Ethephon Treatment Enhanced Postharvest Litchi Fruit Resistance to Peronophythora litchii by Strengthening Antioxidant Capacity and Defense Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material and P. litchii
2.2. Treatments
2.3. Assessment of Disease Index and Pericarp Browning Index
2.4. Measurement of Ethylene Production Rate
2.5. Determination of Reactive Oxygen Species (ROS) Levels, 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Scavenging Activity and Malondialdehyde (MDA) Contents
2.6. Determination of Antioxidant Enzymes Activities
2.7. Determination of Defense Related Enzymes Activities
2.8. RNA Extraction and Gene Expression Analysis
2.9. Statistical Analyses
3. Results
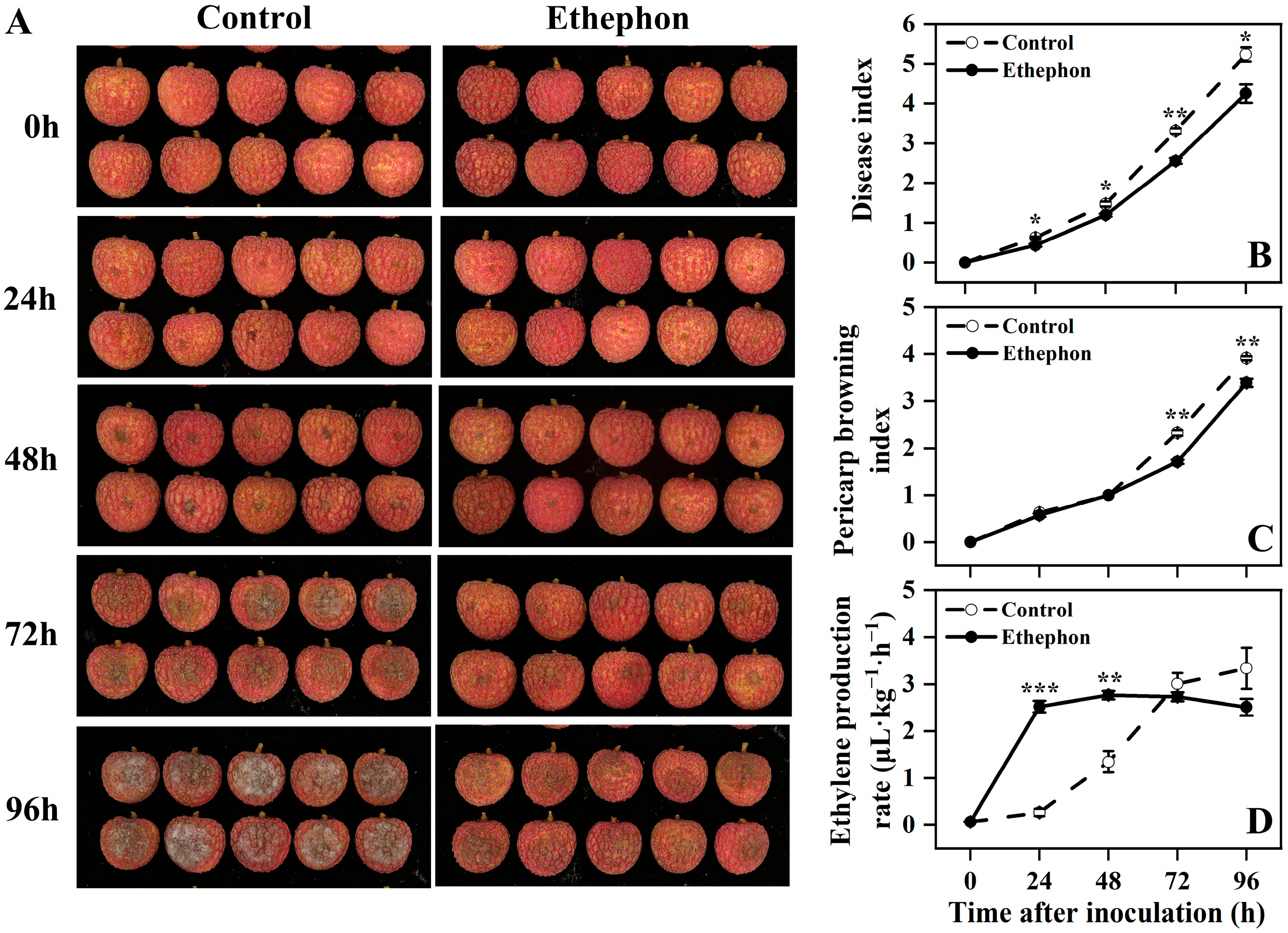
3.1. Effect of Ethephon Treatment on Postharvest Litchi Downy Blight, Disease Index, Pericarp Browning Index, and the Ethylene Production Rate After Inoculation with P. litchii
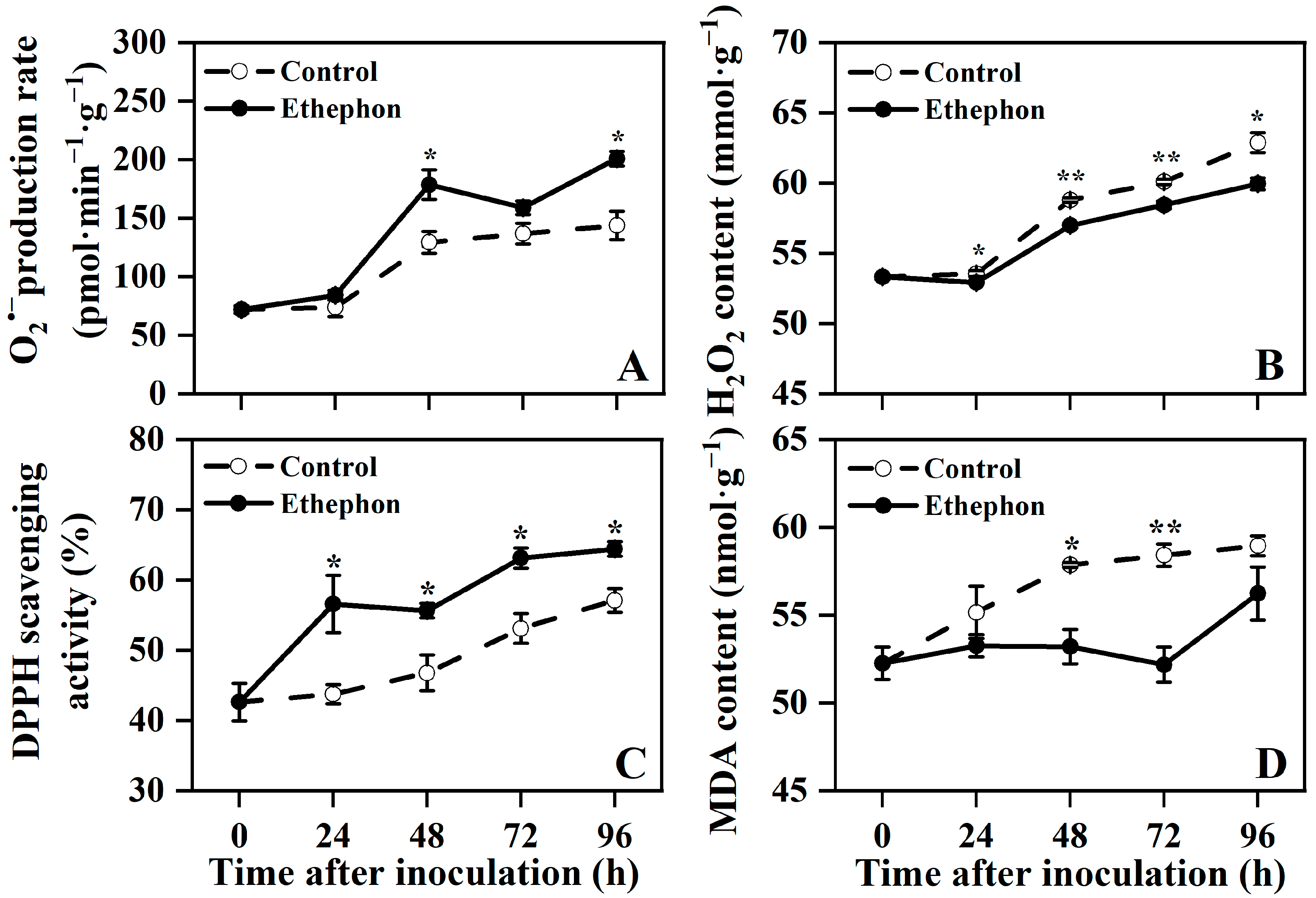
3.2. Effect of Ethephon Treatment on the ROS Levels, DPPH Scavenging Activity, and MDA Content in Postharvest Litchi Fruit
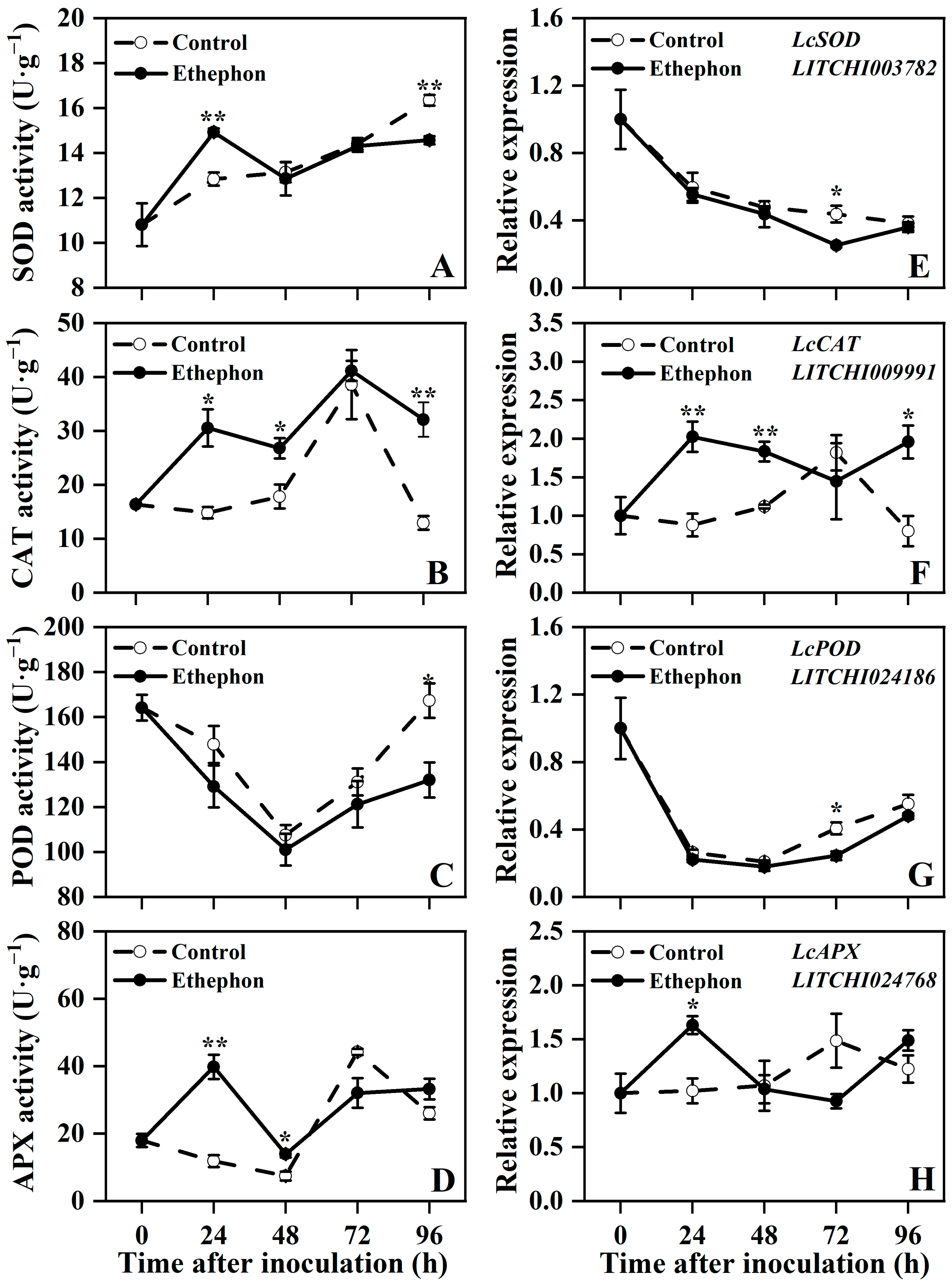
3.3. Effect of Ethephon Treatment on Antioxidant Indices in Postharvest Litchi Fruit
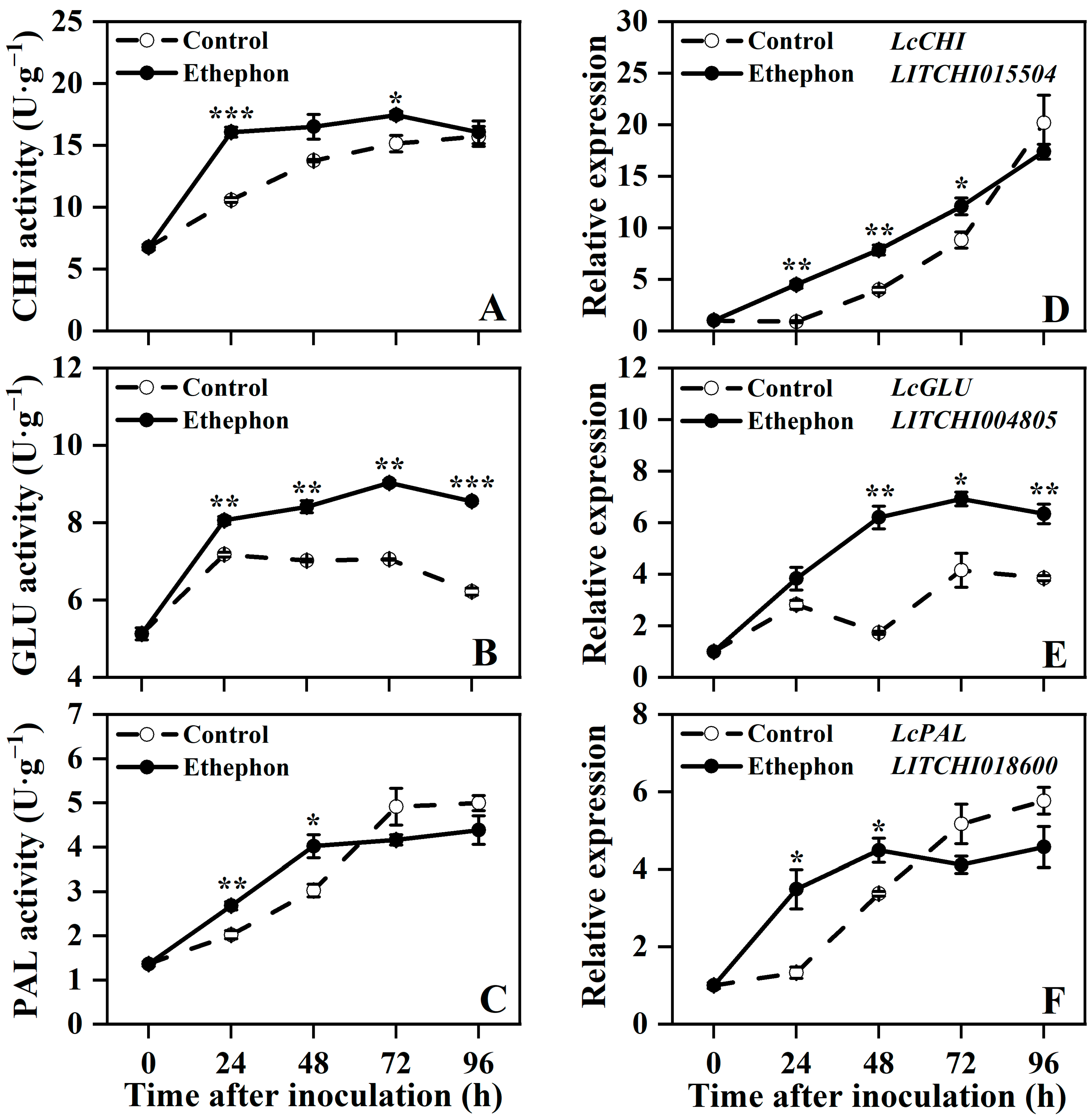
3.4. Effect of Ethephon Treatment on Defense-Related Enzymes Activities and Key Gene Expression in Postharvest Litchi Fruit
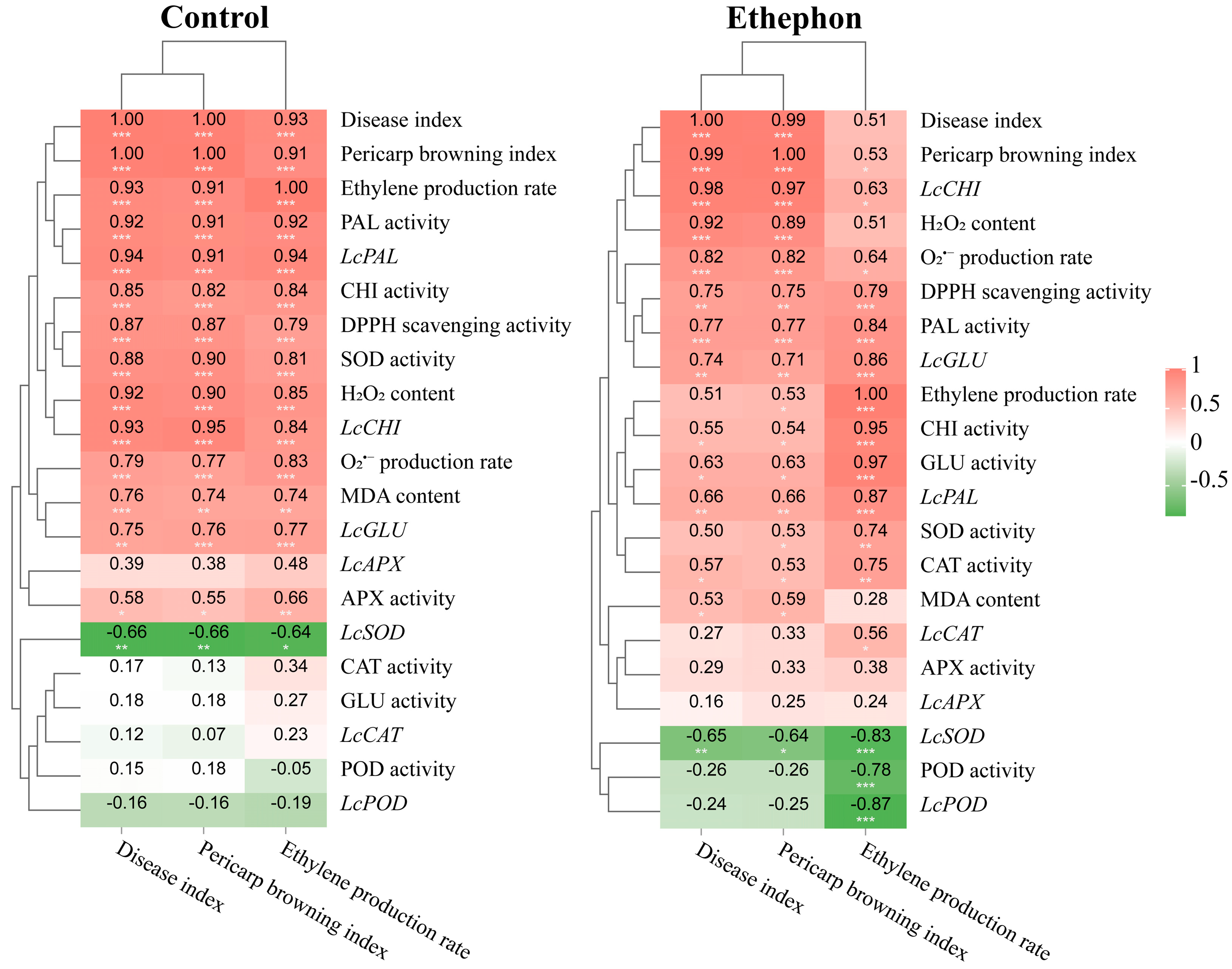
3.5. Correlation and Hierarchical Cluster Analysis of Physiological and Biochemical Indices in Control and Ethephon-Treated Fruits
4. Discussion
4.1. Ethephon Treatment Enhanced Ethylene Signal to Improve Postharvest Litchi Fruit Resistance Against P. litchii Infection
4.2. Ethephon Treatment Inhibited the Development of Litchi Downy Blight by Enhancing Antioxidant Capacity of Postharvest Litchi Fruit
4.3. Ethephon Treatment Inhibited the Development of Litchi Downy Blight by Improving the Defense Capacity of Postharvest Litchi Fruit
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.; Luo, T.; Han, D.; Zhu, D.; Li, Z.; Wu, Z.; Wu, Z. Multi-omics analysis revealed room temperature storage affected the quality of litchi by altering carbohydrate metabolism. Sci. Hortic. 2022, 293, 110663. [Google Scholar] [CrossRef]
- Chen, H.; Yang, S.; Su, Z.; Ou, S.; Pan, Y.; Peng, X. Analysis of the National Litchi Production in 2024 and Management Suggestions. China Trop. Agric. 2024, 21, 8–20. [Google Scholar] [CrossRef]
- Chen, H.; Ou, L.; Li, J.; Su, Z.; Yang, S.; Wu, Z.; Hu, Z. Fruit scientific research in New China in the past 70 years: Litchi. J. Fruit Sci. 2019, 36, 1399–1413. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Liu, G.; Hu, M.; Yun, Z.; Duan, X.; Cai, K.; Jiang, G. Inhibition of downy blight and enhancement of resistance in litchi fruit by postharvest application of melatonin. Food Chem. 2021, 347, 129009. [Google Scholar] [CrossRef]
