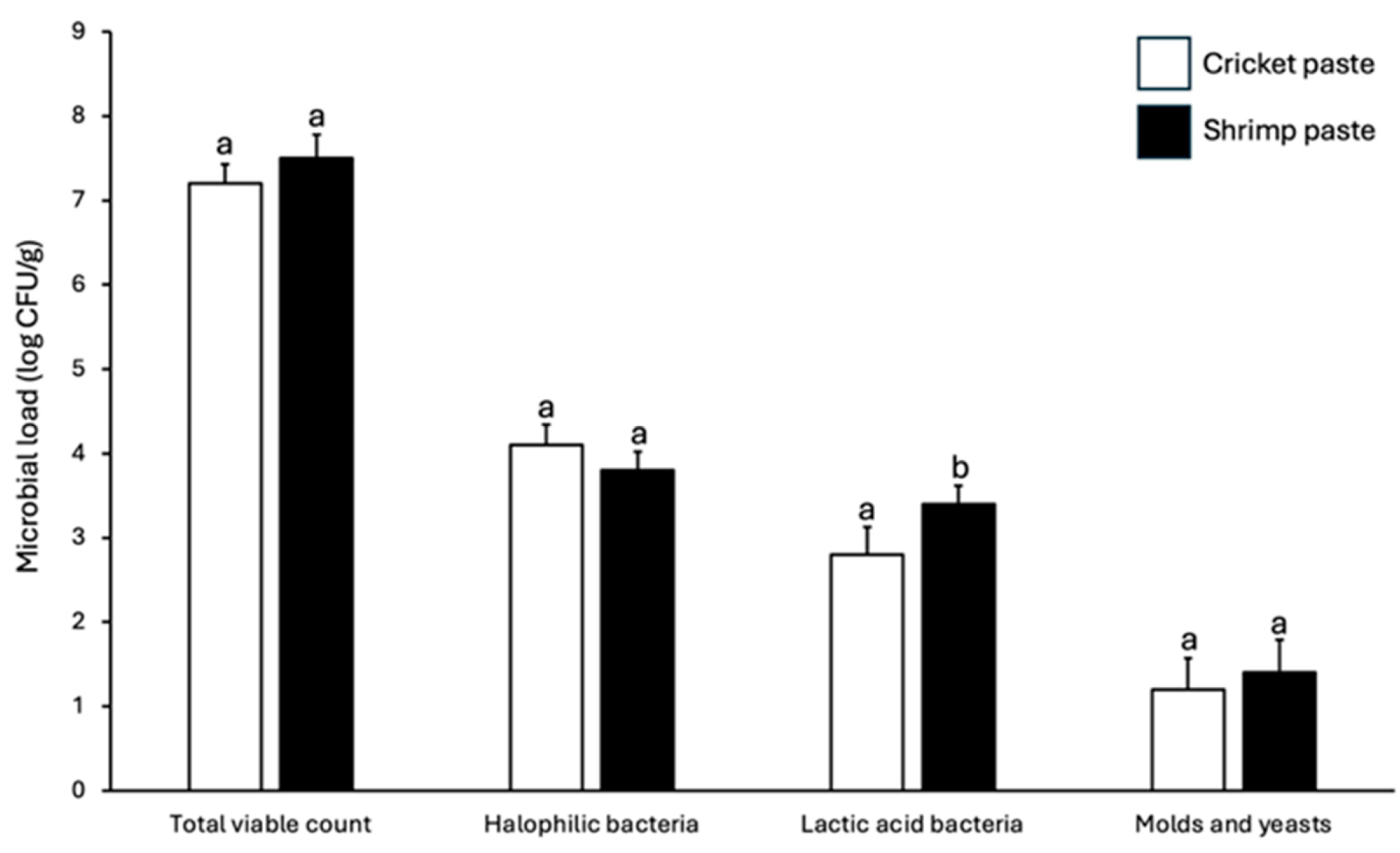
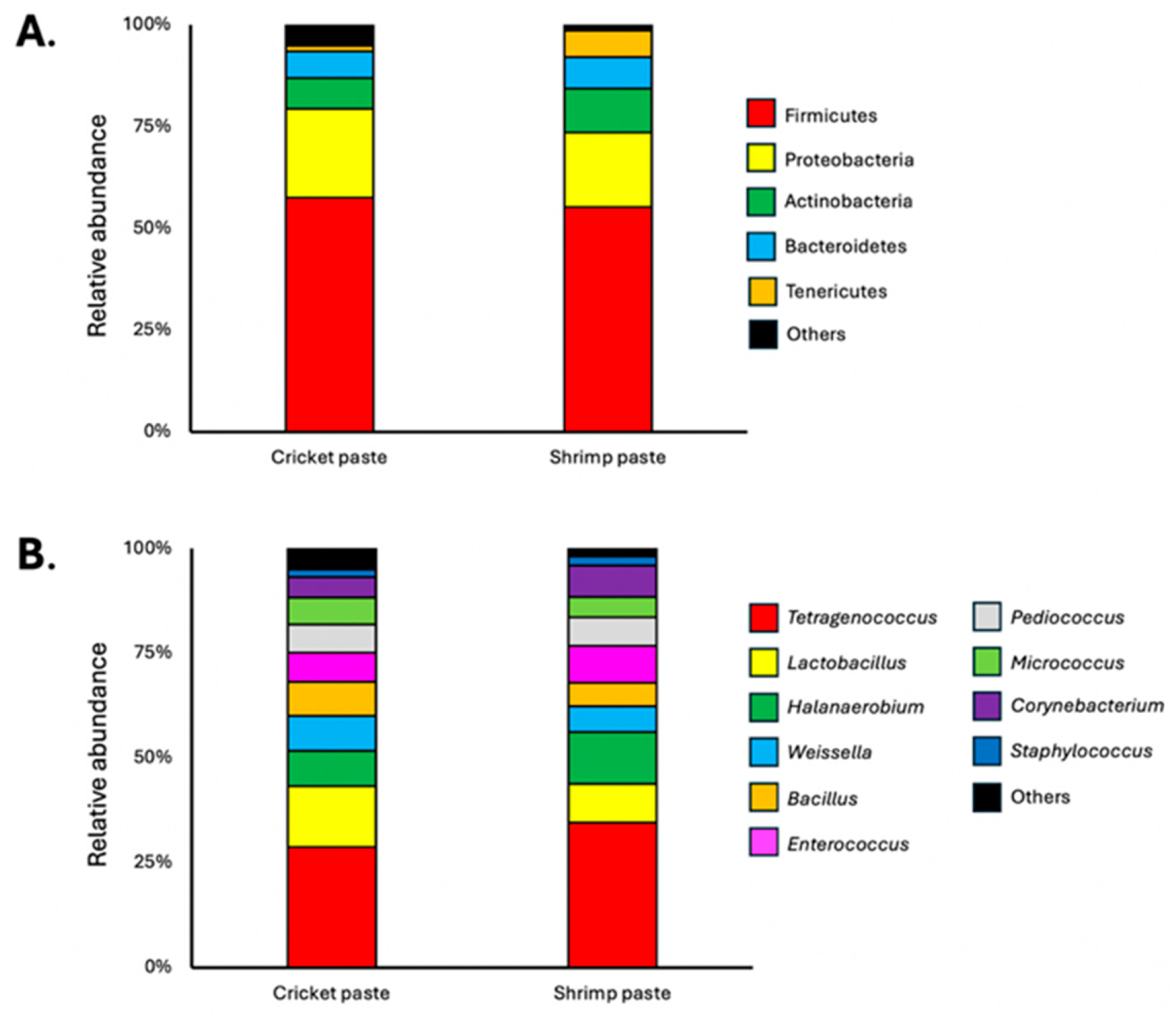
3.3. Microbiological Profile of Cricket Paste
3.4. Sensory Characteristics of Cricket Paste
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manning, D.A. How will minerals feed the world in 2050? Proc. Geol. Assoc. 2015, 126, 14–17. [Google Scholar] [CrossRef]
- Dreoni, I.; Matthews, Z.; Schaafsma, M. The impacts of soy production on multi-dimensional well-being and ecosystem services: A systematic review. J. Clean. Prod. 2022, 335, 130182. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Nascimento, A.; Arruda, A.; Sarinho, A.; Lima, J.; Batista, L.; Dantas, M.F.; Andrade, R. Unlocking the Potential of Insect-Based Proteins: Sustainable Solutions for Global Food Security and Nutrition. Foods 2024, 13, 1846. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. A Review of Traditional, Time-Honoured Foods and Recipes: To Choose to Use or Not to Use. Foods 2025, 14, 3371. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2022, 23, e13366. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, D.Y.; Mariano, E., Jr.; Park, J.; Han, D.; Kim, J.S.; Park, J.W.; Namkung, S.; Hur, S.J. A Review on the Current Research and Industrialization Status of Edible Insect Protein. Food Sci. Anim. Resour. 2025, 45, 981–997. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the World: Distribution, Nutritional Value, and Other Benefits—A Review. Front. Nutr. 2020, 7, 537915. [Google Scholar] [CrossRef] [PubMed]
- Hanboonsong, Y.; Jamjanya, T.; Durst, P.B. Six-Legged Livestock: Edible Insect Farming, Collection and Marketing in Thailand; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Suckling, J.; Druckman, A.; Moore, C.D.; Driscoll, D. The environmental impact of rearing crickets for live pet food in the UK, and implications of a transition to a hybrid business model combining production for live pet food with production for human consumption. Int. J. Life Cycle Assess. 