Identification of Key Aroma Substances in Pomegranate from Different Geographical Origins via Integrated Volatile Profiling and Multivariate Statistical Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. E-Nose Analysis
2.3. E-Tongue Analysis
2.4. HS-GC-IMS Analysis
2.5. HS-SPME-GC-MS Analysis
2.6. ROAV Analysis
2.7. Data Analysis
3. Results and Discussion
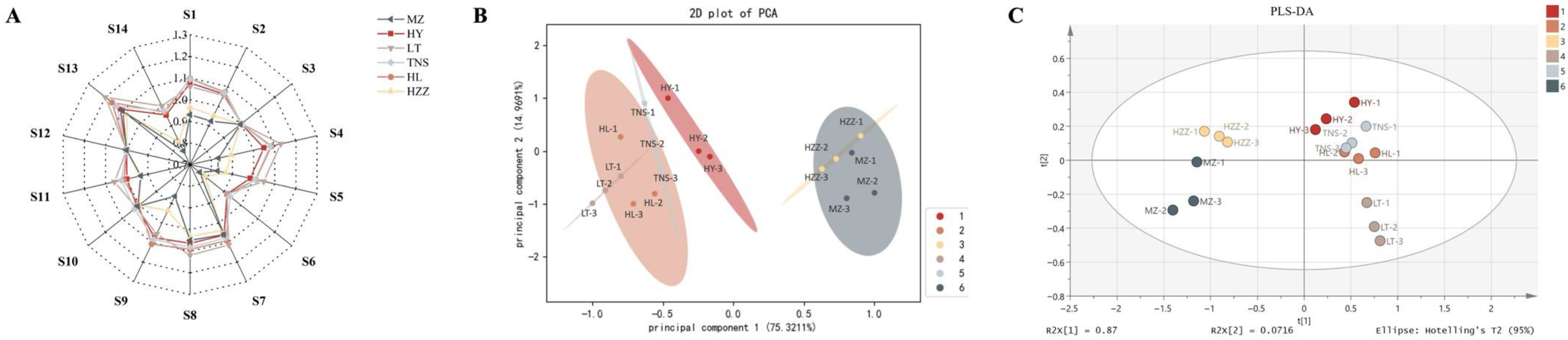
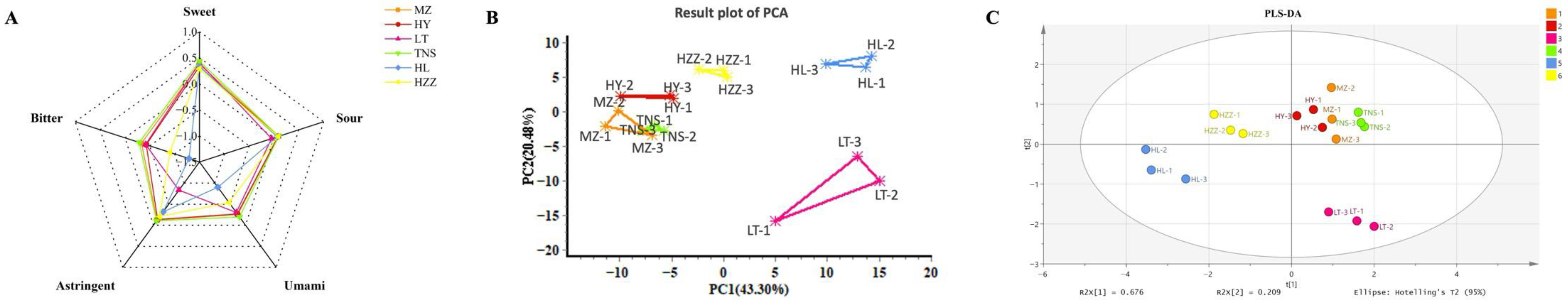
3.1. E-Nose Analysis
3.2. E-Tongue Analysis
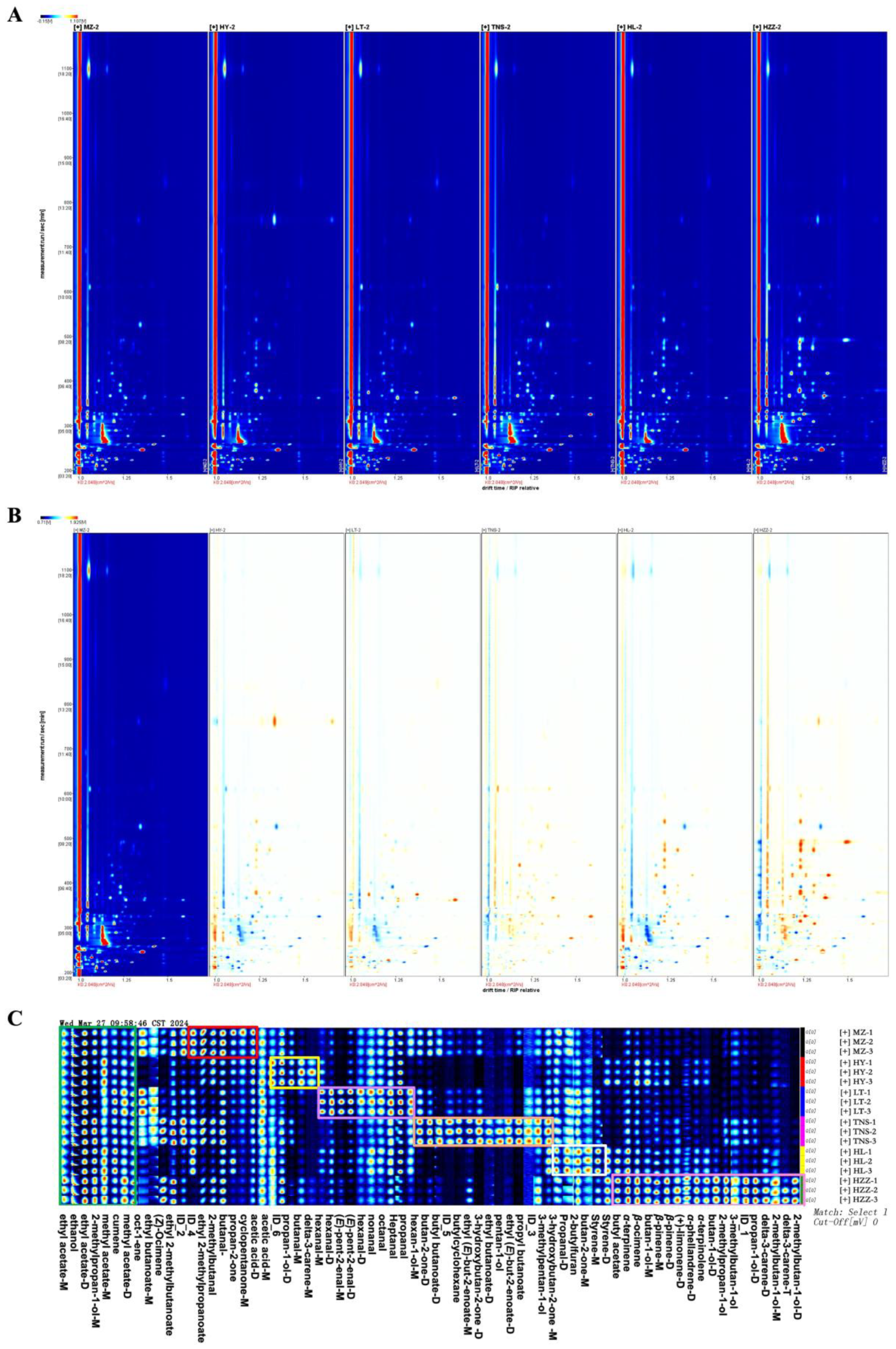
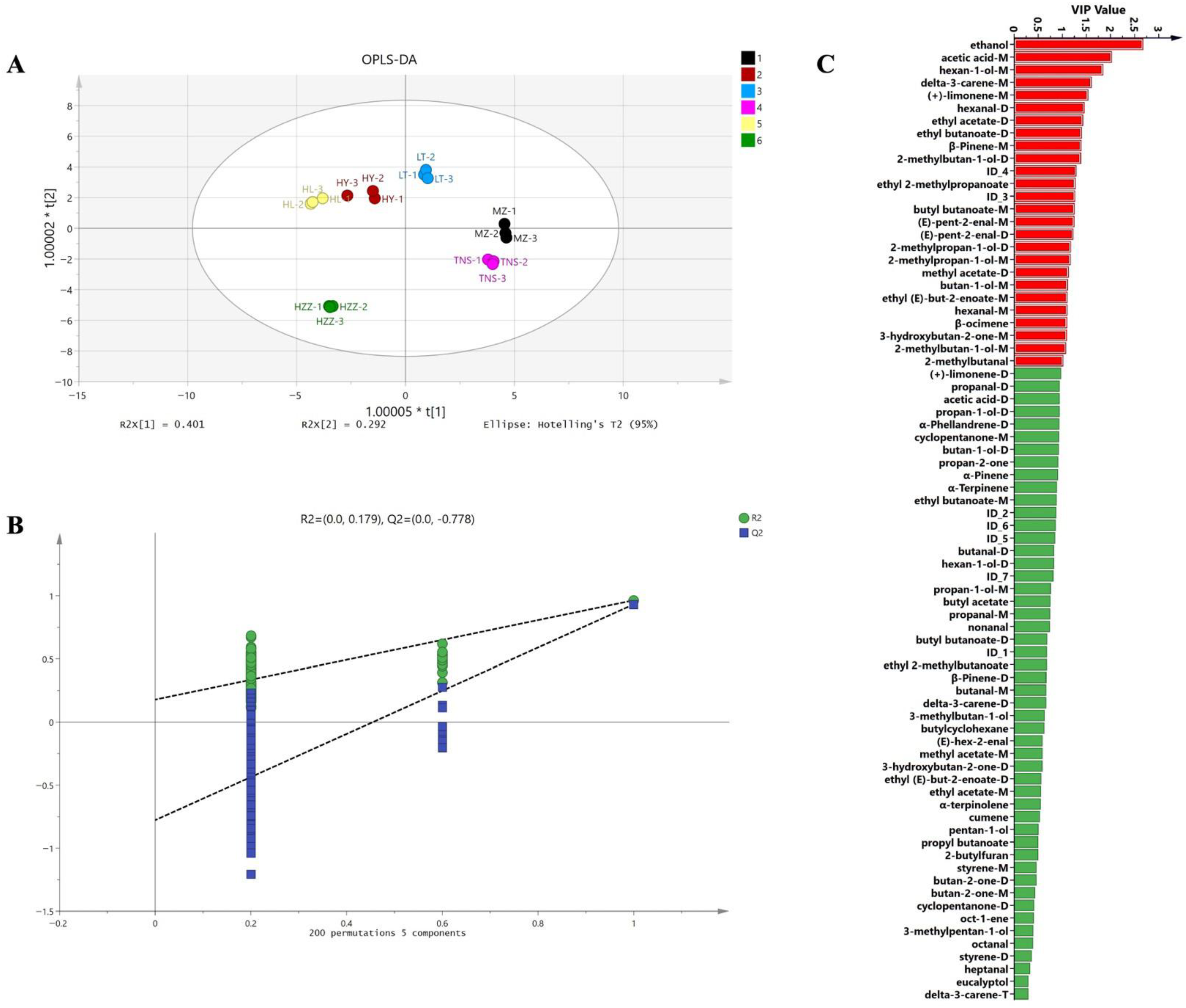
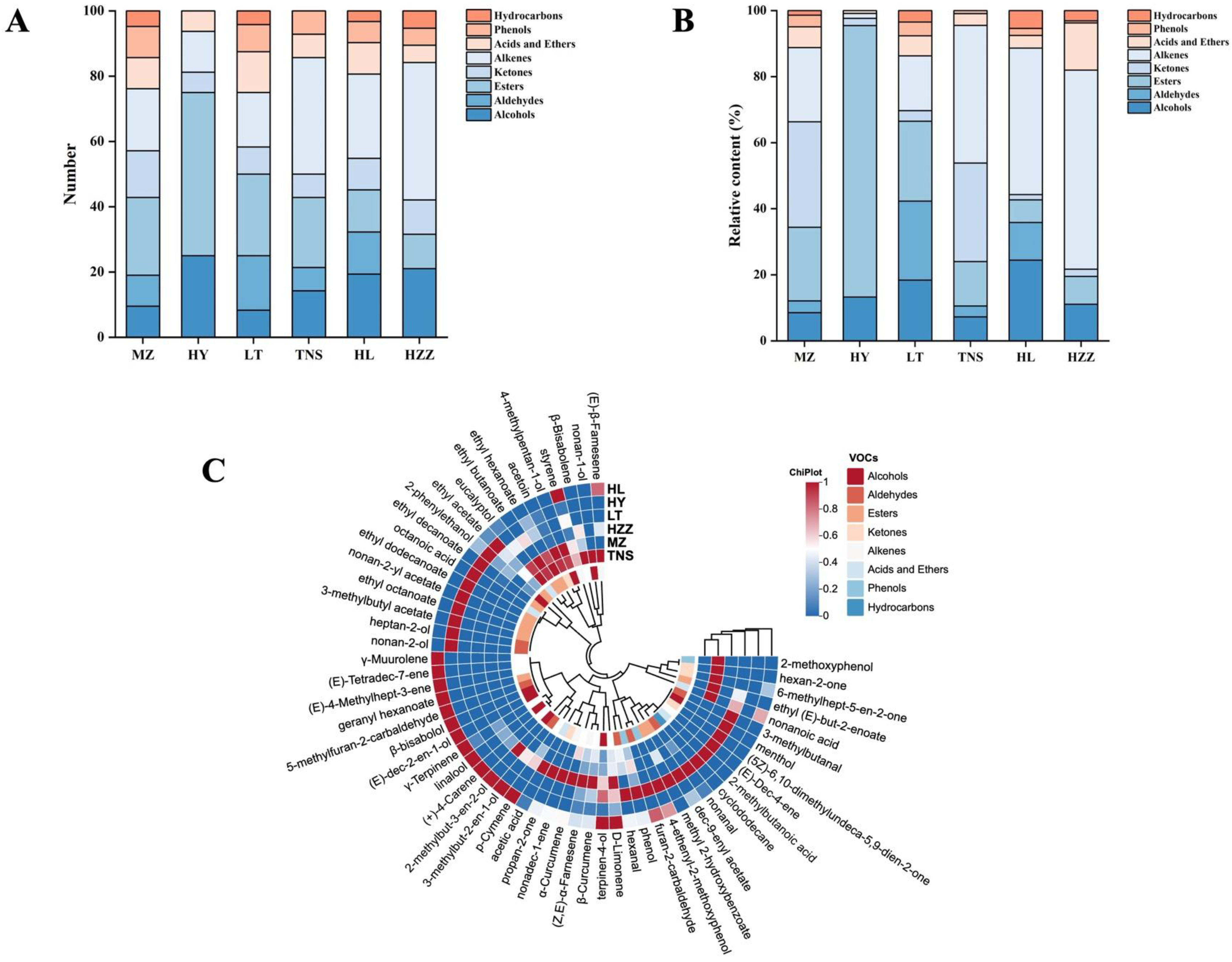
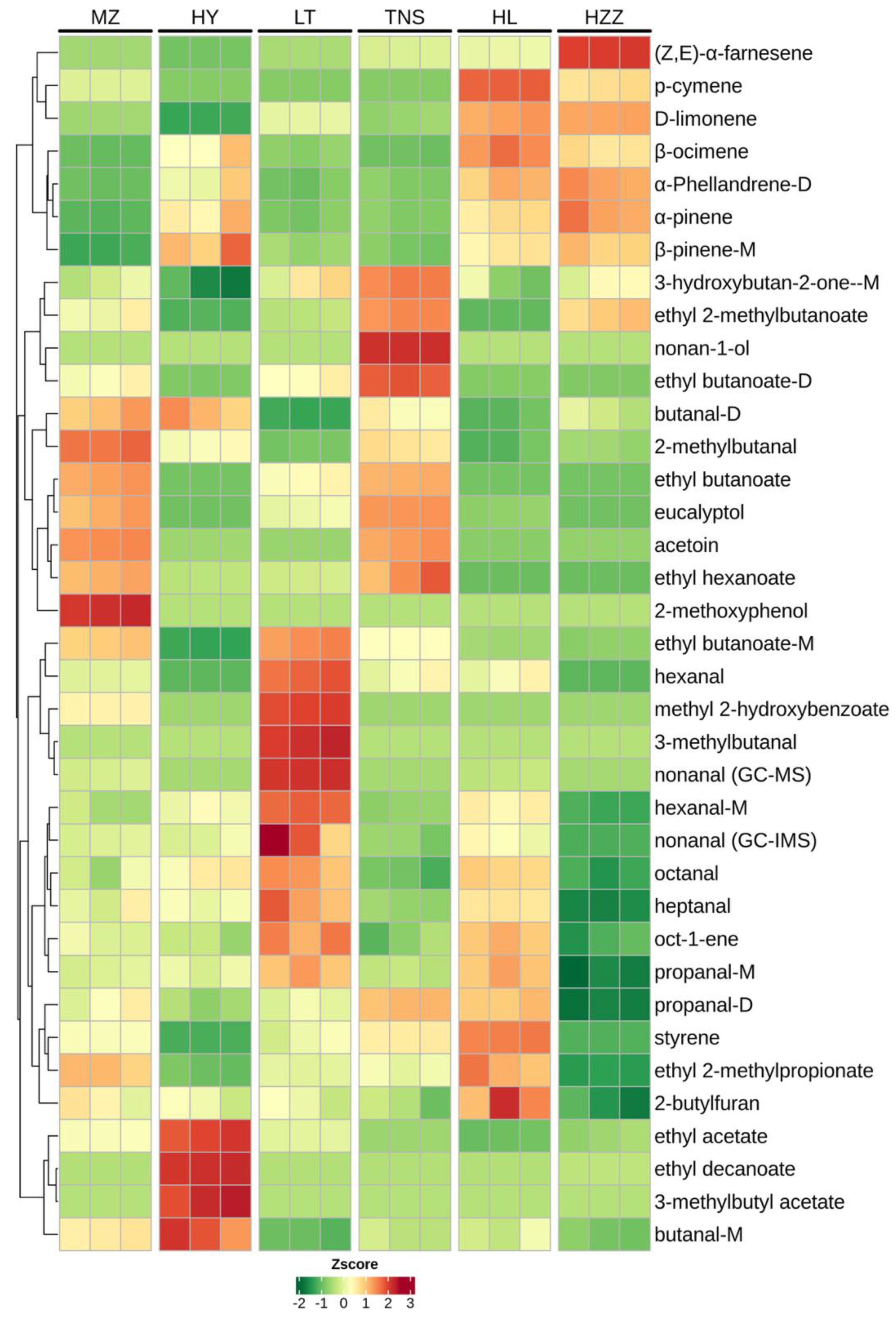
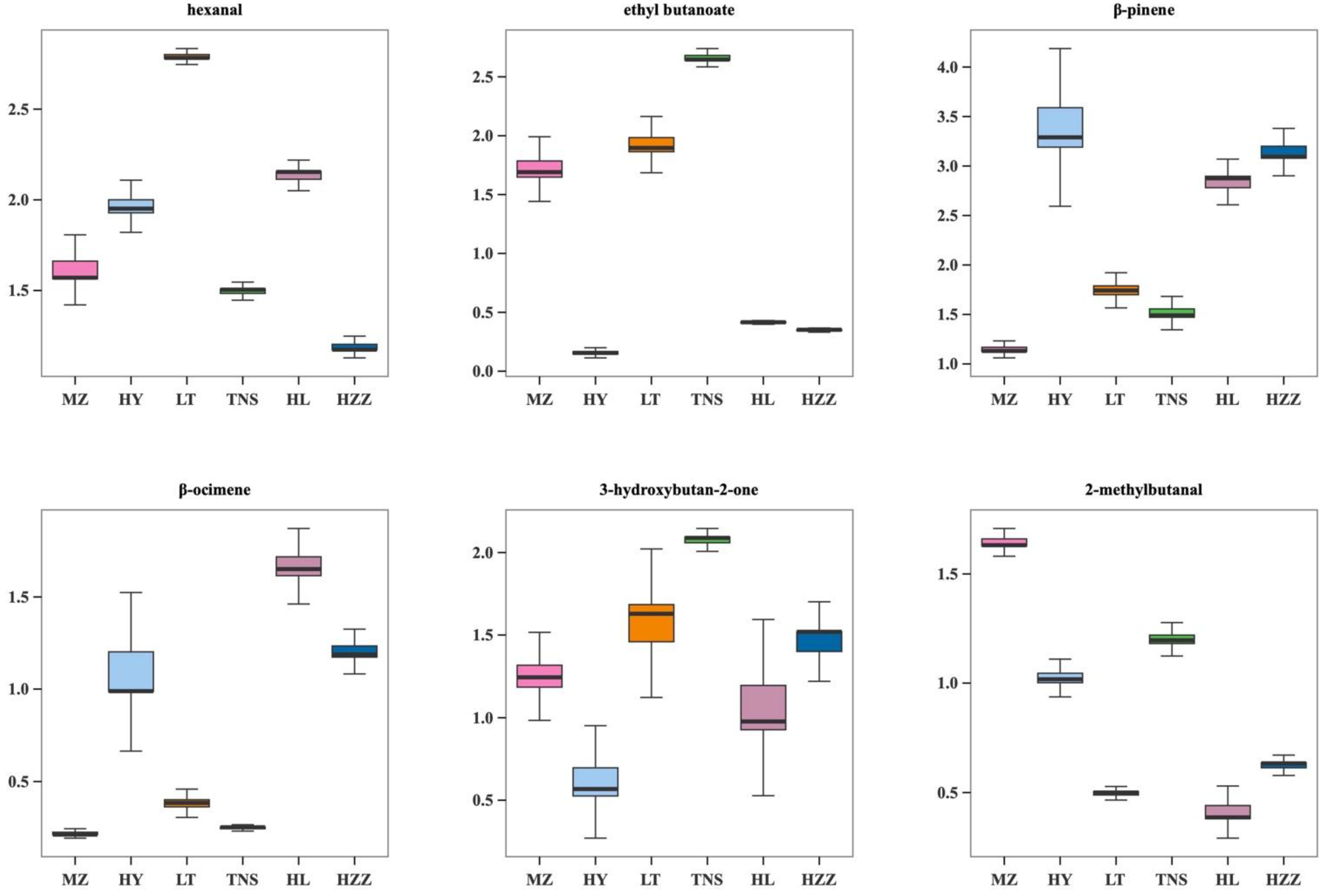
3.3. Volatile Compounds in Pomegranate from Different Origins Were Characterized via HS-GC-IMS
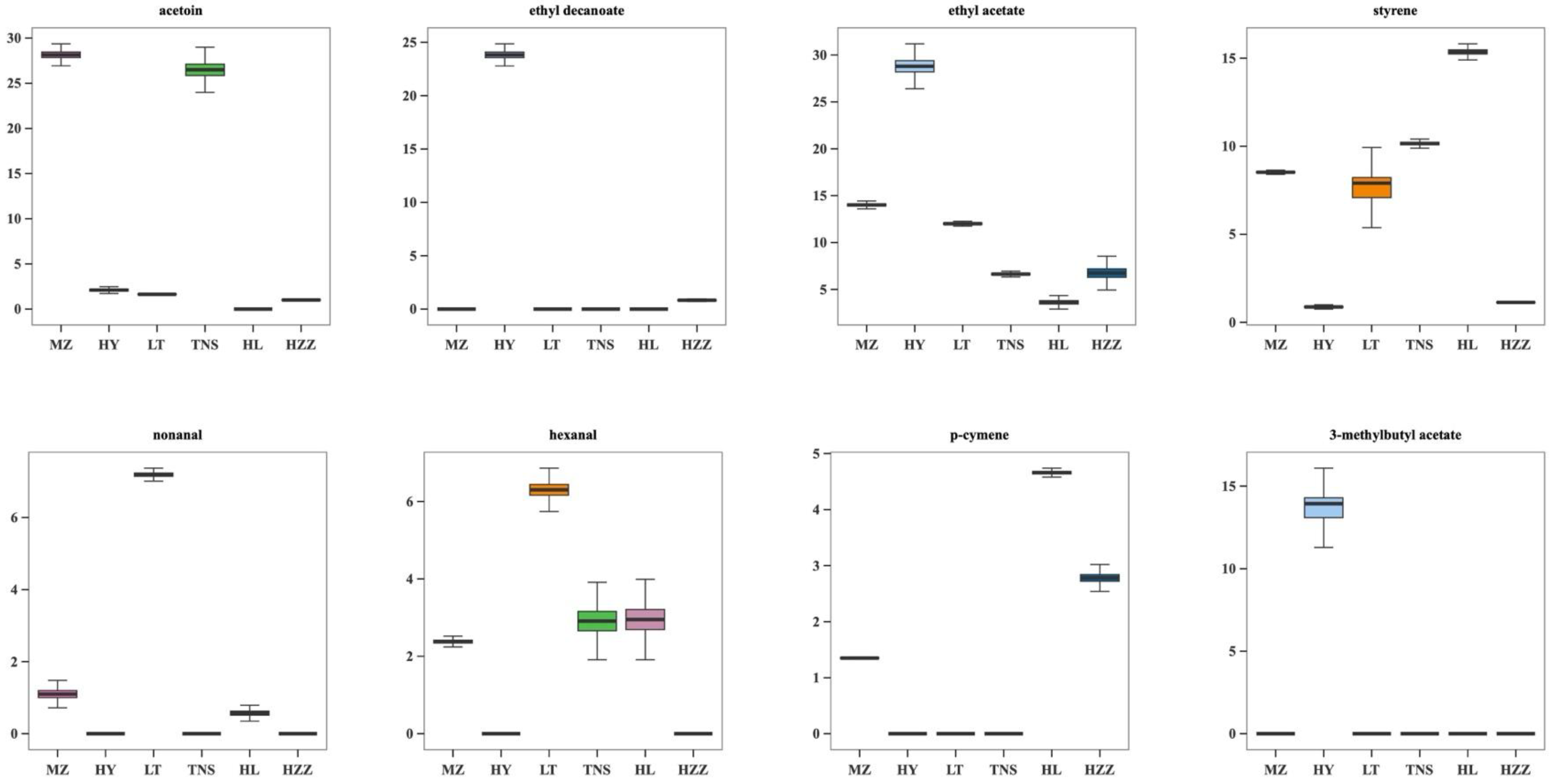
3.4. Volatile Compounds in Pomegranate from Different Origins Were Characterized via HS-SPME-GC-MS
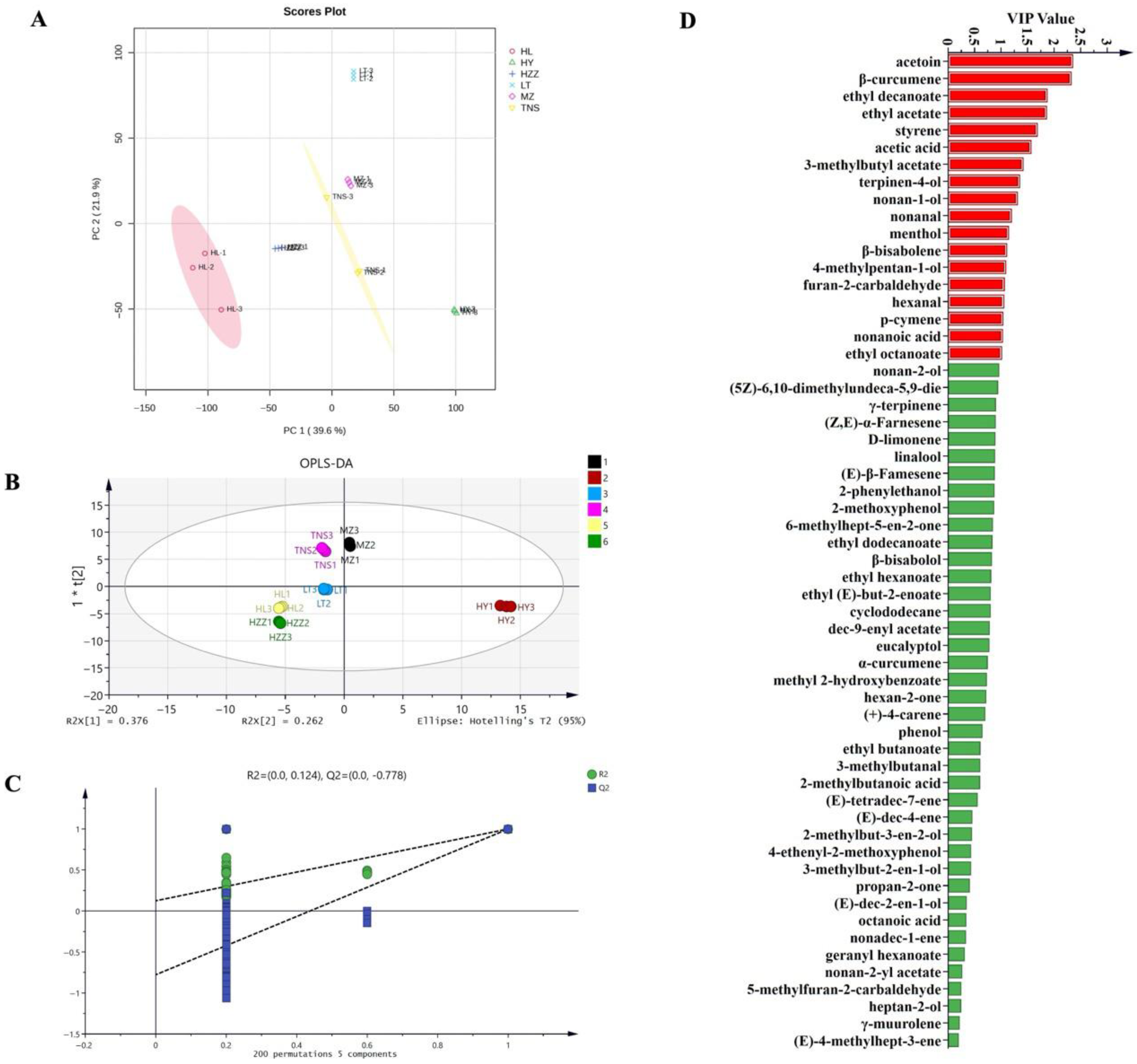
3.5. Key Aroma Substances Analysis in Pomegranate from Different Origins
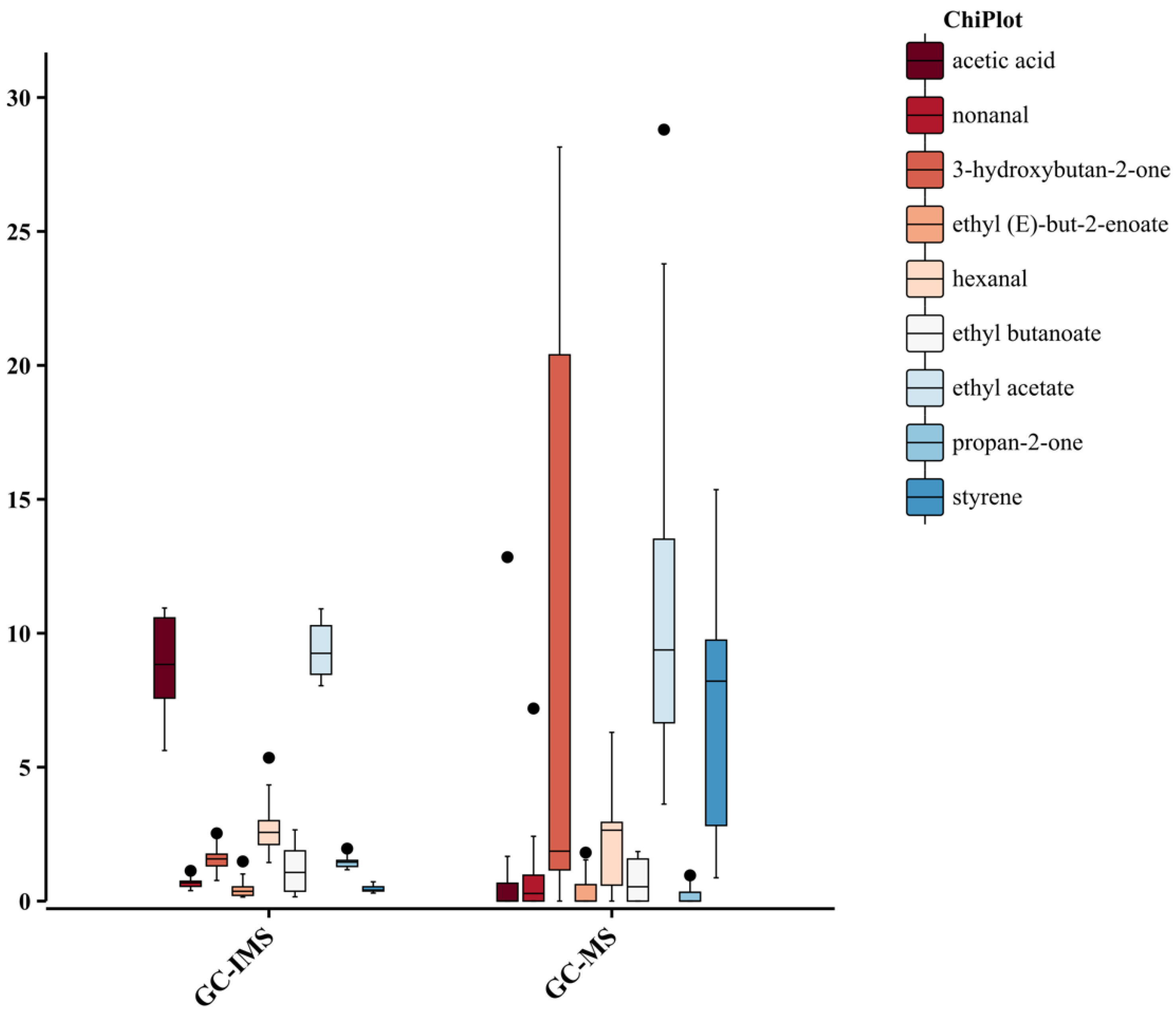
3.6. Comparison of the Ability of HS-GC-IMS and HS-SPME-GC-MS to Identify of Main Biomarkers and Aroma Characteristics of Pomegranate from Different Origins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and Potential Health Benefits of Pomegranate: A Review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef]
- Yuan, Z.; Yin, Y.; Qu, J.; Zhu, L.; Li, Y. Population genetic diversity in Chinese pomegranate (Punica granatum L.) cultivars revealed by fluorescent-AFLP markers. J. Genet. Genom. 2007, 34, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Duo, L.; Wang, J.; Zhula, G.; Yang, J.; Li, Z.; Tu, Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021, 271, 113877. [Google Scholar] [CrossRef]
- Fadavi, A.; Barzegar, M.; Azizi, M.H.; Bayat, M. Note. Physicochemical Composition of Ten Pomegranate Cultivars (Punica granatum L.) Grown in Iran. Food Sci. Technol. Int. 2005, 11, 113–119. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Wang, R.; Ding, Y.; Liu, R.; Xiang, L.; Du, L. Pomegranate: Constituents, Bioactivities and Pharmacokinetics. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 77–87. [Google Scholar]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Wong, T.L.; Strandberg, K.R.; Croley, C.R.; Fraser, S.E.; Venkata, K.C.N.; Fimognari, C.; Sethi, G.; Bishayee, A. Pomegranate bioactive constituents target multiple oncogenic and oncosuppressive signaling for cancer prevention and intervention. Semin. Cancer Biol. 2021, 73, 265–293. [Google Scholar] [CrossRef]
- Tripathi, J.; Chatterjee, S.; Gamre, S.; Chattopadhyay, S.; Variyar, P.S.; Sharma, A. Analysis of free and bound aroma compounds of pomegranate (Punica granatum L.). LWT Food Sci. Technol. 2014, 59, 461–466. [Google Scholar] [CrossRef]
- Feng, T.; Sun, J.; Song, S.; Wang, H.; Yao, L.; Sun, M.; Wang, K.; Chen, D. Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chem. X 2022, 15, 100423. [Google Scholar] [CrossRef]
- Schwartz, E.; Tzulker, R.; Glazer, I.; Bar-Ya’aKov, I.; Wiesman, Z.; Tripler, E.; Bar-Ilan, I.; Fromm, H.; Borochov-Neori, H.; Holland, D.; et al. Environmental Conditions Affect the Color, Taste, and Antioxidant Capacity of 11 Pomegranate Accessions’ Fruits. J. Agric. Food Chem. 2009, 57, 9197–9209. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.; Lloyd, S.; Preece, J.; Moersfelder, J.; Stein-Chisholm, R.; Obando-Ulloa, J. Physicochemical properties and aroma volatile profiles in a diverse collection of California-grown pomegranate (Punica granatum L.) germplasm. Food Chem. 1 2015, 181, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Jeong, H.; Hong, S.J.; Jo, S.M.; Park, H.; Ban, Y.; Youn, M.Y.; Kim, J.K.; Kim, Y.J.; Shin, E.-C. Characterization of taste and aroma profile in pomegranate (Punica granatum L.) seed oil using electronic sensors, GC-MS/olfactometry. Food Biosci. 2024, 61, 104554. [Google Scholar] [CrossRef]
- Xi, B.-N.; Zhang, J.-J.; Xu, X.; Li, C.; Shu, Y.; Zhang, Y.; Shi, X.; Shen, Y. Characterization and metabolism pathway of volatile compounds in walnut oil obtained from various ripening stages via HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2023, 435, 137547. [Google Scholar] [CrossRef]
