Glycine Betaine Treatment Maintains Postharvest Quality of Hupingzao Jujube Fruit by Enhancing the Antioxidant System
Abstract
1. Introduction
2. Material and Methods
2.1. Jujube Samples and Treatments
2.2. Firmness, Respiration Rate, and Weight Loss Assessments
2.3. Color Assessment
2.4. Total Soluble Solids and Titratable Acid Assessments
2.5. The Content of Antioxidant (Ascorbic Acid, Total Flavonoids, Total Phenolics, and Proanthocyanidins) Assessments
2.6. The Malondialdehyde (MDA) Content, Hydrogen Peroxide Content, and the Inhibition Ability of Superoxide Anion Production Assessments
2.7. The Activity of Antioxidant Enzymes Assessments
2.8. Statistical Analysis
3. Results and Discussion
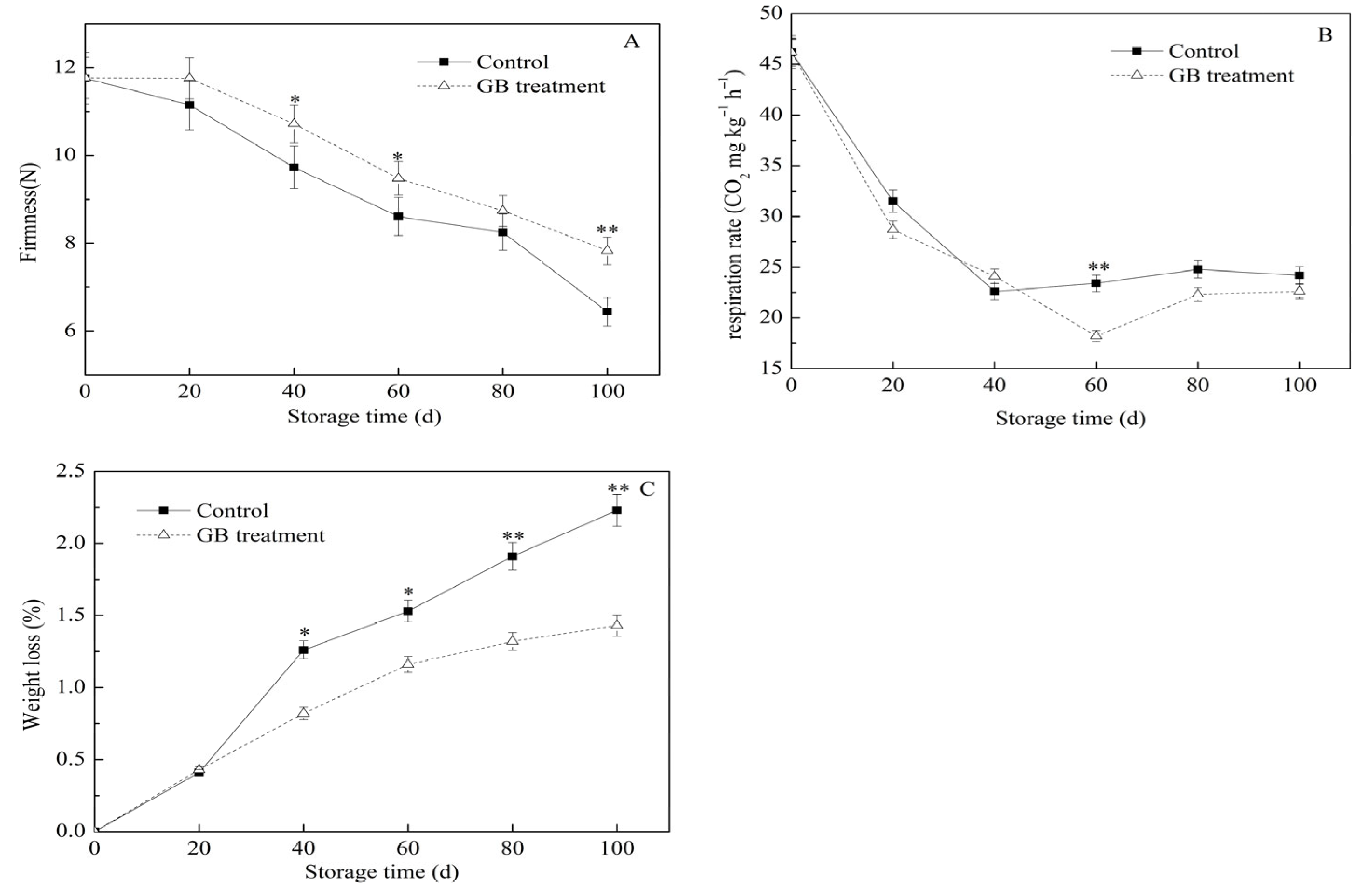
3.1. The Roles of GB Treatment on the Jujube Firmness, Respiration Rate, and Weight Loss Rate
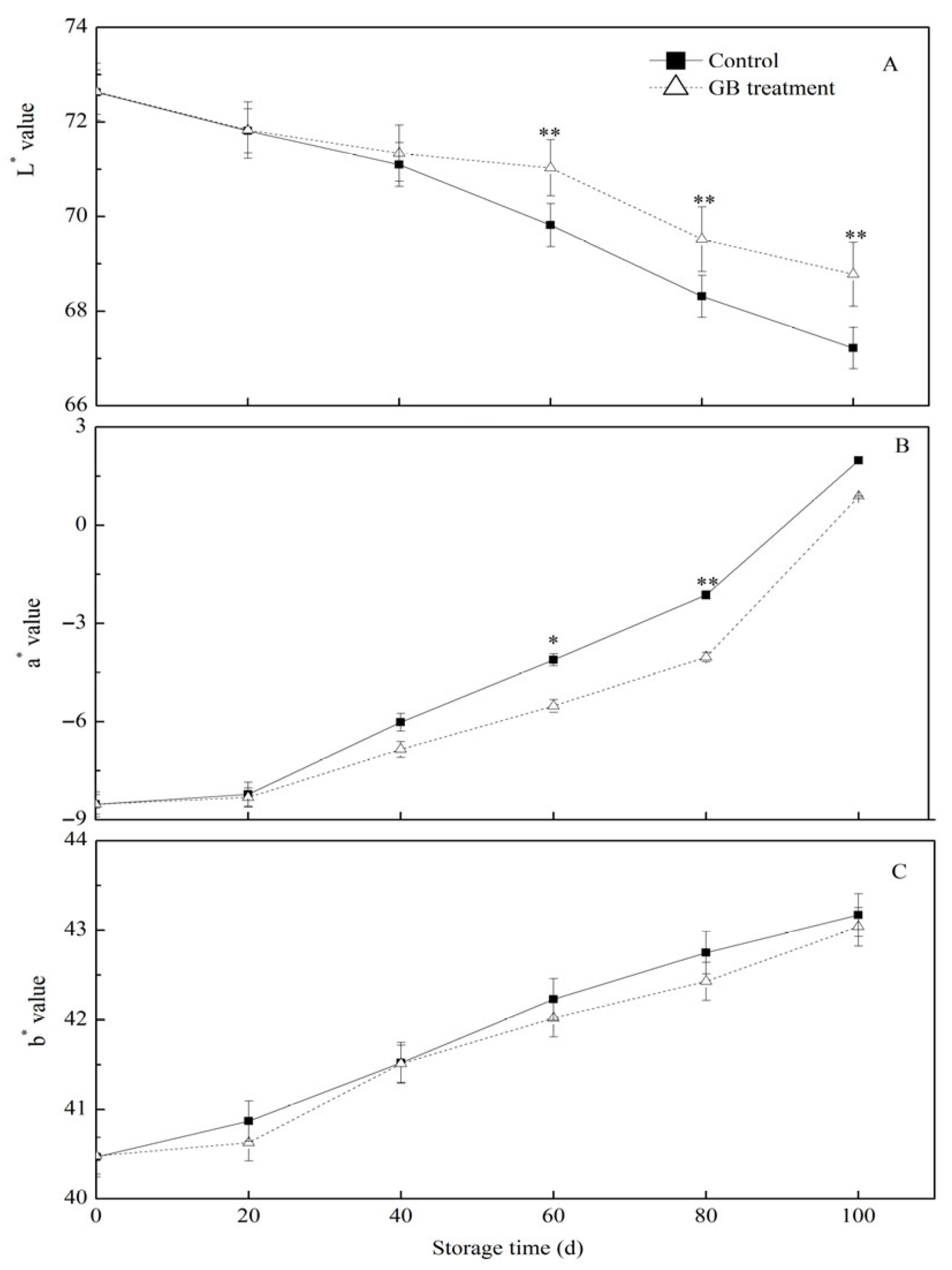
3.2. The Role of GB Treatment on Jujube Fruit Peel Color
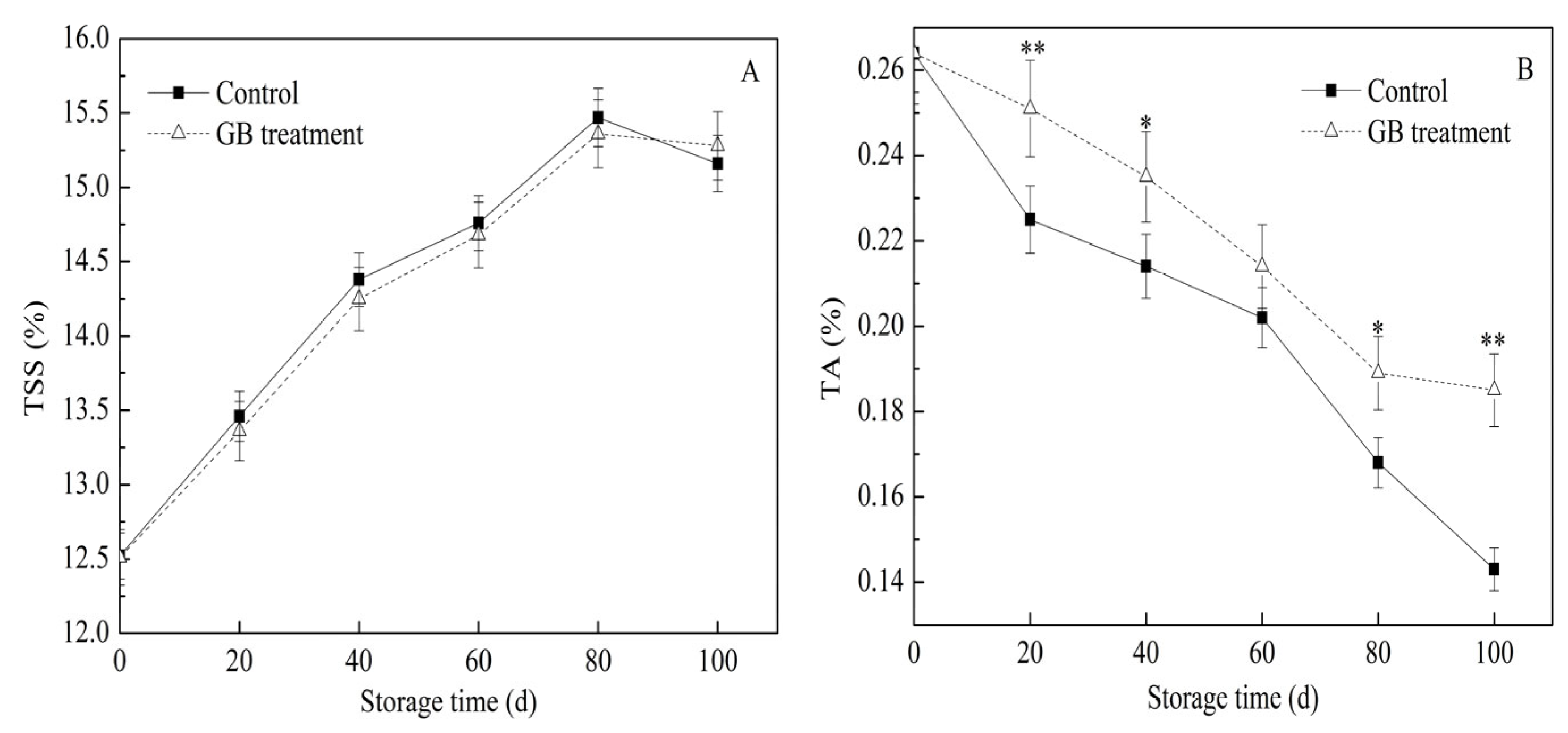
3.3. The Roles of GB Treatment on Jujube Fruit TSS and TA Content
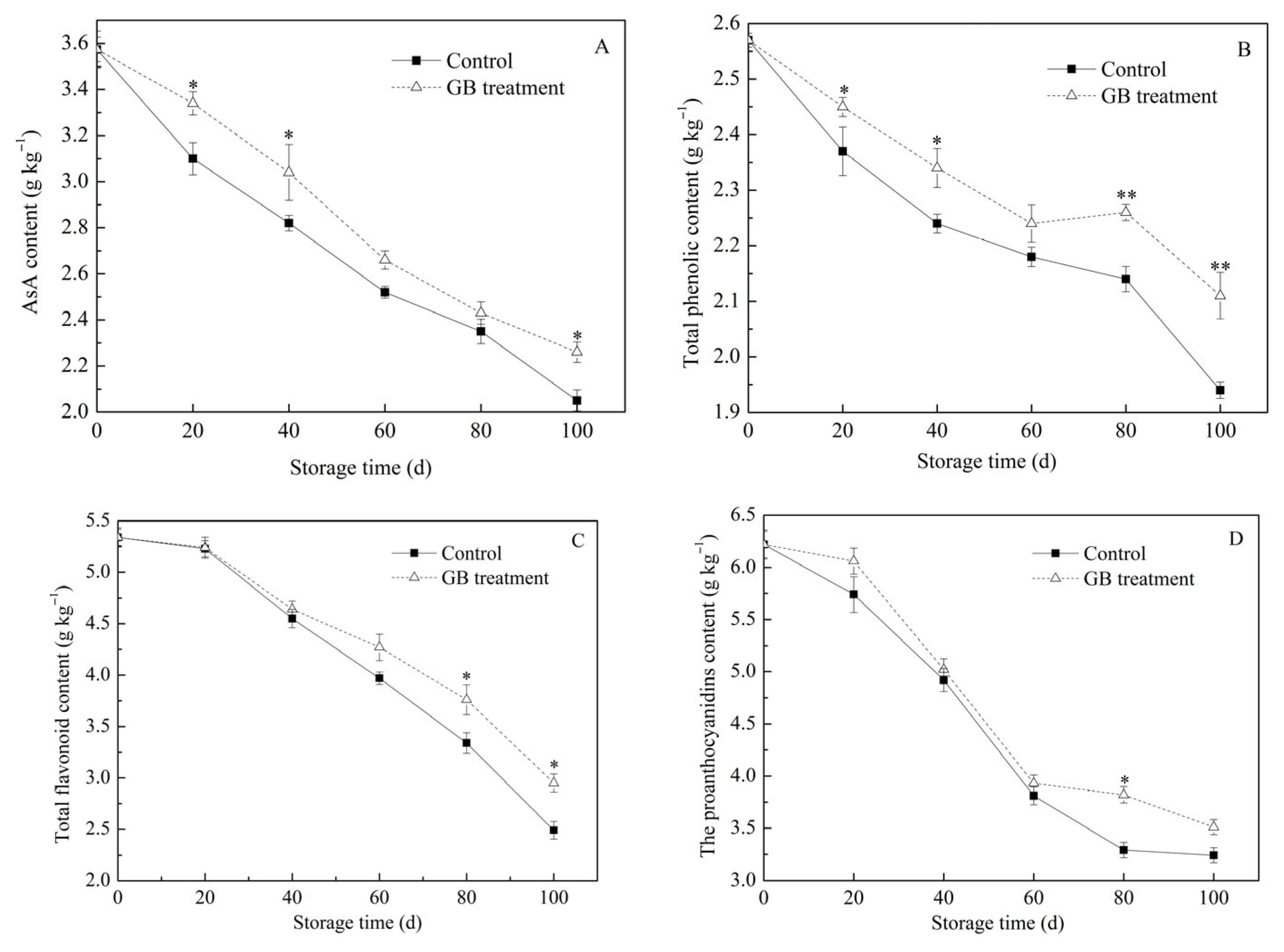
3.4. The Roles of GB Treatment on Jujube Fruit Antioxidants Content
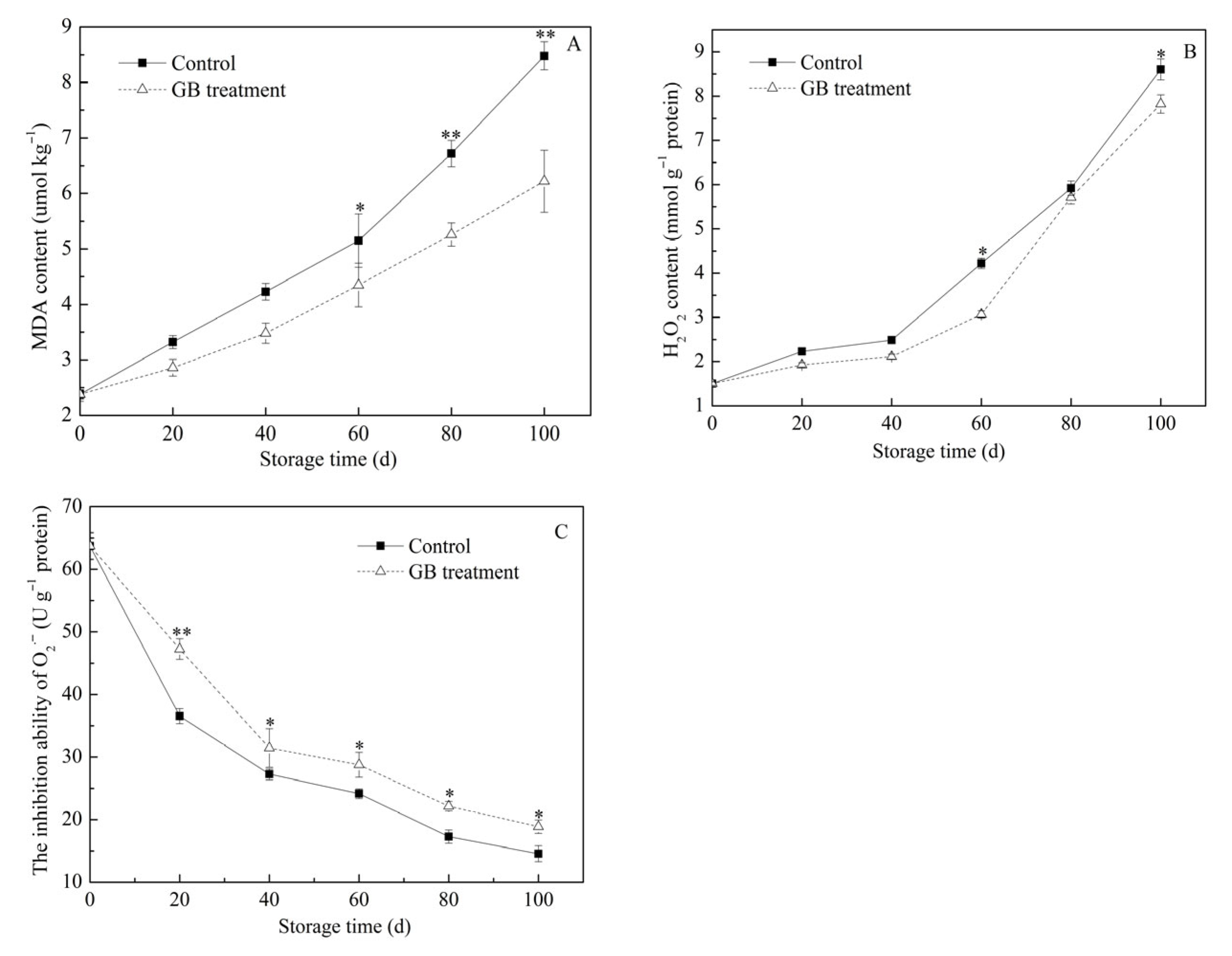
3.5. The Roles of GB Treatment on Jujube Fruit MDA Content, H2O2 Content and the Inhibition Ability of O2−
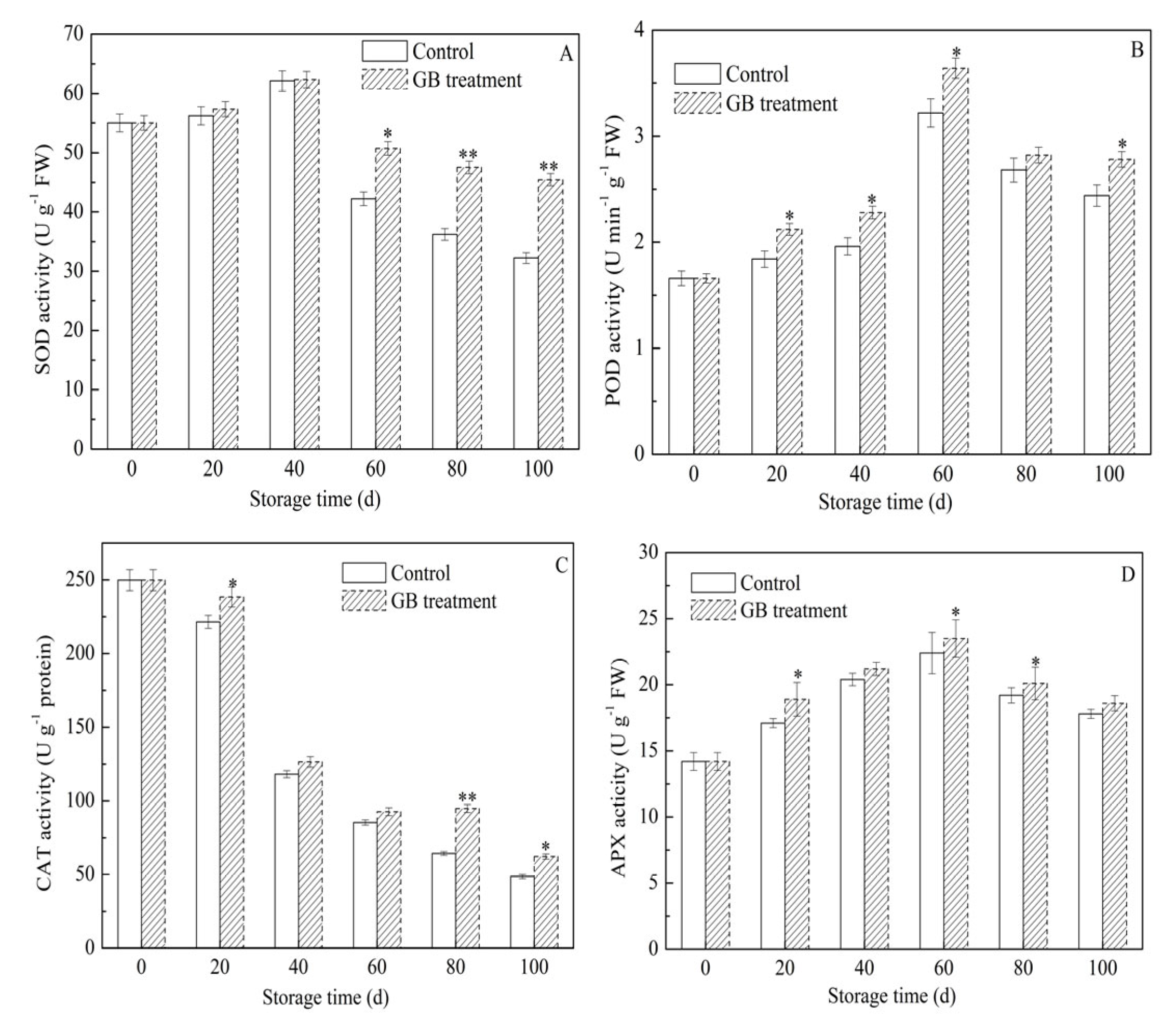