- Huang, S.; Lv, X.; Zheng, L.; Guo, D. Exiguobacterium acetylicum Strain SI17: A Potential Biocontrol Agent against Peronophythora litchii Causing Post-Harvest Litchi Downy Blight. Horticulturae 2024, 10, 888. [Google Scholar] [CrossRef]
- Luo, T.; Li, S.; Han, D.; Guo, X.; Shuai, L.; Wu, Z. The effect of desulfurization on the postharvest quality and sulfite metabolism in pulp of sulfitated “Feizixiao” Litchi (Litchi chinensis Sonn.) fruits. Food Sci. Nutr. 2019, 7, 1715–1726. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Xi, P.; Zhu, Z.; Kong, X.; Wan, L.; Situ, J.; Li, M.; Gao, L.; Jiang, Z. Biological activity of pterostilbene against Peronophythora litchii, the litchi downy blight pathogen. Postharvest Biol. Technol. 2018, 144, 29–35. [Google Scholar] [CrossRef]
- Zheng, L.; Huang, S.; Hsiang, T.; Yu, G.; Guo, D.; Jiang, Z.; Li, J. Biocontrol Using Bacillus amyloliquefaciens PP19 Against Litchi Downy Blight Caused by Peronophythora litchii. Front. Microbiol. 2021, 11, 619423. [Google Scholar] [CrossRef]
- Xing, M.; Zheng, L.; Deng, Y.; Xu, D.; Xi, P.; Li, M.; Kong, G.; Jiang, Z. Antifungal Activity of Natural Volatile Organic Compounds against Litchi Downy Blight Pathogen Peronophythora litchii. Molecules 2018, 23, 358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Situ, J.-j.; Zhu, Q.-f.; Xi, P.-g.; Zheng, Y.; Liu, H.-x.; Zhou, X.; Jiang, Z.-d. Identification of volatile organic compounds for the biocontrol of postharvest litchi fruit pathogen Peronophythora litchii. Postharvest Biol. Technol. 2019, 155, 37–46. [Google Scholar] [CrossRef]
- Xiang, Y.; Su, Z.; Li, T.; Xu, D.; Hu, M.; Sun, J.; Jiang, Y.; Zhang, Z. Inhibitory activity and action mode of apple polyphenols against Peronophythora litchii that causes litchi downy blight. Postharvest Biol. Technol. 2022, 194, 112095. [Google Scholar] [CrossRef]
- Luo, J.; Li, Z.; Wang, J.; Weng, Q.; Chen, S.; Hu, M. Antifungal Activity of Isoliquiritin and Its Inhibitory Effect against Peronophythora litchi Chen through a Membrane Damage Mechanism. Molecules 2016, 21, 237. [Google Scholar] [CrossRef]
- Gong, L.; Li, T.; Chen, F.; Duan, X.; Yuan, Y.; Zhang, D.; Jiang, Y. An inclusion complex of eugenol into β-cyclodextrin: Preparation, and physicochemical and antifungal characterization. Food Chem. 2016, 196, 324–330. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Zhang, Z.; Jiang, G.; Feng, L.; Duan, X.; Jiang, Y. 6-Benzylaminopurine improves the quality of harvested litchi fruit. Postharvest Biol. Technol. 2018, 143, 137–142. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Li, B.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular mechanisms underlying multi-level defense responses of horticultural crops to fungal pathogens. Hortic. Res. 2022, 9, uhac066. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Pei, Y.; Xu, X.; Du, X.; Xue, Q.; Gao, Z.; Shu, P.; Wu, Y.; Liu, Z.; Jian, Y.; et al. Ethylene-MPK8-ERF.C1-PR module confers resistance against Botrytis cinerea in tomato fruit without compromising ripening. New Phytol. 2024, 242, 592–609. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Lin, Y.; Shi, J.; Lin, Y. A novel chitosan formulation treatment induces disease resistance of harvested litchi fruit to Peronophythora litchii in association with ROS metabolism. Food Chem. 2018, 266, 299–308. [Google Scholar] [CrossRef]