- Yan, N.-N.; Xu, C.-J.; Xiong, S.-L.; Li, X.; Gu, S.-Y.; Zhu, Z.-Y.; Liu, Y.; Zhu, N.; Zhou, Y.; Xiao, H. Comprehensive Characterization and Comparison of Aroma Profiles of Tricholoma matsutake Soup During the Cooking Process by HS-GC-IMS and HS-SPME-GC-MS. Foods 2025, 14, 1478. [Google Scholar] [CrossRef]
- Huang, G.-L.; Liu, T.-T.; Mao, X.-M.; Quan, X.-Y.; Sui, S.-Y.; Ma, J.-J.; Sun, L.-X.; Li, H.-C.; Shao, Q.-S.; Wang, Y.-N. Insights into the volatile flavor and quality profiles of loquat (Eriobotrya japonica Lindl.) during shelf-life via HS-GC-IMS, E-nose, and E-tongue. Food Chem. X 2023, 20, 100886. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Zhu, W.; Luan, H.; Bu, Y.; Li, X.; Li, J.; Ji, G. Flavor characteristics of shrimp sauces with different fermentation and storage time. LWT 2019, 110, 142–151. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhu, Y.; Ben, A.; Qi, J. A novel strategy for discriminating different cultivation and screening odor and taste flavor compounds in Xinhui tangerine peel using E-nose, E-tongue, and chemometrics. Food Chem. 2022, 384, 132519. [Google Scholar] [CrossRef]
- van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Chen, X.; Chen, H.; Xiao, J.; Liu, J.; Tang, N.; Zhou, A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, Y.; Zheng, N.; Wang, J.; Liu, H. HS-GC-IMS and HS-SPME/GC-MS coupled with E-nose and E-tongue reveal the flavors of raw milk from different regions of China. Curr. Res. Food Sci. 2024, 8, 100673. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Gui, M.; Zhang, Y.; Li, Y.; Li, M.; Gu, Y.; Song, X.; Shi, W. Characterization of key aroma compounds from squids of different origins using HS-GC-IMS and MMSE-GC-MS. J. Food Meas. Charact. 2025, 19, 5898–5908. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Stein-Chisholm, R. HS-GC–MS volatile compounds recovered in freshly pressed ‘Wonderful’ cultivar and commercial pomegranate juices. Food Chem. 2016, 190, 643–656. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Iv, E.C.; Adhikari, K.; Carbonell-Barrachina, Á.A. Sensory and Physicochemical Characterization of Juices Made with Pomegranate and Blueberries, Blackberries, or Raspberries. J. Food Sci. 2010, 75, S398–S404. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, H.; Kong, J.; Hu, Z.; Hu, Y.; Zeng, J.; Chen, X.; Zhang, H.; Chen, J.; Xu, J. Flavor characterization of Citri Reticulatae Pericarpium (Citrus reticulata ‘Chachiensis’) with different aging years via sensory and metabolomic approaches. Food Chem. 2024, 443, 138616. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Liu, L.-X.; Zhang, L.-Y.; Liu, J.; Gao, C.-J.; Liu, Y.-G. Geographical differentiation of garlic based on HS-GC-IMS combined with multivariate statistical analysis. Anal. Methods 2024, 16, 465–473. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, Á.; Vázquez-Araújo, L.; Hernández, F.; Martínez, J.J.; Legua, P.; Carbonell-Barrachina, Á.A. Volatile Composition of Pomegranates from 9 Spanish Cultivars Using Headspace Solid Phase Microextraction. J. Food Sci. 2011, 76, S114–S120. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Porat, R. The flavor of pomegranate fruit: A review. J. Sci. Food Agric. 2014, 94, 21–27. [Google Scholar] [CrossRef]
- Yang, T.W.; Li, T.; Zhang, J.; Li, J.Q.; Liu, Y.; Wang, H.G. Rapid Identification of Bolete Mushrooms by UV Spectroscopy Combined with Euclidean Distance and Principal Component Analysis. Food Sci. 2014, 35, 105–109. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.D.; Meng, F.Y.; Wang, B.; Wang, Y. Comparative analysis of the volatile flavor compounds of Monascus-fermented cheese with different ripening periods by SPME-GC-MS, SPME-GC×GC-MS, and HS-GC-IMS. Food Biosci. 2024, 62, 105045. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Martínez, J.J.; Vázquez-Araújo, L.; Burló, F.; Melgarejo, P.; A Carbonell-Barrachina, Á. Volatile composition and sensory quality of Spanish pomegranates (Punica granatum L.). J. Sci. Food Agric. 2011, 91, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Araújo, L.; Chambers, E.; Adhikari, K.; Carbonell-Barrachina, A.A. Physico-chemical and sensory properties of pomegranate juices with pomegranate albedo and carpellar membranes homogenate. LWT Food Sci. Technol. 2011, 44, 2119–2125. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.; Zhan, P.; Wang, P.; Tian, H. Characterization of the key aroma compounds in four varieties of pomegranate juice by gas chromatography-mass spectrometry, gas chromatography-olfactometry, odor activity value, aroma recombination, and omission tests. Food Sci. Hum. Wellness 2023, 12, 151–160. [Google Scholar] [CrossRef]