3.6. The Roles of GB Treatment on Jujube Fruit Antioxidant Enzyme Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Song, Y.Q.; Li, J.; Cheng, Y.Y.; Xue, X.F.; Li, L.L. Development of the cuticular membrane and biomechanical properties in Hupingzao (Ziziphus jujuba Mill. ‘Hupingzao’). Sci. Hortic. 2018, 229, 25–32. [Google Scholar] [CrossRef]
- Feng, Z.H.; Gao, Z.F.; Jiao, X.; Shi, J.F.; Wang, R.F. Widely targeted metabolomic analysis of active compounds at different maturity stages of ‘Hupingzao’ jujube. J. Food Compos. Anal. 2020, 88, 103417. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Chen, C.; Zhang, S.; Zhao, X.; Wu, C.; Kou, X.; Xue, Z. Glycine betaine inhibits postharvest softening and quality decline of winter jujube fruit by regulating energy and antioxidant metabolism. Food Chem. 2023, 410, 135445. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Duan, J.-A.; Qian, D.; Tang, Y.; Wu, D.; Su, S.; Wang, H.; Zhao, Y. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chem. 2015, 167, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wang, P.J.; Yan, Z.W.; Ren, Y.S.; Dong, K.H.; Song, Z.; Zhang, J.; Zhang, C.X. Growth performance, nutrient digestibility, carcass traits, body composition, and meat quality of goat fed Chinese jujube (Ziziphus jujuba Mill.) fruit as a replacement for maize in diet. Anim. Feed Sci. Technol. 2018, 246, 127–136. [Google Scholar] [CrossRef]