- Xing, M.; Zhao, J.; Zhang, J.; Wu, Y.; Khan, R.A.A.; Li, X.; Wang, R.; Li, T.; Liu, T. 6-Pentyl-2H-pyran-2-one from Trichoderma erinaceum Is Fungicidal against Litchi Downy Blight Pathogen Peronophythora litchii and Preservation of Litchi. J. Agric. Food Chem. 2023, 71, 19488–19500. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xi, P.; Lin, Z.; Huang, J.; Lu, S.; Jiang, Z.; Qiao, F. Efficacy and potential mechanisms of benzothiadiazole inhibition on postharvest litchi downy blight. Postharvest Biol. Technol. 2021, 181, 111660. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Huang, X.; Wang, H.; Wu, H.; Zhao, M.; Li, J. Involvement of HD-ZIP I transcription factors LcHB2 and LcHB3 in fruitlet abscission by promoting transcription of genes related to the biosynthesis of ethylene and ABA in litchi. Tree Physiol. 2019, 39, 1600–1613. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Di, J.; Liu, Y.; Wang, Y. Involvement of ethylene in glutamate-mediated tomato fruit resistance to Alternaria alternata. Postharvest Biol. Technol. 2022, 190, 111940. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L.; Wu, C.; Shan, W.; Cai, D.; Lin, Z.; Wei, W.; Chen, J.; Lu, W.; Kuang, J. Methionine oxidation and reduction of the ethylene signaling component MaEIL9 are involved in banana fruit ripening. J. Integr. Plant Biol. 2022, 65, 150–166. [Google Scholar] [CrossRef]
- Wang, Y.; An, H.; Yang, Y.; Yi, C.; Duan, Y.; Wang, Q.; Guo, Y.; Yao, L.; Chen, M.; Meng, J.; et al. The MpNAC72/MpERF105-MpMYB10b module regulates anthocyanin biosynthesis in Malus ‘Profusion’ leaves infected with Gymnosporangium yamadae. Plant J. 2024, 118, 1569–1588. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, M.; Wang, L.; Tong, P.; Xing, Q.; Qi, H. An ethylene response factor negatively regulates red light induced resistance of melon to powdery mildew by inhibiting ethylene biosynthesis. Int. J. Biol. Macromol. 2025, 307, 141867. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Y.; Xian, Q.; Chen, X.; Xu, J. Transcriptome analysis reveals ethylene-mediated defense responses to Fusarium oxysporum f. sp. cucumerinum infection in Cucumis sativus L. BMC Plant Biol. 2020, 20, 334. [Google Scholar] [CrossRef]
- Nunez-Pastrana, R.; Fabiola Arcos-Ortega, G.; Armando Souza-Perera, R.; Alberto Sanchez-Borges, C.; Elena Nakazawa-Ueji, Y.; Javier Garcia-Villalobos, F.; Alberto Guzman-Antonio, A.; Juan Zuniga-Aguilar, J. Ethylene, but not salicylic acid or methyl jasmonate, induces a resistance response against Phytophthora capsici in Habanero pepper. Eur. J. Plant Pathol. 2011, 131, 669–683. [Google Scholar] [CrossRef]