- Yi, Z.; Feng, T.; Zhuang, H.; Ye, R.; Li, M.; Liu, T. Comparison of Different Extraction Methods in the Analysis of Volatile Compounds in Pomegranate Juice. Food Anal. Methods 2016, 9, 2364–2373. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Tietel, Z.; Porat, R.; Ulrich, D. Identification of aroma-active compounds in ‘wonderful’ pomegranate fruit using solvent-assisted flavour evaporation and headspace solid-phase micro-extraction methods. Eur. Food Res. Technol. 2012, 235, 277–283. [Google Scholar] [CrossRef]
- Xu, S.; Shi, D.; Ma, F.; Tao, G.; Xu, J.; Meng, L.; Chen, H.; Cao, S.; Lin, D.; Fei, Q.; et al. Discrimination and Characterization of the Aroma Profile in Four Strawberry Varieties Cultivated Under Substrates. Foods 2025, 14, 1464. [Google Scholar] [CrossRef]
- Guo, W.-Z.; Ma, C.; Li, Y.-Y.; Liu, L.-X.; Yu, C.; Ren, J.-J.; Lei, S.-M.; Peng, S.-L.; Liu, Y.-G. Geographical authentication of rose cakes via integrated HS-SPME-GC-MS, GC-IMS, and chemometric profiling. Anal. Methods 2025, 17, 7079–7095. [Google Scholar] [CrossRef]

| Sample Number | Sample Producing Area | Altitude | Latitude | Longitude |
|---|---|---|---|---|
| MZ | Mengzi, Yunnan | 1300–1800 m | 23°15′~23°34′ | 103°19′~103°31′ |
| HY | Huaiyuan, Anhui | ≤330 m | 32°43′~33°19′ | 116°45′~117°09′ |
| LT | Lintong, Shanxi | 400–1000 m | 34°16′~34°44′ | 109°5′~109°27′ |
| TNS | Hetian, Xinjiang | ≤1200 m | 36°99′~37°01′ | 80°73′~80°88′ |
| HL | Huili, Sichuan | 1000–1800 m | 26°5′~27°12′ | 101°52′~102°38′ |
| HZZ | Zaozhuang, Shandong | 500–700 m | 34°35′~34°51′ | 117°22′~117°49′ |
| Compounds | CAS# | RI | Dt [a.u.] | Relative Content (%) | Odor Description * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MZ | HY | LT | TNS | HL | HZZ | |||||
| acetic acid-M | C64197 | 1489 | 1.05459 | 9.66 ± 0.72 ab | 9.89 ± 0.68 a | 8.7 ± 0.15 bc | 6.79 ± 0.18 d | 7.62 ± 0.29 cd | 5.25 ± 0.05 e | acid, fruit |
| acetic acid-D | C64197 | 1489 | 1.15933 | 1.26 ± 0.32 a | 1.05 ± 0.14 ab | 0.83 ± 0.02 bc | 0.6 ± 0.08 cd | 0.52 ± 0.05 cd | 0.37 ± 0.05 e | acid, fruit |
| nonanal | C124196 | 1402.2 | 1.48852 | 0.67 ± 0.02 b | 0.69 ± 0.04 b | 1.13 ± 0.22 a | 0.51 ± 0.04 bc | 0.76 ± 0.04 b | 0.39 ± 0.00 c | floral, green, lemon |
| hexan-1-ol-M | C111273 | 1367.8 | 1.3359 | 0.57 ± 0.08 c | 3.84 ± 0.52 a | 0.9 ± 0.31 bc | 0.46 ± 0.03 c | 0.86 ± 0.06 bc | 1.32 ± 0.05 b | flower, grass, herb |
| hexan-1-ol-D | C111273 | 1367.8 | 1.66154 | 0.13 ± 0.03 b | 0.77 ± 0.16 a | 0.15 ± 0.02 b | 0.08 ± 0.01 b | 0.13 ± 0.03 b | 0.18 ± 0.02 b | flower, grass, herb |
| 3-hydroxybutan-2-one-M | C513860 | 1296 | 1.06057 | 1.25 ± 0.13 b | 0.63 ± 0.18 c | 1.55 ± 0.23 b | 2.07 ± 0.04 a | 1.09 ± 0.28 bc | 1.44 ± 0.14 b | butter, creamy |
| 3-hydroxybutan-2-one-D | C513860 | 1295.8 | 1.35071 | 0.2 ± 0.01 bc | 0.14 ± 0.00 d | 0.22 ± 0.02 bc | 0.46 ± 0.03 a | 0.18 ± 0.03 bc | 0.26 ± 0.06 b | butter, creamy |
| butyl butanoate-M | C109217 | 1240.1 | 1.34511 | 1.56 ± 0.15 b | 0.39 ± 0.20 c | 1.71 ± 0.12 ab | 2.03 ± 0.10 a | 0.43 ± 0.06 c | 0.23 ± 0.03 c | floral |
| butyl butanoate-D | C109217 | 1241.1 | 1.8405 | 0.22 ± 0.05 b | 0.09 ± 0.01 c | 0.26 ± 0.04 b | 0.51 ± 0.07 a | 0.1 ± 0.01 c | 0.06 ± 0.01 c | floral |
| 3-methylbutan-1-ol | C123513 | 1215.1 | 1.25243 | 0.4 ± 0.06 bc | 0.31 ± 0.08 cd | 0.19 ± 0.01 d | 0.5 ± 0.04 b | 0.45 ± 0.01 b | 0.78 ± 0.04 a | burnt, floral |
| (+)-limonene-M | C138863 | 1205.6 | 1.23244 | 0.82 ± 0.03 e | 2.01 ± 0.25 c | 1.32 ± 0.09 d | 1.13 ± 0.06 de | 3.14 ± 0.14 b | 4.11 ± 0.12 a | citrus |
| (+)-limonene-D | C138863 | 1205 | 1.3072 | 0.7 ± 0.04 d | 1.63 ± 0.13 b | 1.05 ± 0.07 c | 1.13 ± 0.01 c | 1.81 ± 0.11 ab | 1.96 ± 0.02 a | citrus |