2020, 25, 1693–1709. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Itterbeeck, J.; Heetkamp, M.J.; Van Den Brand, H.; Van Loon, J.J.; Van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, F.; Lu, Y.; Wang, J.; Chen, J.; Yu, Y.; Tao, X.; Xiao, Y.; Peng, Y. A review on edible insects in China: Nutritional supply, environmental benefits, and potential applications. Curr. Res. Food Sci. 2023, 7, 100596. [Google Scholar] [CrossRef] [PubMed]
- Kröger, T.; Dupont, J.; Büsing, L.; Fiebelkorn, F. Acceptance of Insect-Based Food Products in Western Societies: A Systematic Review. Front. Nutr. 2021, 8, 759885. [Google Scholar] [CrossRef]
- Surya, R. Fermented foods of Southeast Asia other than soybean-or seafood-based ones. J. Ethn. Foods 2024, 11, 27. [Google Scholar] [CrossRef]
- Castro-López, C.; Santiago-López, L.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Liceaga, A.; Garcia, H.; Hernandez, A. An insight to fermented edible insects: A global perspective and prospective. Food Res. Int. 2020, 137, 109750. [Google Scholar] [CrossRef]
- Kurnianto, M.A.; Adirama, S.I.; Xu, W.; Winarti, S.; Rini, D.M. Enhancing the Quality of Traditional Indonesian Shrimp Paste (Terasi) Through Tetragenococcus halophilus 54M106-3 Inoculation: Physicochemical, Sensory, and Bioactivity Insights. Foods 2025, 14, 2419. [Google Scholar] [CrossRef]
- Surya, R.; Tedjakusuma, F. Diversity of sambals, traditional Indonesian chili pastes. J. Ethn. Foods 2022, 9, 25. [Google Scholar] [CrossRef]
- Surya, R.; Nugroho, D.; Kamal, N.; Tedjakusuma, F. Effects of fermentation time on chemical, microbiological, antioxidant, and organoleptic properties of Indonesian traditional shrimp paste, terasi. Int. J. Gastron. Food Sci. 2023, 31, 100643. [Google Scholar] [CrossRef]