- Veselova, S.; Nuzhnaya, T.; Maksimov, I. The Role of Salicylic, Jasmonic Acid and Ethylene in the Development of the Resistance/Susceptibility of Wheat to the SnTox1-Producing Isolate of the Pathogenic Fungus Stagonospora nodorum (Berk.). Plants 2024, 13, 2546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Li, B.-Q.; Qin, G.-Z.; Li, L.; Tian, S.-P. Defense response of tomato fruit at different maturity stages to salicylic acid and ethephon. Sci. Hortic. 2011, 129, 183–188. [Google Scholar] [CrossRef]
- Tunsagool, P.; Jutidamrongphan, W.; Phaonakrop, N.; Jaresitthikunchai, J.; Roytrakul, S.; Leelasuphakul, W. Insights into stress responses in mandarins triggered by Bacillus subtilis cyclic lipopeptides and exogenous plant hormones upon Penicillium digitatum infection. Plant Cell Rep. 2019, 38, 559–575. [Google Scholar] [CrossRef]
- Dong, T.; Zheng, T.; Fu, W.; Guan, L.; Jia, H.; Fang, J. The Effect of Ethylene on the Color Change and Resistance to Botrytis cinerea Infection in ‘Kyoho’ Grape Fruits. Foods 2020, 9, 892. [Google Scholar] [CrossRef]
- Zhuo, M.-G.; Wang, T.-Y.; Huang, X.-M.; Hu, G.-B.; Zhou, B.-Y.; Wang, H.-C.; Abbas, F. ERF transcription factors govern anthocyanin biosynthesis in litchi pericarp by modulating the expression of anthocyanin biosynthesis genes. Sci. Hortic. 2024, 337, 113464. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Wu, B.; Song, Z.; Shi, J. Methionine represses gray mold of tomato by keeping nitric oxide at an appropriate level via ethylene synthesis and signal transduction. Food Chem. 2024, 461, 140942. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, R.; Sheng, J.; Shen, L. SlERF2 Is Associated with Methyl Jasmonate-Mediated Defense Response against Botrytis cinereain Tomato Fruit. J. Agric. Food Chem. 2018, 66, 9923–9932. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, K.; Sadeghnezhad, E.; Jiu, S.; Zhu, X.; Dong, T.; Liu, Z.; Guan, L.; Jia, H.; Fang, J. Chitinase family genes in grape differentially expressed in a manner specific to fruit species in response to Botrytis cinerea. Mol. Biol. Rep. 2020, 47, 7349–7363. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yi, Y.; Ai, Y.; Hou, W.; Wang, L.; Wang, H.; Min, T. Ethephon and 1-methylcyclopropene regulate storage quality and browning of fresh-cut Chinese water chestnuts. Postharvest Biol. Technol. 2023, 200, 112331. [Google Scholar] [CrossRef]
- Yan, Y.; Li, M.; Ding, Z.; Yang, J.; Xie, Z.; Ye, X.; Tie, W.; Tao, X.; Chen, G.; Huo, K.; et al. The regulation mechanism of ethephon-mediated delaying of postharvest physiological deterioration in cassava storage roots based on quantitative acetylproteomes analysis. Food Chem. 2024, 458, 140252. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kashash, Y.; Goldenberg, L.; Sabag, A.; Doron-Faigenboim, A.; Porat, R. Effects of 1-methylcyclopropene on postharvest storage performance and the transcriptome of cactus pear fruit. Int. J. Food Sci. Technol. 2017, 52, 1801–1809. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, L.; Lin, H.; Lin, Y.; Lin, Y.; Wang, H. Paper-containing 1-methylcyclopropene treatment suppresses fruit decay of fresh Anxi persimmons by enhancing disease resistance. Food Qual. Saf. 2021, 5, fyab007. [Google Scholar] [CrossRef]