| (Z)-Ocimene | C3338554 | 1195.3 | 1.29581 | 0.09 ± 0.01 b | 0.04 ± 0.00 c | 0.13 ± 0.01 a | 0.1 ± 0.01 b | 0.04 ± 0.0 c | 0.04 ± 0.01 c | floral, grass |
| cumene | C98828 | 1192.8 | 1.16786 | 0.37 ± 0.02 d | 0.45 ± 0.03 c | 0.57 ± 0.02 b | 0.38 ± 0.00 d | 0.64 ± 0.01 a | 0.29 ± 0.00 e | - |
| heptanal | C111717 | 1194.2 | 1.33794 | 0.18 ± 0.02 b | 0.18 ± 0.01 b | 0.24 ± 0.02 a | 0.13 ± 0.00 c | 0.2 ± 0.00 b | 0.08 ± 0.00 d | citrus, fat, green |
| ethyl (E)-but-2-enoate-M | C623701 | 1173.2 | 1.18502 | 0.46 ± 0.07 b | 0.17 ± 0.01 c | 0.48 ± 0.02 b | 1.22 ± 0.14 a | 0.18 ± 0.01 c | 0.12 ± 0.00 c | tropical fruit |
| ethyl (E)-but-2-enoate-D | C623701 | 1173.4 | 1.58227 | 0.05 ± 0.01 b | 0.04 ± 0.01 b | 0.06 ± 0.00 b | 0.26 ± 0.07 a | 0.04 ± 0.00 b | 0.03 ± 0.01 b | tropical fruit |
| α-phellandrene-D | C99832 | 1169 | 1.23339 | 0.33 ± 0.01 c | 0.96 ± 0.23 b | 0.37 ± 0.05 c | 0.42 ± 0.03 c | 1.27 ± 0.09 a | 1.38 ± 0.07 a | citrus, fresh, mint, pepper, wood |
| butan-1-ol-D | C71363 | 1152.2 | 1.39021 | 0.11 ± 0.00 de | 0.08 ± 0.00 e | 0.14 ± 0.02 d | 0.24 ± 0.02 c | 0.48 ± 0.02 b | 1.17 ± 0.02 a | - |
| butan-1-ol-M | C71363 | 1152 | 1.18658 | 0.92 ± 0.02 e | 0.99 ± 0.02 e | 1.29 ± 0.06 c | 1.16 ± 0.03 d | 2.3 ± 0.03 a | 1.69 ± 0.01 b | - |
| ID_1 | unidentified | 1152.5 | 1.26304 | 0.22 ± 0.02 d | 0.17 ± 0.01 e | 0.23 ± 0.01 d | 0.43 ± 0.02 b | 0.27 ± 0.02 c | 0.76 ± 0.01 a | - |
| cyclopentanone-M | C120923 | 1136.7 | 1.11246 | 0.66 ± 0.19 ab | 0.58 ± 0.08 ab | 0.4 ± 0.03 bc | 0.21 ± 0.02 c | 0.75 ± 0.20 a | 0.14 ± 0.02 c | mint, cool |
| cyclopentanone-D | C120923 | 1137 | 1.3434 | 0.12 ± 0.07 a | 0.06 ± 0.01 ab | 0.03 ± 0.00 ab | 0.03 ± 0.01 b | 0.09 ± 0.03 ab | 0.03 ± 0.00 b | mint, cool |
| 2-butylfuran | C4466244 | 1136.7 | 1.1936 | 0.44 ± 0.03 b | 0.41 ± 0.02 b | 0.41 ± 0.03 b | 0.36 ± 0.03 bc | 0.54 ± 0.05 a | 0.29 ± 0.03 c | wet hay |
| delta-3-carene-M | C13466789 | 1127.6 | 1.23417 | 1.55 ± 0.03 b | 2.84 ± 0.81 a | 0.58 ± 0.05 cd | 1.34 ± 0.10 bc | 0.4 ± 0.07 d | 0.53 ± 0.06 cd | lemon |
| delta-3-carene-D | C13466789 | 1130.8 | 1.3091 | 0.11 ± 0.00 b | 0.1 ± 0.01 b | 0.15 ± 0.02 b | 0.15 ± 0.01 b | 0.48 ± 0.02 a | 0.58 ± 0.15 a | lemon |
| delta-3-carene-T | C13466789 | 1131 | 1.76814 | 0.06 ± 0.01 b | 0.09 ± 0.02 b | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.09 ± 0.01 b | 0.18 ± 0.07 a | lemon |
| β-Pinene-M | C127913 | 1115 | 1.23183 | 1.15 ± 0.05 d | 3.42 ± 0.41 a | 1.74 ± 0.09 c | 1.52 ± 0.09 cd | 2.83 ± 0.12 b | 3.16 ± 0.13 ab | pine, polish, wood |
| β-Pinene-D | C127913 | 1115.2 | 1.30639 | 0.21 ± 0.01 c | 0.73 ± 0.09 a | 0.3 ± 0.01 c | 0.25 ± 0.02 c | 0.53 ± 0.02 b | 0.59 ± 0.03 b | pine, polish, wood |
| (E)-pent-2-enal-M | C1576870 | 1110.7 | 1.09998 | 0.68 ± 0.06 c | 0.45 ± 0.01 e | 2.05 ± 0.09 a | 0.61 ± 0.05 cd | 1.23 ± 0.02 b | 0.53 ± 0.03 de | green, fruit, herb |
| (E)-pent-2-enal-D | C1576870 | 1110.4 | 1.37305 | 0.13 ± 0.01 c | 0.09 ± 0.01 c | 1.34 ± 0.16 a | 0.16 ± 0.01 bc | 0.32 ± 0.02 b | 0.32 ± 0.01 b | green, fruit, herb |
| 2-methylpropan-1-ol-M | C78831 | 1102.7 | 1.178 | 1.16 ± 0.07 c | 0.77 ± 0.06 d | 0.59 ± 0.04 e | 1.43 ± 0.02 b | 1.66 ± 0.06 a | 1.38 ± 0.02 b | apple, bitter, wine |
| 2-methylpropan-1-ol-D | C78831 | 1102.4 | 1.37305 | 0.59 ± 0.04 c | 0.28 ± 0.02 e | 0.37 ± 0.00 d | 1.25 ± 0.04 b | 0.66 ± 0.02 c | 2.1 ± 0.05 a | apple, bitter, wine |
| hexanal-M | C66251 | 1097 | 1.26304 | 1.63 ± 0.11 c | 1.97 ± 0.07 b | 2.79 ± 0.02 a | 1.49 ± 0.03 c | 2.13 ± 0.05 b | 1.19 ± 0.03 d | apple, fat, fresh, green |
| hexanal-D | C66251 | 1097 | 1.5876 | 0.67 ± 0.12 c | 0.86 ± 0.05 b | 2.56 ± 0.09 a | 0.56 ± 0.01 c | 0.93 ± 0.01 b | 0.25 ± 0.06 d | apple, fat, fresh, green |
| butylcyclohexane | C1678939 | 1077.3 | 1.26148 | 0.37 ± 0.06 a | 0.05 ± 0.00 b | 0.07 ± 0.01 b | 0.46 ± 0.04 a | 0.09 ± 0.00 b | 0.09 ± 0.01 b | - |
| ethyl 2-methylbutanoate | C7452791 | 1061.8 | 1.24588 | 0.37 ± 0.05 c | 0.07 ± 0.00 e | 0.23 ± 0.01 d | 0.61 ± 0.01 a | 0.09 ± 0.00 e | 0.5 ± 0.03 b | fruit |
| propan-1-ol-M | C71238 | 1050.1 | 1.1148 | 0.5 ± 0.01 b | 0.94 ± 0.03 a | 0.53 ± 0.01 b | 0.52 ± 0.02 b | 0.99 ± 0.04 a | 0.53 ± 0.00 b | alcohol, candy, pungent |