- Tedjakusuma, F.; Linggadiputra, J.; Cahya, A.D.; Surya, R. Development of cricket flour-enriched cookies. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1115, p. 012092. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Boonchuen, P. Changes in volatile compounds and quality characteristics of salted shrimp paste stored in different packaging containers. Fermentation 2022, 8, 69. [Google Scholar] [CrossRef]
- Daroonpunt, R.; Uchino, M.; Tsujii, Y.; Kazami, M.; Oka, D.; Tanasupawat, S. Chemical and physical properties of Thai traditional shrimp paste (Ka-pi). J. Appl. Pharm. Sci. 2016, 6, 058–062. [Google Scholar] [CrossRef]
- Namwong, S.; Tanasupawat, S.; Lee, K.C.; Lee, J.-S. Oceanobacillus kapialis sp. nov., from fermented shrimp paste in Thailand. Int. J. Syst. Evol. Microbiol. 2009, 59, 2254–2259. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Sampavapol, P.; Osako, K.; Faithong, N. Chemical composition and physical properties of salted shrimp paste (Kapi) produced in Thailand. Int. Aquat. Res. 2014, 6, 155–166. [Google Scholar] [CrossRef]
- Smith, S.D. Quantifying color variation: Improved formulas for calculating hue with segment classification. Appl. Plant Sci. 2014, 2, 1300088. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Surya, R.; Megumi, E.; Rombot, O.; Nugroho, D.; Tedjakusuma, F. Supplementation of red alga (Porphyra) improves nutritional profile, protein digestibility and sensory acceptance of tempeh. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2024; p. 012078. [Google Scholar]
- Lie, E.; Surya, R. Effects of matcha supplementation on in vitro digestibility and sensory acceptance of soy milk. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2024; p. 012085. [Google Scholar]