- Ge, Y.; Hu, K.-D.; Wang, S.-S.; Hu, L.-Y.; Chen, X.-Y.; Li, Y.-H.; Yang, Y.; Yang, F.; Zhang, H. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE 2017, 12, 1556–1562. [Google Scholar] [CrossRef]
- Jiang, L.; Situ, J.; Deng, Y.Z.; Wan, L.; Xu, D.; Chen, Y.; Xi, P.; Jiang, Z. PlMAPK10, a Mitogen-Activated Protein Kinase (MAPK) in Peronophythora litchii, Is Required for Mycelial Growth, Sporulation, Laccase Activity, and Plant Infection. Front. Microbiol. 2018, 9, 426. [Google Scholar] [CrossRef]
- Guo, X.; Li, Q.; Luo, T.; Han, D.; Zhu, D.; Wu, Z. Postharvest Calcium Chloride Treatment Strengthens Cell Wall Structure to Maintain Litchi Fruit Quality. Foods 2023, 12, 2478. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Luo, T.; Han, D.; Zhu, D.; Jiang, Z.; Wu, Z. Integrated transcriptomics, proteomics, and metabolomics analysis reveals the mechanism of litchi pulp deterioration during long-term cold storage. Postharvest Biol. Technol. 2023, 195, 112140. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Qu, H.; Yun, Z.; Wang, H.; Huang, Z.; Huang, H.; Jiang, Y. Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. 2015, 171, 191–199. [Google Scholar] [CrossRef]
- Guo, X.; Li, Q.; Luo, T.; Xu, D.; Zhu, D.; Li, J.; Han, D.; Wu, Z. Zinc Oxide Nanoparticles Treatment Maintains the Postharvest Quality of Litchi Fruit by Inducing Antioxidant Capacity. Foods 2024, 13, 3357. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shaheen, T.; Shahid, M. Pre-storage methionine treatment inhibits postharvest enzymatic browning of cold stored ‘Gola’ litchi fruit. Postharvest Biol. Technol. 2018, 140, 100–106. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Chen, X.; Chen, Z.; Zhang, Z.; Li, T.; Qu, H.; Jiang, Y. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2021, 336, 127618. [Google Scholar] [CrossRef]
- Wu, S.; Zhen, C.; Wang, K.; Gao, H. Effects of Bacillus Subtilis CF-3 VOCs Combined with Heat Treatment on the Control of Monilinia fructicola in Peaches and Colletotrichum gloeosporioides in Litchi Fruit. J. Food Sci. 2019, 84, 3418–3428. [Google Scholar] [CrossRef]
- Mauch, F.; Hadwiger, L.A.; Boller, T. Ethylene: Symptom, Not Signal for the Induction of Chitinase and beta-1,3-Glucanase in Pea Pods by Pathogens and Elicitors. Plant Physiol. 1984, 76, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, G.; Huang, W.; Li, W.; Feng, D.; Liu, L.; Xi, P.; Jiang, Z.; Kong, G. Autophagy-Related Gene PlATG6a Is Involved in Mycelial Growth, Asexual Reproduction and Tolerance to Salt and Oxidative Stresses in Peronophythora litchii. Int. J. Mol. Sci. 2022, 23, 1839. [Google Scholar] [CrossRef]