| propan-1-ol-D | C71238 | 1048.9 | 1.25758 | 0.37 ± 0.02 d | 0.39 ± 0.02 d | 0.33 ± 0.01 d | 0.77 ± 0.00 b | 0.6 ± 0.04 c | 1.5 ± 0.02 a | alcohol, candy, pungent |
| ethyl butanoate-M | C105544 | 1048.9 | 1.21233 | 0.8 ± 0.02 b | 0.11 ± 0.01 e | 0.95 ± 0.04 a | 0.61 ± 0.00 c | 0.34 ± 0.01 d | 0.29 ± 0.01 d | fruit, butter |
| ethyl butanoate-D | C105544 | 1047.3 | 1.5837 | 0.92 ± 0.12 b | 0.05 ± 0.01 c | 0.98 ± 0.09 b | 2.05 ± 0.04 a | 0.08 ± 0.01 c | 0.06 ± 0 c | fruit, butter |
| ID_2 | unidentified | 1024.5 | 1.19672 | 2.29 ± 0.09 b | 1.59 ± 0.07 d | 1.67 ± 0.04 cd | 2.35 ± 0.01 a | 1.27 ± 0.03 e | 1.75 ± 0.06 c | - |
| ethanol | C64175 | 943.7 | 1.13899 | 36.66 ± 0.2 a | 31.21 ± 1.06 c | 31.23 ± 0.25 c | 34.03 ± 0.30 b | 27.73 ± 0.03 d | 34.08 ± 0.08 b | alcohol |
| ethyl 2-methylpropanoate | C97621 | 991.3 | 1.20609 | 1.18 ± 0.06 a | 0.32 ± 0.04 c | 0.74 ± 0.01 b | 0.79 ± 0.05 b | 1.28 ± 0.14 a | 0.13 ± 0.01 d | fruit |
| ID_3 | unidentified | 965.4 | 1.47603 | 0.64 ± 0.05 b | 0.06 ± 0.00 c | 0.14 ± 0.00 c | 1.51 ± 0.10 a | 0.11 ± 0.00 c | 0.05 ± 0.01 c | - |
| 2-methylbutanal | C96173 | 921.8 | 1.4222 | 1.65 ± 0.04 a | 1.03 ± 0.04 c | 0.5 ± 0.02 e | 1.2 ± 0.04 b | 0.42 ± 0.07 e | 0.62 ± 0.03 d | almond, nut, fermented |
| butan-2-one-M | C78933 | 909.5 | 1.06877 | 0.27 ± 0.01 bc | 0.17 ± 0.00 d | 0.31 ± 0.01 ab | 0.23 ± 0.02 c | 0.33 ± 0.01 a | 0.17 ± 0.04 d | fragrant, fruit, pleasant |
| butan-2-one-D | C78933 | 908.8 | 1.26226 | 0.18 ± 0.00 c | 0.04 ± 0.00 e | 0.27 ± 0.02 a | 0.15 ± 0.01 d | 0.2 ± 0.00 b | 0.05 ± 0.01 e | fragrant, fruit, pleasant |
| ethyl acetate-M | C141786 | 901.2 | 1.11246 | 0.98 ± 0.02 b | 1.11 ± 0.02 a | 1.02 ± 0.02 b | 0.84 ± 0.02 c | 1.18 ± 0.03 a | 0.86 ± 0.05 c | aromatic, brandy, grape |
| ethyl acetate-D | C141786 | 893.5 | 1.35354 | 7.48 ± 0.03 c | 8.9 ± 0.14 b | 9.35 ± 0.14 bc | 7.2 ± 0.08 c | 9.73 ± 0.08 a | 7.63 ± 0.34 c | aromatic, brandy, grape |
| ID_4 | unidentified | 860.8 | 1.07111 | 2.55 ± 0.07 a | 0.7 ± 0.05 c | 1.22 ± 0.06 b | 2.44 ± 0.07 a | 0.75 ± 0.01 c | 0.72 ± 0.05 c | - |
| methyl acetate-M | C79209 | 846.7 | 1.03905 | 0.49 ± 0.02 de | 0.82 ± 0.02 b | 0.71 ± 0.01 c | 0.45 ± 0.02 e | 0.89 ± 0.02 a | 0.53 ± 0.01 d | ester, green |
| methyl acetate-D | C79209 | 845.5 | 1.20826 | 0.93 ± 0.01 e | 1.48 ± 0.02 c | 2.22 ± 0.04 a | 1.13 ± 0.02 d | 1.64 ± 0.05 b | 1.6 ± 0.06 b | ester, green |
| oct-1-ene | C111660 | 841.6 | 1.16972 | 0.59 ± 0.01 b | 0.56 ± 0.02 bc | 0.69 ± 0.02 a | 0.54 ± 0.02 cd | 0.66 ± 0.01 a | 0.5 ± 0.02 d | - |
| propan-2-one | C67641 | 836 | 1.12921 | 1.96 ± 0.01 a | 1.24 ± 0.03 d | 1.45 ± 0.06 b | 1.54 ± 0.11 b | 1.46 ± 0.05 b | 1.17 ± 0.06 d | pungent |
| butanal-M | C123728 | 832.3 | 1.10066 | 0.86 ± 0.01 b | 1.06 ± 0.06 a | 0.6 ± 0.01 e | 0.72 ± 0.02 cd | 0.75 ± 0.04 c | 0.63 ± 0.02 de | |
| butanal-D | C123728 | 831.3 | 1.29777 | 0.92 ± 0.06 a | 0.93 ± 0.08 a | 0.27 ± 0.01 d | 0.72 ± 0.05 b | 0.33 ± 0.03 d | 0.56 ± 0.07 c | banana, green, pungent |
| propanal-M | C123386 | 819.1 | 1.07717 | 0.87 ± 0.02 b | 0.9 ± 0.04 b | 1.16 ± 0.05 a | 0.81 ± 0.02 b | 1.16 ± 0.05 a | 0.46 ± 0.04 c | |
| propanal-D | C123386 | 819.1 | 1.15534 | 0.74 ± 0.08 b | 0.52 ± 0.04 c | 0.68 ± 0.04 b | 0.95 ± 0.02 a | 0.93 ± 0.03 a | 0.22 ± 0.02 d | floral, pungent |
| ID_5 | unidentified | 795.6 | 1.13182 | 0.93 ± 0.02 b | 0.18 ± 0.01 e | 0.58 ± 0.01 c | 1.05 ± 0.02 a | 0.12 ± 0.01 f | 0.24 ± 0.01 d | - |
| ID_6 | unidentified | 1428 | 1.09417 | 2.14 ± 0.12 bc | 2.55 ± 0.23 ab | 2.76 ± 0.20 a | 2.1 ± 0.08 bc | 2.47 ± 0.26 ab | 1.75 ± 0.09 d | - |
| octanal | C124130 | 1298.7 | 1.41383 | 0.22 ± 0.03 c | 0.28 ± 0.02 b | 0.34 ± 0.02 a | 0.16 ± 0.01 d | 0.31 ± 0.01 ab | 0.14 ± 0.01 d | citrus, fat, green |
| β-Ocimene | C13877913 | 1248.5 | 1.23231 | 0.22 ± 0.01 c | 1.13 ± 0.25 b | 0.38 ± 0.04 c | 0.25 ± 0.01 c | 1.67 ± 0.10 a | 1.21 ± 0.06 b | floral |
| (E)-hex-2-enal | C6728263 | 1226.7 | 1.19301 | 0.18 ± 0.02 c | 0.34 ± 0.03 b | 0.52 ± 0.02 a | 0.13 ± 0.01 cd | 0.34 ± 0.04 b | 0.11 ± 0.00 d | green, fruit, grass |
| pentan-1-ol | C71410 | 1267.9 | 1.25106 | 0.12 ± 0.02 b | 0.1 ± 0.01 bc | 0.13 ± 0.01 b | 0.27 ± 0.02 a | 0.11 ± 0.01 b | 0.07 ± 0.01 c | fruit, green |