- Rujirapong, C.; Siripongvutikorn, S.; Usawakesmanee, W.; Wanikorn, B. Quality of reduced sodium shrimp paste from shrimp head as alternative source. Food Sci. Technol. 2022, 42, e36921. [Google Scholar] [CrossRef]
- Krivorotova, T.; Sereikaite, J. Determination of fructan exohydrolase activity in the crude extracts of plants. Electron. J. Biotechnol. 2014, 17, 329–333. [Google Scholar] [CrossRef]
- El Hosry, L.; Elias, V.; Chamoun, V.; Halawi, M.; Cayot, P.; Nehme, A.; Bou-Maroun, E. Maillard Reaction: Mechanism, Influencing Parameters, Advantages, Disadvantages, and Food Industrial Applications: A Review. Foods 2025, 14, 1881. [Google Scholar] [CrossRef] [PubMed]
- Sandulachi, E. Water activity concept and its role in food preservation. Meridian Ing. 2012, 20, 40–48. [Google Scholar]
- Hamad, S.H. Factors affecting the growth of microorganisms in food. Prog. Food Preserv. 2012, 405–427. [Google Scholar]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Rael, L.T.; Thomas, G.W.; Craun, M.L.; Curtis, C.G.; Bar-Or, R.; Bar-Or, D. Lipid peroxidation and the thiobarbituric acid assay: Standardization of the assay when using saturated and unsaturated fatty acids. BMB Rep. 2004, 37, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Oktariani, A.F.; Ramona, Y.; Sudaryatma, P.E.; Dewi, I.A.M.M.; Shetty, K. Role of Marine Bacterial Contaminants in Histamine Formation in Seafood Products: A Review. Microorganisms 2022, 10, 1197. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An Overview of Histamine and Other Biogenic Amines in Fish and Fish Products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef]
- FDA. FDA Issues Draft Compliance Policy Guide for Decomposition and Histamine in Scombrotoxin (Histamine)-Forming Fish and Fishery Products. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-issues-draft-compliance-policy-guide-decomposition-and-histamine-scombrotoxin-histamine-forming (accessed on day month year).