- Shi, F.; He, P.; Li, Z.G.; Wei, W.; Meng, H.F.; Wang, D.X.; Wang, Y. Effect of cold water and cold electrolyzed functional water treatments on the postharvest quality of cold stored jujube fruit (Ziziphus jujuba Mill. “Hupingzao”). J. Food Process. Preserv. 2022, 46, e16580. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, S.; Dong, Y.; Zhi, H.H.; Zong, W. Tea polyphenols incorporated into alginate-based edible coating for quality maintenance of Chinese winter jujube under ambient temperature. LWT Food Sci. Technol. 2016, 70, 155–161. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhu, X.; Hou, Y.Y.; Wang, X.Y.; Li, X.H. Effects of nitricoxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. cv. Dongzao) fruit during storage. Postharvest Bio. Technol. 2019, 156, 110954. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Park, E.J.; Jeknic, Z.; Chen, T.H. Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef]
- Shan, T.; Jin, P.; Zhang, Y.; Huang, Y.; Wang, X.; Zheng, Y. Exogenous glycine betaine treatment enhances chilling tolerance of peach fruit during cold storage. Postharvest Biol. Technol. 2016, 114, 104–110. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, T.; Zuo, J.; Gao, L.; Fan, L. Amelioration of postharvest chilling injury in sweet pepper by glycine betaine. Postharvest Biol. Technol. 2016, 112, 114–120. [Google Scholar] [CrossRef]
- Wang, L.; Shan, T.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019, 272, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Sun, Y.; Ge, W.; Wei, B.; Cheng, S.; Ji, S. Exogenous glycine betaine treatment alleviates low temperature-induced pericarp browning of ‘Nanguo’ pears by regulating antioxidant enzymes and proline metabolism. Food Chem. 2020, 306, 125626. [Google Scholar] [CrossRef]