- Yin, C.; Xie, L.; Wu, Y.; Qu, H.; Yang, B.; Gong, L.; Jiang, Y.; Li, T. Involvement of miRNAs-mediated senescence and salicylic acid defense in postharvest litchi downy blight. Food Chem. 2023, 404, 134662. [Google Scholar] [CrossRef]
- Lin, X.; Lin, Y.; Liao, Z.; Niu, X.; Wu, Y.; Shao, D.; Shen, B.; Shen, T.; Wang, F.; Ding, H.; et al. Preservation of Litchi Fruit with Nanosilver Composite Particles (Ag-NP) and Resistance against Peronophythora litchi. Foods 2022, 11, 2934. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Niu, Q.; Gao, L.; Wang, Q.; Lan, C.; Jiang, Z.; Qiao, F. Biocontrol potential of Burkholderia gladioli STPT16 on postharvest decay of litchi fruit caused by Peronophythora litchii and Colletotrichum fructicola. Food Control 2025, 168, 110956. [Google Scholar] [CrossRef]
- Xing, M.; Xu, D.; Wu, Y.; Liu, T.; Xi, P.; Wang, R.; Zhao, J.; Jiang, Z. Biocontrol of Litchi Downy Blight Dependent on Streptomyces abikoensis TJGA-19 Fermentation Filtrate Antagonism Competition with Peronophythora litchii. Fermentation 2023, 9, 1011. [Google Scholar] [CrossRef]
- Situ, J.; Zheng, L.; Xu, D.; Gu, C.; Xi, P.; Deng, Y.; Hsiang, T.; Jiang, Z. Screening of effective biocontrol agents against postharvest litchi downy blight caused by Peronophythora litchii. Postharvest Biol. Technol. 2023, 198, 112249. [Google Scholar] [CrossRef]
- Sabuz, A.A.; Chowdhury, M.G.F.; Molla, M.M.; Khan, M.H.H.; Miaruddin, M. Effect of Ethephon on ripening and postharvest quality of mango. Bangladesh J. Agric. Res. 2019, 44, 453–467. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khatoon, R.; Hossain, M.F.B.; Rahman, M.T.; Alam, S.N. Influence of ethephon on ripening and quality of winter tomato fruit harvested at different maturity stages. Bangladesh J. Agric. Res. 2016, 40, 567–580. [Google Scholar] [CrossRef]
- GB 2763-2021; National Food Safety Standards—Maximum Residue Limits for Pesticides in Food. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2021.
- Lu, Y.; Wang, X.; Lou, Y.; Cheng, J. Role of ethylene signaling in the production of rice volatiles induced by the rice brown planthopper Nilaparvata lugens. Chin. Sci. Bull. 2006, 51, 2457–2465. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Zhou, X.; Situ, J.; Xie, L.; Xi, P.; Yang, B.; Kong, G.; Jiang, Z. Peronophythora litchii RXLR effector P. litchii avirulence homolog 202 destabilizes a host ethylene biosynthesis enzyme. Plant Physiol. 2023, 193, 756–774. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Yu, J. Delay of pericarp browning by chondroitin sulfate attributes of enhancing antioxidant activity and membrane integrity in litchi fruit after harvest. Postharvest Biol. Technol. 2025, 227, 113612. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Wang, P.; Li, H.; Wang, G.; Wang, S.; Cao, X.; Sun, J.; Cao, L.; Zhang, L.; et al. The resistance of litchi fruit to Peronophythora litchii is associated with lignin biosynthesis and reactive oxygen species metabolism. Sci. Hortic. 2025, 349, 114254. [Google Scholar] [CrossRef]