| 3-methylpentan-1-ol | C589355 | 1353.5 | 1.32182 | 0.12 ± 0.01 b | 0.1 ± 0.03 b | 0.19 ± 0.03 a | 0.21 ± 0.02 a | 0.1 ± 0.01 b | 0.06 ± 0.01 b | fruit |
| α-terpinolene | C586629 | 1286.7 | 1.23608 | 0.12 ± 0.02 c | 0.32 ± 0.05 b | 0.18 ± 0.01 c | 0.13 ± 0.00 c | 0.49 ± 0.02 a | 0.47 ± 0.03 a | pine |
| styrene-M | C100425 | 1266 | 1.06685 | 0.21 ± 0.01 d | 0.3 ± 0.02 c | 0.4 ± 0.01 b | 0.28 ± 0.03 c | 0.48 ± 0.02 a | 0.28 ± 0.02 c | - |
| styrene-D | C100425 | 1266.4 | 1.47187 | 0.09 ± 0.01 d | 0.13 ± 0.02 c | 0.17 ± 0.01 b | 0.11 ± 0.00 cd | 0.24 ± 0.02 a | 0.09 ± 0.01 d | - |
| α-terpinene | C99865 | 1183.4 | 1.23244 | 0.11 ± 0.00 c | 0.66 ± 0.16 b | 0.25 ± 0.02 c | 0.14 ± 0.00 c | 1.09 ± 0.09 a | 0.75 ± 0.03 b | lemon |
| butyl acetate | C123864 | 1084.5 | 1.25013 | 0.24 ± 0.02 b | 0.12 ± 0.03 c | 0.31 ± 0.01 b | 0.3 ± 0.02 b | 0.67 ± 0.01 a | 0.27 ± 0.08 b | apple, banana |
| propyl butanoate | C105668 | 1143.5 | 1.27774 | 0.08 ± 0.01 b | 0.05 ± 0.01 c | 0.08 ± 0.01 b | 0.24 ± 0.00 a | 0.05 ± 0.00 c | 0.05 ± 0.00 c | apricot, fruit, pineapple |
| α-pinene | C80568 | 1031.7 | 1.23215 | 0.41 ± 0.01 c | 1.19 ± 0.18 b | 0.52 ± 0.04 c | 0.54 ± 0.03 c | 1.18 ± 0.06 b | 1.47 ± 0.11 a | cedarwood, pine |
| ID_7 | unidentified | 1023.9 | 1.31129 | 0.21 ± 0.01 c | 0.11 ± 0.01 c | 0.21 ± 0.03 c | 0.43 ± 0.01 b | 0.77 ± 0.02 a | 0.77 ± 0.13 a | - |
| 2-methylbutan-1-ol-D | C137326 | 1215.8 | 1.49253 | 0.22 ± 0.02 c | 0.24 ± 0.03 bc | 0.23 ± 0.01 bc | 0.34 ± 0.00 b | 0.34 ± 0.03 b | 2.32 ± 0.09 a | green, wine |
| 2-methylbutan-1-ol-M | C137326 | 1214.7 | 1.23582 | 0.48 ± 0.05 e | 0.8 ± 0.10 c | 0.56 ± 0.04 de | 0.66 ± 0.03 cd | 1.42 ± 0.08 b | 2.05 ± 0.05 a | green, wine |
| No. | Compounds | CAS | RI | Relative Content (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | HY | LT | TNS | HL | HZZ | ||||
| Alcohols | |||||||||
| 1 | terpinen-4-ol | 562-74-3 | 1617 | 2.55 ± 0.08 d | 0.54 ± 0.05 f | 9.66 ± 0.05 b | 1.86 ± 0.4 e | 11.75 ± 0.25 a | 7.12 ± 0.11 c |
| 2 | menthol | 15356-70-4 | 1544 | ND | ND | 6.05 ± 0.04 a | ND | ND | ND |
| 3 | 4-methylpentan-1-ol | 626-89-1 | 1316 | 5.87 ± 0.4 a | ND | ND | 5.38 ± 0.03 b | ND | ND |
| 4 | linalool | 78-70-6 | 1556 | ND | ND | ND | ND | 3.7 ± 0.11 a | 0.83 ± 0.02 b |
| 5 | 2-phenylethanol | 60-12-8 | 1935 | ND | 5.43 ± 0.05 a | 1.07 ± 0.12 c | 0.81 ± 0.02 d | 1.51 ± 0.36 b | 1.44 ± 0.26 b |
| 6 | β-bisabolol | 15352-77-9 | 2162 | ND | ND | ND | ND | 2.94 ± 0.6 a | ND |
| 7 | (E)-dec-2-en-1-ol | 18409-18-2 | 1819 | ND | ND | ND | ND | 0.57 ± 0.32 a | ND |
| 8 | 2-methylbut-3-en-2-ol | 115-18-4 | 1048 | ND | ND | ND | ND | 0.91 ± 0.03 a | 0.92 ± 0.02 a |
| 9 | nonan-1-ol | 143-08-8 | 1664 | ND | ND | ND | 4.62 ± 0.02 a | ND | ND |
| 10 | 3-methylbut-2-en-1-ol | 556-82-1 | 1327 | ND | ND | ND | ND | 0.93 ± 0.03 a | 0.51 ± 0.04 b |
| 11 | heptan-2-ol | 543-49-7 | 1328 | ND | 0.41 ± 0.01 a | ND | ND | ND | ND |
| 12 | nonan-2-ol | 628-99-9 | 1521 | ND | 6.28 ± 0.07 a | ND | ND | ND | ND |
| Aldehydes | |||||||||
| 13 | 3-methylbutanal | 590-86-3 | 918 | ND | ND | 1.69 ± 0.08 a | ND | ND | ND |
| 14 | hexanal | 66-25-1 | 1093 | 2.38 ± 0.07 c | ND | 6.30 ± 0.28 a | 2.91 ± 0.5 b | 2.95 ± 0.52 b | ND |
| 15 | nonanal | 124-19-6 | 1389 | 1.1 ± 0.19 b | ND | 7.19 ± 0.09 a | ND | 0.57 ± 0.11 c | ND |
| 16 | furan-2-carbaldehyde | 98-01-1 | 1482 | ND | ND | 5.25 ± 0.22 a | ND | 4.45 ± 0.08 b | ND |
| 17 | 5-methylfuran-2-carbaldehyde | 620-02-0 | 1570 | ND | ND | ND | ND | 0.27 ± 0.01 a | ND |
| Esters | |||||||||
| 18 | ethyl acetate | 141-78-6 | 894 | 14.01 ± 0.21 b | 28.8 ± 1.12 a | 12.01 ± 0.13 c | 6.63 ± 0.15 d | 3.62 ± 0.36 e | 6.74 ± 0.9 d |
| 19 | ethyl butanoate | 105-54-4 | 1030 | 1.85 ± 0.08 a | ND | 1.07 ± 0.09 b | 1.74 ± 0.03 a | ND | ND |
| 20 | ethyl (E)-but-2-enoate | 623-70-1 | 1154 | 1.81 ± 0.07 a | ND | 0.82 ± 0.07 b | ND | ND | ND |
| 21 | ethyl hexanoate | 123-66-0 | 1241 | 3.21 ± 0.15 b | 0.93 ± 0.04 d | 1.20 ± 0.06 c | 3.63 ± 0.6 a | ND | ND |
| 22 | dec-9-enyl acetate | 50816-18-7 | 1722 | ND | ND | 3.15 ± 0.06 a | ND | 0.98 ± 0.08 b | ND |
| 23 | methyl 2-hydroxybenzoate | 119-36-8 | 1751 | 1.00 ± 0.01 b | ND | 2.39 ± 0.06 a | ND | ND | ND |