- Chen, L.L.; Shan, W.; Cai, D.L.; Chen, J.Y.; Lu, W.J.; Su, X.G.; Kuang, J.F. Postharvest application of glycine betaine ameliorates chilling injury in cold-stored banana fruit by enhancing antioxidant system. Sci. Hortic. 2021, 287, 110264. [Google Scholar] [CrossRef]
- Habibi, F.; Guillen, F.; Serrano, M.; Valero, D. Postharvest treatment with glycine betaine enhances chilling tolerance of blood orange fruit by increasing antioxidant defence systems and osmoregulation during cold storage. Sci. Hortic. 2022, 305, 111352. [Google Scholar] [CrossRef]
- Silva, W.B.; Silva, G.M.C.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, Y.; Tang, Y.; Yang, W.; Guo, M.; Chen, G. Transcriptome sequencing reveals mechanism of improved antioxidant capacity and maintained postharvest quality of winter jujube during cold storage after salicylic acid treatment. Postharvest Biol. Technol. 2022, 189, 111929. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, W.; Wang, X.; Cao, J.; Jiang, W. Polyphenol composition and antioxidant capacity in pulp and peel of apricot fruits of various varieties and maturity stages at harvest. Int. J. Food Sci. Technol. 2018, 53, 327–336. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, W.; Liu, Y.; Sang, Y.; Yang, W.; Guo, M.; Cheng, S.; Chen, G. Effect of composite coating treatment and low-temperature storage on the quality and antioxidant capacity of Chinese jujube (Zizyphus jujuba cv. Junzao). Sci. Hortic. 