- Ruan, Z.; Zhu, Y.; Liu, M.; Zhou, Y.; Su, X.; Jiang, Y.; Jiang, G. Tyr-Asp treatment delays senescence in litchi fruit by enhancing redox balance and maintaining energy homeostasis. Postharvest Biol. Technol. 2025, 223, 113420. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, J.; Wu, Y.; Wen, Y.; Lin, Y.; Chen, Y.; Lin, H. Bacillus amyloliquefaciens LY-1 culture broth enhances the storage properties of fresh litchi through acting on ROS metabolism. Food Chem. 2025, 480, 143811. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, R.; Jiang, Z.; Cheng, Z.; Li, W.; Shao, Y. Combined effect of Debaryomyces hansenii and Bacillus atrophaeus on the physicochemical attributes, defense-related enzyme activity, and transcriptomic profile of stored litchi fruit. Biol. Control 2022, 172, 104975. [Google Scholar] [CrossRef]
- Lv, J.; Tai, R.; Cao, Y.; Ge, Y.; Chen, J.; Li, J. Genome-wide identification and comparative expression analysis of ascorbate peroxidase (APX) gene family in apple fruit under 1-methylcyclopropene (1-MCP) and ethephon treatments during ripening. Sci. Hortic. 2024, 329, 113016. [Google Scholar] [CrossRef]
- Shu, P.; Li, Y.; Xiang, L.; Sheng, J.; Shen, L. Ethylene enhances tolerance to chilling stress in tomato fruit partially through the synergistic regulation between antioxidant enzymes and ATP synthases. Postharvest Biol. Technol. 2022, 193, 112065. [Google Scholar] [CrossRef]
- Li, Q.; Xian, B.; Yu, Q.; Jia, R.; Zhang, C.; Zhong, X.; Zhang, M.; Fu, Y.; Liu, Y.; He, H.; et al. The CsAP2-09-CsWRKY25-CsRBOH2 cascade confers resistance against citrus bacterial canker by regulating ROS homeostasis. Plant J. 2024, 118, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Yang, F.; Chai, S.; Wang, L.; de Dios, V.R.; Tan, W.; Yao, Y. Ethylene activates poplar defense against Dothiorella gregaria Sacc by regulating reactive oxygen species accumulation. Physiol. Plant 2022, 174, e13726. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Li, J.; Ali, M.; Wang, Y.; Liu, X.; Li, F.; Li, X. GABA primes defense responses against Botrytis cinerea in tomato fruit by modulating ethylene and JA signaling pathways. Postharvest Biol. Technol. 2024, 208, 112665. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, T.; Hu, C.; Liu, D.; He, J. Exogenous Nitric Oxide-Induced Postharvest Gray Spot Disease Resistance in Loquat Fruit and Its Possible Mechanism of Action. Int. J. Mol. Sci. 2023, 24, 4369. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Peng, D.; Shao, Y.; Li, R.; Li, W. Bacillus siamensis N-1 improves fruit quality and disease resistance by regulating ROS homeostasis and defense enzyme activities in pitaya. Sci. Hortic. 2024, 329, 112975. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Y.; Chen, Y.; Jiang, S.; Ye, J.; Xu, F.; Lou, Y.; Ding, P.; Ouaziz, M.; Shao, X. Agaro-oligosaccharides enhanced the Monilinia fructicola resistance of peach fruit by regulating antioxidative and phenylpropanoid metabolism. Postharvest Biol. Technol. 2024, 217, 113126. [Google Scholar] [CrossRef]