| 24 | geranyl hexanoate | 10032-02-7 | 1726 | ND | ND | ND | ND | 0.42 ± 0.04 a | ND |
| 25 | 3-methylbutyl acetate | 123-92-2 | 1137 | ND | 13.94 ± 1.7 a | ND | ND | ND | ND |
| 26 | ethyl octanoate | 106-32-1 | 1430 | ND | 6.99 ± 0.11 a | ND | ND | ND | ND |
| 27 | nonan-2-yl acetate | 14936-66-4 | 1425 | ND | 0.48 ± 0.01 a | ND | ND | ND | ND |
| 28 | ethyl decanoate | 110-38-3 | 1645 | ND | 23.82 ± 0.52 a | ND | ND | ND | 0.83 ± 0.06 b |
| 29 | ethyl dodecanoate | 106-33-2 | 1835 | ND | 4.71 ± 0.06 a | ND | ND | ND | ND |
| Ketones | |||||||||
| 30 | acetoin | 513-86-0 | 1277 | 28.15 ± 0.61 a | 2.10 ± 0.19 c | 1.63 ± 0.03 d | 26.49 ± 1.25 b | ND | 1.01 ± 0.01 e |
| 31 | hexan-2-one | 591-78-6 | 1078 | 1.37 ± 0.06 a | ND | ND | ND | ND | ND |
| 32 | 6-methylhept-5-en-2-one | 110-93-0 | 1348 | 1.83 ± 0.07 a | ND | ND | ND | 0.56 ± 0.08 b | ND |
| 33 | propan-2-one | 67-64-1 | 821 | ND | ND | ND | ND | 0.44 ± 0.01 b | 0.96 ± 0.01 a |
| 34 | (5Z)-6,10-dimethylundeca-5,9-dien-2-one | 3879-26-3 | 1813 | ND | ND | 1.13 ± 0.01 a | ND | ND | ND |
| Alkenes | |||||||||
| 35 | (Z,E)-α-Farnesene | 26560-14-5 | 1737 | 1.15 ± 0.01 d | 0.54 ± 0.02 e | 1.26 ± 0.01 d | 2.06 ± 0.06 c | 2.39 ± 0.06 b | 5.96 ± 0.07 a |
| 36 | D-Limonene | 5989-27-5 | 1205 | 2.82 ± 0.03 c | 1.45 ± 0.05 e | 4.03 ± 0.03 b | 2.69 ± 0.12 d | 6.39 ± 0.2 a | 6.35 ± 0.04 a |
| 37 | (E)-dec-4-ene | 19398-89-1 | 982 | ND | ND | 0.97 ± 0.01 a | ND | ND | ND |
| 38 | α-Curcumene | 644-30-4 | 1784 | ND | ND | ND | 1.68 ± 0.05 b | 1.47 ± 0.08 c | 2.86 ± 0.07 a |
| 39 | β-Curcumene | 28976-67-2 | 1756 | 9.59 ± 0.07 d | 3.25 ± 0.21 e | 13.51 ± 0.06 c | 17.91 ± 0.4 b | 17.52 ± 0.07 b | 41.52 ± 0.03 a |
| 40 | (E)-4-methylhept-3-ene | 4485-16-9 | 885 | ND | ND | ND | ND | 0.18 ± 0.01 a | ND |
| 41 | β-Bisabolene | 495-61-4 | 1743 | 1.71 ± 0.05 c | ND | ND | 5.26 ± 0.12 a | ND | 2.90 ± 0.1 b |
| 42 | (E)-tetradec-7-ene | 10374-74-0 | 1365 | ND | ND | ND | ND | 1.35 ± 0.15 a | ND |
| 43 | nonadec-1-ene | 18435-45-5 | 1922 | ND | ND | ND | ND | 0.31 ± 0.05 b | 0.64 ± 0.02 a |
| 44 | γ-Muurolene | 30021-74-0 | 1704 | ND | ND | ND | ND | 0.21 ± 0.01 a | ND |
| 45 | γ-Terpinene | 99-85-4 | 1255 | ND | 0.38 ± 0.01 c | ND | ND | 3.74 ± 0.12 a | 0.70 ± 0.05 b |
| 46 | (E)-β-Famesene | 18794-84-8 | 1673 | ND | ND | ND | 2.16 ± 0.02 a | 1.78 ± 0.11 b | 0.90 ± 0.01 c |
| 47 | (+)-4-Carene | 29050-33-7 | 1128 | ND | ND | ND | ND | 2.31 ± 0.04 a | 0.47 ± 0.02 b |
| 48 | styrene | 100-42-5 | 1225 | 8.52 ± 0.06 c | 0.87 ± 0.06 f | 7.90 ± 0.64 d | 10.15 ± 0.13 b | 15.36 ± 0.23 a | 1.13 ± 0.02 e |
| Acids and Ethers | |||||||||
| 49 | acetic acid | 64-19-7 | 1471 | ND | ND | ND | ND | 0.89 ± 0.06 b | 12.84 ± 0.19 a |
| 50 | nonanoic acid | 112-05-0 | 2184 | 3.08 ± 0.05 a | ND | 2.08 ± 0.04 b | ND | 2.12 ± 0.15 b | ND |
| 51 | 2-methylbutanoic acid | 116-53-0 | 1688 | ND | ND | 1.67 ± 0.15 a | ND | ND | ND |
| 52 | octanoic acid | 124-07-2 | 2035 | ND | 0.81 ± 0.07 a | ND | ND | ND | ND |
| 53 | eucalyptol | 470-82-6 | 1216 | 2.94 ± 0.24 b | ND | 1.47 ± 0.12 c | 3.21 ± 0.02 a | 0.34 ± 0.05 d | ND |
| Phenols | |||||||||
| 54 | phenol | 108-95-2 | 2037 | 1.51 ± 0.3 b | ND | 2.63 ± 0.06 a | 0.82 ± 0.02 d | 1.18 ± 0.13 c | 0.58 ± 0.05 e |
| 55 | 2-methoxyphenol | 90-05-1 | 1859 | 1.98 ± 0.06 a | ND | ND | ND | ND | ND |
| 56 | 4-ethenyl-2-methoxyphenol | 7786-61-0 | 2180 | ND | ND | 0.92 ± 0.07 a | ND | 0.67 ± 0.06 b | ND |
| Hydrocarbons | |||||||||
| 57 | cyclododecane | 294-62-2 | 1519 | ND | ND | 2.95 ± 0.05 a | ND | ND | ND |
| 58 | p-Cymene | 99-87-6 | 1280 | 1.35 ± 0.01 c | ND | ND | ND | 4.66 ± 0.04 a | 2.78 ± 0.12 b |
| Compounds | Threshold # (mg/kg) | ROAV | Odor Description * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | HY | LT | TNS | HL | HZZ | ||||
| HS-GC-IMS | nonanal | 0.0011 | 10.37 | 43.13 | 28.14 | 4.79 | 11.88 | 4.47 | floral, green, lemon |
| 3-hydroxybutan-2-one | 0.0140 | 1.52 | 3.09 | 3.03 | 1.53 | 1.34 | 1.30 | butter, creamy, green pepper | |
| heptanal | 0.0028 | 1.09 | 4.42 | 2.35 | 0.48 | 1.23 | 0.36 | citrus, fat, green, nut | |
| α-phellandrene | 0.0400 | 0.14 | 1.65 | 0.25 | 0.11 | 0.55 | 0.43 | citrus, fresh, mint, pepper, wood | |