2021, 288, 110372. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Li, N.; Lin, H.T.; Lin, M.S.; Chen, Y.H.; Wang, H.; Ritenour, M.; Lin, Y.F. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chem. 2020, 306, 125627. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Hou, Y.; Pan, Y.; Shi, L.; Li, X. Effects of harvest maturity stage on postharvest quality of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage. Sci. Hortic. 2021, 277, 109778. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Wei, M.; Ge, Y.; Tang, Q.; Xue, W.; Zhang, S.; Wang, W.; Lv, J. Effects of trisodium phosphate treatment after harvest on storage quality and sucrose metabolism in jujube fruit. J. Sci. Food Agric. 2019, 99, 5526–5532. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Hou, J.B.; Zhang, L.; Jiang, C.N.; Ge, Y.H. Effect of exogenous glycine betaine treatment on storage quality of “Nanguo pears”. Packag. Food Mach. 2021, 39, 31–37. [Google Scholar] [CrossRef]
- Chu, Y.; Wei, S.; Ding, Z.; Mei, J.; Xie, J. Application of ultrasound and curing agent during osmotic dehydration to improve the quality properties of freeze-dried yellow peach (Amygdalus persica) slices. Agriculture 2021, 11, 1069. [Google Scholar] [CrossRef]
- Kou, X.H.; He, Y.; Li, Y.; Chen, X.; Feng, Y.; Xue, Z. Effect of abscisicacid (ABA) and chitosan/nano-silica/sodium alginate composite film on the color development and quality of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao). Food Chem. 2019, 270, 385–394. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhu, X.; Hou, Y.Y.; Wang, X.Y.; Li, X.H. Postharvest nitric oxide treatment delays the senescence of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage by regulating reactive oxygen species metabolism. Sci. Hortic. 2020, 261, 109009. [Google Scholar] [CrossRef]
- Jiang, X.J.; Lin, H.T.; Shi, J.; Neethirajan, S.; Lin, Y.F.; Chen, Y.; Wang, H.; Lin, Y.X. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 2018, 252, 134–141. [Google Scholar] [CrossRef]
- Wang, L.; Bokhary, S.U.F.; Xie, B.; Hu, S.; Jin, P.; Zheng, Y. Biochemical and molecular effects of glycine betaine treatment on membrane fatty acid metabolism in cold stored peaches. Postharvest Biol. Technol. 2019, 154, 58–69. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, X.M.; Guo, Y.X.; Yao, S.X.; Zeng, K.F. Effects of methionine treatment on storage quality and antioxidant activity of postharvest jujube fruit. J. Integr. Agric. 2023, 22, 2893–2904. [Google Scholar] [CrossRef]
- Hernández, F.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A.; Legua, P. Physico-chemical, nutritional, and volatile composition and sensory profile of Spanish jujube (Ziziphus jujuba Mill.) fruits. J. Sci. Food Agric. 2015, 96, 2682–2691. [Google Scholar] [CrossRef]