- Song, Y.; Ren, Y.; Xue, Y.; Lu, D.; Yan, T.; He, J. Putrescine (1,4-Diaminobutane) enhances antifungal activity in postharvest mango fruit against Colletotrichum gloeosporioides through direct fungicidal and induced resistance mechanisms. Pest. Biochem. Physiol. 2023, 195, 105581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, H.; Lin, Y.; Shi, J.; Xue, S.; Hung, Y.-C.; Chen, Y.; Wang, H. Effects of biocontrol bacteria Bacillus amyloliquefaciens LY-1 culture broth on quality attributes and storability of harvested litchi fruit. Postharvest Biol. Technol. 2017, 132, 81–87. [Google Scholar] [CrossRef]
- Belhadj, A.; Telef, N.; Cluzet, S.; Bouscaut, J.; Corio-Costet, M.-F.; Mérillon, J.-M. Ethephon Elicits Protection Against Erysiphe necator in Grapevine. J. Agric. Food Chem. 2008, 56, 5781–5787. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-Q.; Fu, D.-Q.; Zhu, B.-Z.; Lu, C.-W.; Luo, Y.-B. Overexpression of the ethylene response factor SIERF1 gene enhances resistance of tomato fruit to Rhizopus nigricans. Postharvest Biol. Technol. 2013, 75, 28–36. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, W.; Fan, Y.C.; Ye, J.L.; Li, G.H. Effects of pre- and post-treatment with ethephon on gum formation of peach gummosis caused by Lasiodiplodia theobromae. Plant Pathol. 2014, 63, 1306–1315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Luo, T.; Guo, X.; Li, J.; Li, Q.; Chen, Y.; Ou, W.; Han, D.; Wu, Z. Ethephon Treatment Enhanced Postharvest Litchi Fruit Resistance to Peronophythora litchii by Strengthening Antioxidant Capacity and Defense Systems. Foods 2025, 14, 3493. https://doi.org/10.3390/foods14203493
Zhu D, Luo T, Guo X, Li J, Li Q, Chen Y, Ou W, Han D, Wu Z. Ethephon Treatment Enhanced Postharvest Litchi Fruit Resistance to Peronophythora litchii by Strengthening Antioxidant Capacity and Defense Systems. Foods. 2025; 14(20):3493. https://doi.org/10.3390/foods14203493
Chicago/Turabian StyleZhu, Difa, Tao Luo, Xiaomeng Guo, Jingyi Li, Qiao Li, Yongqi Chen, Wenbo Ou, Dongmei Han, and Zhenxian Wu. 2025. "Ethephon Treatment Enhanced Postharvest Litchi Fruit Resistance to Peronophythora litchii by Strengthening Antioxidant Capacity and Defense Systems" Foods 14, no. 20: 3493. https://doi.org/10.3390/foods14203493
APA StyleZhu, D., Luo, T., Guo, X., Li, J., Li, Q., Chen, Y., Ou, W., Han, D., & Wu, Z. (2025). Ethephon Treatment Enhanced Postharvest Litchi Fruit Resistance to Peronophythora litchii by Strengthening Antioxidant Capacity and Defense Systems. Foods, 14(20), 3493. https://doi.org/10.3390/foods14203493