| 2-butylfuran | 0.0050 | 1.50 | 5.64 | 2.25 | 0.74 | 1.86 | 0.73 | wet hay | |
| β-pinene | 0.1400 | 0.14 | 1.68 | 0.34 | 0.11 | 0.35 | 0.28 | pine, polish, wood | |
| hexanal | 0.073 | 0.38 | 1.86 | 1.05 | 0.21 | 0.5 | 0.21 | apple, fat, fresh, green | |
| ethyl 2-methylbutanoate | 0.000063 | 100.00 | 76.39 | 100.00 | 100.00 | 24.55 | 100.00 | apple, ester, green apple, kiwi, strawberry | |
| ethyl butanoate-M | 0.0030 | 4.54 | 2.52 | 8.67 | 2.1 | 1.95 | 1.22 | apple, butter, cheese, pineapple, strawberry | |
| ethyl butanoate-D | 0.0030 | 5.22 | 1.15 | 8.95 | 7.06 | 0.46 | 0.25 | apple, butter, cheese, pineapple, strawberry | |
| ethyl 2-methylpropionate | 0.0002 | 91.33 | 100 | 92.13 | 37.09 | 100 | 7.45 | fruit, sweet | |
| 2-methylbutanal | 0.0044 | 6.39 | 16.09 | 3.11 | 2.82 | 1.64 | 1.78 | almond, nut, fermented | |
| oct-1-ene | 0.0005 | 20.09 | 77.00 | 37.80 | 11.15 | 22.69 | 12.60 | - | |
| butanal-M | 0.0020 | 7.32 | 36.44 | 8.22 | 3.72 | 6.45 | 3.97 | banana, green, pungent | |
| butanal-D | 0.0020 | 7.83 | 31.97 | 3.7 | 3.72 | 2.84 | 3.53 | banana, green, pungent | |
| propanal-M | 0.0151 | 0.98 | 4.10 | 2.10 | 0.55 | 1.32 | 0.38 | floral, pungent | |
| propanal-D | 0.0151 | 0.83 | 2.37 | 1.23 | 0.65 | 1.06 | 0.18 | floral, pungent | |
| octanal | 0.0069 | 0.54 | 2.79 | 1.35 | 0.24 | 0.77 | 0.26 | citrus, fat, green, pungent | |
| β-ocimene | 0.0340 | 0.11 | 2.28 | 0.31 | 0.08 | 0.84 | 0.45 | floral | |
| α-pinene | 0.0410 | 0.17 | 2.00 | 0.35 | 0.14 | 0.49 | 0.45 | cedarwood, pine | |
| HS-SPME-GC-MS | nonan-1-ol | 0.087 | ND | ND | ND | 2.81 | ND | ND | floral, green, fat |
| 3-methylbutanal | 0.0012 | ND | ND | 58.63 | ND | ND | ND | - | |
| hexanal | 0.073 | 1.16 | ND | 3.59 | 2.11 | 5.58 | ND | apple, fat, fresh, green | |
| nonanal | 0.008 | 4.91 | ND | 37.42 | ND | 9.84 | ND | floral, green, lemon | |
| ethyl acetate | 0.005 | 100 | 2.42 | 100 | 70.08 | 100 | 16.24 | aromatic, brandy, grape | |
| ethyl butanoate | 0.003 | 22.01 | ND | 14.85 | 30.65 | ND | ND | apple, butter, cheese, pineapple, strawberry | |
| ethyl hexanoate | 0.005 | 22.91 | 0.08 | 9.99 | 38.37 | ND | ND | fruit | |
| methyl 2-hydroxybenzoate | 0.04 | 0.89 | ND | 2.49 | ND | ND | ND | ||
| 3-methylbutyl acetate | 0.002 | ND | 2.93 | ND | ND | ND | ND | apple, banana, pear | |
| ethyl decanoate | 0.0001 | ND | 100 | ND | ND | ND | 100 | brandy, grape, pear | |
| acetoin | 0.014 | 71.76 | 0.06 | 4.85 | 100 | ND | 0.87 | butter, creamy, green pepper | |
| (Z,E)-α-farnesene | 0.087 | 0.47 | ND | 0.60 | 1.25 | 3.79 | 5.08 | floral, wood | |
| D-limonene | 0.034 | 2.96 | 0.02 | 4.93 | 4.18 | 25.96 | 2.25 | citrus, mint | |
| styrene | 0.065 | 4.68 | 0.01 | 5.06 | 8.25 | 32.64 | 0.21 | - | |
| eucalyptol | 0.023 | 4.56 | ND | 2.42 | 7.38 | 2.04 | ND | ||
| 2-methoxyphenol | 0.0016 | 44.16 | ND | ND | ND | ND | ND | burnt, phenol, wood | |
| p-cymene | 0.01 | 0.48 | ND | ND | ND | 6.44 | 0.33 | citrus, fresh | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, W.; Qu, H.; Zhang, L.; Liu, L.; Hu, X.; Liu, Y. Identification of Key Aroma Substances in Pomegranate from Different Geographical Origins via Integrated Volatile Profiling and Multivariate Statistical Analysis. Foods 2025, 14, 3546. https://doi.org/10.3390/foods14203546
Zhang Y, Guo W, Qu H, Zhang L, Liu L, Hu X, Liu Y. Identification of Key Aroma Substances in Pomegranate from Different Geographical Origins via Integrated Volatile Profiling and Multivariate Statistical Analysis. Foods. 2025; 14(20):3546. https://doi.org/10.3390/foods14203546
Chicago/Turabian StyleZhang, Yanzhen, Wenzhu Guo, Haitao Qu, Lihua Zhang, Lingxiao Liu, Xiaojie Hu, and Yunguo Liu. 2025. "Identification of Key Aroma Substances in Pomegranate from Different Geographical Origins via Integrated Volatile Profiling and Multivariate Statistical Analysis" Foods 14, no. 20: 3546. https://doi.org/10.3390/foods14203546
APA StyleZhang, Y., Guo, W., Qu, H., Zhang, L., Liu, L., Hu, X., & Liu, Y. (2025). Identification of Key Aroma Substances in Pomegranate from Different Geographical Origins via Integrated Volatile Profiling and Multivariate Statistical Analysis. Foods, 14(20), 3546. https://doi.org/10.3390/foods14203546