- Zhang, W.; Kang, J.; Yang, W.; Guo, H.; Guo, M.; Chen, G. Incorporation of 1-methylcyclopropene and salicylic acid improves quality and shelf life of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) through regulating reactive oxygen species metabolism. Front. Nutr. 2022, 9, 940494. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Ramezanian, A.; Guillen, F.; Serrano, M.; Valero, D. Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with gamma-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 2020, 306, 125634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, H.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. Multiple 1-MCP treatment more effectively alleviated postharvest nectarine chilling injury than conventional one-time 1-MCP treatment by regulating ROS and energy metabolism. Food Chem. 2020, 330, 127256. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guill’en, F.; Serrano, M.; Valero, D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Habibi, F.; Valero, D.; Serrano, M.; Guill’en, F. Exogenous application of glycine betaine maintains bioactive compounds, antioxidant activity and physicochemical attributes of blood orange fruit during prolonged cold storage. Front. Nutr. 2022, 9, 873915. [Google Scholar] [CrossRef]
- Tang, J.; Chen, H.; Lin, H.; Hung, Y.-C.; Xie, H.; Chen, Y. Acidic electrolyzed water treatment delayed fruit disease development of harvested longans through inducing the disease resistance and maintaining the ROS metabolism systems. Postharvest Biol. Technol. 2021, 171, 111349. [Google Scholar] [CrossRef]