- Kitagawa, G.; Mizuta, T.; Akamatsu, M.; Ifuku, S. High-yield chitin extraction and nanochitin production from cricket legs. Carbohydr. Polym. Technol. Appl. 2025, 10, 100816. [Google Scholar] [CrossRef]
- da Costa, W.K.A.; de Souza, G.T.; Brandão, L.R.; de Lima, R.C.; Garcia, E.F.; dos Santos Lima, M.; de Souza, E.L.; Saarela, M.; Magnani, M. Exploiting antagonistic activity of fruit-derived Lactobacillus to control pathogenic bacteria in fresh cheese and chicken meat. Food Res. Int. 2018, 108, 172–182. [Google Scholar] [CrossRef]
- Agüero, N.d.L.; Frizzo, L.S.; Ouwehand, A.C.; Aleu, G.; Rosmini, M.R. Technological Characterisation of Probiotic Lactic Acid Bacteria as Starter Cultures for Dry Fermented Sausages. Foods 2020, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Jonas-Levi, A.; Martinez, J.-J.I. The high level of protein content reported in insects for food and feed is overestimated. J. Food Compos. Anal. 2017, 62, 184–188. [Google Scholar] [CrossRef]
- Xu, M.-L.; Gao, Y.; Han, X.X. Structure information analysis and relative content determination of protein and chitin from yellow mealworm larvae using Raman spectroscopy. Int. J. Biol. Macromol. 2024, 272, 132787. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Kentra, E.; Starkute, V.; Klupsaite, D.; Mockus, E.; Zokaityte, G.; Cernauskas, D.; Rocha, J.M.; Guiné, R.P.F. Characterisation of Lacto-Fermented Cricket (Acheta domesticus) Flour and Its Influence on the Quality Parameters and Acrylamide Formation in Wheat Biscuits. Fermentation 2023, 9, 153. [Google Scholar] [CrossRef]
- Ma, X.; Sang, X.; Yan, C.; Zhang, Y.; Bi, J.; Zhang, G.; Hao, H.; Hou, H. Dynamics of Bacterial Composition and Association with Quality Formation and Biogenic Amines Accumulation during Fish Sauce Spontaneous Fermentation. Appl. Env. Microbiol. 2022, 88, e0069022. [Google Scholar] [CrossRef]
- Herlina, V.T.; Setiarto, R.H.B. Terasi, exploring the Indonesian ethnic fermented shrimp paste. J. Ethn. Foods 2024, 11, 7. [Google Scholar] [CrossRef]
- Maeda, M.; Shibata, A.; Biswas, G.; Korenaga, H.; Kono, T.; Itami, T.; Sakai, M. Isolation of lactic acid bacteria from kuruma shrimp (Marsupenaeus japonicus) intestine and assessment of immunomodulatory role of a selected strain as probiotic. Mar. Biotechnol. 2014, 16, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Srygley, R.B.; Healy, F.; Swaminath, K.; Mueller, U.G. Spatial Structure of the Mormon Cricket Gut Microbiome and its Predicted Contribution to Nutrition and Immune Function. Front. Microbiol. 2017, 8, 801. [Google Scholar] [CrossRef]
- Yao, W.; Yang, L.; Shao, Z.; Xie, L.; Chen, L. Identification of salt tolerance-related genes of Lactobacillus plantarum D31 and T9 strains by genomic analysis. Ann. Microbiol. 2020, 70, 10. [Google Scholar] [CrossRef]
- Vasilica, B.T.B.; Chiș, M.S.; Alexa, E.; Pop, C.; Păucean, A.; Man, S.; Igual, M.; Haydee, K.M.; Dalma, K.E.; Stănilă, S.; et al. The Impact of Insect Flour on Sourdough Fermentation-Fatty Acids, Amino-Acids, Minerals and Volatile Profile. Insects 2022, 13, 576. [Google Scholar] [CrossRef]
- Udomsil, N.; Rodtong, S.; Choi, Y.J.; Hua, Y.; Yongsawatdigul, J. Use of Tetragenococcus halophilus as a starter culture for flavor improvement in fish sauce fermentation. J. Agric. Food Chem. 2011, 59, 8401–8408. [Google Scholar] [CrossRef]