- Yang, W.; Kang, J.; Liu, Y.; Guo, M.; Chen, G. Effect of salicylic acid treatment on antioxidant capacity and endogenous hormones in winter jujube during shelf life. Food Chem. 2022, 397, 133788. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, T.; Farooq, S.U.; Jin, P.; Zheng, Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, F.; Wu, J.; Geng, Z.; Xing, Y.; Zhang, Y.; Li, Z.; Wang, T.; Wang, Y. Glycine Betaine Treatment Maintains Postharvest Quality of Hupingzao Jujube Fruit by Enhancing the Antioxidant System. Foods 2025, 14, 3385. https://doi.org/10.3390/foods14193385
Shi F, Wu J, Geng Z, Xing Y, Zhang Y, Li Z, Wang T, Wang Y. Glycine Betaine Treatment Maintains Postharvest Quality of Hupingzao Jujube Fruit by Enhancing the Antioxidant System. Foods. 2025; 14(19):3385. https://doi.org/10.3390/foods14193385
Chicago/Turabian StyleShi, Fei, Jinbin Wu, Zifan Geng, Yuqing Xing, Yulei Zhang, Zhigang Li, Tengfei Wang, and Yu Wang. 2025. "Glycine Betaine Treatment Maintains Postharvest Quality of Hupingzao Jujube Fruit by Enhancing the Antioxidant System" Foods 14, no. 19: 3385. https://doi.org/10.3390/foods14193385
APA StyleShi, F., Wu, J., Geng, Z., Xing, Y., Zhang, Y., Li, Z., Wang, T., & Wang, Y. (2025). Glycine Betaine Treatment Maintains Postharvest Quality of Hupingzao Jujube Fruit by Enhancing the Antioxidant System. Foods, 14(19), 3385. https://doi.org/10.3390/foods14193385