- Udomsil, N.; Chen, S.; Rodtong, S.; Yongsawatdigul, J. Improvement of fish sauce quality by combined inoculation of Tetragenococcus halophilus MS33 and Virgibacillus sp. SK37. Food Control 2017, 73, 930–938. [Google Scholar] [CrossRef]

| Unit | Cricket Paste | Shrimp Paste | |
|---|---|---|---|
| Color analysis | |||
| L* (lightness) | - | 32.5 ± 2.1 b | 42.7 ± 1.8 a |
| a* (red-green) | - | 7.3 ± 0.29 b | 12.3 ± 0.6 a |
| b* (yellow-blue) | - | 14.8 ± 1.2 b | 9.6 ± 0.7 a |
| ΔE | - | 9.9 ± 1.5 | 0 |
| Chemical analysis | |||
| pH | - | 6.3 ± 0.1 b | 6.5 ± 0.1 a |
| Water activity (aw) | - | 0.74 ± 0.01 b | 0.78 ± 0.01 a |
| Moisture content | %w/w | 42.2 ± 1.5 a | 48.4 ± 1.7 b |
| TCA-soluble peptides | mg tyrosine equivalent/g | 8.62 ± 0.49 b | 12.15 ± 0.58 a |
| Total volatile base nitrogen | mg N/100 g | 80.61 ± 2.13 b | 92.74 ± 4.23 a |
| TBARS | ppm MDA | 1.94 ± 0.13 b | 3.27 ± 0.15 a |
| Reducing sugars | mg GE/g | 16.69 ± 1.09 a | 9.42 ± 0.77 b |
| Histamine | ppm | 2.37 ± 0.09 b | 50.51 ± 1.65 a |
| Acrylamide | ppb | 52.76 ± 5.64 b | 41.19 ± 3.83 a |
| Unit | Cricket Paste | Shrimp Paste | |
|---|---|---|---|
| Ash/minerals | %w/w | 12.76 ± 3.85 a | 15.22 ± 4.41 a |
| Protein (crude) | %w/w | 77.58 ± 3.17 b | 72.46 ± 2.82 a |
| Protein (chitin-corrected) | %w/w | 67.38 ± 2.58 a | 65.29 ± 2.19 a |
| Fat | %w/w | 1.39 ± 0.22 a | 8.36 ± 0.75 b |
| Carbohydrate (including chitin) | %w/w | 18.47 ± 1.27 b | 11.13 ± 0.89 a |
| Protein digestibility | % | 80.41 ± 6.13 b | 69.67 ± 4.19 a |
| Lipid digestibility | % | 71.15 ± 5.33 a | 74.26 ± 4.65 a |
| Sample | No. of Panelists | No. of Correct Judgments to Achieve (α = 0.05, p = 1/3) | No. of Correct Judgements |
|---|---|---|---|
| Cricket paste vs. shrimp paste | 84 | 36 | 62 |
| Cricket chili paste vs. shrimp chili paste | 84 | 36 | 49 |
| Cricket Chili Paste | Shrimp Chili Paste | |
|---|---|---|
| Appearance | 7.16 ± 0.95 a | 7.22 ± 0.83 a |
| Aroma | 7.28 ± 1.27 a | 7.49 ± 1.17 a |
| Taste | 6.95 ± 0.92 a | 7.26 ± 0.85 a |
| Overall rate | 7.19 ± 1.39 a | 7.36 ± 1.08 a |
| Overall rank | 1.58 ± 0.13 a | 1.42 ± 0.17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surya, R.; Tedjakusuma, F.; Petsong, K.; Thinthasit, A.; Nugroho, D. Development of Fermented Cricket Paste and Its Characteristic Comparison with Traditional Fermented Shrimp Paste (terasi). Foods 2025, 14, 3562. https://doi.org/10.3390/foods14203562
Surya R, Tedjakusuma F, Petsong K, Thinthasit A, Nugroho D. Development of Fermented Cricket Paste and Its Characteristic Comparison with Traditional Fermented Shrimp Paste (terasi). Foods. 2025; 14(20):3562. https://doi.org/10.3390/foods14203562
Chicago/Turabian StyleSurya, Reggie, Felicia Tedjakusuma, Kantiya Petsong, Aphinya Thinthasit, and David Nugroho. 2025. "Development of Fermented Cricket Paste and Its Characteristic Comparison with Traditional Fermented Shrimp Paste (terasi)" Foods 14, no. 20: 3562. https://doi.org/10.3390/foods14203562
APA StyleSurya, R., Tedjakusuma, F., Petsong, K., Thinthasit, A., & Nugroho, D. (2025). Development of Fermented Cricket Paste and Its Characteristic Comparison with Traditional Fermented Shrimp Paste (terasi). Foods, 14(20), 3562. https://doi.org/10.3390/foods14203562






